Abstract
Acrylamide (AA) is formed in heat treated carbohydrate rich foods in the so-called Maillard reaction. AA is readily absorbed in the body and converted to glycidamide (GA) by epoxidation by the CYP2E1 (cytochrome P450 2E) enzyme. Both AA and GA may be detoxified through direct conjunction to glutathione by glutathione-S-transferases and GA by hydrolysis to glyceramide. Recently, we reported that biomarkers of AA exposure reflect intake of major food sources of AA; there were large interindividual variations in the blood ratio of GA-Hb/AA-Hb (GA- and AA-hemoglobin adducts). In this study we investigated whether the ratio of GA-Hb/AA-Hb in subjects could be related to polymorphic differences in genes coding for metabolizing enzymes CYP2E1, EPHX1 (microsomal epoxide hydrolase), GSTM1, GSTT1, and GSTP1, all being expected to be involved in the activation and detoxification of AA-associated adducts. We found significant associations between GSTM1 and GSTT1 genotypes and the ratio of GA-Hb/AA-Hb (p = 0.039 and p = 0.006, respectively). The ratio of GA-Hb/AA-Hb in individuals with the combined GSTM1- and GSTT1-null variants was significantly (p = 0.029) higher than those with the wild-type genotypes. Although the number of subjects was small, there were also significant associations with other combinations; CYP2E1 (Val179Val) plus GSTM1-null (p = 0.022); CYP2E1 (Val/Val), GSTM1-null plus GSTT1-null (p = 0.047); and CYP2E1 (Val/Val), GSTT1 null, EPHX1 (Tyr113Tyr) plus EPHX1 (His139Arg) (p = 0.018). Individuals with these combined genotypes had significantly higher blood ratio of GA-Hb/AA-Hb than other combinations. The observed associations correspond with what would be expected from the relative roles of these enzymes in activation and detoxification of AA, except for individuals with the EPHX1 (His139Arg) variant. The internal dose of genotoxic metabolite and also the concentration of AA in blood seem to be affected by these polymorphic genes. The genotypes and their combination may constitute useful biomarkers for the assessment of individual susceptibility to AA intake, and could add to the precision of epidemiological studies of dietary cancer.
Keywords: cytochrome P450 2E1, glutathione-S-transferase, SNPs, polymorphisms, glycidamide, acrylamide, biotransformation
The Maillard reaction is responsible for the formation of browning products contributing to color, odor and taste of processed food (Ho, 2006). Maillard browning reaction products from mainly glucose and asparagine are responsible for the formation of acrylamide (AA) in foods (Mottram et al., 2002; Stadler et al., 2002). The presence of AA in foods caused considerable concern because AA is classified by IARC (1994) as probably carcinogenic to humans (2A). AA is readily absorbed and metabolized in the body. A major detoxification pathway is glutathione conjugation, resulting in urinary excretion of N-acetyl-S-(3-amino-3-oxopropyl) cysteine, a mercapturic acid derivative (MA-AA) (Sumner et al., 1992, 1997). An alternative metabolic pathway is the CYP2E1 (cytochrome P450 2E1) dependent oxidation of AA, from which the genotoxic epoxide glycidamide (GA) is formed (Ghanayem et al., 2005; Sumner et al., 1999). GA reacts readily with DNA and other macromolecules forming adducts (Dearfield et al., 1995; Segerback et al., 1995) and this is probably the main pathway responsible for the carcinogenic effect of AA. GA may, however, also be further metabolized by epoxide hydrolase (EPHX1) to glyceramide, which is excreted in urine (Kirman et al., 2003) or conjugated to glutathione, and these reactions represent deactivation pathways and therefore compete with the formation of GA adducts. Following a stepwise conversion, the GA-glutathione conjugates are excreted as the urinary MA derivatives N-acetyl-S-(3-amino-2-hydroxy-3-oxopropyl)cysteine (MA-GA3) and N-acetyl-S-(carbamoyl-2-hydroxyethyl)cysteine (MA-GA2) (Sumner et al., 1992). There are nonenzymatic pathways for the transformation of AA and GA but these are considered to be less significant in vivo. AA may also interact covalently in vivo with proteins forming different types of macromolecular adducts (Doerge et al., 2005; Tornqvist et al., 2002). Hemoglobin (Hb) adducts of AA and GA represent an integral of AA and GA concentrations present in the circulation over the lifetime of the erythrocytes (about 4 months) and reflect therefore the average exposure to AA over the last months (Hagmar et al., 2005).
Genes coding for enzymes involved in the biotransformation of carcinogens can be used as markers of individual susceptibility to cancer because different activity of these enzymes may increase or decrease the conversion of a xenobiotic into its reactive metabolite. A large number of chemical carcinogens are biotransformed to more toxic metabolites by phase I activation enzymes, such as CYPs or to nontoxic compounds by phase II detoxification enzymes, such as glutathione-S-transferase (GSTs). Many enzymes involved in either activation or detoxification of chemical carcinogen metabolism are polymorphically expressed, with the alleles presenting different enzymatic activities (Autrup, 2000). CYP2E1 is an enzyme responsible for the metabolic activation of many carcinogens, including AA; this inducible and major CYP isoform is mainly expressed in the liver, but is also found at significant levels in other human tissues. The important role of CYP2E1 in epoxidation of AA to GA and formation of GA-DNA adducts has been demonstrated 100 by using CYP2E1-null mice, and when such mice were exposed to AA, higher levels of AA-adducts were observed compared with wild-type mice (Ghanayem et al., 2005; Sumner et al., 1999).
The microsomal EPHX1 is involved in the metabolism of highly reactive epoxide intermediates and presumably also GA, to the less reactive and water-soluble glyceramide. It has been shown that the activity of the enzyme EPHX1 is affected by two polymorphisms; a substitution of 113Tyr to 113His in exon 3 corresponds to a 40% decrease in the enzyme activity, whereas a substitution of 139His to 139Arg in exon 4 corresponds to a 25% increase in the enzyme activity (Benhamou et al., 1998; Hassett et al., 1994). It has been shown that the resulting activity of EPHX1 depends on its expression level, measured with benzo[a]pyrene-4,5-oxide and cis-stilbene oxide as substrates (Hosagrahara et al., 2004; Omiecinski et al., 2000).
GSTs constitute a superfamily of at least 13 isoenzymes belonging to five different families, in humans designated alpha, gamma, mu, pi, and theta (Mannervik et al., 1992; Meyer et al., 1991). GSTs are known to play important roles in the defense system against damaging effects of oxidative stress as well as reactive electrophiles. In humans, there are three GST genes, GSTM1, GSTT1, and GSTP1, with known functional polymorphisms (Ali-Osman et al., 1997; Board, 1981; Pemble et al., 1994; Peter et al., 1989). Because the specificity and also expression level of the GST isoenzymes affect the biotransformation of most toxic electrophiles, the existence of different variants of the enzymes may influence the detoxification efficiency. The homozygous deletion polymorphisms of the GSTM1 and GSTT1 genes, as well as the GSTP1 105Val allele polymorphism, are known to alter enzyme activities (Cotton et al., 2000; Hayes and Strange, 1995). It has been suggested that individuals who are environmentally exposed to carcinogens and also carry genetic variants of genes for phase I or phase II enzymes, have a higher risk of developing cancer (Autrup, 2000). This may also apply to AA, a possibility which is explored in the present work.
Recently, we reported on methods to determine the Hb adducts of AA and GA in blood (Bjellaas et al., 2007). These biomarkers of exposure to AA were quantified in blood samples from volunteers and correlated to dietary intake as recorded in food frequency questionnaires (Bjellaas et al., 2007). The relationship between the estimated dietary intake of AA and Hb adducts in this cohort has been described in detail elsewhere (Bjellaas et al., 2007). We found significant correlations between the biomarkers and major known food sources of AA. The molar ratio between GA-derived and AA-derived Hb adducts varied considerably (ninefold) between the individuals (Bjellaas et al., 2007), and the varying ratio of GA-Hb/AA-Hb among individuals could not be explained by the AA intake alone. This could reflect individual differences in metabolizing capacity. Recently, it was reported that there are large variations in AA-Hb and GA-Hb adducts between individuals, and between subpopulations in Europe (Vesper et al., 2008). We have therefore characterized our study subjects with respect to polymorphisms in CYP2E1, EPHX1, GSTM1, GSTT1, and GSTP1, and explored associations between given combinations of these polymorphisms and the amount of AA and GA adducts as well as their ratio.
METHODS
Subjects.
Forty-seven nonsmoking employees at the Norwegian Institute of Public Health accepted an invitation to participate in the study. In addition, six smokers were recruited through personal invitation. There were no exclusion criteria, but samples from four participants (two male and two female) were left out of the study because all of the blood had been used for adduct measurement. In total, the study population consisted of 49 participants, 18 males, and 31 females. A detailed description of the subjects was also given in our previous report (Bjellaas et al., 2007). Two males and four females were smokers (12% of the study population); the median number of cigarettes per day was 14 (range 7–21). All individuals examined were Caucasians. The Regional Ethics Committee and the Data Inspectorate approved the study, and all participants gave their written informed consent.
Determination of hemoglobin adducts of AA and GA.
Hb adducts of AA and GA were determined as phenylthiohydantoin derivatives and quantified using liquid chromatography with an ion trap tandem mass spectrometric detection in negative electrospray ionization mode. The preparation of blood samples and determination of Hb adducts have been described in detail previously (Bjellaas et al., 2007), and the same methods for the determination of Hb adducts were used recently (Olesen et al., 2008).
Genotyping.
Genomic DNA was extracted from frozen whole blood from the 49 subjects using BloodPrep Chemistry on the ABI PRISM 6100 Nucleic Acid PrepStation (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions, and the quantity and quality of isolated DNA was determined using a NanoDrop Spectrophotometer (Thermo Scientific, Waltham, MA). The presence or absence of GSTM1 and GSTT1 genes were determined with PCR, using GSTM2 as a positive control. The primers used for GSTT1 were 5′-CAT CTC CTT AGC TG ACCT CGT AG-3′ and 5′-GGC ATC AGC TTC TGC TTT ATG-3′, generating a 412-base pair (bp) fragment (Abdel-Rahman et al., 1996). For GSTM1, the primers were 5′-CTG CCC TAC TTG ATT GAT G-3′ and 5′-CTG GAT TGT AGC AGA TCA TGC-3′, generating a 276-bp fragment (Ryberg et al., 1994). The primers for GSTM2 were 5′- CTG CCC TAC TTG ATT GAT G-3′ and 5′-GAC TCA CTC TGA GCA TAG CAC-3′, generating a 165-bp fragment (Ryberg et al., 1994). The absence of a 412- or a 276-bp electrophoretic band in the PCR product indicates the presence of a GSTM1-null or a GSTT1-null genotype, respectively. The presence of a 165-bp band of GSTM2 indicates successful PCR assay.
The TaqMan single nucleotide polymorphism (SNP) Genotyping Assay was used for CYP2E1, EPHX1, and GSTP1 according to the manufacturer's protocol (Applied Biosystems). Briefly, each reaction mixture (13.75 μl) of polymerase chain reaction contained 50 ng of DNA, 12.50 μl of 2× TaqMan Universal PCR Master Mix, No AmpErase UNG, and 1.25 μl of 20× TaqMan SNP Genotyping Assay Mix (primers and probes specific for each polymorphism) (Applied Biosystems). Amplification was done under the following conditions: 50°C for 2 min, 95°C for 10 min followed by 50 cycles of 92°C for 15 s and 60°C for 1.5 min. Data were analyzed using Allelic Discrimination Program (Applied Biosystems). For each polymorphism, all 49 DNA samples were genotyped at least twice to confirm the results. The primer and probe used were: CYP2E1 Val179Ile polymorphism (rs6413419), CYP2E1 Phe421Phe polymorphism (rs2515641), EPHX1 Tyr113His polymorphism (rs1051740), EPHX1 His139Arg polymorphism (rs2234922), and GSTP1 Ile105Val polymorphism (rs1695), and all reagents and the 7500Fast Real-Time PCR System are from Applied Biosystems (Applied Biosystems).
Statistical analysis.
The difference in mean molar ratios between the GA and AA Hb adducts in blood was analyzed with the nonparametric, Mann–Whitney U test. The Chi-squared goodness-of-fit test was used to examine the distributions of subjects with the polymorphic genotypes between genders, and this test was also used to compare the allele proportions and to determine whether the CYP2E1, EPHX1, and GSTP1 allelic frequencies conformed with the Hardy-Weinberg equilibrium. Deviations from Hardy-Weinberg equilibrium for GSTM1 and GSTT1 individuals were not tested, because heterozygous individuals could not be distinguished from wild-type by the PCR method used. The level of significance was chosen as p < 0.05. All statistical analyses were performed using SPSS for Windows version 14 (SPSS, Inc., Chicago, IL).
RESULTS
Participants
The relevant characteristics of the study subjects are presented in Table 1. Mean age was 41 years (range 24–60) and 45 years (range 26–65) for the 31 females and the 18 males, respectively. The distributions of smoking status among the genders were approximately similar (Table 1).
TABLE 1.
Characteristics of the Study Subjects
| Gender | n (%) |
| Male | 18 (36.7) |
| Female | 31 (63.3) |
| Age (years) | Mean (range) |
| Male | 45 (26–65) |
| Female | 41 (24–60) |
| All subjects | 43 (24–65) |
| Smoking status | Smokers (%) | Nonsmokers (%) |
| Male | 2 (11.1) | 16 (88.9) |
| Female | 4 (12.9) | 27 (87.1) |
| All subjects | 6 (12.2) | 43 (87.8) |
| Ratio of GA-Hb |
AA-Hb (pmol/g globin) |
GA-Hb (pmol/g globin) |
||||
| Mean ± SE | Median (range) | Mean ± SE | Median (range) | Mean ± SE | Median (range) | |
| Male | 0.48 ± 0.05 | 0.46 (0.12–0.91) | 55.30 ± 8.00 | 47 (18–154) | 24.20 ± 3.50 | 21 (7–67) |
| Female | 0.56 ± 0.04 | 0.50 (0.24–1.08) | 53.20 ± 8.60 | 40 (19–211) | 29.00 ± 4.50 | 18 (8–99) |
| Smokers | 0.52 ± 0.10 | 0.45 (0.29–0.98) | 154.00 ± 19.00 * | 166 (99–211) * | 76.50 ± 10.74* | 83 (29–99)* |
| Nonsmokers | 0.54 ± 0.03 | 0.49 (0.12–1.08) | 40.00 ± 2.25 | 37 (18–97) | 20.41 ± 1.34 | 18 (7–46) |
| All subjects | 0.53 ± 0.03 | 0.49 (0.12–1.08) | 53.10 ± 6.20 | 37 (18–211) | 27.30 ± 3.10 | 19 (7–99) |
Note. *Significantly higher levels of hemoglobin adducts in smokers compared with nonsmokers (p < 0.001 for both), but no significant difference of GA-Hb/AA-Hb ratio in blood between smokers and nonsmokers (p = 0.602). No significant difference of GA-Hb/AA-Hb ratio in blood between male and female (p = 0.163).
Biomarkers of Exposure in Blood
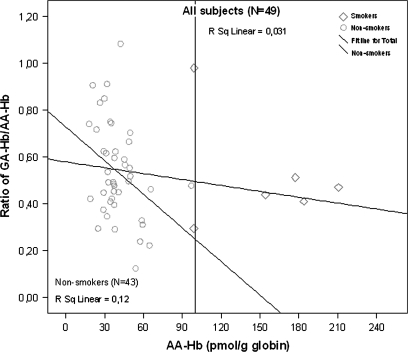
The Hb adducts of AA (AA-Hb) and GA (GA-Hb) were detected in all blood samples analyzed, and the mean concentrations for nonsmokers were 40.00 ± 2.25 pmol/g globin and 20.41 ± 1.34 pmol/g globin for AA-Hb and GA-Hb, respectively. For smokers, the mean adduct levels were 154.00 ± 19.00 pmol/g globin and 76.50 ± 10.74 pmol/g globin for AA-Hb and GA-Hb, respectively. The smokers had significantly higher concentrations of Hb adducts in comparison with nonsmokers (p < 0.001 for both). There were no significant differences between males and females in the ratio of GA-Hb versus AA-Hb adduct levels (Table 1). There was no (all subjects) or a very small (nonsmokers only) association between the ratio of GA-Hb/AA-Hb versus AA-Hb (Fig. 1). The ratio of GA-HB/AA-Hb data for nonsmokers and for all subjects were normally distributed (Supplementary Figs. 1A–D).
FIG. 1.
The ratio of GA-Hb/AA-Hb versus AA-Hb, for nonsmokers and for all subjects. For all study subjects, there was no association (R2 = 0.031); excluding smokers a very weak association was observed (R2 = 0.12).
Genotype Distributions and Association with the Biomarkers of AA Exposure in Blood
The genotype distributions and allele frequencies of the study subjects are shown in Table 2. The frequencies of the CYP2E1 179Ile allele and CYP2E1 421Phe (T) allele were 0.10 and 0.35, respectively (Table 2). CYP2E1 (Val179Ile) which is a mis-sense transition mutation, has not been shown to have any effect on the enzymatic activity (Fairbrother et al., 1998; Hanioka et al., 2003), whereas CYP2E1 (Phe421Phe) is a silent intronic mutation. The frequencies of the EPHX1 113His allele, EPHX1 139Arg allele, and GSTP1 105Val allele were 0.43, 0.33, and 0.49, respectively. The genotype distributions in this study were in Hardy-Weinberg equilibrium, except for CYP2E1 Phe421Phe allele, and the frequencies of the alleles are in agreement with those found in other Caucasian populations (The International HapMap Project, 2003; Thorisson et al., 2005).
TABLE 2.
Genotype Distributions and Allele Frequencies of the Subjects
| Frequency |
|||||
| Genotype/allele | Male, n (%) | Female, n (%) | p Value | Nonsmokers, n = 43 (%) | All subjects, n = 49 (%) |
| CYP2E1 (Val179Ile) | |||||
| Val/Val | 14 (77.8) | 30 (96.8) | 39 (90.7) | 44 (89.8) | |
| Val/Ile | 4 (22.2) | 1 (3.2) | 4 (9.3) | 5 (10.2) | |
| Ile/Ile | 0 (0.0) | 0 (0.0) | 0.054 | 0 (0.0) | 0 (0.0) |
| Val/Ile + Ile/Ile | 4 (22.2) | 1 (3.2) | 4 (9.3) | 5 (10.2) | |
| CYP2E1 (Phe421Phe) | |||||
| Phe/Phe (C/C) | 11 (61.1) | 21 (67.7) | 30 (69.8) | 32 (65.3) | |
| Phe/Phe (T/C) | 6 (33.3) | 6 (19.4) | 10 (23.3) | 12 (24.5) | |
| Phe/Phe (T/T) | 1 (5.6) | 4 (12.9) | 0.542 | 3 (7.0) | 5 (10.2) |
| Phe/Phe (C/T+T/T) | 7 (38.9) | 10 (32.3) | 13 (30.2) | 17 (34.7) | |
| EPHX1 (Tyr113His) | |||||
| Tyr/Tyr | 7 (38.9) | 21(67.7) | 27 (62.8) | 28 (57.1) | |
| Tyr/His | 8 (44.4) | 8 (25.8) | 12 (27.9) | 16 (32.7) | |
| His/His | 3 (16.7) | 2 (6.5) | 0.812 | 4 (9.3) | 5 (10.2) |
| Tyr/His + His/His | 11 (61.1) | 10 (32.3) | 16 (37.2) | 21 (42.9) | |
| EPHX1 (His139Arg) | |||||
| His/His | 14 (77.8) | 19 (61.3) | 27 (62.8) | 33 (67.3) | |
| His/Arg | 3 (16.7) | 9 (29.0) | 12 (27.9) | 12 (24.5) | |
| Arg/Arg | 1 (5.6) | 3 (9.7) | 0.790 | 4 (9.3) | 4 (8.2) |
| His/Arg + Arg/Arg | 4 (22.3) | 12 (38.7) | 16 (37.2) | 16 (32.7) | |
| GSTP1 (Ile105Val) | |||||
| Ile/Ile | 7 (38.9) | 18 (58.1) | 23 (53.5) | 25 (51.0) | |
| Ile/Val | 8 (44.4) | 8 (25.8) | 13 (30.2) | 16 (32.7) | |
| Val/Val | 3 (16.7) | 5 (16.1) | 0.375 | 7 (16.3) | 8 (16.3) |
| Ile/Val + Val/Val | 11 (61.1) | 13 (41.9) | 20 (46.5) | 24 (49.0) | |
| GSTM1 | |||||
| Positive | 13 (72.2) | 27 (87.1) | 37 (86.0) | 40 (81.6) | |
| Null | 5 (27.8) | 4 (12.9) | 0.259 | 6 (14.0) | 9 (18.4) |
| GSTT1 | |||||
| Positive | 11 (61.1) | 13 (41.9) | 23 (53.5) | 24 (49.0) | |
| Null | 7 (38.9) | 18 (58.1) | 0.244 | 20 (46.5) | 25 (51.0) |
Note. There are no significant differences in genotype frequencies between genders, and also no significant differences in genotype distributions between the nonsmokers subjects and all subjects.
Large variations have been reported in the frequencies of GSTT1 and GSTM1 deletion among different ethnicities. In our study the frequency of GSTT1 null genotype was 51%, and that of GSTM1 null genotype was 18% (Table 2). The range of values reported for Caucasian populations is 10–20% for GSTT1 and 40–50% for GSTM1 (Garte et al., 2001; Nelson et al., 1995; Rebbeck, 1997). Our study subjects hence show a genotype distribution which is unexpected and different from these ranges.
Because GA is the genotoxic metabolite of AA, a most relevant biomarker of exposure to AA is the molar ratio of GA-Hb and AA-Hb. We therefore tested the association of this biomarker with the various polymorphisms. For the CYP2E1 genotypes, we found no significant association with the ratio of GA-Hb/AA-Hb levels (Table 3). For the EPHX1 genotype, we found that individuals with the EPHX1 139Arg allele in exon 4, corresponding to increased enzyme activity, had significantly higher ratio of GA-Hb/AA-Hb compared with individuals with the wild-type allele 139His (p = 0.007) (Table 3). The 139Arg allele also gave strongly reduced absolute AA-Hb concentrations, which is contrary to what was expected. For the GST genotypes, we found a statistically significant association with the GSTM1 and GSTT1 genotypes (p = 0.039 and p = 0.006, respectively); the GA-Hb/AA-Hb ratio for the null alleles were increased (Table 3). In addition the absolute concentrations of GA-Hb were significantly increased for the null genotypes for both enzymes.
TABLE 3.
Genotype Distributions and their Association with the Biomarkers of AA Exposure in Blood
| Genotype | No. of subjects | Mean ratio of GA-Hb/AA-Hb ± SE | p Value | Mean AA-Hb (pmol/g globin) ± SE | p Value | Mean GA-Hb (pmol/g globin) ± SE | p Value |
| CYP2E1 (Val179Ile) | |||||||
| Val/Val | 44 | 0.52 ± 0.03 | 52.10 ± 5.70 | 25.82 ± 3.10 | |||
| Val/Ile | 5 | 0.70 ± 0.08 | 0.061 | 70.56 ± 35.46 | 0.987 | 40.04 ± 15.00 | 0.096 |
| Ile/Ile | 0 | — | — | — | |||
| CYP2E1 (Phe421Phe) | |||||||
| Phe/Phe (C/C) | 32 | 0.51 ± 0.03 | 47.00 ± 4.62 | 22.56 ± 2.12 | |||
| Phe/Phe (T/C) | 12 | 0.58 ± 0.06 | 0.224 | 63.50 ± 18.31 | 0.969 | 32.63 ± 8.76 | 0.490 |
| Phe/Phe (T/T) | 5 | 0.58 ± 0.12 | 0.846 | 76.63 ± 30.00 | 0.780 | 44.60 ± 17.48 | 0.589 |
| Phe/Phe (C/T + T/T) | 17 | 0.58 ± 0.05 | 0.275 | 67.35 ± 15.15 | 0.925 | 36.15 ± 7.85 | 0.411 |
| EPHX1 (Tyr113His) | |||||||
| Tyr/Tyr | 28 | 0.54 ± 0.04 | 42.53 ± 3.01 | 23.20 ± 3.12 | |||
| Tyr/His | 16 | 0.53 ± 0.04 | 0.971 | 75.80 ± 16.60 | 0.655 | 36.80 ± 7.45 | 0.211 |
| His/His | 5 | 0.53 ± 0.16 | 0.752 | 48.10 ± 13.75 | 0.865 | 19.70 ± 4.27 | 0.981 |
| Tyr/His + His/His | 21 | 0.53 ± 0.05 | 0.928 | 69.20 ± 13.17 | 0.772 | 32.72 ± 5.93 | 0.292 |
| EPHX1 (His139Arg) | |||||||
| His/His | 33 | 0.48 ± 0.03 | 64.00 ± 8.54 | 30.13 ± 4.48 | |||
| His/Arg | 12 | 0.65 ± 0.07 | 0.023* | 34.03 ± 2.50 | 0.007** | 22.30 ± 2.94 | 0.849 |
| Arg/Arg | 4 | 0.62 ± 0.07 | 0.073 | 31.31 ± 4.50 | 0.039** | 18.65 ± 2.56 | 0.463 |
| His/Arg + Arg/Arg | 16 | 0.64 ± 0.05 | 0.007* | 33.34 ± 2.13 | 0.002** | 21.40 ± 2.30 | 0.620 |
| GSTP1 (Ile105Val) | |||||||
| Ile/Ile | 25.0 | 0.55 ± 0.04 | 45.90 ± 6.70 | 24.45 ± 4.10 | |||
| Ile/Val | 16.0 | 0.50 ± 0.05 | 0.781 | 68.12 ± 13.53 | 0.132 | 32.16 ± 6.60 | 0.112 |
| Val/Val | 8.0 | 0.56 ± 0.06 | 0.853 | 50.92 ± 15.00 | 0.984 | 26.33 ± 6.25 | 0.397 |
| Ile/Val + Val/Val | 24 | 0.52 ± 0.04 | 0.913 | 62.40 ± 10.30 | 0.270 | 30.22 ± 4.81 | 0.108 |
| GSTM1 | |||||||
| Positive | 40 | 0.50 ± 0.03 | 47.10 ± 5.46 | 22.00 ± 2.50 | |||
| Null | 9 | 0.68 ± 0.08 | 0.039* | 84.44 ± 21.00 | 0.053 | 50.74 ± 10.20 | 0.001*** |
| GSTT1 | |||||||
| Positive | 24 | 0.45 ± 0.03 | 46.00 ± 5.76 | 19.41 ± 2.46 | |||
| Null | 25 | 0.61 ± 0.04 | 0.006* | 62.26 ± 11.00 | 0.335 | 35.46 ± 5.46 | 0.001*** |
Note. *Significant associations between GSTT1, GSTM1 and EPHX1 139Arg genotypes and GA-Hb/AA-Hb ratio levels in blood, p < 0.05. **Significant associations between EPHX1 139Arg allele and AA-Hb adduct concentration, p < 0.05. ***Significant associations between GSTM1 and GSTT1 genotypes and GA-Hb adduct concentration, p < 0.05. The numbers in italics indicate the p-value for each calculated association.
The ratio and the absolute concentrations of GA-Hb and AA-Hb adducts were also analyzed with respect to their association with the combined genotypes. We found significant associations between individuals with the double combination variant of GSTM1-null plus GSTT1-null (p = 0.029), and CYP2E1 (Val/Val) plus GSTM1 null (p = 0.022); both the ratio of GA-Hb/AA-Hb and the absolute GA-Hb adduct concentration for these combined variants were significantly higher in comparison with individuals which did not carry these genotype combinations (Table 4). The triple combination CYP2E1 (Val/Val), GSTM1 null and GSTT1 null, was also significantly associated with both increased adduct ratios and the absolute GA-Hb adduct concentration, p = 0.047 and p = 0.033, respectively (Table 4). There was also a significant association between the combination CYP2E1 (Val179Val), GSTT1 null, EPHX1 (Tyr113Tyr), and EPHX1 (His139Arg), and the ratio of GA-Hb/AA-Hb adduct level, p = 0.018 (Table 4). This means that individuals with these combined genotypes have significantly higher blood ratio of GA-Hb/AA-Hb levels, compared with individuals which did not carry these genotypes. Because the smokers had significantly higher AA-Hb and GA-Hb adduct concentration than nonsmokers, we reanalyzed the data for nonsmoking subjects. When the data for the six smokers were excluded and the associations were analyzed for the remaining individuals (43 nonsmokers), we observed similar trends as those for the whole study subjects, but the associations were slightly weaker with higher p values (Table 5). This suggests that the adduct level ratios were not affected by the high AA-Hb concentrations in smokers. The other polymorphic combinations were not statistically significant (data not shown). Attempts to correlate the AA-Hb level with the intake of AA-rich food, from food frequency questionnaires, without taking into account the polymorphisms in biotransformation enzymes, were unsuccessful. In a new study, using a revised database for AA content in various food items, a positive association was indeed found (Brantsaeter et al., 2008).
TABLE 4.
Association between Combined Genotypes and Biomarkers of AA Exposure in Blood
| Joint effects | No. of subjects | Mean ratio of GA-Hb/AA-Hb ± SE | p Value | Mean AA-Hb (pmol/g globin) ± SE | p Value | Mean GA-Hb (pmol/g globin) ± SE | p Value |
| GSTM1 and GSTT1 | |||||||
| Ref | 42 | 0.50 ± 0.03 | 49.70 ± 5.80 | 23.32 ± 2.62 | |||
| GSTM1-null + GSTT1-null | 7 | 0.71 ± 0.09 | 0.029* | 79.60 ± 24.50 | 0.196 | 51.00 ± 12.80 | 0.006** |
| CYP2E1 and GSTM1 | |||||||
| Ref | 41 | 0.50 ± 0.03 | 51.10 ± 6.65 | 23.90 ± 3.10 | |||
| CYP2E1 (Val/Val) + GSTM1-null | 8 | 0.70 ± 0.09 | 0.022* | 68.70 ± 15.57 | 0.175 | 44.71 ± 9.30 | 0.003** |
| CYP2E1, GSTM1, and GSTT1 | |||||||
| Ref | 44 | 0.52 ± 0.03 | 52.90 ± 6.65 | 25.10 ± 3.03 | |||
| CYP2E1 (Val/Val) + GSTM1-null + GSTT1-null | 5 | 0.74 ± 0.12 | 0.047* | 63.40 ± 14.50 | 0.236 | 46.54 ± 13.74 | 0.033** |
| CYP2E1, GSTT1, and EPHX1 | |||||||
| Ref. | 45 | 0.51 ± 0.03 | 55.64 ± 6.61 | 27.20 ± 3.40 | |||
| CYP2E1(Val/Val) + GSTT1-null + EPHX1 (Tyr/Tyr) + EPHX1 (His/Arg) | 4 | 0.79 ± 0.11 | 0.018* | 35.10 ± 2.90 | 0.385 | 28.10 ± 6.00 | 0.347 |
Note. *Significant associations between individuals with the combination variant of GSTM1 null and GSTT1 null, CYP2E1 (Val179Val) and GSTM1 null, CYP2E1 (Val/Val), GSTM1 null and GSTT1 null, and CYP2E1 (Val179Val), GSTT1 null, EPHX1 (Tyr113Tyr) and EPHX1 (His139Arg), and GA-Hb/AA-Hb ratio levels, p < 0.05. **Significant association between individuals with the combination variant of GSTM1 null and GSTT1 null, CYP2E1 (Val179Val) and GSTM1 null, and CYP2E1 (Val/Val), GSTM1 null and GSTT1 null, and GA-Hb adduct concentration, p < 0.05. The presence of the combination of the other genotypes was taken as the reference (or wild-type) group. The numbers in italics indicate the p-value for each calculated association.
TABLE 5.
Association between Genotypes and Biomarkers of AA Exposure in Blood for Nonsmoker Subjects (n = 43)
| Genotype | No. of subjects | Mean ratio of GA-Hb/AA-Hb ± SE | p Value | Mean AA-Hb (pmol/g globin) ± SE | p Value | Mean GA-Hb (pmol/g globin) ± SE | p Value |
| EPHX1 (His139Arg) | |||||||
| His/His | 27 | 0.47 ± 0.03 | 44.00 ± 3.14 | 19.83 ± 1.67 | |||
| His/Arg + Arg/Arg | 16 | 0.64 ± 0.05 | 0.008* | 33.34 ± 2.13 | 0.014** | 21.40 ± 2.30 | 0.593 |
| GSTM1 | |||||||
| Positive | 37 | 0.51 ± 0.03 | 38.50 ± 2.01 | 18.50 ± 1.10 | |||
| Null | 6 | 0.70 ± 0.09 | 0.042* | 49.40 ± 10.20 | 0.344 | 32.22 ± 4.70 | 0.004*** |
| GSTT1 | |||||||
| Positive | 20 | 0.46 ± 0.03 | 39.00 ± 3.00 | 16.41 ± 1.50 | |||
| Null | 23 | 0.62 ± 0.04 | 0.012* | 41.20 ± 3.60 | 0.638 | 24.44 ± 2.03 | 0.002*** |
| Joint genotype effects | |||||||
| GSTM1 and GSTT1 | |||||||
| Ref | 38 | 0.51 ± 0.03 | 38.80 ± 2.00 | 18.90 ± 1.13 | |||
| GSTM1-null + GSTT1-null | 5 | 0.71 ± 0.11 | 0.078 | 49.50 ± 12.50 | 0.543 | 32.20 ± 5.80 | 0.021*** |
| CYP2E1 and GSTM1 | |||||||
| Ref | 37 | 0.51 ± 0.03 | 38.50 ± 2.00 | 18.50 ± 1.20 | |||
| CYP2E1 (Val/Val) + GSTM1-null | 6 | 0.70 ± 0.09 | 0.042* | 49.40 ± 10.20 | 0.344 | 32.22 ± 4.70 | 0.004*** |
| CYP2E1, GSTM1, and GSTT1 | |||||||
| Ref | 39 | 0.52 ± 0.03 | 38.52 ± 2.00 | 19.02 ± 1.11 | |||
| CYP2E1 (Val/Val) + GSTM1-null + GSTT1-null | 4 | 0.66 ± 0.14 | 0.244 | 54.50 ± 14.80 | 0.244 | 34.00 ± 7.10 | 0.031*** |
| CYP2E1, GSTT1, and EPHX1 | |||||||
| Ref. | 39 | 0.51 ± 0.03 | 40.51 ± 2.45 | 19.63 ± 1.31 | |||
| CYP2E1(Val/Val) + GSTT1-null + EPHX1 (Tyr/Tyr) + EPHX1 (His/Arg) | 4 | 0.79 ± 0.11 | 0.021* | 35.06 ± 2.90 | 0.614 | 28.10 ± 6.00 | 0.120 |
Note. *Significant association between individuals with the genotype or combination genotype and GA-Hb/AA-Hb adduct ratio levels, p < 0.05. **Significant association between individuals with the genotype or combination genotype and AA-Hb adduct concentration, p < 0.05. ***Significant association between individuals with the genotype or combination genotype and GA-Hb adduct concentration, p < 0.05. The presence of the combination of the other genotypes was taken as the reference (or wild-type) group. The numbers in italics indicate the p-value for each calculated association.
DISCUSSION
In rodents, AA is metabolized by CYP2E1 to the genotoxic epoxide GA which forms adducts with DNA and proteins (Ghanayem et al., 2005; Sumner et al., 1999). Conjugation with glutathione is a major detoxification pathway for both AA and GA. Genetic polymorphisms affecting enzymes involved in AA biotransformation could be important determinants of the individual variability of AA metabolism and therefore the individual susceptibility; this also concerns many other human xenobiotic metabolizing enzymes (Schoket et al., 2001).
We recently reported major interindividual variations in the dietary intake of AA estimated from food frequency questionnaires (Bjellaas et al., 2007). These were however neither associated with the observed levels of AA-Hb nor the GA-Hb in blood (data not shown). The AA-Hb adduct concentration will be a marker for the internal AA dose, whereas the resulting GA-Hb concentration is a marker of the internal genotoxic dose. By relating the GA-Hb concentration to the AA-Hb concentration in assessing the role of biotransformation enzymes for the internal genotoxic dose, we compensate both for variations in external AA exposure and also for variations in the influence of enzymatic removal of AA. Hence, the ratio of GA-Hb to AA-Hb is expected to be a biomarker of AA-related genotoxic exposure. This ratio varied considerably (ninefold) between the individuals in this study.
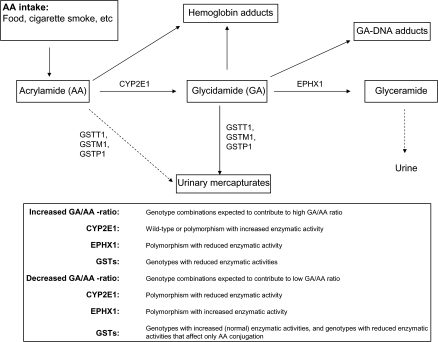
Our primary aim was to investigate whether the observed interindividual variations could be related to polymorphic differences in the genes CYP2E1, EPHX1, GSTM1, GSTT1, and GSTP1, either alone or in combination. It would be expected that the net level of GA-Hb—resulting from endogenously formed GA—results from a combination of metabolic reactions as depicted in Figure 2. Maximum GA is expected with the most efficient CYP2E1 genotype, and—at the same time—the least efficient genotypes of GSTM1, GSTP1, GSTT1, and the least efficient genotype of EPHX1. The minimum GA levels would be expected with the inverse genotype combinations.
FIG. 2.
Metabolism of AA and GA. The association between AA and its metabolite, with drug metabolizing enzymes.
We observed that individuals with GSTM1- and GSTT1-null phenotype have significantly higher ratio of GA-Hb/AA-Hb compared with individuals without these genotypes, and these individuals may therefore be prone to increased risk resulting from the decreased enzymatic activity in removal of both AA and GA. The null genotypes were both associated with increased absolute AA- and GA-Hb adduct concentrations.
Analysis of the combined effects of various combinations of genotypes (Table 4) indicates that individuals with some specific genotype combinations had significantly higher ratio of GA-Hb/AA-Hb in the blood. These combinations were (1) GSTM1- and GSTT1- null; (2) CYP2E1 179Val and GSTM1 null; (3) CYP2E1 179Val, GSTM1 null and GSTT1 null; (4) CYP2E1 179Val, GSTT1 null, EPHX1 113Tyr and EPHX1 139Arg. The observed associations may be explained by the biological roles of these enzymes in activation/deactivation as discussed above (Fig. 2). However, the combination (4) was unexpected because the EPHX1 genotype combinations are assumed to give increased enzymatic activity (Benhamou et al., 1998; Hassett et al., 1994) and a concomitant reduced concentration of GA. The EPHX1 139Arg allele unexpectedly, contrary to our expectations of high activity (Fig. 2), was associated with an increased relative concentration of GA-Hb. In individuals with the EPHX1 139Arg (high activity) alleles, the AA-Hb adduct concentrations were significantly lower (about 50%) than in individuals with the EPHX1 139His (low activity) allele (Table 3). The reason for this is unknown. The exceptional low AA-Hb concentration would be important for a resulting high GA-Hb/AA-Hb ratio. It should be noted that an EPHX1 polymorphisms with a low activity can be compensated for by increased expression (Hosagrahara et al., 2004; Omiecinski et al., 2000). Other combinations of the alleles studied here did not exhibit any indication of significant association (data not shown).
It has previously been shown that the lack of the GSTT1 gene increases the genotoxic effects of styrene-7,8-oxide in human whole-blood lymphocyte cultures, suggesting that GSTT1 is involved in the detoxification of styrene oxide in humans (Ollikainen et al., 1998). A positive association has also been reported for ethylene oxide and GSTT1 (Fost et al., 1995). Recently, Paulsson et al. (2005) studied the Hb-adduct levels after in vitro incubation of blood from various donors with AA or GA, and reported that the polymorphisms in the GSTT1 or GSTM1 genes did not affect the adduct levels in blood (Paulsson et al., 2005). This was however a short-term in vitro exposure whereas our results are from humans, chronically exposed to AA primarily via the diet. The GSTM1 gene is one of the most studied genes related to metabolic polymorphisms and cancer risk. The GSTM1 null genotype has been shown to increase the frequency of chromosome aberrations after in vitro N-nitrosamine exposure, and in addition, it has been shown to modulate PAH DNA adduct levels (Ryberg et al., 1997; Salama et al., 1999).
Interactions between several polymorphic variants have been reported for other xenobiotics. Ryberg et al. (1997) studied the interaction between GSTM1 null and GSTP1 105Val genotypes, and observed that individuals with this combination had significantly higher PAH DNA adduct levels in their lung tissue than the other genotype combinations (Ryberg et al., 1997). Another study has shown that the GSTM1 null and GSTP1 105Val allele combination was associated with a four times increased risk of lung cancer in individuals exposed to environmental tobacco smoke (Wenzlaff et al., 2005). The GSTM1 genotype has been shown to modulate the levels of PAH DNA adducts, in an interaction with other genotypes, particularly with GSTP1 and CYP1A1 (Butkiewicz et al., 1998; Ichiba et al., 1994; Ryberg et al., 1997). In our study of AA, we observed a similar interaction between the CYP-dependent activation and the GST-dependent deactivation.
The intake of AA via food may vary markedly between individuals as indicated by food frequency questionnaires (Brantsaeter et al., 2008). This variation, but also the genotype combination as suggested in our study, would be expected to contribute to the overall risk of individuals for AA-associated disease such as neurotoxicity and cancer. In particular this applies to the GSTM1 null genotype, which in addition to increasing the relative and absolute internal dose of GA also tends to increase the internal AA dose (p = 0.053). A larger study group would needed to confirm these findings, because the data in the present work are based on relatively few individuals. The gene-environment interaction may therefore be taken into account in the analysis of epidemiological data.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
Norwegian Research Council and the European Commission, Priority 5 on Food Quality and Safety (Contract n°FOOD-CT-2003-506820 HEATOX).
Supplementary Material
Acknowledgments
This publication reflects the authors' views and not necessarily those of the EC. The information in this document is provided as is and no guarantee or warranty is given that the information is fit for any particular purpose. The user thereof uses the information at its sole risk and liability. We thank Mathias Eriksen and Khalid Mohammad for their excellent technical assistance.
References
- Abdel-Rahman SZ, el Zein RA, Anwar WA, Au WW. A multiplex PCR procedure for polymorphic analysis of GSTM1 and GSTT1 genes in population studies. Cancer Lett. 1996;107:229–233. doi: 10.1016/0304-3835(96)04832-x. [DOI] [PubMed] [Google Scholar]
- Ali-Osman F, Akande O, Antoun G, Mao JX, Buolamwini J. Molecular cloning, characterization, and expression in Escherichia coli of full-length cDNAs of three human glutathione S-transferase Pi gene variants. Evidence for differential catalytic activity of the encoded proteins. J. Biol. Chem. 1997;272:10004–10012. doi: 10.1074/jbc.272.15.10004. [DOI] [PubMed] [Google Scholar]
- Autrup H. Genetic polymorphisms in human xenobiotica metabolizing enzymes as susceptibility factors in toxic response. Mutat. Res. 2000;464:65–76. doi: 10.1016/s1383-5718(99)00167-9. [DOI] [PubMed] [Google Scholar]
- Benhamou S, Reinikainen M, Bouchardy C, Dayer P, Hirvonen A. Association between lung cancer and microsomal epoxide hydrolase genotypes. Cancer Res. 1998;58:5291–5293. [PubMed] [Google Scholar]
- Bjellaas T, Olesen PT, Frandsen H, Haugen M, Stolen LH, Paulsen JE, Alexander J, Lundanes E, Becher G. Comparison of estimated dietary intake of acrylamide with hemoglobin adducts of acrylamide and glycidamide. Toxicol. Sci. 2007;98:110–117. doi: 10.1093/toxsci/kfm091. [DOI] [PubMed] [Google Scholar]
- Board PG. Biochemical genetics of glutathione-S-transferase in man. Am. J. Hum. Genet. 1981;33:36–43. [PMC free article] [PubMed] [Google Scholar]
- Brantsaeter AL, Haugen M, Mul A, Bjellaas T, Becher G, Klaveren JV, Alexander J, Meltzer HM. Exploration of different methods to assess dietary acrylamide exposure in pregnant women participating in the Norwegian Mother and Child Cohort Study (MoBa) Food Chem. Toxicol. 2008;46:2808–2814. doi: 10.1016/j.fct.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Butkiewicz D, Grzybowska E, Hemminki K, Ovrebo S, Haugen A, Motykiewicz G, Chorazy M. Modulation of DNA adduct levels in human mononuclear white blood cells and granulocytes by CYP1A1 CYP2D6 and GSTM1 genetic polymorphisms. Mutat. Res. 1998;415:97–108. doi: 10.1016/s1383-5718(98)00064-3. [DOI] [PubMed] [Google Scholar]
- Cotton SC, Sharp L, Little J, Brockton N. Glutathione S-transferase polymorphisms and colorectal cancer: A HuGE review. Am. J. Epidemiol. 2000;151:7–32. doi: 10.1093/oxfordjournals.aje.a010124. [DOI] [PubMed] [Google Scholar]
- Dearfield KL, Douglas GR, Ehling UH, Moore MM, Sega GA, Brusick DJ. Acrylamide: A review of its genotoxicity and an assessment of heritable genetic risk. Mutat. Res. 1995;330:71–99. doi: 10.1016/0027-5107(95)00037-j. [DOI] [PubMed] [Google Scholar]
- Doerge DR, da Costa GG, McDaniel LP, Churchwell MI, Twaddle NC, Beland FA. DNA adducts derived from administration of acrylamide and glycidamide to mice and rats. Mutat. Res. 2005;580:131–141. doi: 10.1016/j.mrgentox.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Fairbrother KS, Grove J, de WI, Steimel DT, Day CP, Crespi CL, Daly AK. Detection and characterization of novel polymorphisms in the CYP2E1 gene. Pharmacogenetics. 1998;8:543–552. doi: 10.1097/00008571-199812000-00011. [DOI] [PubMed] [Google Scholar]
- Fost U, Tornqvist M, Leutbecher M, Granath F, Hallier E, Ehrenberg L. Effects of variation in detoxification rate on dose monitoring through adducts. Hum. Exp. Toxicol. 1995;14:201–203. doi: 10.1177/096032719501400208. [DOI] [PubMed] [Google Scholar]
- Garte S, Gaspari L, Alexandrie AK, Ambrosone C, Autrup H, Autrup JL, Baranova H, Bathum L, Benhamou S, Boffetta P, et al. Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol. Biomarkers Prev. 2001;10:1239–1248. [PubMed] [Google Scholar]
- Ghanayem BI, McDaniel LP, Churchwell MI, Twaddle NC, Snyder R, Fennell TR, Doerge DR. Role of CYP2E1 in the epoxidation of acrylamide to glycidamide and formation of DNA and hemoglobin adducts. Toxicol. Sci. 2005;88:311–318. doi: 10.1093/toxsci/kfi307. [DOI] [PubMed] [Google Scholar]
- Hagmar L, Wirfalt E, Paulsson B, Tornqvist M. Differences in hemoglobin adduct levels of acrylamide in the general population with respect to dietary intake, smoking habits and gender. Mutat. Res. 2005;580:157–165. doi: 10.1016/j.mrgentox.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Hanioka N, Tanaka-Kagawa T, Miyata Y, Matsushima E, Makino Y, Ohno A, Yoda R, Jinno H, Ando M. Functional characterization of three human cytochrome p450 2E1 variants with amino acid substitutions. Xenobiotica. 2003;33:575–586. doi: 10.1080/0049825031000086400. [DOI] [PubMed] [Google Scholar]
- Hassett C, Aicher L, Sidhu JS, Omiecinski CJ. Human microsomal epoxide hydrolase: Genetic polymorphism and functional expression in vitro of amino acid variants. Hum. Mol. Genet. 1994;3:421–428. doi: 10.1093/hmg/3.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JD, Strange RC. Potential contribution of the glutathione S-transferase supergene family to resistance to oxidative stress. Free Radic. Res. 1995;22:193–207. doi: 10.3109/10715769509147539. [DOI] [PubMed] [Google Scholar]
- Ho CT. Maillard reaction and health aspects. Mol. Nutr. Food Res. 2006;50:1099–1100. [Google Scholar]
- Hosagrahara VP, Rettie AE, Hassett C, Omiecinski CJ. Functional analysis of human microsomal epoxide hydrolase genetic variants. Chem. Biol. Interact. 2004;150:149–159. doi: 10.1016/j.cbi.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC. IARC: Acrylamide. IARC Monogr. Eval. Carcinog. Risks Hum. 1994;60:389–433. 389–433. [PMC free article] [PubMed] [Google Scholar]
- Ichiba M, Hagmar L, Rannug A, Hogstedt B, Alexandrie AK, Carstensen U, Hemminki K. Aromatic DNA adducts, micronuclei and genetic polymorphism for CYP1A1 and GST1 in chimney sweeps. Carcinogenesis. 1994;15:1347–1352. doi: 10.1093/carcin/15.7.1347. [DOI] [PubMed] [Google Scholar]
- Kirman CR, Gargas ML, Deskin R, Tonner-Navarro L, Andersen ME. A physiologically based pharmacokinetic model for acrylamide and its metabolite, glycidamide, in the rat. J. Toxicol. Environ. Health A. 2003;66:253–274. doi: 10.1080/15287390306368. [DOI] [PubMed] [Google Scholar]
- Mannervik B, Awasthi YC, Board PG, Hayes JD, Di Ilio C, Ketterer B, Listowsky I, Morgenstern R, Muramatsu M, Pearson WR, et al. Nomenclature for human glutathione transferases. Biochem. J. 1992;282:305–306. doi: 10.1042/bj2820305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer DJ, Coles B, Pemble SE, Gilmore KS, Fraser GM, Ketterer B. Theta, a new class of glutathione transferases purified from rat and man. Biochem. J. 1991;274:409–414. doi: 10.1042/bj2740409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottram DS, Wedzicha BL, Dodson AT. Acrylamide is formed in the Maillard reaction. Nature. 2002;419:448–449. doi: 10.1038/419448a. [DOI] [PubMed] [Google Scholar]
- Nelson HH, Wiencke JK, Christiani DC, Cheng TJ, Zuo ZF, Schwartz BS, Lee BK, Spitz MR, Wang M, Xu X, et al. Ethnic differences in the prevalence of the homozygous deleted genotype of glutathione S-transferase theta. Carcinogenesis. 1995;16:1243–1245. doi: 10.1093/carcin/16.5.1243. [DOI] [PubMed] [Google Scholar]
- Olesen PT, Olsen A, Frandsen H, Frederiksen K, Overvad K, Tjonneland A. Acrylamide exposure and incidence of breast cancer among postmenopausal women in the Danish Diet, Cancer and Health Study. Int. J. Cancer. 2008;122:2094–2100. doi: 10.1002/ijc.23359. [DOI] [PubMed] [Google Scholar]
- Ollikainen T, Hirvonen A, Norppa H. Influence of GSTT1 genotype on sister chromatid exchange induction by styrene-7,8-oxide in cultured human lymphocytes. Environ. Mol. Mutagen. 1998;31:311–315. doi: 10.1002/(sici)1098-2280(1998)31:4<311::aid-em2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Omiecinski CJ, Hassett C, Hosagrahara V. Epoxide hydrolase—Polymorphism and role in toxicology. Toxicol. Lett. 2000;112-113:365–370. doi: 10.1016/s0378-4274(99)00235-0. [DOI] [PubMed] [Google Scholar]
- Paulsson B, Rannug A, Henderson AP, Golding BT, Tornqvist M, Warholm M. In vitro studies of the influence of glutathione transferases and epoxide hydrolase on the detoxification of acrylamide and glycidamide in blood. Mutat. Res. 2005;580:53–59. doi: 10.1016/j.mrgentox.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Pemble S, Schroeder KR, Spencer SR, Meyer DJ, Hallier E, Bolt HM, Ketterer B, Taylor JB. Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterization of a genetic polymorphism. Biochem. J. 1994;300:271–276. doi: 10.1042/bj3000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter H, Deutschmann S, Muelle A, Gansewendt B, Bolt M, Hallier E. Different affinity of erythrocyte glutathione-S-transferase to methyl chloride in humans. Arch. Toxicol. Suppl. 1989;13:128–132. doi: 10.1007/978-3-642-74117-3_17. [DOI] [PubMed] [Google Scholar]
- Rebbeck TR. Molecular epidemiology of the human glutathione S-transferase genotypes GSTM1 and GSTT1 in cancer susceptibility. Cancer Epidemiol. Biomarkers Prev. 1997;6:733–743. [PubMed] [Google Scholar]
- Ryberg D, Kure E, Lystad S, Skaug V, Stangeland L, Mercy I, Borresen AL, Haugen A. p53 mutations in lung tumors: Relationship to putative susceptibility markers for cancer. Cancer Res. 1994;54:1551–1555. [PubMed] [Google Scholar]
- Ryberg D, Skaug V, Hewer A, Phillips DH, Harries LW, Wolf CR, Ogreid D, Ulvik A, Vu P, Haugen A. Genotypes of glutathione transferase M1 and P1 and their significance for lung DNA adduct levels and cancer risk. Carcinogenesis. 1997;18:1285–1289. doi: 10.1093/carcin/18.7.1285. [DOI] [PubMed] [Google Scholar]
- Salama SA, Abdel-Rahman SZ, Sierra-Torres CH, Hamada FA, Au WW. Role of polymorphic GSTM1 and GSTT1 genotypes on NNK-induced genotoxicity. Pharmacogenetics. 1999;9:735–743. [PubMed] [Google Scholar]
- Schoket B, Papp G, Levay K, Mrackova G, Kadlubar FF, Vincze I. Impact of metabolic genotypes on levels of biomarkers of genotoxic exposure. Mutat. Res. 2001;482:57–69. doi: 10.1016/s0027-5107(01)00210-x. [DOI] [PubMed] [Google Scholar]
- Segerback D, Calleman CJ, Schroeder JL, Costa LG, Faustman EM. Formation of N-7-(2-carbamoyl-2-hydroxyethyl)guanine in DNA of the mouse and the rat following intraperitoneal administration of [14C]acrylamide. Carcinogenesis. 1995;16:1161–1165. doi: 10.1093/carcin/16.5.1161. [DOI] [PubMed] [Google Scholar]
- Stadler RH, Blank I, Varga N, Robert F, Hau J, Guy PA, Robert MC, Riediker S. Acrylamide from Maillard reaction products. Nature. 2002;419:449–450. doi: 10.1038/419449a. [DOI] [PubMed] [Google Scholar]
- Sumner SC, Fennell TR, Moore TA, Chanas B, Gonzalez F, Ghanayem BI. Role of cytochrome P450 2E1 in the metabolism of acrylamide and acrylonitrile in mice. Chem. Res. Toxicol. 1999;12:1110–1116. doi: 10.1021/tx990040k. [DOI] [PubMed] [Google Scholar]
- Sumner SC, MacNeela JP, Fennell TR. Characterization and quantitation of urinary metabolites of [1,2,3–13C]acrylamide in rats and mice using 13C nuclear magnetic resonance spectroscopy. Chem. Res. Toxicol. 1992;5:81–89. doi: 10.1021/tx00025a014. [DOI] [PubMed] [Google Scholar]
- Sumner SC, Selvaraj L, Nauhaus SK, Fennell TR. Urinary metabolites from F344 rats and B6C3F1 mice coadministered acrylamide and acrylonitrile for 1 or 5 days. Chem. Res. Toxicol. 1997;10:1152–1160. doi: 10.1021/tx9602123. [DOI] [PubMed] [Google Scholar]
- The International HapMap Project. The International HapMap Project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- Thorisson GA, Smith AV, Krishnan L, Stein LD. The International HapMap Project Web site. Genome Res. 2005;15:1592–1593. doi: 10.1101/gr.4413105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornqvist M, Fred C, Haglund J, Helleberg H, Paulsson B, Rydberg P. Protein adducts: Quantitative and qualitative aspects of their formation, analysis and applications. J. Chromatogr. B. 2002;778:279–308. doi: 10.1016/s1570-0232(02)00172-1. [DOI] [PubMed] [Google Scholar]
- Vesper HW, Slimani N, Hallmans G, Tjonneland A, Agudo A, Benetou V, Bingham S, Boeing H, Boutron-Ruault MC, Bueno-de-Mesquita HB, et al. Cross-sectional study on acrylamide hemoglobin adducts in subpopulations from the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. J. Agric. Food Chem. 2008;56:6046–6053. doi: 10.1021/jf703750t. [DOI] [PubMed] [Google Scholar]
- Wenzlaff AS, Cote ML, Bock CH, Land SJ, Schwartz AG. GSTM1, GSTT1 and GSTP1 polymorphisms, environmental tobacco smoke exposure and risk of lung cancer among never smokers: A population-based study. Carcinogenesis. 2005;26:395–401. doi: 10.1093/carcin/bgh326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.