Abstract
Despite pharmacological and immunohistochemical evidence for GABA as a neurotransmitter in the olivocochlear efferent bundle, a clear functional role of GABA in the inner ear has not emerged. To explore the role of metabotropic GABAB receptors, we characterized the cochlear phenotype of mice with targeted deletion of the GABAB1 subunit and determined its tissue localization using a mouse line expressing a GFP-tagged GABAB1 subunit under the endogenous promoter. Immunostaining revealed GABAB1 expression in both type I and type II ganglion cells and in their synaptic terminals under inner and outer hair cells, respectively. No GABAB1 expression was observed in hair cells. Mean cochlear thresholds, measured via both auditory brainstem responses and distortion product otoacoustic emissions (DPOAEs), were elevated by ∼10 dB in GABAB1-deficient mice, consistent with outer hair cell dysfunction. Olivocochlear efferent function, assessed via DPOAE suppression during efferent electrical stimulation, was unaffected by GABAB1 deletion. GABAB1-deficient mice showed increased resistance to permanent acoustic injury, with mean threshold shifts ∼25 dB smaller than wild-types after exposure to 8–16-kHz noise at 100 dB for 2 h. In contrast, there was no vulnerability difference to temporary acoustic injury following exposure to the same noise at 94 dB for 15 min. Our results suggest that GABAergic signaling in type II afferent neurons may be required for normal outer hair cell amplifier function at low sound levels and may also modulate outer hair cell responses to high-level sound.
Keywords: inner ear, feedback, efferent
Introduction
Hair cells and sensory neurons of the inner ear receive efferent feedback from the olivocochlear (OC) system (Guinan 1996). This feedback circuitry includes two major types of fibers: medial (M)OC fibers targeting predominately the outer hair cells (OHCs), but also making synaptic contact with the OHCs’ afferent innervation (Liberman et al. 1990); and lateral (L)OC fibers targeting mainly the unmyelinated dendrites of cochlear nerve fibers near their afferent synapses with inner hair cells (IHCs), but also synapsing with the IHCs themselves.
The MOC system comprises a sound-evoked negative feedback loop, which when activated, rapidly elevates cochlear thresholds by decreasing the normal contribution of OHC electromotility to the amplification of sound-induced motion of the sensory epithelium (Guinan 1996). Electrically evoked MOC activity also produces a slower, more long-lasting enhancement of cochlear sound-evoked responses (Maison et al. 2007). It is not known if LOC activity is sound-evoked; however, electrical stimulation of LOC system causes either slow enhancement or slow suppression of sound-evoked neural responses from cochlear nerve fibers, depending on which functional subgroup is activated (Groff and Liberman 2003).
In mouse, peripheral terminals of both LOC and MOC neurons colocalize markers for both cholinergic and GABAergic transmission (Maison et al. 2003). However, only the cholinergic effects on OHCs are well understood. Many lines of evidence converge to support the notion that acetylcholine (ACh) release from MOC terminals induces Ca2+ entry through α9/α10 nicotinic ACh receptors expressed by OHCs; this in turn leads to activation of Ca2+-activated K+ channels (Fuchs and Murrow 1992; Elgoyhen et al. 1994, 2001; Oliver et al. 2000) and the fast suppressive effects of MOC activation on cochlear responses (Sridhar et al. 1997).
The effects of GABAergic transmission and patterns of expression of GABA receptor subtypes in the cochlear targets of efferent fibers are much more poorly understood, and particularly little attention has been paid to GABAB receptors and metabotropic GABAergic transmission in the cochlea. One electrophysiological study of isolated cochlear neurons from neonatal mice (Lin et al. 2000) reports a GABA-induced increase in intracellular Ca2+ that is mimicked by the GABAB agonist baclofen and is resistant to blockade by bicuculline (a GABAA receptor blocker). The same report shows by RT-PCR that GABAB receptor expression is maintained at post-natal ages beyond those that could be tested electrophysiologically.
To gain insight into the role of GABAB receptors in the adult cochlea, we investigated cochlear structure and function in a mutant mouse line lacking the GABAB1 subunit, a necessary constituent of functional GABAB receptors (Prosser et al. 2001; Schuler et al. 2001). To study the cellular localization of the GABAB receptors in the adult inner ear, we examined a transgenic mouse line in which a GFP-tagged GABAB1 is expressed under the endogenous promoter in a transgene derived from a Bacterial Artificial Chromosome. We found that GABAB receptors are expressed in all cochlear sensory neurons innervating IHCs and OHCs, but not in the hair cells themselves. Loss of GABAB function leads to a small (∼10 dB) threshold elevation suggestive of OHC dysfunction. GABAB1-null ears are unchanged in their vulnerability to temporary noise-induced threshold shifts; however, the loss of GABAB1 increases their resistance to permanent acoustic injury. Our results suggest a possible role for the unmyelinated sensory fibers innervating OHCs, via their reciprocal synapses with the hair cells (Nadol 1981; Thiers et al. 2002), in coordinating OHC electromotility and its normal contribution to cochlear sensitivity.
Materials and methods
Mutant lines Mutant mice lacking GABAB1 expression were generated by (1) creating one mouse line in BALB/c, in which critical exons (VII and VIII) in the GABAB1 gene were flanked by lox511 sites; and (2) crossing these mice carrying the floxed allele with another BALB/c line carrying the Cre recombinase under control of the CMV promoter (Cre-deleter line). Offspring have been maintained in the BALB/c strain as described elsewhere (Haller et al. 2004). The GABAB1 “reporter” mouse was created via oocyte pronuclear injection of a GABAB1-GFP BAC and backcrossing into GABAB1 knockout mice (Schuler et al. 2001). The BAC expresses a GABAB1 subunit with the GFP fused to its C-terminus. This fusion-protein heterodimerizes with GABAB2 and reconstitutes functional pre- and post-synaptic GABAB receptors in the GABAB1 knockout background (Casanova et al. 2005). For the present study of both mutant lines, heterozygote breeders were obtained, and homozygous mutants and wild-type littermates were identified among the offspring by genotyping.
Animals and groups For physiological studies of the GABAB1 mutant line, a total of 96 wild-type and homozygote mutant animals were studied at 6–8 weeks of age. For assessment of baseline cochlear responses, auditory brainstem responses (ABRs) and distortion product otoacoustic emissions (DPOAEs) were measured from 56 animals: 28 wild-types and 28 homozygous mutants. ABRs were measured from one ear; DPOAEs were measured from both ears, because the DPOAE test is quicker. Subsets of these 56 animals were noise-exposed within a week after initial test, and cochlear responses were re-measured at appropriate post-exposure times: Eight wild-types and nine mutants were used for studies of permanent threshold shifts; seven wild-types and eight mutants were used for studies of temporary threshold shift. For functional assessment of the olivocochlear efferent system, an additional 12 animals of each genotype were studied. For the studies of the effects of strychnine blockade, an additional 16 animals were studied: four wild-types and 12 homozygous mutants. For histological studies of the GABAB1 mutant line, selected animals from the unexposed group (eight knockouts and three wild-types) were killed immediately after initial ABR and DPOAE testing, and both cochleas were extracted: One ear of each was embedded in plastic and serially sectioned for hair cell counts and assessment of histopathology in all structures of the cochlear duct; the other ear was processed as a whole mount for immunohistochemical assessment of the afferent and efferent innervation. For immunohistochemical studies of the GABAB1 reporter mouse, nine ears from nine animals were studied as cochlear whole mounts, with a variety of antibody combinations and compared to two wildype mice without the reporter construct (negative controls). For analysis of the pattern of immunostaining for GABA itself, four ears from three normal CBA/CaJ mice were prepared as cochlear whole mounts.
ABR and DPOAE measurements Mice were anesthetized with xylazine (20 mg/kg i.p.) and ketamine (100 mg/kg i.p.). Needle electrodes were inserted at vertex and pinna, with a ground near the tail. ABRs were evoked with 5-ms tone pips (0.5-ms rise-fall with a cos2 onset envelope delivered at 35/s). The response was amplified, filtered, and averaged in a LabVIEW-driven data-acquisition system. Sound level was raised in 5-dB steps from 10 dB below threshold up to 80 dB SPL. At each sound level, 1,024 responses were averaged (with stimulus polarity alternated), using an “artifact reject,” whereby response waveforms were discarded when peak-to-peak amplitude exceeded 15 μV. On visual inspection of stacked waveforms, “ABR threshold” was defined as the lowest SPL level at which any wave could be detected, usually corresponding to the level step just below that at which the peak-to-peak response amplitude rose significantly above the noise floor (approximately 0.25 μV). For amplitude vs. level functions, the wave I peak was identified by visual inspection at each sound level and the peak-to-peak amplitude computed. DPOAEs at 2f1–f2 were recorded in response to primary tones: f1 and f2 with f2/f1 = 1.2 and f2 level at 10 dB < f1 level. Ear-canal sound pressure was amplified and digitally sampled at 4-μs intervals. Fast Fourier transforms were computed and averaged, and 2f1–f2 DPOAE amplitude and surrounding noise floor were extracted. Iso-response contours were interpolated from plots of amplitude vs. sound level, performed in 5-dB steps of f1 level. “DPOAE Threshold” is defined as the f1 level required to produce a DPOAE of 0 dB SPL. For strychnine experiments only, animals were paralyzed as was done for the medial olivocochlear assay (see below), and amplitude vs. level functions for DPOAEs were repetitively measured before and after the injection.
Medial olivocochlear assay A craniotomy and cerebellar aspiration exposed the floor of the fourth ventricle. Shocks were applied through a pair of silver wires placed at the brainstem midline, at an appropriate rostro-caudal location based on surface landmarks. Shock threshold for facial twitches was determined, then paralysis was induced with α-D-tubocurarine (1.25 mg/kg i.p.), and the animal was connected to a respirator. Shock levels were twice the twitch threshold. During the MOC suppression assay, f2 level was set to produce a DPOAE ∼10–15 dB > noise floor. The primary tones were presented continuously, and DPOAE amplitudes were measured roughly every 5 s, before, during, and after a 70-s period during which a shock train (150-ms monophasic pulses at 300/s) was delivered to the brainstem electrodes. Magnitude of MOC-mediated suppression was defined as the difference in decibel between the mean pre-shock DPOAE amplitude and the mean of the amplitudes obtained during the first three during-shock measures.
Acoustic overexposure Animals were exposed free-field, awake and unrestrained, in a small reverberant chamber. Acoustic trauma consisted of a 2-h exposure to an 8–16-kHz octave band noise presented at 100 dB SPL (for permanent injury) or a 15-min exposure to the same noise at 94 dB SPL (for temporary injury). The exposure stimulus was generated by a custom white-noise source, filtered (Brickwall Filter with a 60 dB/octave slope), amplified (Crown power amplifier), and delivered (JBL compression driver) through an exponential horn fitted securely to a hole in the top of a reverberant box. Sound exposure levels were measured at four positions within each cage using a 0.25″ Bruel and Kjaer condenser microphone: Sound pressure was found to vary by <0.5 dB across these measurement positions.
Histological analysis Cochlear histopathology in the GABAB1 mutants was assessed via serial sections of osmium-stained plastic sections. Tissue was fixed by intracardial perfusion with 0.01 M phosphate-buffered saline (PBS) followed by 2.5% glutaraldehyde with 1.25% paraformaldehyde. Cochleas were post-fixed at 4°C overnight then decalcified in 120 mM EDTA for 3–4 days. Ears were then osmicated for 1 h in 1% OsO4, dehydrated in ethanols, embedded in Araldite, and sectioned at 40 μm. All sections through each ear were evaluated with DIC optics. Hair cells in all sections were examined with a high-NA ×100 objective and locations of missing cells noted. All structures of the cochlear duct and modiolus, including the organ of Corti, stria, limbus, spiral ligament, and spiral ganglion, were examined with a ×2x objective, and the presence of any histopathology was noted.
Cochlear immunohistochemistry After intracardial perfusion with buffered 4% paraformaldehyde (or 4% paraformaldehyde with 0.2% glutaraldehyde for the anti-GABA antibody) followed by intralabyrinthine perfusion with the same fixative, cochleas were decalcified as described above. For cochlear whole mounts, the organ of Corti was dissected into six or seven segments, cryoprotected in 30% sucrose in PBS for 15 min, permeabilized by freezing (in sucrose) on dry ice, then allowed to thaw and rinse in PBS. For frozen sections, decalcified cochleas were cryoprotected overnight in 20% sucrose in PBS, then incubated in 1:1 OCT/40% sucrose for 6 h, transferred to pure OCT, and put on a shaker overnight. Cochleas were positioned in TissueTek molds, frozen on dry ice, and sectioned on a cryostat. Labeling of sectioned material was performed in a humidified box; labeling of whole mounts was performed on a shaker. In either case, tissue was first blocked for 1 h at room temperature in 5% normal horse serum with 0.3% Triton X-100 for additional permeabilization. Tissue was then incubated overnight at room temperature in the primary antibodies diluted in 1% normal horse serum. Primary antibodies included rabbit anti-GFP (1:10,000, Molecular Probes), mouse anti-NF200 (1:50,000, ICN Biomedicals, #69705), mouse anti-peripherin (1:500, Chemicon, #MAB1527), mouse anti-CtBP2 (BD TransductionLabs, # 612044), rabbit anti-VAT (1:1,000, Sigma, #V5387), sheep anti-GAD (1:10,000, Oertel, lot# 1440-4), and mouse anti-GABA (1:1,000, Holstein et al. 2004a). Two 1-h secondary incubations followed, both at room temperature: The first was a cocktail of biotin-conjugated donkey anti-mouse (1:200, Jackson ImmunoResearch, #715-065-150) plus species-appropriate fluorescent-conjugated secondary (AlexaFluor 488, Invitrogen, 1:1,000), and the second was a mix of streptavidin-conjugated fluorophore (AlexaFluor 568, Invitrogen, 1:1,000) plus an AlexaFluor 488-conjugate complementary to that used in the previous step (1:1,000). Whole mounts were then wet-mounted in Vectashield (Vector Labs) and coverslipped; sectioned material was also coverslipped with Vectashield.
Immunohistochemistry analysis Each sensory epithelium to be studied as a cochlear whole mount was dissected into five or six pieces. Care was taken to insure that the entire epithelium was retrieved. Prior to the confocal session, each piece was photographed at low power, and the images were ported to ImageJ, where a custom plug-in1 was used to measure the length of the organ of Corti in each piece, compute the total cochlear length, and then superimpose the computed locations of seven log-spaced cochlear frequencies from 5.6 to 45.2 kHz (based on the published cochlear frequency map for the mouse (Muller et al. 2005). Using this cochlear “roadmap”, confocal z-stacks were then typically obtained at four to five frequency regions along the cochlear spiral using a high-NA immersion objective in the Leica confocal.
Results
Cellular localization of GABAB1 subunits and GABA in the mouse cochlea
To determine the cell types and cochlear regions expressing the GABAB1 gene, we studied a transgenic mouse line in which a GFP fusion protein is coupled to the functional GABAB receptors (Casanova et al. 2005). Cochlear function in these transgenics was indistinguishable from wild-type animals using both DPOAE and ABR measures (data not shown). We double-immunostained cochlear whole mounts and sections with (1) anti-GFP, to detect GFP-tagged GABAB1 subunits (GABAB1-GFP), and (2) markers of afferent and efferent neurons in the inner ear. In total, ten cochleas from ten transgenic animals and three control ears from three wild-type littermates were examined. In each ear, high-power confocal z-stacks were obtained from four evenly spaced positions spanning the cochlear spiral.
We found GABAB1 expression only in the cell bodies and unmyelinated peripheral terminals of cochlear afferent fibers, including both type I neurons contacting IHCs and type II neurons contacting OHCs. All neurons of both types appeared to be positive for the GABAB1-GFP, and there was no obvious gradient in expression level along the cochlear spiral. No GABAB1-GFP signal was seen in cochlear hair cells in the transgenic animals, and no GFP signal was seen anywhere in the wild-type control littermates.
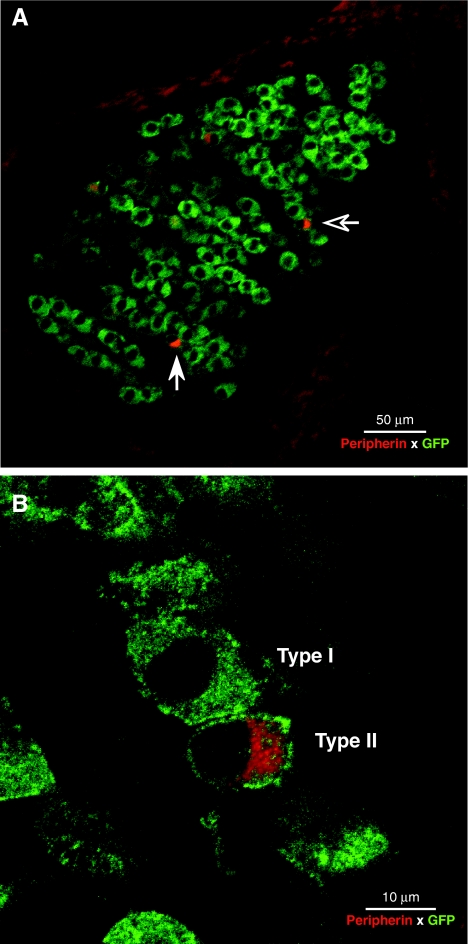
Analysis of immunostained cochlear sections showed that cell bodies of cochlear sensory neurons were immunopositive for GFP (Fig. 1); staining of the axonal processes was weaker than that in the somata. Using peripherin as a selective marker for the type II neurons (Hafidi, 1998), which comprise only 5–10% of the cochlear nerve (Spoendlin 1972), we saw that GABAB1-GFP signal was present in both major classes of cochlear afferents (Fig. 1B).
FIG. 1.
GABAB1 receptors are expressed in type I and type II ganglion cells. In these double-immunostained sections, anti-GFP (green) is used to enhance the endogenous GFP signal in the GABAB1-GFP reporter mouse; anti-peripherin (red) is used to identify type II ganglion cells. A Cross-section through the spiral ganglion from the lower apical turn showing type I and type II neurons (arrows). B High-power view of the type II cell shown by the unfilled arrow in A.
Analysis of staining patterns in whole mounts of the organ of Corti yielded the same conclusions. The expression of GABAB1 receptors within the peripheral unmyelinated terminals of type I and type II afferent fibers is shown in Figure 2. Since both the IHC and OHC areas of the mammalian organ of Corti also receive a rich efferent innervation from the olivocochlear (OC) bundle (Guinan 1996), we confirmed the afferent nature of the GABAB1-positive terminals in two ways: (1) immunostaining for CtBP2, a component of synaptic ribbons, which sit within the hair cells directly opposite the afferent synapses (Khimich et al. 2005), and (2) immunostaining for the GABA-synthetic enzyme glutamic acid decarboxylase (GAD; Fig. 2C), which, in mouse, labels almost all OC efferent terminals in both OHC and IHC areas (Maison et al. 2003). OC terminals also colocalize cholinergic markers such as vesicular acetylcholine transporter (VAT); thus, both GAD- and VAT-positive terminals disappear after the OC bundle is cut (Maison et al. 2003).
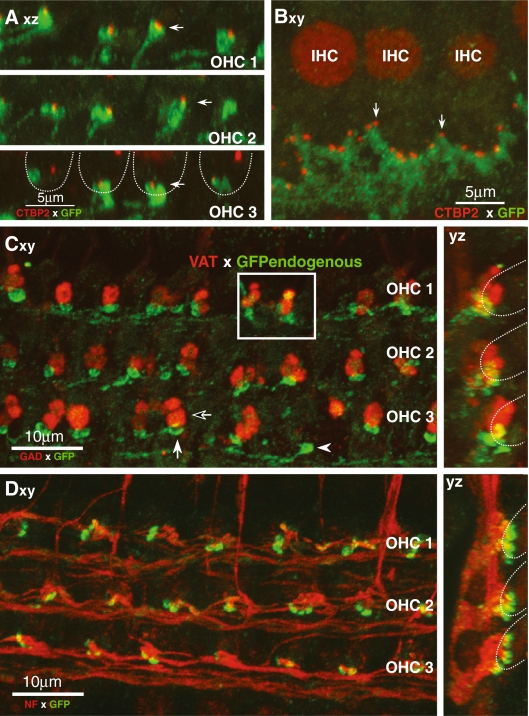
FIG. 2.
GABAB1 receptors are expressed by terminals of type I and type II ganglion cells on IHCs or OHCs, respectively. Anti-GFP (green) is used to enhance the endogenous signal from the GABAB1-GFP reporter, except in the inset of C, where the endogenous GFP signal is shown. Images are 2-D projections from confocal z-stacks of cochlear whole mounts. The projection view is indicated in each panel: xy surface view; yz cross-section view; xz view from tunnel of Corti. In several panels, the approximate positions of OHCs are indicated by dashed lines. A, B Proximity of GABAB1-positive terminals on OHCs (A) or IHCs (B) to synaptic ribbons immunostained with CtBP2 (red). Axz projections through four OHCs from each of the three rows. Filled arrows indicate terminal clusters closely associated with synaptic ribbons. Bxy projection of three IHCs from 20 serial images spanning 5 μm in the z dimension. Arrows point to two synaptic ribbons. C GABAB1 containing terminals on OHCs (filled arrow) are complementary to OC terminals (unfilled arrow) expressing GAD (red). D Neurofilament antibody (NF-200; red) reveals spiraling type II axons terminating in GABAB1-positive terminals under three rows of OHCs.
In the OHC area (Fig. 2A), the type II terminals and their pre-terminal stalks were immunopositive for GABAB1-GFP, indicative of receptor localization, and almost every terminal cluster was in close proximity to a synaptic ribbon (e.g., filled arrows in Fig. 2A). Further proof of the afferent nature of the GABAB1-positive terminals in the OHC area is seen by double-staining for GAD (Fig. 2C): The smaller GABAB1-positive terminals under OHCs are distinct from the larger GAD-positive (efferent) terminals. Their small size and connection to the spiraling fibers under the OHCs (Fig. 2D) are further evidence that the GABAB1-positive terminals contacting the OHCs belong to the type II afferent fibers. The GABAB1-GFP protein is also localized along the spiraling unmyelinated portions of the type II projections in the organ of Corti (Fig. 2C). In cases where the endogenous GFP was imaged directly (without immunotagging and signal amplification), the signal also appeared diffusely spread across the entire terminal swelling (inset to Fig. 2C).
In the IHC area, the bases of the hair cells are studded with GABAB1-positive (green) terminals and their pre-terminal stalks, which, in turn, are in intimate association with a row of synaptic ribbons (red) within the IHC itself (Fig. 2B). Double-staining with anti-GAD antibodies further confirmed the afferent nature of these GABAB1-positive terminals in the IHC area (data not shown). In addition, cochlear whole mounts were immunostained with an antibody raised against GABA conjugated to keyhole limpet hemocyanin using a glutaraldehyde bridge and extensively characterized for lack of cross-reactivity (Holstein et al. 2004b). In toadfish vestibular organs, this antibody revealed a small subpopulation of GABAergic hair cells among the majority population of glutamatergic cells (Holstein et al. 2004b). As shown in Figure 3, in the mouse cochlea, this antibody brightly stains only terminals in the IHC area. Double-staining with an anti-VAT antibody shows that the anti-GABA immunostaining is restricted to the inner spiral bundle, where the OC efferents are found (data not shown). The lack of anti-GABA immunostaining in the OHC area suggests that if GABA is synthesized in the GAD-positive OHC efferents, the concentration is much lower in the adult ear. There was no obvious apex to base gradient in the intensity of staining in the IHC area (four cochleas from three control mice were examined). Based on comparison to other cochleas immunostained for vesicular acetylcholine transporter (data not shown), which labels almost all efferent terminals in the IHC area (Darrow et al. 2006), the GABA is also present in the great majority of efferent terminals.
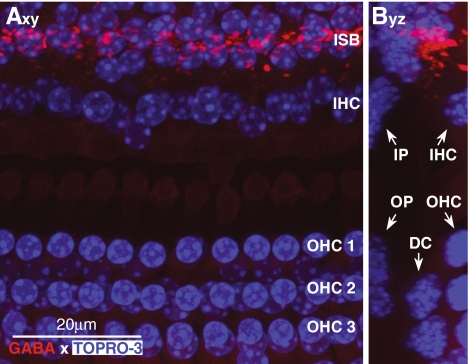
FIG. 3.
GABA immunoreactivity (red) is seen only in the efferent terminals in the inner spiral bundle (ISB) in the region under the inner hair cells. Nuclei are stained with TOPRO-3 (blue). Axy projection of a confocal z-stack of a whole mount from roughly the 16 kHz region of the cochlea. Byz projection (side view) of the same stack. IP Inner pillar cells, OP outer pillar cells, DC Deiters’ cells.
Baseline cochlear function and morphology without GABAB receptors
We used two tests to assess baseline cochlear function in the GABAB1-null ears: tone-pip evoked ABRs and DPOAEs. Given that wave 1 of the ABR represents the summed activity of the type I cochlear ganglion cells (Melcher and Kiang 1996) and that the DPOAE requires neither IHCs nor cochlear nerve fibers for its normal generation (Liberman et al. 1997), the comparison of response thresholds by these two measures can provide insight into the locus of any dysfunction.
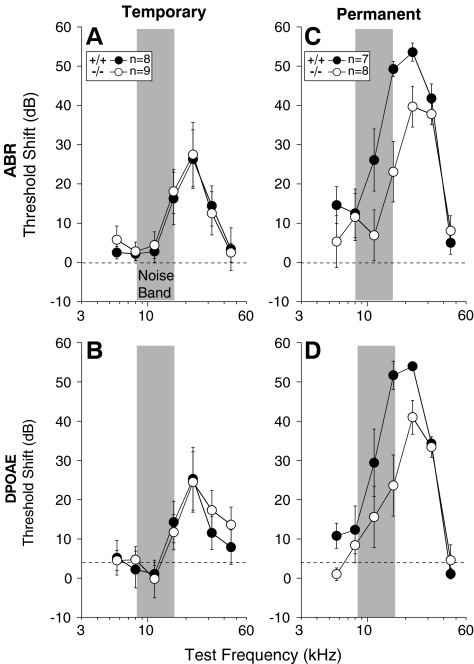
There was a small, but statistically significant, elevation of mean cochlear thresholds in the GABAB1-null ears compared to wild-type (Fig. 4). This phenotype was seen across all cochlear frequency regions and was similar in magnitude whether measured by ABR or DPOAE.
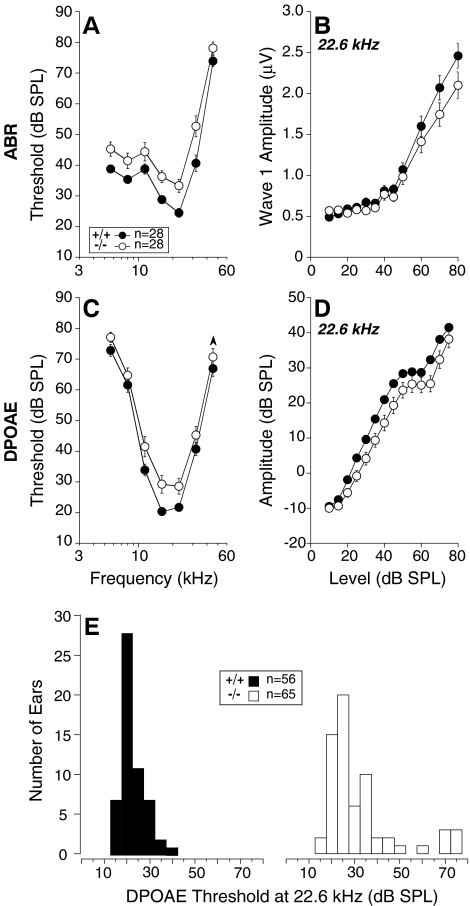
FIG. 4.
Cochlear thresholds are elevated, and response amplitudes are reduced in GABAB1 nulls, as measured by either ABRs (A, B) or DPOAEs (C, D, E). A,C Mean thresholds (±SEM) for wild-type (+/+) vs. GABAB1-null (−/−) ears. ABR thresholds were identified by visual inspection of the stacked level-series waveforms. DPOAE threshold was defined by interpolation as the sound pressure of the f2 primary required to evoke a DPOAE of 0 dB SPL. B, D Mean amplitude vs. level functions (±SEM) for responses evoked by 22.6 kHz tone pips (B) or primaries with f2 at 22.6 kHz (D). ABR amplitudes are for Wave 1 (i.e., cochlear nerve response). E Distributions of DPOAE thresholds at 22.6 kHz for wild-type (left) and GABAB1 nulls (right). A–D Data from the right ears of 28 animals of each genotype. E DPOAE data from both ears of these 56 animals, plus from an additional nine GABAB1-null ears from nine animals studied with strychnine injection.
With respect to ABR thresholds, the difference between wild-types and GABAB1 nulls was significant by two-way ANOVA [F(1,54) = 11.667, p = 0.001]. The same was true for DPOAE thresholds [F(1,54) = 7.611, p = 0.008]. Thresholds in Figure 4 were obtained at 6 weeks of age; thresholds were re-measured at 12 weeks, and no further deterioration was seen (data not shown).
As shown by Figure 4E, the threshold distribution in the GABAB1 nulls differed from wild-types in two ways: There were (1) an upward shift in the major peak and (2) a small group of GABAB1 nulls with exceptionally high thresholds. Another indication of phenotypic heterogeneity in the GABAB1 nulls was that a subset of animals (six of 30 GABAB1 nulls genotyped) showed clear circling behavior consistent with vestibular pathophysiology and previous reports (Schuler et al. 2001). Among the GABAB1 nulls, circling animals had higher mean cochlear thresholds than non-circlers, especially at high frequencies (24 dB at 32 kHz; data not shown).
Consistent with the small mean threshold effects of the GABAB1 gene deletion, cochlear morphology at the light-microscopic level in the mutant ears was similar to that in wild-types (Fig. 5). Analysis of serial sections through eight GABAB1-null ears and three age-matched wild-types was undertaken, including examination of the entire organ of Corti from base to apex with oil-immersion objectives under differential interference contrast optics. This analysis revealed scattered loss of outer hair cells in the lower basal turn from both genotypes. However, there were no systematic differences between genotypes in the degree of hair cells loss, in ganglion cell density, or in the overall appearance of the organ of Corti, stria vascularis, spiral ligament, or spiral limbus. One of the analyzed GABAB1-null ears was part of the subset showing exceptionally high DPOAE thresholds (75 dB at 22.6 kHz: Fig. 4E): Even in this ear, no obvious morphological abnormalities were apparent at the light-microscopic level.
FIG. 5.
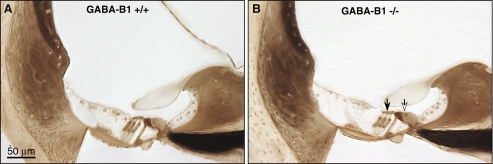
Light micrographs of osmium-stained plastic sections through the cochlear duct from the upper basal turn in a wild-type (A) and a GABAB1-null ear (B). Inner and outer hair cells are indicated by the unfilled and filled arrows in B, respectively.
In addition to this analysis of sectioned plastic-embedded cochleas, ears from six GABAB1-null animals and three wild-type littermates were examined as cochlear whole-mounts double-immunostained for neurofilament (to show all the unmyelinated terminals of afferents and efferent fibers in the organ of Corti) and the cholinergic marker VAT (to show all efferent terminals in both IHC and OHC areas). One additional GABAB1 null was double-stained for neurofilament and the GABAergic marker GAD. This analysis, which included comparison of 20 high-power confocal z-stacks from mutant ears and ten from wild-types, failed to reveal any consistent abnormalities in the afferent or efferent innervation of ear and also further illustrated the lack of hair cell loss in the mutant ears.
Response to activation of the olivocochlear efferent bundle
Since the only known source of GABAergic input to the organ of Corti is the OC efferent bundle (Eybalin 1993), we wanted to determine if efferent function was affected by the loss of GABAB receptors. The classic approach to in vivo assessment of OC function involves comparing cochlear sound-evoked responses before, during, and after applying a train of shocks to the OC bundle at the floor of the fourth ventricle (Galambos 1956; Sridhar et al. 1995).
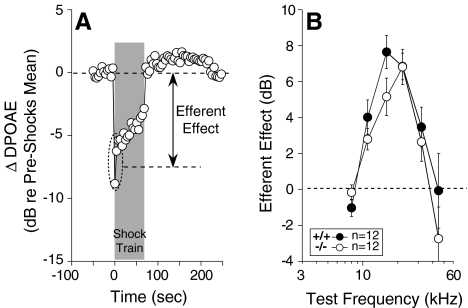
As shown in Figure 6A, OC activation normally evokes a rapid suppression of DPOAEs, which adapts somewhat during the continuous 70-s shock epoch. The rapid suppressive effect is mediated by ACh, which is present in OC cochlear terminals and binds to α9/α10 ACh receptors on OHCs (Vetter et al. 1999). The magnitude of fast suppression normally shows a strong dependence on stimulus frequency, peaking in response to 16–22 kHz stimuli in mouse (Fig. 6B). The magnitude of fast OC-mediated suppression was not significantly affected by loss of GABAB1 function (Fig. 6B). Correspondingly, the density of OC terminals on OHCs was also unaffected in the GABAB1-null ears, as described above.
FIG. 6.
Effects of shock-evoked activation of OC efferents are unaffected in GABAB1-null ears. To assay OC effects, DPOAE amplitudes evoked by low-level tones are measured before, during, and after delivering a 70-s shock train to the OC bundle at the floor of the fourth ventricle. A One “run” of the assay shows DPOAE amplitudes, normalized to the mean pre-shock value. Efferent effect is defined as the difference between the pre-shock mean and the mean DPOAE amplitude for the first three measures taken after shock-train onset (dashed ellipse in A). B Mean efferent effect size (±SEM) for wild-type vs. GABAB1-null ears: Numbers of animals in each group are shown in the key.
After shock-train termination, cochlear responses in wild-type mice rebound to supranormal levels and then slowly return to baseline (Fig. 6A). The post-shock enhancement of cochlear responses in wild-type mice is unaffected by strychnine, a potent blocker of α9/α10 receptors, or by targeted deletion of either of these cholinergic receptors (Maison et al. 2007). The mechanism underlying slow enhancement is unknown; however, present results suggest that GABAB receptors are not required, as enhancement magnitude was also unaffected by GABAB1 deletion (data not shown).
Response to acoustic overstimulation
Given evidence for GABAB-mediated inhibition of glutamate-induced Ca2+ influx in isolated spiral ganglion neurons (Lin et al. 2000) and the suggestion that GABAergic transmission between efferents and cochlear afferents might play a role in reducing the ear’s excitotoxic response to acoustic overstimulation, we investigated the effect of GABAB1 deletion on the response of the ear to acoustic injury.
Since the mechanisms underlying temporary and permanent noise-induced threshold shifts are different (Saunders et al. 1985), we used two noise exposures. The first was designed to produce ∼20–30 dB of temporary threshold shift when measured 6 h post exposure and to recover completely by 1 week post exposure. When age- and sex-matched groups of animals were exposed to this noise band, the temporary threshold shift was not different between wild-types and mutants, as assessed by ABRs (Fig. 7A) and DPOAEs (Fig. 7B). The exposure in the second set of experiments (on a different cohort of animals) was designed to produce ∼40–50 dB of permanent threshold shift when measured 1 week post exposure. As shown in Figure 7C and D, in contrast to expectations, the mutant ears were more resistant to noise exposure than wild-types: Differences in threshold shift between the genotypes were significant by two-way ANOVA, both for ABRs [F(1,13) = 8.674, p = 0.011] and for DPOAEs [F(1,13) = 5.434, p = 0.036]. The fact that the differences were seen in both DPOAE- and ABR-based measures suggests that the site of enhanced resistance is the OHCs.
FIG. 7.
Vulnerability to temporary acoustic injury (A, B) is unaffected by loss of GABAB1; however, GABAB1 nulls are more resistant to permanent noise-induced hearing loss than wild-types (C, D). Each panel shows mean thresholds (±SEM) for the group, as indicated in the symbol key (numbers of ears tested are indicated: one ear of each animal by ABR and both ears by DPOAEs). A, B Temporary cochlear dysfunction was measured 6 h after exposure to an 8–16-kHz octave band of noise at 94 dB for 15 min. C, D Permanent acoustic injury was measured 1 week after exposure to the same noise band at 100 dB for 2 h.
Strychnine blockade of cholinergic effects
OC-mediated activation of α9/α10 ACh receptors on OHCs protects the ear from permanent acoustic injury (Maison et al. 2002) and also suppresses near-threshold cochlear responses (Vetter et al. 1999). Thus, the phenotype in the GABAB1 nulls could arise via enhancement of spontaneous and sound-evoked OC activity. Such a disinhibition of the OC system might, in turn, result from effects of GABAB1 deletion on the central circuitry driving the OC system.
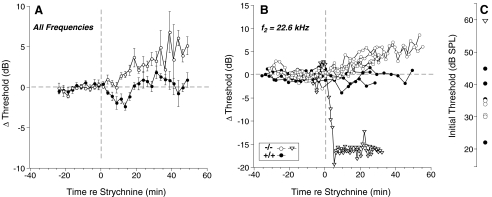
To test this idea, we injected strychnine, among the most potent blockers of the α9/α10 ACh receptor complexes (Elgoyhen et al. 1994, 2001), at the lowest concentration that completely blocks the suppression of cochlear responses evoked by shocking the OC bundle (Maison et al. 2007). As shown in the mean data of Figure 8A, blockade of OC cholinergic effects in wild-type animals caused a transient enhancement in threshold sensitivity followed by a return to baseline. The mean effect of strychnine in the GABAB1 nulls was a slow, small (∼5 dB) elevation of DPOAE thresholds, thus ruling out hyperexcitability of the OC reflex as a basis for the phenotype in the GABAB1 nulls. Intriguingly, in one GABAB1-null ear (triangles in Fig. 8B), strychnine evoked a dramatic reversal of the threshold-shift phenotype. This ear also showed the highest pre-strychnine thresholds (Fig. 8C; see also Fig. 4E), and the threshold recovery (close to 20 dB) was comparable to the pre-exposure threshold disparity between this animal and the other null (and wild-type) ears.
FIG. 8.
Effects of strychnine blockade of cholinergic OC effects on DPOAE thresholds, which were repeatedly measured for at least 20 min before and after strychnine injection (10 mg/kg). Threshold is plotted as shift re mean pre-injection threshold. A Mean threshold shift (±SEM) for all runs at all f2 frequencies (11.3, 16, or 22.6 kHz) from four wild-type ears and 12 GABAB1-null ears. B Data for individual runs from four wild-type and five GABAB1-null ears for f2 = 22 kHz, including one mutant in which strychnine caused a dramatic threshold improvement. C Absolute DPOAE thresholds measured before injection for the same nine cases in B. Symbol key in B applies to A and C.
Discussion
Cochlear expression of GABAB receptors and sources of GABAergic inputs
Using expression of a GFP-GABAB1 fusion protein under the endogenous GABAB1 promoter, we show here, in the adult mouse cochlea, that GABAB1 is expressed only in the type I and type II ganglion cells, their unmyelinated dendrites within the cochlea, and their afferent terminals contacting IHCs and OHCs, respectively. RT-PCR studies have shown GABAB expression in tissue extracted from the modiolus, expected to include spiral ganglion cells, but not hair cells (Lin et al. 2000). There are a few tissue localization studies (using immunolabeling or in situ hybridization) reporting expression of GABAA receptors in apical-turn hair cells and in spiral ganglion cells or their terminals under IHCs (e.g., Maison et al. 2006), but no previous studies describe the cellular expression patterns of GABAB receptors in the cochlea.
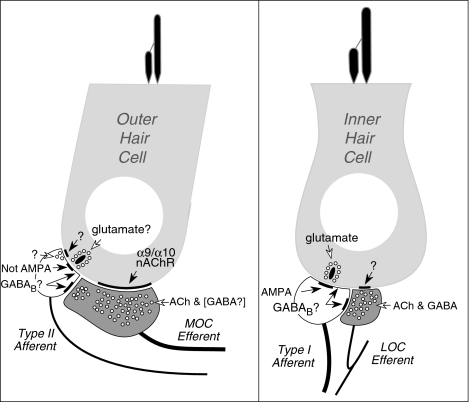
As schematized in Figure 9, the only source of GABAergic input to the mammalian cochlea is the OC efferent system. Although the OC system is primarily thought of as a cholinergic pathway and although the best-studied peripheral effects of OC activation are mediated via ACh and nicotinic ACh receptors in OHCs (Vetter et al. 1999), there is evidence that (at least) a subset of the OC efferent fibers are immunopositive for GABA as well as for the GABA-synthetic enzyme, GAD (Eybalin et al. 1988; Nitecka and Sobkowicz 1996; Maison et al. 2003). In mouse, the GABAergic component of the OC system in the IHC area is present throughout the cochlear spiral and immunoreactivity to both GAD (Maison et al. 2003), and GABA (Nitecka and Sobkowicz 1996) have been verified. In the OHC area, the longitudinal distribution may be species dependent: In guinea pig, OHC efferents immunopositive for GABA are predominately seen in the apical half of the cochlea (Fex et al. 1986), whereas in the squirrel monkey (Thompson et al. 1986) and human (Schrott-Fischer et al. 2002), efferent fibers in the OHC area are GABA positive throughout the cochlea. In mouse, OHC efferents colocalize GAD and cholinergic markers such as VAT throughout the cochlear spiral (Maison et al. 2003); however, results from the present study fail to show GABA-like immunoreactivity in the OHC area (Fig. 3).
FIG. 9.
Schematic of the afferent and efferent innervation of outer and inner hair cells summarizing some of the known and unknown transmitters and receptors involved in synaptic transmission. See text for further details.
The known cochlear targets of OC fibers include both classes of sensory cells (IHCs and OHCs) as well as both classes of afferent neurons, the type I and type II ganglion cells innervating IHCs and OHCs, respectively (Liberman 1980; Liberman et al. 1990). Immunohistochemical studies at the electron microscopic level in guinea pig confirm that GABAergic terminals contact all four of these peripheral targets, although in both IHC and OHC areas, the preferred targets of GABAergic terminals may be the dendrites of type I and type II ganglion cells, respectively (Eybalin et al. 1988). Although there is recent evidence in vestibular end-organs from the toadfish that some hair cells use GABA as an afferent transmitter (Holstein et al. 2004a,b), the anti-GABA antibody utilized in the toadfish study stains only OC efferents in the mouse cochlea (Fig. 3).
In the mammalian cochlea, the IHC/type I afferent synapse is clearly glutamatergic and excitatory: There is both pharmacological (Ruel et al. 2000) and immunohistochemical (Matsubara et al. 1996) evidence that fast transmission at this synapse is mediated by AMPA-type glutamate receptors. The afferent synapse between OHCs and type II neurons is less well understood. Immunohistochemistry suggests that AMPA-type glutamate receptors are absent (Matsubara et al. 1996; Thiers et al. 2008). Direct pharmacological data are lacking, because reliable electrophysiological recordings from the unmyelinated axons of type II neurons have yet to be achieved (Brown 1994; Robertson et al. 1999). Indirect pharmacological evidence for lack of AMPA-type receptors in OHCs is provided by the observation that cochlear perfusion of AMPA agonists evokes swelling of afferent dendrites in the IHC area (Pujol and Puel 1999), reminiscent of the excitotoxic effects seen after acoustic overexposure (Robertson 1983), whereas no histologic consequences of either manipulation are seen beneath the OHCs (Pujol and Lenoir 1986). There is limited in situ hybridization data suggesting that both type I and type II afferents express metabotropic glutamate receptors (e.g., mGluR1: Peng et al. 2004); however, the main afferent transmitter at the OHC/type II synapse remains a matter of speculation.
GABAB1 subunits are obligatory components of the functional GABAB receptor, which can be located both pre- and post-synaptically (for review, see Bettler and Tiao 2006; Ulrich and Bettler 2007). GABAB receptor activation involves G-protein-mediated modulation of (1) presynaptic Ca2+ channels, where vesicle release can be inhibited, and (2) post-synaptic K+ channels, where slow inhibitory potentials can be evoked by activating Kir3-type channels. Based on present results and current understanding of cochlear neuronal circuitry (summarized in Fig. 9), GABAB receptors on IHC afferents could be responding post-synaptically to GABA released by OC efferent terminals. Potential sources of GABA in the OHC area in mouse are less clear, given the lack of immunostaining of the OHC efferents with the anti-GABA antibody (Fig. 3). However, recent work in cerebellar Purkinje cells suggests that the GABAB receptor, whose extracellular domain has sequence homology to that of the Ca2+-sensing receptor, can bind Ca2+ and in the absence of GABA and can modulate the glutamate sensitivity of mGluR1 receptors (Tabata et al. 2004). Thus, GABAB1 signaling in type I or type II terminals could directly modulate (glutamatergic) afferent transmission from the hair cells without GABAergic input from efferent terminals.
Functional studies of cochlear GABAergic transmission via GABAA or GABAB receptors
In vitro, studies of isolated OHCs (Sziklai et al. 1996) report GABA-induced effects on stiffness and electromotility with a GABAA pharmacology (blocked by bicuculline). However, in vivo, the effects of activating OC efferents to the OHC region do not include any GABAergic components: (1) The classic suppressive effects of OC activation on OHCs are cholinergic and mediated by α9/α10 nicotinic ACh receptors (Vetter et al. 1999), and (2) a slower OC-mediated response enhancement that persists in α9- or α10-null ears (Maison et al. 2007) is unaffected by deletion of a variety of GABAA receptors (Maison et al. 2006) or by deletion of GABAB signaling (as shown here).
In vitro studies of spiral ganglion cells report GABA-mediated Cl− currents consistent with GABAA signaling (Malgrange 1997). Correspondingly, in vivo cochlear perfusion studies have reported that GABA agonists reduce glutamate-induced activity in afferent terminals in the IHC area (Arnold et al., 1998); however, sound-evoked responses could not be studied in these preparations. In vivo, activation of OC efferents to the IHC area (Groff and Liberman 2003) evokes a slow-onset and slow-offset inhibition of spontaneous and sound-evoked neural activity; however, the pharmacology has not yet been studied.
The only prior suggestion of GABAB signaling in cochlear tissues is an in vitro study (Lin et al. 2000) showing that GABA application in neonatal spiral ganglion cells induces a Ca2+-entry spike, which is mimicked by baclofen and blocked by saclofen in a dose-dependent manner. Baclofen also suppressed glutamate-induced Ca2+ entry, presumably by preventing the depolarization-induced increases in Ca2+ flux through voltage-dependent Ca2+ channels. The authors speculated that GABAB receptors in ganglion cells might help control glutamate-induced excitotoxicity, a hallmark of the acute response of the cochlea to acoustic overstimulation (Liberman and Mulroy 1982), although the balance between direct effects (increasing Ca2+ entry) and indirect effects (decreasing Ca2+ entry) was not discussed. According to this view, deletion of GABAB receptors should increase the ear’s vulnerability to acute threshold shifts, without changing the chronic effects of permanent noise-induced threshold shift. In fact, we saw no change in vulnerability to temporary acoustic injury and enhanced resistance to permanent acoustic trauma (Fig. 7), as discussed below.
The present report is the first in vivo study showing effects of GABAB-mediated signaling on cochlear function. Since functional GABAB receptors require both the GABAB1 and GABAB2 subunit and since the mutant line studied here does not express either the GABAB1a or the GABAB1b subunit isoforms (Haller et al. 2004), all GABAB-mediated signaling should be eliminated in the mutants. By analogy to GABAB effects on post-synaptic targets elsewhere in the brain and in the more closely analogous vestibular organs (Holstein et al. 2004a), GABAB receptor activation should be inhibitory, e.g., by increasing conductance of K+ir channels (Ulrich and Bettler 2007), and loss of GABAB function in the IHC area might be expected to increase excitability of the cochlear nerve. In contrast, the cochlear phenotype observed (Fig. 4), i.e., a frequency-independent increase in cochlear thresholds of roughly equal magnitude in neural responses (ABRs) and OHC-based responses (DPOAEs), suggests that all the dysfunction can be explained based on changes in the OHC area. A decrease in the contributions of OHC electromotility to cochlear mechanical amplification would be reflected in both measures, whereas even massive loss of IHCs and their type I afferents does not alter DPOAE responses (Liberman et al. 1997). It is possible that subtler changes in type I physiology, e.g., in spontaneous activity, were present in GABAB nulls.
Peripheral vs. central effects of GABAB deletion: a role for type II afferents in OHC function
Previous studies in guinea pig describe an idiopathic threshold elevation in a subset of animals that can be “rescued” by cutting the OC efferent pathway (Rajan 1989). It was suggested that the threshold shift in these animals was due to abnormally high levels of spontaneous activity in OC neurons that inhibited OHC function via the classic cholinergic actions of this efferent feedback. The putative hyperexcitability in the central circuitry of the OC pathway was of unknown origin. In the present study, the effects of GABAB deletion on OHC function could arise because GABAB signaling (either in type II neurons or in brainstem auditory pathways) normally inhibits the central circuitry driving the medial OC neurons projecting to OHCs. Enhancement of spontaneous activity in these OC fibers would raise baseline DPOAE thresholds, and enhancement of sound-evoked activity would render the ears more resistant to acoustic injury (Maison and Liberman 2000), both of which were observed in the GABAB1-null ears in the present study.
Strychnine is a potent blocker of the α9/α10 nicotinic ACh receptor complexes on OHCs that mediate both these effects (Kujawa et al. 1994; Maison et al. 2007). Based on dose–response curves for strychnine blockade of shock-evoked OC effects (Maison et al. 2007), we chose the lowest effective concentration (10 mg/kg) in order to maximize the specificity of the strychnine effects. Strychnine blockade in the GABAB1-null ears did not, in general, reverse the threshold-shift phenotype (Fig. 8A); thus, the observed effects of GABAB1 deletion appear to arise from changes intrinsic to the cochlea. The dramatic strychnine-mediated threshold rescue in one ear (Fig. 8B) is suggestive of the de-efferentation rescue of idiopathic threshold shifts cited above and is intriguing, given that this ear was also the only one of the strychnine-treated ears from the small subset (roughly 10%) of GABAB1 nulls with extremely high baseline thresholds (Figs. 4E and 8C). However, the rarity of the phenomenon makes it difficult to study systematically. The slow decline in threshold sensitivity elicited by strychnine in most of the GABAB1-null ears (Fig. 8A) does not simply reflect a slow deterioration in animal condition because all animals were paralyzed and artificially ventilated, and no changes in heart rate were observed. There is no ready explanation for such a strychnine-mediated effect in the GABAB1 nulls.
The baseline threshold elevation, in the GABAB1-null ears, suggests a role for type II neurons and their GABAB receptors in the local control of OHC function. Recent ultrastructural studies suggest that type II “afferent” synapses with OHCs are reciprocal in nature (Thiers et al. 2002; Thiers et al. 2008). Thus, they may form a local neural network mediating direct communication among OHCs and or nearby OC terminals without central processing of afferent signals. As summarized in Figure 9, the transmitters and/or receptors involved in the “efferent” component of this reciprocal interaction between OHCs and type II terminals are as poorly understood as the afferent component. Indeed, the physiology and function of these highly branched cochlear afferents are virtually unknown, since their relative rarity (5–10% of all cochlear nerve fibers) and lack of myelination render them difficult to study (Brown 1994; Robertson et al. 1999). This local network may coordinate the electromotile properties of groups of OHCs, perhaps by controlling the resting length of OHCs and thus the set-point of the stereociliary bundles at their apical surfaces (Fettiplace 2006). It is also possible that GABAB signaling modulates this local network by post-synaptic modulation of glutamate transmission at the afferent synapse. Regardless of the exact nature of any putative type II/OHC interactions, the present observation, that deletion of GABAB receptors from type II terminals leads to OHC dysfunction, provides the most direct evidence to date that the type II pathway is not just a simple relay to the brainstem.
Acknowledgments
The skillful assistance of Leslie Dodds and Martin Gassmann is gratefully acknowledged. This work was supported by NIH-NIDCD grants RO1 DC0188, R01 DC006677, P30 DC05029, and the Swiss Science Foundation.
Footnotes
The plug-in can be examined and downloaded: (http://research.meei.harvard.edu/Otopathology/3dmodels/other_tools.html
References
- Arnold T, Oestreicher E, Ehrenberger K, Felix D. GABAA receptor modulates the activity of inner hair cell afferents in guinea pig cochlea. Hear Res. 125:147–153, 1998. [DOI] [PubMed]
- Bettler B, Tiao JY. Molecular diversity, trafficking and subcellular localization of GABAB receptors. Pharmacol. Ther. 110:533–543, 2006. [DOI] [PubMed]
- Brown MC. Antidromic responses of single units from the spiral ganglion. J. Neurophysiol. 71:1835–47, 1994. [DOI] [PubMed]
- Casanova E, Vigot R, Haller C, Barbieri S, Abdelaziz S, Guet N, Biermann B, Bradadia A, Gassmann M, Bettler B. Life imaging of GABAB receptors in neurons. Abstract of the 6th EMBL Mouse Molecular Genetics Meeting, Heidelberg, Germany, p 68, 2005.
- Darrow KN, Simons EJ, Dodds L, Liberman MC. Dopaminergic innervation of the mouse inner ear: evidence for a separate cytochemical group of cochlear efferent fibers. J. Comp. Neurol. 498:403–414, 2006. [DOI] [PMC free article] [PubMed]
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. Alpha 9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell. 79:705–715, 1994. [DOI] [PubMed]
- Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, Boulter J. Alpha10: a determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc. Natl. Acad. Sci. U S A. 98:3501–3506, 2001. [DOI] [PMC free article] [PubMed]
- Eybalin M. Neurotransmitters and Neuromodulators of the Mammalian Cochlea. Physiol. Rev. 73:309–373, 1993. [DOI] [PubMed]
- Eybalin M, Parnaud C, Geffard M, Pujol R. Immunoelectron microscopy identifies several types of GABA-containing efferent synapses in the guinea-pig organ of Corti. Neuroscience. 24:29–38, 1988. [DOI] [PubMed]
- Fettiplace R. Active hair bundle movements in auditory hair cells. J. Physiol. 576:29–36, 2006. [DOI] [PMC free article] [PubMed]
- Fex J, Altschuler RA, Kachar B, Wenthold RJ, Zempel JM. GABA visualized by immunocytochemistry in the guinea pig cochlea in axons and endings of efferent neurons. Brain Res. 366:106–117, 1986. [DOI] [PubMed]
- Fuchs PA, Murrow BW. A novel cholinergic receptor mediates inhibition of chick cochlear hair cells. Proc. Roy Soc. London B. 248:35–40, 1992. [DOI] [PubMed]
- Galambos R. Suppression of auditory activity by stimulation of efferent fibers to the cochlea. J. Neurophysiol. 19:424–437, 1956. [DOI] [PubMed]
- Groff JA, Liberman MC. Modulation of cochlear afferent response by the lateral olivocochlear system: activation via electrical stimulation of the inferior colliculus. J. Neurophysiol. 90:3178–3200, 2003. [DOI] [PubMed]
- Guinan JJ. The physiology of olivocochlear efferents. In: Dallos PJ, Popper AN, Fay RR (eds) The Springer Verlag Handbook of Auditory Research: The Cochlea. New York, Springer, p. 435–502, 1996.
- Hafidi A. Peripherin-like immunoreactivity in type II spiral ganglion cell body and projections. Brain Res. 805:181–190, 1998. [DOI] [PubMed]
- Haller C, Casanova E, Muller M, Vacher CM, Vigot R, Doll T, Barbieri S, Gassmann M, Bettler B. Floxed allele for conditional inactivation of the GABAB(1) gene. Genesis. 40:125–130, 2004. [DOI] [PubMed]
- Holstein GR, Rabbitt RD, Martinelli GP, Friedrich VL, Jr., Boyle RD, Highstein SM. Convergence of excitatory and inhibitory hair cell transmitters shapes vestibular afferent responses. Proc. Natl. Acad. Sci. U S A. 101:15766–15771, 2004a. [DOI] [PMC free article] [PubMed]
- Holstein GR, Martinelli GP, Henderson SC, Friedrich VL, Jr., Rabbitt RD, Highstein SM. Gamma-aminobutyric acid is present in a spatially discrete subpopulation of hair cells in the crista ampullaris of the toadfish Opsanus tau. J. Comp. Neurol. 471:1–10, 2004b. [DOI] [PubMed]
- Khimich D, Nouvian R, Pujol R, Tom Dieck S, Egner A, Gundelfinger ED, Moser T. Hair cell synaptic ribbons are essential for synchronous auditory signalling. Nature. 434:889–894, 2005. [DOI] [PubMed]
- Kujawa SG, Glattke TJ, Fallon M, Bobbin RP. A nicotinic-like receptor mediates suppression of distortion product otoacoustic emissions by contralateral sound. Hear. Res. 74:1–13, 1994. [DOI] [PubMed]
- Liberman MC. Efferent synapses in the inner hair cell area of the cat cochlea: An electron microscopic study of serial sections. Hear. Res. 3:189–204, 1980. [DOI] [PubMed]
- Liberman MC, Mulroy MJ. Acute and chronic effects of acoustic trauma: cochlear pathology and auditory nerve pathophysiology. In: Hamernik RP, Henderson D, Salvi R (eds) New Perspectives on Noise-Induced Hearing Loss. 105–136, 1982.
- Liberman MC, Dodds LW, Pierce S. Afferent and efferent innervation of the cat cochlea: quantitative analysis with light and electron microscopy. J. Comp. Neurol. 301:443–460, 1990. [DOI] [PubMed]
- Liberman MC, Chesney CP, Kujawa SG. Effects of selective inner hair cell loss on DPOAE and CAP in carboplatin-treated chinchillas. Audit. Neurosci. 3:255–268, 1997.
- Lin X, Chen S, Chen P. Activation of metabotropic GABAB receptors inhibited glutamate responses in spiral ganglion neurons of mice. NeuroReport. 57:957–961, 2000. [DOI] [PubMed]
- Maison SF, Liberman MC. Predicting vulnerability to acoustic injury with a non-invasive assay of olivocochlear reflex strength. J. Neuroscience. 20:4701–4707, 2000. [DOI] [PMC free article] [PubMed]
- Maison SF, Luebke AE, Liberman MC, Zuo J. Efferent protection from acoustic injury is mediated via alpha9 nicotinic acetylcholine receptors on outer hair cells. J. Neurosci. 22:10838–10846, 2002. [DOI] [PMC free article] [PubMed]
- Maison SF, Adams JC, Liberman MC. Olivocochlear innervation in mouse: immunocytochemical maps, crossed vs. uncrossed contributions and colocalization of ACh, GABA, and CGRP. J. Comp. Neurol. 455:406–416, 2003. [DOI] [PMC free article] [PubMed]
- Maison SF, Rosahl TW, Homanics GE, Liberman MC. Functional role of GABAergic innervation of the cochlea: phenotypic analysis of mice lacking GABA(A) receptor subunits alpha 1, alpha 2, alpha 5, alpha 6, beta 2, beta 3, or delta. J. Neurosci. 26:10315–10326, 2006. [DOI] [PMC free article] [PubMed]
- Maison SF, Vetter DE, Liberman MC. A novel effect of cochlear efferents: in vivo response enhancement does not require alpha9 cholinergic receptors. J. Neurophysiol. 97:3269–3278, 2007. [DOI] [PubMed]
- Malgrange B. Diazepam-Insensitive GABAA receptors on postnatal spiral ganglion neurones in culture. Neuroreport. 8:591–596, 1997. [DOI] [PubMed]
- Matsubara A, Laake JH, Davanger S, Usami SI, Ottersen OP. Organization of AMPA receptor subunits at a glutamate synapse: quantitative immunogold analysis of hair cell synapses in the rat organ of Corti. J. Neurosci. 16:4457–4467, 1996. [DOI] [PMC free article] [PubMed]
- Melcher JR, Kiang NYS. Generators of the brainstem auditory evoked potential in cat. III. Identified cell populations. Hear. Res. 93:52–71, 1996. [DOI] [PubMed]
- Muller M, von Hunerbein K, Hoidis S, Smolders JW. A physiological place-frequency map of the cochlea in the CBA/J mouse. Hear. Res. 202:63–73, 2005. [DOI] [PubMed]
- Nadol JB, Jr. Reciprocal synapses at the base of outer hair cells in the organ of corti of man. Ann. Otol. Rhinol. Laryngol. 90:12–17, 1981. [DOI] [PubMed]
- Nitecka LM, Sobkowicz HM. The GABA/GAD innervation within the inner spiral bundle in the mouse cochlea. Hear. Res. 99:91–105, 1996. [DOI] [PubMed]
- Oliver D, Klocker N, Schuck J, Baukrowitz T, Ruppersberg JP, Fakler B. Gating of Ca2+-activated K+ channels controls fast inhibitory synaptic transmission at auditory outer hair cells. Neuron. 26:595–601, 2000. [DOI] [PubMed]
- Peng BG, Li QX, Ren TY, Ahmad S, Chen SP, Chen P, Lin X. Group I metabotropic glutamate receptors in spiral ganglion neurons contribute to excitatory neurotransmissions in the cochlea. Neuroscience. 123:221–230, 2004. [DOI] [PubMed]
- Prosser HM, Gill CH, Hirst WD, Grau E, Robbins M, Calver A, Soffin EM, Farmer CE, Lanneau C, Gray J, Schenck E, Warmerdam BS, Clapham C, Reavill C, Rogers DC, Stean T, Upton N, Humphreys K, Randall A, Geppert M, Davies CH, Pangalos MN. Epileptogenesis and enhanced prepulse inhibition in GABAB(1)-deficient mice. Mol. Cell. Neurosci. 17:1059–1070, 2001. [DOI] [PubMed]
- Pujol R, Lenoir M. The four types of synapses in the organ of Corti. New York, Raven, 1986.
- Pujol R, Puel JL. Excitotoxicity, synaptic repair and functional recovery in the mammalian cochlea: a review of recent findings. Ann. NY. Acad. Sci. 884:249–254, 1999. [DOI] [PubMed]
- Rajan R. Tonic activity of the crossed olivocochlear bundle in guinea pigs with idiopathic losses in auditory sensitivity. Hear. Res. 39:299–308, 1989. [DOI] [PubMed]
- Robertson D. Functional significance of dendritic swelling after loud sounds in the guinea pig cochlea. Hear. Res. 9:263–278, 1983. [DOI] [PubMed]
- Robertson D, Sellick PM, Patuzzi R. The continuing search for outer hair cell afferents in the guinea pig spiral ganglion. Hear. Res. 136:151–158, 1999. [DOI] [PubMed]
- Ruel J, Bobbin RP, Vidal D, Pujol R, Puel JL. The selective AMPA receptor antagonist GYKI 5387 blocks action potential generation and excitotoxicity in the guinea pig cochlea. Neuropharmacology. 39:1959–1973, 2000. [DOI] [PubMed]
- Saunders JC, Dear SP, Schneider ME. The anatomical consequences of acoustic injury: a review and tutorial. J. Acoust. Soc. Am. 78:833–860, 1985. [DOI] [PubMed]
- Schrott-Fischer A, Kammen-Jolly K, Scholtz AW, Gluckert R, Eybalin M. Patterns of GABA-like immunoreactivity in efferent fibers of the human cochlea. Hear. Res. 174:75–85, 2002. [DOI] [PubMed]
- Schuler V, Luscher C, Blanchet C, Klix N, Sansig G, Klebs K, Schmutz M, Heid J, Gentry C, Urban L, Fox A, Spooren W, Jaton AL, Vigouret J, Pozza M, Kelly PH, Mosbacher J, Froestl W, Kaslin E, Korn R, Bischoff S, Kaupmann K, van der Putten H, Bettler B. Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)). Neuron. 31:47–58, 2001. [DOI] [PubMed]
- Spoendlin HH. Innervation densities of the cochlea. Acta. Otolaryng. 73:235–248, 1972. [DOI] [PubMed]
- Sridhar TS, Liberman MC, Brown MC, Sewell WF. A novel cholinergic “slow effect” of efferent stimulation on cochlear potentials in the guinea pig. J. Neuroscience. 15:3667–3678, 1995. [DOI] [PMC free article] [PubMed]
- Sridhar TS, Brown MC, Sewell WF. Unique postsynaptic signaling at the hair cell efferent synapse permits calcium to evoke changes on two time scales. J. Neuroscience. 17:428–437, 1997. [DOI] [PMC free article] [PubMed]
- Sziklai I, He DZZ, Dallos P. Effect of acetylcholine and GABA on the transfer function of electromotility in isolated hair cells. Hear. Res. 95:87–99, 1996. [DOI] [PubMed]
- Tabata T, Araishi K, Hashimoto K, Hashimotodani Y, van der Putten H, Bettler B, Kano M. Ca2+ activity at GABAB receptors constitutively promotes metabotropic glutamate signaling in the absence of GABA. Proc. Natl. Acad. Sci. U S A. 101:16952–16957, 2004. [DOI] [PMC free article] [PubMed]
- Thiers FA, Burgess BJ, Nadol JB, Jr. Reciprocal innervation of outer hair cells in a human infant. J. Assoc. Res. Otolaryngol. 3:269–278, 2002. [DOI] [PMC free article] [PubMed]
- Thiers FA, Nadol JB, Jr, Liberman MC. Reciprocal synapses between outer hair cells and their afferent terminals: evidence for a local neural network in the mammalian cochlea. J Assoc Res Otolaryngol (in press), 2008. [DOI] [PMC free article] [PubMed]
- Thompson GC, Cortez AM, Igarashi M. GABA-like immunoreactivity in the squirrel monkey organ of Corti. Brain Res. 372:72–79, 1986. [DOI] [PubMed]
- Ulrich D, Bettler B. GABA(B) receptors: synaptic functions and mechanisms of diversity. Curr. Opin. Neurobiol. 17:298–303, 2007. [DOI] [PubMed]
- Vetter DE, Liberman MC, Mann J, Barhanin J, Boulter J, Brown MC, Saffiote-Kolman J, Heinemann SF, Elgoyhen AB. Role of alpha9 nicotinic ACh receptor subunits in the development and function of cochlear efferent innervation. Neuron. 23:93–103, 1999. [DOI] [PubMed]