Abstract
The molecular understanding of diseases has been accelerated in recent years, producing many new potential therapeutic targets. A noninvasive delivery system that can target specific anatomical sites would be a great boost for many therapies, particularly those based on manipulation of gene expression. The use of microbubbles controlled by ultrasound as a method for delivery of drugs or genes to specific tissues is promising. It has been shown by our group and others that ultrasound increases cell membrane permeability and enhances uptake of drugs and genes. One of the important mechanisms is that microbubbles act to focus ultrasound energy by lowering the threshold for ultrasound bioeffects. Therefore, clear understanding of the bioeffects and mechanisms underlying the membrane permeability in the presence of microbubbles and ultrasound is of paramount importance. (Neth Heart J 2009;17:82-6.)
Keywords: ultrasound, microbubbles, cell membrane permeability, bioeffects, local therapy
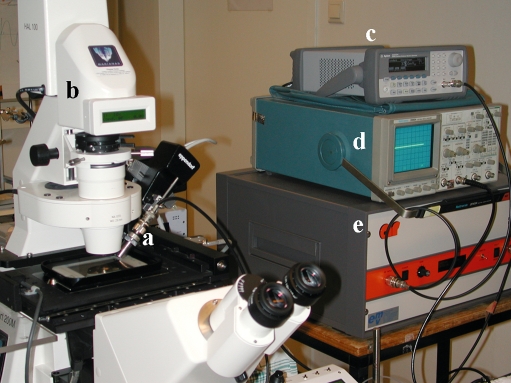
In the last few years, many new therapeutic targets have emerged as a consequence of the continuously growing understanding of the molecular basics of diseases. Conventional administration of drugs, such as injection and oral medications, are often not applicable for proteins, silencing RNAs, DNA and other biotherapeutics.1 Therapeutic systems need to be improved to increase efficacy and safety by targeting specific cells or organs, in order to minimise possible side effects. Ultrasound in combination with contrast agents, i.e. microbubbles, is a promising technique for delivery of therapeutic compounds.2 Microbubbles are encapsulated gasfilled bubbles (1-10 μm in diameter), and originally designed to improve conventional ultrasoundscanning. When subjected to ultrasound, microbubbles start oscillating at the frequency of the ultrasound, under influence of positive and negative pressure differences in the ultrasonic wave.3 Recent discoveries have opened up promising emerging applications. Due to their acoustic behaviour microbubbles cause increased permeability of surrounding cells. This opens a window for ultrasound-targeted local delivery and enhanced cellular uptake of therapeutic compounds.4 However, it is still unclear exactly how cells that are subjected to ultrasound and microbubbles internalise therapeutic compounds, and which cellular responses ultrasound and microbubbles evoke. To get more insight into these mechanisms we studied the biological effects of ultrasound and microbubbles at the cellular level. By mounting an ultrasound transducer on a live-cell fluorescence microscope (figure 1), we were able to look in detail into cells and record their responses during exposure to ultrasound and microbubbles.
Figure 1.
Experimental set-up. Ultrasound transducer (a) is mounted on the live-cell fluorescence microscope (b) to study the effects of ultrasound-exposed microbubbles in detail at the cellular level. The transducer is connected to an arbitrary wave-form generator (c) and a linear 60-dB power amplifier (e). The ultrasound signal was monitored by a synchronised oscilloscope (d).
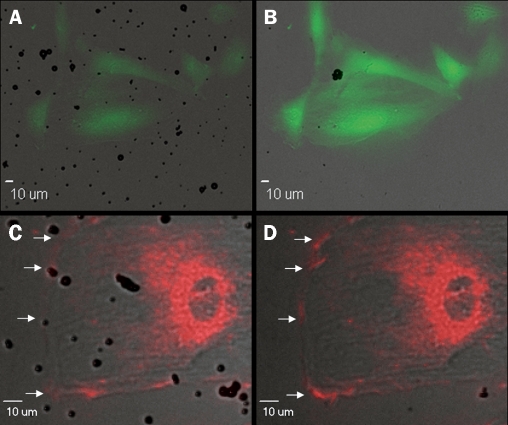
Several studies suggest that ultrasound and microbubbles induce formation of transient pores in cell membrane, termed sonoporation. Sonoporation is proposed to be the mechanism by which ultrasound-exposed microbubbles lead to increased permeability of the cell membrane for extracellular molecules.5-8We demonstrated the occurrence of sonoporation by the influx of calcium ions in cardiomyoblast cells (figures 2A and B).9 Although the size of ions is not in proportion to the size of drugs or genes, it did demonstrate formation of transient pores, as well as rapid resealing of the cell membrane. Furthermore, we found that ultrasound and microbubbles cause an increase in intracellular levels of hydrogen peroxide (H2O2).
Figure 2.
Calcium influx and hyperpolarisation. Fluorescent images from a time-lapse recording. (A, B) Cells loaded with Fluo4, a green fluorescent probe sensitive for free cytosolic calcium. (C, D) Cell loaded with Di-4-ANEPPS, a red fluorescent probe sensitive for changes in membrane potential. An increase in fluorescence corresponds to hyperpolarisation of the cell membrane (indicated by arrows). (A, C) Levels of fluorescence before ultrasound is switched on. (B, D) Increased levels of fluorescence during ultrasound exposure.
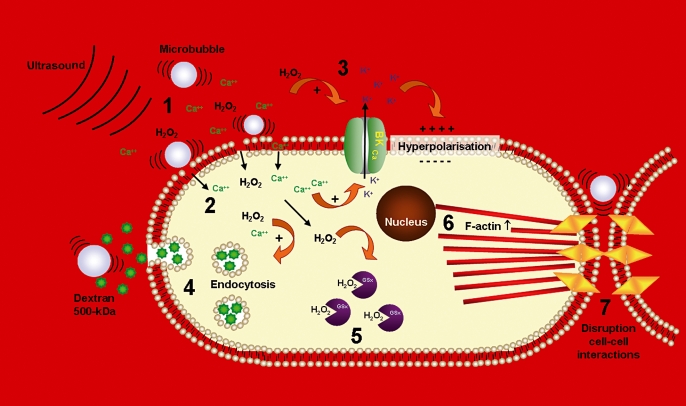
When scavenging H2O2 with catalase, we found that the increased levels of H2O2 were partially responsible for the influx of calcium ions. A schematic overview of all of the unravelled bioeffects is shown in figure 3.
Figure 3.
Schematic overview of unravelled bioeffects and mechanisms. Ultrasound and microbubbles induced generation of H2O2 (1). There was a causal relationship between H2O2 and the formation of transient pores in the cell membrane with a concomitant calcium influx (2). The calcium ions activated the large-conductance potassium channels, thereby causing local hyperpolarisation of the cell membrane (3). Besides formation of transient pores, ultrasound and microbubbles induced uptake of macromolecules (dextran 500 kDa) via endocytosis (4). Ultrasound and microbubbles further affected ROS homeostasis, and caused a decrease in total gluthation (GSx) levels (5). Other unravelled effects of ultrasound and microbubbles were rearrangement and increased number of F-actin stress cables (6), and disruption of cell-cell interactions (7).
It can be imagined that a sudden influx of calcium ions is likely to have consequences for intracellular calcium homeostasis, as calcium ions are important second messengers in numerous cell-signalling pathways. For example, one of these consequences may be the occurrence of premature ventricular contractions (PVCs). It has been reported that patients, as well as rats, undergoing contrast-enhanced echocardiography may suffer from PVCs.10-12 We hypothesised that the influx of calcium ions may cause depolarisation and a subsequent calcium-induced calcium release from the sarcoplasmic reticulum, thereby evoking a PVC. In contrast with our hypothesis, we found the cell membrane to hyperpolarise (figures 2C and D).13 This hyperpolarisation was clearly a local event, occurring only where a microbubble ‘hits’ the cell during ultrasound exposure. The ultrasound and microbubble-evoked influx of calcium ions activated large conductance, outwardly rectifying potassium channels (BKCa channels). These channels overcompensated the influx of positive calcium ions with an efflux of positive potassium ions, thereby causing a hyperpolarisation of the cell membrane. How may the occurrence of PVCs then be explained? In the literature, a calcium spark, arising locally at the cell membrane, which activates K+ channels in smooth muscle causing the muscle to relax is described.14 But when elementary-release events deeper in the cell are activated to cause a global calcium signal, the muscle contracts.15 In our experimental setting, we applied ultrasound with low acoustic pressure (<0.5 MPa), which does not cause microbubbles to violently collapse. However, in the situation of the reported PVCs during contrast-enhanced echocardiography, these PVCs occurred during ultrasound pulses with high acoustic pressures. These so-called ‘flashes’ cause microbubbles to violently burst, thereby probably permeabilising not only the endothelium, but also the adjacent cardiomyocytes, resulting in a calcium overload of the cardiomyocytes and subsequent activation of the contractile apparatus.
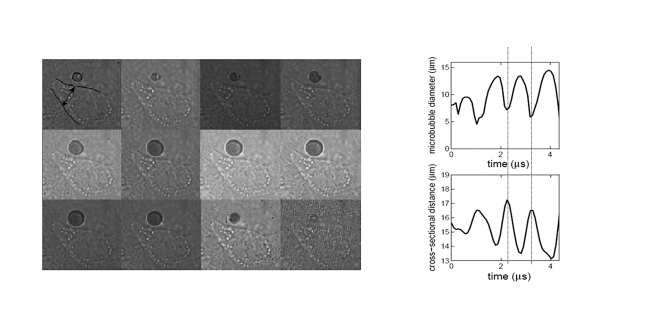
As mentioned before, the general assumption at the moment is that ultrasound and microbubbles induce formation of small pores in the membrane. Using an ultrafast camera, i.e. the Brandaris128, optical recordings with 10 million frames per second demonstrated a single oscillating microbubble causing displacement of the cell membrane (figure 4), and increased permeability of the cell for propidium iodide (PI).16 This small compound (0.7 kDa), which cannot normally enter the cell, entered the cell during ultrasound and microbubble exposure, most likely through transient pores in the membrane as described by van Wamel et al.8 However, we further hypothesised that an additional mechanism, namely endocytosis, is involved in the uptake of especially larger molecules. To study this, we chose fluorescent dextrans as a model for drug delivery. The molecular weight of the dextrans ranged from 4.4 to 500-kDa, covering approximately the whole range of therapeutic compounds; pharmaceutical drugs are generally smaller than 4.4-kDa, proteins may range between 4 and 500-kDa, and plasmid DNA often exceeds 500-kDa. The first indication of uptake via endocytosis was shown by a difference in cellular distribution of the small and large dextrans. The 4.4-kDa dextran showed a homogeneous distribution throughout the cytosol, indicating uptake via transient pores in the cell membrane.17 The 500-kDa, in contrast, showed a clear localisation in vesicle-like structures, indicating that it might be taken up via endocytosis.18 The role of endocytosis in ultrasound and microbubble-enhanced uptake was further confirmed by repeating the dextran-delivery experiments with cells deprived from ATP, and in the presence of inhibitors of the three main routes of endocytosis, i.e. clathrin- and caveolin-mediated endocytosis and macropinocytosis.
Figure 4.
Deformation of the cell membrane by an insonified microbubble. A sequence of 12 images (a selection out of a 128-image sequence) is shown, in which the first cycle of 1 MHz ultrasound applied on a Sonovue™ microbubble close to an endothelial cell is observed. The interaction between the oscillating microbubble and the cell results in cell deformation. In the right panel the deformation in relation to the oscillating microbubble is depicted. Under the rarefaction effect of ultrasound, the microbubble expands to a maximum of 15 μm, whereby it presses against the cell. This maximal expansion is translated to a displacement of the cell membrane up to 2.3 μm (~15%). The compression of the microbubble is also translated into a displacement of the cell membrane. Thus, microbubble vibrations are translated into pushing and pulling of the cell membrane.
Furthermore, co-localisation of 500-kDa dextran with clathrin, a marker for clathrin-mediated endocytosis, was demonstrated. All these experiments confirmed the role of endocytosis. The contribution of either endocytosis or pore formation to the uptake of extracellular molecules could not be expressed in absolute numbers, but is dependent on the size of the molecule. The smaller the molecule, the greater the contribution of pore formation, and the larger the molecule, the greater the contribution of endocytosis, especially when the size of the molecule exceeds the size of the transient pores.19 This should be taken into account when designing new effective therapies using ultrasound and microbubble-targeted delivery. The compartmentalisation of therapeutic compounds, and most importantly genes, may affect therapy efficiency and should be taken into consideration when measuring drug action following ultrasound and microbubble-targeted delivery.
In addition to ultrasound and microbubble-targeted delivery of dextrans, experiments were also performed to deliver plasmid DNA to endothelial cells. To be precise, fluorescently labelled plasmid DNA encoding for green fluorescent protein (GFP). Using this construct the uptake and localisation of the plasmid could be detected by the fluorescent label, followed by determining transfection efficiency 24 hours after ultrasound and microbubble exposure by measuring GFP expression. We found that also labelled plasmid DNA was localised in vesicle-like structures, comparable with 500-kDa dextran. Furthermore, approximately 50% of the cells exposed to ultrasound and microbubbles internalised fluorescent plasmids, but only as few as 2% actually showed expression of GFP. This result is no longer surprising in the light of the above-described results. Plasmid DNA is a large molecule, most likely to be taken up via endocytosis. This means that DNA molecules need to escape from the endosomes to be able to reach the nucleus for transcription.20
Summary
Ultrasound and microbubble-targeted delivery provides opportunities for new therapies due to its low toxicity, low immunogenicity, noninvasive nature, local application and its cost-effectiveness. The bioeffects found in our studies provide important new insights into the mechanisms of ultrasound and microbubble-targeted delivery of therapeutic compounds and will lead to the rational design of new drug or gene therapies involving ultrasound and microbubbles.
References
- 1.Schlicher RK, Radhakrishna H, Tolentino TP, Apkarian RP, Zarnitsyn V, et al. Mechanism of intracellular delivery by acoustic cavitation. Ultrasound Med Biol 2006;32:915-24. [DOI] [PubMed] [Google Scholar]
- 2.Mayer CR, Bekeredjian R. Ultrasonic gene and drug delivery to the cardiovascular system. Adv Drug Deliv Rev 2008;60:1177-92. [DOI] [PubMed] [Google Scholar]
- 3.Dijkmans PA, Juffermans LJM, Musters RJP, van Wamel A, ten Cate F, van Gilst W, et al. Microbubbles and ultrasound: from diagnosis to therapy. Eur J Echocardiography 2004;5:245-56. [DOI] [PubMed] [Google Scholar]
- 4.Bekeredjian R, Chen S, Grayburn PA, Shohet RV. Augmentation of cardiac protein delivery using ultrasound targeted microbubble destruction. Ultrasound Med Biol 2005;31:687-91. [DOI] [PubMed] [Google Scholar]
- 5.Tachibana K, Uchida T, Ogawa K, Yamashita N, Tamura K. Induction of cell-membrane porosity by ultrasound. Lancet 1999;353:1409. [DOI] [PubMed] [Google Scholar]
- 6.Miller DL, Pislaru SV, Greenleaf JE. Sonoporation: mechanical DNA delivery by ultrasonic cavitation. Somat Cell Mol Genet 2002;27:115-34. [DOI] [PubMed] [Google Scholar]
- 7.Ohl CD, Arora M, Ikink R, de Jong N, Versluis M, Delius M, et al. Sonoporation from jetting cavitation bubbles. Biophys J 2006;91:4285-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Wamel A, Kooiman K, Harteveld M, Emmer M, Ten Cate FJ, Versluis M, et al. Vibrating microbubbles poking individual cells: Drug transfer into cells via sonoporation. J Control Release 2006;112:149-55. [DOI] [PubMed] [Google Scholar]
- 9.Juffermans LJ, Dijkmans PA, Musters RJ, Visser CA, Kamp O. Transient permeabilization of cell membranes by ultrasound-exposed microbubbles is related to formation of hydrogen peroxide. Am J Physiol Heart Circ Physiol 2006;291:H1595-601. [DOI] [PubMed] [Google Scholar]
- 10.van der Wouw PA, Brauns AC, Bailey SE, Powers JE, Wilde AA. Premature ventricular contractions during triggered imaging with ultrasound contrast. J Am Soc Echocardiogr 2000;13:288-94. [DOI] [PubMed] [Google Scholar]
- 11.Li P, Armstrong WF, Miller DL. Impact of myocardial contrast echocardiography on vascular permeability: comparison of three different contrast agents. Ultrasound Med Biol 2004;30:83-91. [DOI] [PubMed] [Google Scholar]
- 12.Dijkmans PA, van Dijk J, Juffermans LJM, Musters RJP, Spreeuwenberg, Visser CA, et al. Safety and feasibility of real-time adenosine myocardial contrast echocardiography with emphasis on induction of arrhythmias: a study in healthy volunteers and patients with stable coronary artery disease. Echocardiography 2009. In press. [DOI] [PubMed] [Google Scholar]
- 13.Juffermans LJ, Kamp O, Dijkmans PA, Visser CA, Musters RJ. Lowintensity ultrasound-exposed microbubbles provoke local hyperpolarization of the cell membrane via activation of BK(Ca) channels. Ultrasound Med Biol 2008;34:502-8. [DOI] [PubMed] [Google Scholar]
- 14.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, et al. Relaxation of arterial smooth muscle by calcium sparks. Science 1995;270:633-7. [DOI] [PubMed] [Google Scholar]
- 15.Berridge MJ, Bootman MD, Lipp P. Calcium–a life and death signal. Nature 1998;395:645-8. [DOI] [PubMed] [Google Scholar]
- 16.van Wamel A, Bouakaz A, Versluis M, de Jong N. Micromanipulation of endothelial cells: ultrasound-microbubbles-cell interaction. Ultrasound Med Biol 2004;30:1255-8. [DOI] [PubMed] [Google Scholar]
- 17.Seksek O, Biwersi J, Verkman AS. Translational diffusion of macromoleculesized solutes in cytoplasm and nucleus. J Cell Biol 1997;138:131-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller DL, Quddus J. Sonoporation of monolayer cells by diagnostic ultrasound activation of contrast-agent gas bodies. Ultrasound Med Biol 2003;26:661-7. [DOI] [PubMed] [Google Scholar]
- 19.Meijering BDM, Juffermans LJM, van Wamel A, Henning RH, Zuhorn IS, Emmer M, et al. Ultrasound and microbubble-targeted delivery of macromolecules is regulated by induction of endocytosis and pore formation. Revision submitted 2008. [DOI] [PubMed] [Google Scholar]
- 20.Meijering BDM, Henning RH, Deelman LE. Ultrasound and microbubble mediated gene therapy: effectiveness of siRNA versus plasmid DNA delivery. Submitted 2008. [Google Scholar]