Abstract
Sulindac (SLD) is a nonsteroidal anti-inflammatory drug (NSAID) that has been associated with a greater incidence of idiosyncratic hepatotoxicity in human patients than other NSAIDs. One hypothesis regarding idiosyncratic adverse drug reactions is that interaction of a drug with a modest inflammatory episode precipitates liver injury. In this study, we tested the hypothesis that lipopolysaccharide (LPS) interacts with SLD to cause liver injury in rats. SLD (50 mg/kg) or its vehicle was administered to rats by gavage 15.5 h before LPS (8.3 × 105 endotoxin unit/kg) or its saline vehicle (i.v.). Thirty minutes after LPS treatment, SLD or vehicle administration was repeated. Rats were killed at various times after treatment, and serum, plasma, and liver samples were taken. Neither SLD nor LPS alone caused liver injury. Cotreatment with SLD/LPS led to increases in serum biomarkers of both hepatocellular injury and cholestasis. Histological evidence of liver damage was found only after SLD/LPS cotreatment. As a result of activation of hemostasis induced by SLD/LPS cotreatment, fibrin and hypoxia were present in liver tissue before the onset of hepatotoxicity. Heparin treatment reduced hepatic fibrin deposition and hypoxia and protected against liver injury induced by SLD/LPS cotreatment. These results indicate that cotreatment with nontoxic doses of LPS and SLD causes liver injury in rats, and this could serve as a model of human idiosyncratic liver injury. The hemostatic system is activated by SLD/LPS cotreatment and plays an important role in the development of SLD/LPS-induced liver injury.
Keywords: idiosyncratic adverse drug reactions, inflammation, sulindac, hepatotoxicity, hemostasis, hypoxia
Idiosyncratic adverse drug reactions (IADRs) have no obvious relation to dose, are unrelated to the pharmacologic effect of the drug and are currently unpredictable. Typically, IADRs occur in a small fraction of the patients who are treated with a specific drug. The liver is a frequent target of IADRs. Some IADRs result in permanent disability or death. They are also a major issue for drug development because they are not currently predicted by animal toxicity studies and become apparent only after the drug is extensively used in humans.
Sulindac (SLD) is a prodrug in the therapeutic class of nonsteroidal anti-inflammatory drugs (NSAIDs) and is used to treat osteoarthritis and rheumatoid arthritis. It is metabolized in the liver to its active form, SLD sulfide, and performs its pharmacological function by inhibiting cyclooxygenases 1 and 2. SLD is associated with idiosyncratic liver injury, which presents as cholestatic, hepatocellular and mixed patterns (Aithal and Day, 2007). Among the NSAIDs, SLD is associated with the greatest risk for liver injury in human patients (Garcia Rodriguez et al., 1994).
The mechanisms behind drug-induced idiosyncratic liver injury are unclear. One hypothesis is that inflammatory stress increases sensitivity to drug-induced liver injury (Ganey et al., 2004). Exposure to inflammagens is variable and episodic, and this might explain why IADRs are variable in time of onset and occur in some patients at doses that are safe in most people. Previous studies suggested that mild inflammation induced by bacterial lipopolysaccharide (LPS) could potentiate hepatotoxicity in rodents from IADR-associated drugs such as chlorpromazine (Buchweitz et al., 2002), ranitidine (Luyendyk et al., 2003), and trovafloxacin (Shaw et al., 2007). In one of these LPS/drug interaction models, the hemostatic system proved to be important in the liver pathogenesis (Luyendyk et al., 2005). This system comprises coagulation and fibrinolytic components. In coagulation, thrombin plays a critical role by cleaving fibrinogen to fibrin that can form occlusive clots in sinusoids. Meanwhile, plasminogen activator inhibitor-1 (PAI-1) can inhibit fibrin clearance by the fibrinolytic system by inhibiting the generation of plasmin from plasminogen.
To evaluate the utility of LPS/drug interaction models to study mechanism(s) of idiosyncratic liver injury, a broad range of drugs needs to be tested. Therefore, the purpose of this study was to test the hypothesis that modest inflammation induced by LPS interacts with SLD to cause liver injury in rats. When the results demonstrated a hepatotoxic interaction, the role of the hemostatic system was explored.
MATERIALS AND METHODS
Materials.
Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich (St Louis, MO). The activity of LPS (Lot 075K4038) derived from Eschericia coli serotype O55:B5 was 3.3 × 106 endotoxin units (EU)/mg as determined by a Limulus amebocyte lysate endpoint assay kit purchased from Cambrex Corp. (Kit 50-650U; East Rutherford, NJ). The reagents for the determination of alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyltransferase (GGT), and total bilirubin were purchased from Thermo Corp. (Waltham, MA). Alkaline phosphatase (ALP) reagent was purchased from BioAssay Systems (Hayward, CA), and the kit for total bile acid determination was purchased from Diazyme Laboratories (Poway, CA).
Animals.
Male, Sprague-Dawley rats (Crl:CD(SD)IGS BR; Charles River, Portage, MI) weighing 250–370 g or 150–200 g were used for in vivo or in vitro studies, respectively. Animals were allowed to acclimate for 1 week in a 12-h light/dark cycle prior to use in experiments. They were fed standard chow (Rodent Chow/Tek 8640; Harlan Teklad, Madison, WI) and allowed access to water ad libitum.
Experimental protocol.
Two administrations of SLD were used in the studies (Supplemental Fig. 1). In a dose-response study, rats were given the first administration of SLD (10, 20, 50, 100, or 300 mg/kg, p.o.) or its vehicle (0.5% methyl cellulose), and food was removed for 24 h. Fifteen and a half hours after the first administration of SLD, LPS (8.25 × 105 EU/kg, i.v.) or its saline vehicle was administered. Half an hour later, a second administration of SLD (same dose) or its vehicle was given. Rats were anesthetized with isoflurane and killed at various times after the second administration of SLD. For subsequent studies, 50 mg/kg was chosen as the dose of SLD. For all studies, blood was drawn from the vena cava of anesthetized rats, and part was transferred into vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ) containing sodium citrate for preparation of plasma. The rest of the blood was allowed to clot at room temperature for preparation of serum. The exterior of the liver was rinsed with saline, and a portion of the left medial lobe was snap frozen in cooled methylbutane for immunohistochemistry. Three slices of the left lateral lobe about 3–4 mm thick were fixed in 10% buffered formalin for histological analysis. In experiments designed to evaluate the role of the hemostatic system, anticoagulant heparin (3000 Units/kg, s.c.) or its saline vehicle was given to rats 0 and 6 h after the second administration of SLD.
Evaluation of liver injury.
Hepatic parenchymal cell injury was assessed by measuring the activities of ALT and AST in serum. Cholestatic injury markers, including the activities of ALP and GGT, as well as the concentrations of total bilirubin and bile acids in serum, were also assessed (see above).
Formalin-fixed liver slices were embedded in paraffin and cut into 6-μm sections. Hematoxylin and eosin (H&E) staining was performed, and sections were examined under 100× magnification using a light microscope. Eight, randomly chosen microscope fields for each slide were evaluated for midzonal necrosis and assigned a score of 0–5. 0 represents no liver injury, and 1–5 represent lesions ranging from single cell necrosis (1) to necrotic area encompassing greater than 30% of the field (5). The average score was determined for each rat.
Evaluation of serum tumor necrosis factor-α concentrations.
The concentration of tumor necrosis factor (TNF)-α in serum taken at 1 h after the second administration of SLD was measured using an enzyme-linked immunosorbent assay (ELISA) kit purchased from BD Biosciences (San Diego, CA).
Evaluation of hemostasis and fibrin deposition.
Thrombin-antithrombin dimer (TAT) concentration in plasma was used as a marker of thrombin activation and evaluated using an ELISA kit (catalog number OWMG15) purchased from Dade Behring, Inc. (Deerfield, IL). The concentration of the active PAI-1 was determined using a kit from Molecular Innovations, Inc. (Southfield, MI).
The immunohistochemistry and quantification for cross-linked fibrin in liver were performed as described previously (Copple et al., 2002). Fibrin monomer is solubilized in this protocol, and only cross-linked fibrin in liver is stained. To investigate whether fibrin deposition occurs before the onset of injury, livers were collected at 4 h and fixed for immunohistochemistry. The fraction of positive pixels averaged from 10 randomly chosen microscope fields was determined for each animal.
Evaluation of liver hypoxia.
Liver hypoxia was evaluated by quantifying pimonidazole (PIM)-protein adducts. PIM is a hypoxia probe which is rapidly reduced under low pO2 conditions to a reactive intermediate that forms PIM-protein adducts. PIM hydrochloride (Hypoxyprobe-1, 120 mg/kg; Chemicon International, Temecula, CA) was given to rats 2 h before sacrifice. Four hours after the second administration of SLD, livers were collected and fixed for immunohistochemistry. The fraction of positive pixels averaged from 10 randomly chosen microscope fields was determined for each rat (Copple et al., 2004).
Hepatocyte isolation and hepatocytotoxicity assessment in vitro.
Hepatocytes (HPCs) were isolated from rat liver as previously described (Tukov et al., 2006). Isolated cells were suspended in Williams’ Medium E (Gibco BRL, Rockville, MD) with 10% fetal bovine serum, and cell viability was evaluated using trypan blue exclusion. The cell viability was always above 80%. The HPCs were suspended and plated randomly at a density of 2.5 × 105 cells/well in 12-well plates (Corning Inc., Corning, NY). After 2.5- to 3-h incubation which allowed HPCs to attach to the plate, serum-containing medium was removed, and serum-free medium was added. HPCs were treated with 60μM SLD sulfide or its vehicle (0.06% dimethyl sulfoxide) and incubated in the presence of 20% or 5% O2 (with 5% CO2 and balance N2). After 8-h incubation, the medium was collected, and the unattached cells were isolated by centrifugation. Both the remaining attached cells and unattached cells were lysed with 1% Triton X-100. ALT activity in the medium, attached cell lysate and unattached cell lysate was determined. Hepatocytotoxicity was assessed by calculating the ALT activity in the medium plus unattached cells as a percentage of the total ALT activity in the well (medium + unattached cell lysate + attached cell lysate).
Statistical analysis.
One-way or two-way ANOVA was used for data analysis, and Tukey's test was employed as a post hoc test. For GGT activity and necrotic lesion score data, an ANOVA on ranks was performed, and Dunn's test was used for multiple comparisons. p < 0.05 was set as the criterion for statistical significance.
RESULTS
Dose-Response and Time Course of Liver Injury
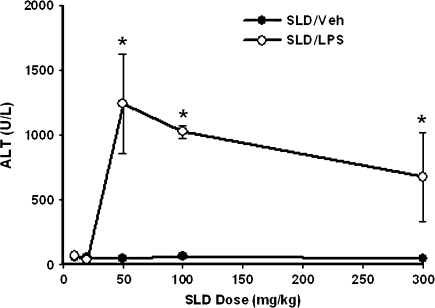
SLD alone did not induce liver injury in rats at any of the doses given. SLD (two administrations) at doses of 10 or 20 mg/kg did not cause hepatotoxicity in LPS-treated rats; however, rats had significant liver injury after cotreatment with 50, 100, or 300 mg/kg SLD plus LPS (Fig. 1). Fifty milligrams per kilogram was chosen as the SLD dose for further study.
FIG. 1.
SLD dose-response in the absence and presence of LPS. Rats were treated with various doses of SLD (10, 20, 50, 100, or 300 mg/kg, p.o.) or its vehicle (0.5% methyl cellulose) at −16 h, and food was removed. At −0.5 h rats received LPS (8.25 × 105 EU/kg, i.v.) or its vehicle (saline), and 30 min later they were given a second administration (same dose as first administration) of SLD. Blood samples were taken at 12 h after the second administration of SLD, and ALT activity was measured. *Significantly different from SLD/Veh group at the same dose. p < 0.05, n = 3.
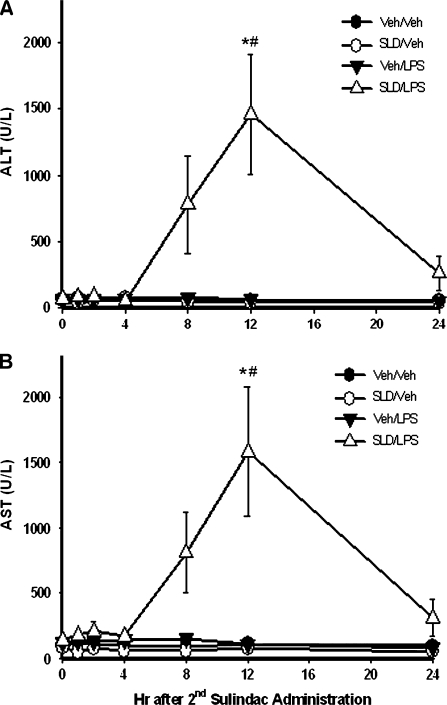
In a time course study, SLD or LPS given alone did not increase serum ALT activity at any time examined (Fig. 2A). In the SLD/LPS-cotreated group, ALT activity remained normal for 4 h but began to increase by 8 h after the second administration of SLD. By 12 h, a significant increase in serum ALT activity was observed. By 24 h, ALT activity had decreased to near normal. The activity of serum AST in rats also reached its peak at 12 h and showed a pattern similar to ALT activity (Fig. 2B).
FIG. 2.
Development of hepatocellular injury induced by SLD/LPS cotreatment. Rats were treated with SLD (50 mg/kg, p.o.) or its vehicle (0.5% methyl cellulose) and LPS or its vehicle as described in Figure 1. Blood samples were taken at various times (0, 1, 2, 4, 8, 12, or 24 h), and ALT (A) and AST (B) activities in serum were measured. *Significantly different from all other groups at the same time. #Significantly different from SLD/LPS group at 0 h. p < 0.05, n = 5–10.
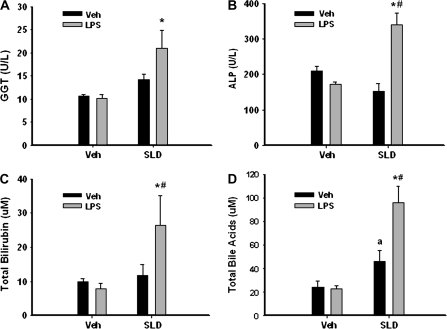
At 12 h, the activities of ALP and GGT as well as the concentrations of total bilirubin and bile acids were also elevated significantly in the sera of SLD/LPS-cotreated rats compared with those of rats treated with SLD or LPS alone (Fig. 3).
FIG. 3.
Markers of hepatic cholestasis induced by SLD/LPS cotreatment. Rats were treated as described in Figure 2, and serum samples were collected at 12 h. Activities of GGT (A) and ALP (B) as well as concentrations of total bilirubin (C) and bile acids (D) in serum at 12 h were evaluated. *Significantly different from Veh/LPS group. #Significantly different from SLD/Veh group. aSignificantly different from Veh/Veh group. p < 0.05, n = 4–11.
Histopathological Findings
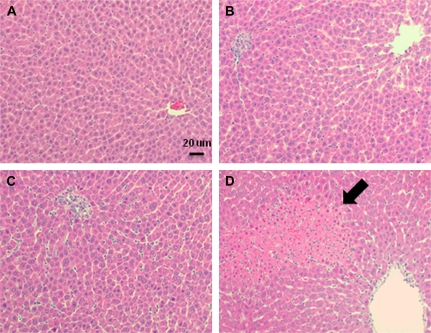
Hepatocellular lesions were not found in livers of rats treated with Veh/Veh, SLD/Veh, or Veh/LPS (Figs. 4A–C). In livers of SLD/LPS-cotreated rats, necrotic foci were present in the midzonal regions (Fig. 4D). These were associated with hemorrhage and neutrophil infiltration. The numbers and sizes of necrotic foci in livers from SLD/LPS-treated rats progressed with time and were consistent with the elevated ALT and AST activities in rat serum (Table 1). Significant liver lesions were observed beginning at 8 h after the second administration of SLD and persisted through 24 h.
FIG. 4.
Liver histopathology in SLD/LPS-cotreated rats. Rats were treated with Veh/Veh (A), SLD/Veh (B), Veh/LPS (C), or SLD/LPS (D) as described in Figure 2. Liver sections were collected at 12 h and were stained with hematoxylin and eosin. The pictures were taken under 200× magnification, and a necrotic area is indicated with an arrow.
TABLE 1.
Midzonal Hepatic Necrosis in Livers of Rats Treated with SLD/LPS
| Treatment | Time after 2nd SLD (h) |
|||
| 4 | 8 | 12 | 24 | |
| Veh/Veh | 0.50 (0.34–0.53) | 0.50 (0.31–0.66) | 0.44 (0.25–0.63) | 0.63 (0.47–0.66) |
| SLD/Veh | 0.38 (0.34–0.88) | 0.88 (0.47–1.03) | 0.75 (0.63–1.00) | 0.75 (0.44–0.88) |
| Veh/LPS | 0.75 (0.47–0.97) | 0.75 (0.56–1.19) | 0.63 (0.44–1.09) | 1.00 (0.81–1.26) |
| SLD/LPS | 0.88 (0.72–1.40) | 1.88 (1.63–3.59)* | 3.37 (2.25–3.88)* | 2.88 (2.25–3.38)* |
Note. Liver sections from rats killed at 4, 8, 12, and 24 h after the second administration of SLD were evaluated and assigned a score of 0–5 as described under Materials and Methods. Data are expressed as median score and 25th and 75th quartiles. *Significantly different from Veh/Veh group at the same time. p < 0.05, n = 5–10.
Effect of SLD/LPS Cotreatment on Serum TNF-α Concentration
SLD alone had no effect on the concentration of TNF-α in serum (Table 2). Treatment with LPS alone caused a significant increase in the serum TNF-α concentration within 1.5 h. SLD significantly enhanced the increase in serum TNF-α induced by LPS.
TABLE 2.
Serum TNF-α Concentration in SLD/LPS-Treated Rats
| Treatment | Veh | SLD |
| Veh | 0.024 ± 0.005 | 0.057 ± 0.018 |
| LPS | 16.9 ± 6.5* | 50.2 ± 10.7*# |
Note. Rats were treated with SLD (50 mg/kg, p.o.) or its vehicle (0.5% methyl cellulose) at −16 h, and food was removed. At −0.5 h rats received LPS (8.25 × 105 EU/kg, i.v.) or its vehicle (saline), and 30 min later they were given a second administration (50 mg/kg) of SLD. Serum samples were collected at 1 h after the second administration of SLD. The concentration of TNF-α (ng/ml) in serum was determined using ELISA. *Significantly different from corresponding group not treated with LPS. #Significantly different from Veh/LPS group. p < 0.05, n = 3–7.
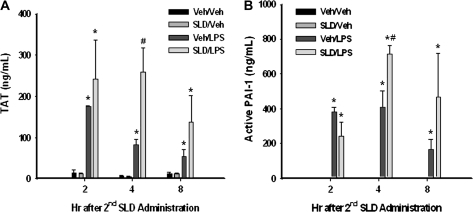
Activation of the Hemostatic System
Thrombin functions as a vital activator of the coagulation system, whereas active PAI-1 is the major endogenous downregulator of the fibrinolytic system. The concentrations of these two regulators were evaluated using ELISA at 1, 4, and 8 h after the second administration of SLD. Both TAT and active PAI-1 concentrations were elevated by LPS alone at 1, 4, and 8 h, whereas SLD was without effect by itself (Fig. 5). At 4 h after the second administration of SLD, SLD significantly enhanced the LPS-induced increases in TAT and active PAI-1 in plasma. Compared with LPS alone, SLD/LPS cotreatment also tended to prolong the elevation in thrombin and active PAI-1 in plasma (Figs. 5A, 5B).
FIG. 5.
Activation of the hemostatic system. Rats were treated with SLD, LPS, or their vehicles as described in Figure 2. They were killed 2, 4, or 8 h after the second administration of SLD, and plasma was collected. Concentrations of TAT (A) and active PAI-1 (B) in plasma were measured. *Significantly different from Veh/Veh group at the same time. #Significantly different from Veh/LPS group at the same time. p < 0.05, n = 3–5.
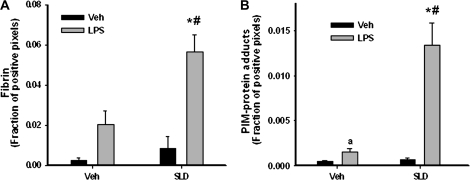
Hepatic fibrin deposition was evaluated 4 h after the second administration of SLD because that is a time just before the onset of liver injury as reflected by serum ALT and AST activities (Fig. 2). SLD alone and LPS alone were associated with small increases in fibrin that were not statistically significant. In contrast, SLD/LPS cotreatment led to a pronounced elevation in fibrin staining that was panlobular in distribution (Fig. 6A and Supplemental Fig. 2).
FIG. 6.
Evaluation of fibrin deposition and hypoxia in liver. Rats were treated as described in Figure 2. (A) Animals were killed at 4 h, and livers were processed for immunohistochemical determination of fibrin deposition. (B) Rats received an additional treatment with PIM hydrochloride (120 mg/kg) 2 h after the second administration of SLD. They were then killed at 4 h, and livers were processed for immunohistochemical determination of hypoxia. In both panels the fraction of positive pixels averaged from 10 randomly chosen microscope fields (100×) was determined for each animal. *Significantly different from Veh/LPS group. #Significantly different from SLD/Veh group. aSignificantly different from Veh/Veh group. p < 0.05, n = 4–6.
As mentioned above, fibrin deposition can lead to hypoxia, which was evaluated by quantifying PIM-protein adducts in livers. PIM-protein adducts were at control levels in livers of SLD-treated rats. LPS treatment caused a modest increase, and SLD/LPS cotreatment led to a greater elevation of PIM-protein adducts, predominantly in midzonal regions of liver lobules by 4 h after the second administration of SLD (Fig. 6B and Supplemental Fig. 3).
Effect of Heparin on Liver Injury Induced by SLD/LPS Cotreatment
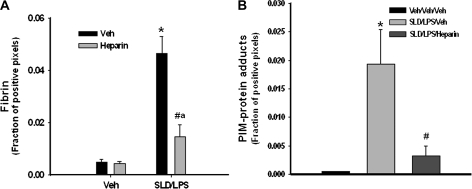
Anticoagulant heparin (3000 units/kg, s.c.), administered concurrently with the second administration of SLD, caused a marked decrease in fibrin deposition in liver as expected (Fig. 7A). PIM-protein adduct staining was also significantly reduced by heparin at 4 h after the second SLD administration (Fig. 7B).
FIG. 7.
Effect of heparin on liver fibrin and hypoxia. SLD and/or LPS were given to rats as described in Figure 2. Heparin (3000 units/kg, s.c.) was given to rats at the same time as the second administration of SLD. Rats were killed at 4 h, and liver sections were fixed and stained immunohistochemically for fibrin (A) or PIM-protein adducts (B). The fraction of positive pixels was determined as described under “Materials and Methods.” *Significantly different from Veh/Veh/Veh group. #Significantly different from SLD/LPS/Veh group. aSignificantly different from Veh/Veh/heparin group. p < 0.05, n = 4–6.
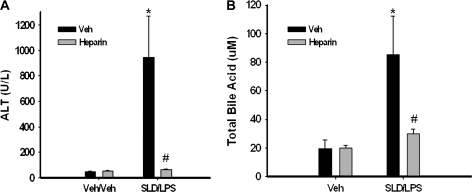
To evaluate the role of hemostatic system in liver injury, heparin was given to rats concurrently with and 6 h after the second administration of SLD, and liver injury was evaluated at 12 h. Heparin was without effect on ALT activity in Veh/Veh-treated rats but significantly attenuated the increase in ALT activity in the sera of SLD/LPS-treated rats (Fig. 8A). The SLD/LPS-induced elevation in total bile acid concentration in serum was also attenuated by heparin (Fig. 8B). Necrotic foci were observed in livers of rats treated with SLD/LPS/Veh, but not in livers of rats treated with SLD/LPS/heparin (Supplemental Fig. 4).
FIG. 8.
Effect of heparin on SLD/LPS-induced liver injury. Rats were treated with SLD/LPS as described in Figure 2. Heparin (3000 units/kg, s.c.) or its vehicle (saline) was given to rats at 0 and 6 h. Rats were killed at 12 h, and ALT activity (A) and concentration of total bile acids (B) in serum was determined. *Significantly different from Veh/Veh/heparin group. #Significantly different from SLD/LPS/Veh group. p < 0.05, n = 3–13.
Effect of Low Oxygen on Hepatocytotoxicity Induced by SLD Sulfide In Vitro
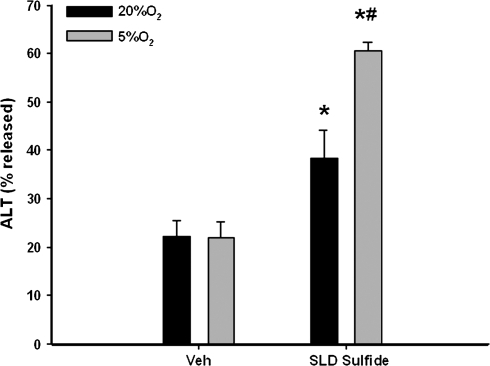
To assess whether hypoxia can affect the killing of HPCs by SLD, the active metabolite of SLD, SLD sulfide (60μM) was administered to primary rat HPCs in vitro. Immediately after treatment, HPCs were incubated in oxygen replete (20% O2) or hypoxic (5% O2) atmospheres. The 5% O2 is equivalent to a nominal pO2 of 30 mm Hg, which is the smallest reported oxygen concentration in blood around hepatic central vein in vivo (Jungermann and Kietzmann, 2000). This degree of hypoxia had no effect on the viability of HPCs after 8 h of incubation. SLD sulfide alone increased ALT activity in the medium, indicating hepatocellular injury. When HPCs treated with SLD sulfide were exposed to 5% O2, cell death caused by SLD sulfide was significantly enhanced compared with incubation in 20% O2 (Fig. 9).
FIG. 9.
Effect of hypoxia on SLD sulfide-induced cytotoxicity. Rat primary HPCs were isolated as described under “Materials and Methods.” They were treated with 60μM SLD sulfide and kept in 20% or 5% oxygen. After 8 h, ALT release was determined. *Significantly different from Veh group at the same oxygen level. #Significantly different from SLD sulfide treatment group at 20% oxygen. p < 0.05, n = 3.
DISCUSSION
SLD is an NSAID that is used for the treatment of arthritis. All of the NSAIDs have been associated with hepatic IADRs in patients (O'Connor et al., 2003). The average risk of serious hepatic injury for NSAIDs is approximately one case in 10,000 patient-years of use, and the risk from SLD is reported to be 5- to 10-fold greater than for NSAIDs as a class (Walker, 1997). The mechanism of NSAID-induced hepatic IADRs is not clearly understood, and several hypotheses have been proposed. It is commonly accepted that large amounts of active metabolites form after exposure to large doses of NSAIDs, and these might lead to protein adduct formation, oxidative stress and mitochondrial injury that could contribute to tissue damage (Boelsterli, 2002). However, evidence supporting these hypotheses is mainly obtained from studies in vitro, and animal models of SLD-induced liver injury are lacking.
Previous studies in rodents suggested that there may be a connection between inflammation and hepatic IADRs for at least some drugs (Buchweitz et al., 2002; Luyendyk et al., 2003; Roth et al., 2003; Shaw et al., 2007). In these drug-LPS interaction models, one administration of the drug was sufficient to produce a hepatotoxic interaction with LPS. In preliminary studies with SLD, we tried several single-administration regimens using various doses and times between SLD and LPS treatment. Although hepatotoxic signals (i.e., increased serum ALT activity) were observed in some rats, these were inconsistent. Changing to a two-administration protocol in which the time between SLD administrations approximated three half lives (Hucker et al., 1973) provided relatively consistent and statistically significant liver injury; moreover, this protocol corresponds to the twice per day treatment regimen typically used therapeutically in human patients. Using this protocol, SLD or LPS alone did not cause any lesions in the liver or increase the clinical chemical biomarkers of liver injury. However, in SLD/LPS-cotreated rats serum markers of both hepatocellular injury and cholestasis were increased significantly, and foci of necrotic parenchymal cells were found in livers. Interestingly, in a study of 91 cases reported to the U.S. Food and Drug Administration, SLD caused hepatocellular injury, cholestatic injury or mixed liver injury in human patients (Tarazi et al., 1993), consistent with observations presented here for rats treated with SLD/LPS. These results raise the possibility that a mild episode of inflammation might render human patients susceptible to SLD-induced liver injury.
Judging by the time course of serum ALT and AST activities (Fig. 2) and histological changes (Table 1), the onset of liver injury was between 4 and 8 h. The serum markers of hepatocellular injury rose until 12 h and declined by 24 h. Despite this decline, histologic evidence of liver injury persisted at 24 h (Table 1). This decline in serum transaminases suggests that injury occurred in the first 12 h, and ALT and AST released from HPCs were cleared from the blood thereafter. The short initial half life of ALT/AST in rat plasma (approximately 5 h) is consistent with this interpretation (Saheki et al., 1990).
LPS has the potential to influence toxicity in a number of ways. For example, it is capable of downregulating the expression of several drug metabolizing enzymes (Morgan, 1989). SLD is bioactivated by HPCs to a more toxic sulfide metabolite by methionine sulfoxide reductase (Etienne et al., 2003; Kitamura and Tatsumi, 1982; Kitamura et al., 1980). It is not known if LPS downregulates the expression of this enzyme; however, in a preliminary study, LPS treatment did not increase the liver concentration of SLD sulfide (unpublished observation).
Cytokines such as TNF-α, interleukin-1 (IL-1), and IL-6 are upregulated after the activation of Toll-like receptor 4 on Kupffer cells by LPS (Luster et al., 1994; Su, 2002). Some of these have been linked to liver injury from drug/LPS interaction (Shaw et al., 2007; Tukov et al., 2007). The observation that fibrin deposited in livers of rats cotreated with SLD and LPS led us to explore the hemostatic system in this study. Cytokines are potent modulators of the hemostatic system. For example, TNF-α and IL-1 activate coagulation by upregulating tissue factor expression and by reducing the fibrinolytic activity of endothelial cells through an increase in PAI-1 (Salgado et al., 1994; Schleef et al., 1988). The plasma concentrations of TAT and active PAI-1 increased rapidly in LPS-treated rats, confirming that LPS induces the activation of the coagulation system and provides conditions for inhibition of the fibrinolytic system (Fig. 5). Although SLD had no effect on these factors when it was given alone, it enhanced the LPS-mediated changes and tended to prolong the activation of the hemostatic system. This could be due to enhanced TNF-α release by SLD (Table 2). In the liver injury induced by ranitidine/LPS cotreatment, ranitidine enhanced the LPS-induced increase in TNF-α concentration through p38-dependent activation of TNF-α converting enzyme (TACE), which cleaves membrane-bound pro-TNF-α to form mature TNF-α (Deng et al., 2008). Whether a similar mechanism is at play in SLD/LPS-cotreated rats is yet to be determined.
SLD/LPS cotreatment significantly increased cross-linked fibrin in livers at 4 h, that is, at a time before the onset of liver injury. Although LPS activated the hemostatic system in rats, it alone was not sufficient to induce marked fibrin deposition in the liver (Fig. 6A). The hemostatic system is activated in endotoxemia-induced liver injury (Hewett and Roth, 1995), and one possible consequence is hypoxia in the liver resulting from disrupted blood flow in the sinusoids. PIM-protein adducts were slightly elevated in livers of rats treated with LPS alone, indicating that mild hypoxia occurred. In contrast, SLD/LPS led to a pronounced increase in PIM-protein adducts, suggesting marked hypoxia (Fig. 6B). Unlike the distribution of fibrin which was panlobular, hypoxia occurred only in the midzonal regions of livers (Supplemental Figs. 2, 3). Interestingly, necrotic foci were also present predominantly in this region (Fig. 4).
It has been reported that hypoxia causes hepatocelluar injury in isolated, perfused rat livers (Lemasters et al., 1981), and liver injury was induced in vivo in rats exposed for a brief period to a low concentration of oxygen (Fassoulaki et al., 1984). In studies presented here, anticoagulant heparin reduced hepatic fibrin deposition and hypoxia induced by SLD/LPS cotreatment (Fig. 7). This suggests that hypoxia might be caused by fibrin clots in liver sinusoids. Moreover, both hepatocellular and bile ductular injury in SLD/LPS-treated rats was significantly attenuated by heparin, supporting the hypothesis that hypoxia induced by fibrin clots plays an important role in the pathogenesis (Fig. 8).
Previous studies suggested that hypoxia can potentiate the toxicity of some xenobiotics towards HPCs (Shen et al., 1982). Compared with SLD or its sulfone metabolite, SLD sulfide is more cytotoxic (Leite et al., 2006). Therefore, we treated rat primary HPCs with SLD sulfide. Hypoxia enhanced its ability to kill these cells (Fig. 9). SLD sulfide, but not SLD or SLD sulfone uncouples the mitochondria of HepG2 cells (Leite et al., 2006). Thus, hypoxia could exacerbate SLD sulfide-mediated mitochondrial dysfunction by diminishing the aerobic metabolism of HPCs. Moreover, hypoxia inducible factor 1 accumulated in cells under hypoxic stress might induce the expression of proapoptotic proteins and cause the stabilization of p53, which increases permeability of the mitochondrial membrane and causes cell death (Greijer and van der Wall, 2004). In the SLD/LPS idiosyncratic liver injury model, hypoxia might enhance the mitochondrial toxicity of SLD, providing a synergistic effect on a mitochondrial pathway to cell death. Although further study is required to test this, SLD/LPS cotreatment in rats could be an animal model supporting the mitochondrial injury hypothesis of NSAID-induced IADRs described above.
The mitochondrial pathway might not be the only contributor to liver injury. Hypoxia can also interplay with inflammatory factors. For example, when stimulated by LPS, neutrophils accumulate in sinusoids and transmigrate through blood vessels to liver parenchyma. At the site of inflammation, activated neutrophils release proteases that can kill rat HPCs (Ho et al., 1996), and hypoxia enhanced neutrophil protease-mediated HPC killing (Luyendyk et al., 2005).
Results with SLD are similar to those observed with diclofenac (DCLF), another NSAID that causes hepatic IADRs in people, in the sense that both drugs interacted with LPS to cause liver injury in rats. However, a large dose of DCLF (100 mg/kg) caused liver injury by itself in rats in the absence of LPS-cotreatment. This was associated with intestinal injury and translocation of bacteria to the liver and was prevented by sterilization of the GI tract (Deng et al., 2006). These results suggested that GI irritation and translocation of bacteria or LPS caused by DCLF contribute to DCLF-induced hepatotoxicity in rats, and a similar mechanism might underlie DCLF IADRs in humans. In contrast, large doses of SLD (up to 300 mg/kg) did not cause liver injury in rats by themselves (Fig. 1). This suggests that, at least in rats, SLD is less irritating to the intestine than DCLF, although the relative gastrointestinal toxicity of SLD and DCLF in humans is still controversial. One study indicated that SLD is less associated with gastrointestinal hospitalizations than DCLF (Garcia Rodriguez et al., 1992), whereas others suggested the opposite (Henry et al., 1993; Savage et al., 1993). The potential implication for human IADRs is that DCLF may be able to provide its own inflammatory stress through GI irritation, whereas SLD toxicity might require an additional inflammatory stress that arises independently of drug treatment. It seems possible that the latter could arise from the very condition that the drug is used to treat, for example, an inflammatory flare of rheumatoid arthritis. Alternatively, an independently occurring inflammatory episode might interact with SLD. The finding that viral hepatitis may predispose patients to NSAID hepatotoxicity supports this possibility (Teoh and Farrell, 2003).
In summary, SLD/LPS cotreatment caused liver injury in rats, which was not produced by SLD or LPS alone. The coagulation system was activated while the fibrinolytic system was inhibited in the cotreated rats. As a consequence, fibrin clots formed in sinusoids, and hypoxia occurred selectively in livers of rats treated with SLD/LPS. Anticoagulant heparin protected rats against liver injury and also attenuated fibrin deposition and liver hypoxia. Hypoxia enhanced the cytotoxicity of SLD sulfide in vitro. The results support the hypothesis that NSAIDs that cause hepatic IADRs in humans interact with an inflammatory stress to cause liver injury in animals. Hypoxia may play an important role in this SLD/LPS idiosyncratic liver injury model through synergistic interplay with the toxic SLD sulfide metabolite. These observations do not exclude a role for other factors, such as cytokines, that are a focus of ongoing investigation.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health (GM075865); and collaborative agreement with Pfizer, Inc.
Supplementary Material
References
- Aithal GP, Day CP. Nonsteroidal anti-inflammatory drug-induced hepatotoxicity. Clin. Liver Dis. 2007;11:563–575. doi: 10.1016/j.cld.2007.06.004. vi–vii. [DOI] [PubMed] [Google Scholar]
- Boelsterli UA. Mechanisms of NSAID-induced hepatotoxicity: Focus on nimesulide. Drug Saf. 2002;25:633–648. doi: 10.2165/00002018-200225090-00003. [DOI] [PubMed] [Google Scholar]
- Buchweitz JP, Ganey PE, Bursian SJ, Roth RA. Underlying endotoxemia augments toxic responses to chlorpromazine: Is there a relationship to drug idiosyncrasy? J. Pharmacol. Exp. Ther. 2002;300:460–467. doi: 10.1124/jpet.300.2.460. [DOI] [PubMed] [Google Scholar]
- Copple BL, Banes A, Ganey PE, Roth RA. Endothelial cell injury and fibrin deposition in rat liver after monocrotaline exposure. Toxicol. Sci. 2002;65:309–318. doi: 10.1093/toxsci/65.2.309. [DOI] [PubMed] [Google Scholar]
- Copple BL, Rondelli CM, Maddox JF, Hoglen NC, Ganey PE, Roth RA. Modes of cell death in rat liver after monocrotaline exposure. Toxicol. Sci. 2004;77:172–182. doi: 10.1093/toxsci/kfh011. [DOI] [PubMed] [Google Scholar]
- Deng X, Lu J, Lehman-McKeeman LD, Malle E, Crandall DL, Ganey PE, Roth RA. p38 mitogen-activated protein kinase-dependent tumor necrosis factor-alpha-converting enzyme is important for liver injury in hepatotoxic interaction between lipopolysaccharide and ranitidine. J. Pharmacol. Exp. Ther. 2008;326:144–152. doi: 10.1124/jpet.108.137497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Stachlewitz RF, Liguori MJ, Blomme EA, Waring JF, Luyendyk JP, Maddox JF, Ganey PE, Roth RA. Modest inflammation enhances diclofenac hepatotoxicity in rats: Role of neutrophils and bacterial translocation. J. Pharmacol. Exp. Ther. 2006;319:1191–1199. doi: 10.1124/jpet.106.110247. [DOI] [PubMed] [Google Scholar]
- Etienne F, Resnick L, Sagher D, Brot N, Weissbach H. Reduction of Sulindac to its active metabolite, sulindac sulfide: Assay and role of the methionine sulfoxide reductase system. Biochem. Biophys. Res. Commun. 2003;312:1005–1010. doi: 10.1016/j.bbrc.2003.10.203. [DOI] [PubMed] [Google Scholar]
- Fassoulaki A, Eger EI, II, Johnson BH, Ferrell LD, Smuckler EA, Harper MH, Eger RR, Cahalan MK. Brief periods of hypoxia can produce hepatic injury in rats. Anesth. Analg. 1984;63:885–887. [PubMed] [Google Scholar]
- Ganey PE, Luyendyk JP, Maddox JF, Roth RA. Adverse hepatic drug reactions: Inflammatory episodes as consequence and contributor. Chem. Biol. Interact. 2004;150:35–51. doi: 10.1016/j.cbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Garcia Rodriguez LA, Walker AM, Perez Gutthann S. Nonsteroidal antiinflammatory drugs and gastrointestinal hospitalizations in Saskatchewan: A cohort study. Epidemiology. 1992;3:337–342. doi: 10.1097/00001648-199207000-00008. [DOI] [PubMed] [Google Scholar]
- Garcia Rodriguez LA, Williams R, Derby LE, Dean AD, Jick H. Acute liver injury associated with nonsteroidal anti-inflammatory drugs and the role of risk factors. Arch. Intern. Med. 1994;154:311–316. doi: 10.1001/archinte.1994.00420030117012. [DOI] [PubMed] [Google Scholar]
- Greijer AE, van der Wall E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J. Clin. Pathol. 2004;57:1009–1014. doi: 10.1136/jcp.2003.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry D, Dobson A, Turner C. Variability in the risk of major gastrointestinal complications from nonaspirin nonsteroidal anti-inflammatory drugs. Gastroenterology. 1993;105:1078–1088. doi: 10.1016/0016-5085(93)90952-9. [DOI] [PubMed] [Google Scholar]
- Hewett JA, Roth RA. The coagulation system, but not circulating fibrinogen, contributes to liver injury in rats exposed to lipopolysaccharide from gram-negative bacteria. J. Pharmacol. Exp. Ther. 1995;272:53–62. [PubMed] [Google Scholar]
- Ho JS, Buchweitz JP, Roth RA, Ganey PE. Identification of factors from rat neutrophils responsible for cytotoxicity to isolated hepatocytes. J. Leukoc. Biol. 1996;59:716–724. doi: 10.1002/jlb.59.5.716. [DOI] [PubMed] [Google Scholar]
- Hucker HB, Stauffer SC, White SD, Rhodes RE, Arison BH, Umbenhauer ER, Bower RJ, McMahon FG. Physiologic disposition and metabolic fate of a new anti-inflammatory agent, cis-5-fluro-2-methyl-1-(p-(methylsulfinyl)-benzylidenyl)-indene-3-acetic acid in the rat, dog, rhesus monkey, and man. Drug Metab. Dispos. 1973;1:721–736. [PubMed] [Google Scholar]
- Jungermann K, Kietzmann T. Oxygen: Modulator of metabolic zonation and disease of the liver. Hepatology. 2000;31:255–260. doi: 10.1002/hep.510310201. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Tatsumi K. In vitro metabolism of sulindac and sulindac sulfide: Enzymatic formation of sulfoxide and sulfone. Jpn. J. Pharmacol. 1982;32:833–838. doi: 10.1254/jjp.32.833. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Tatsumi K, Yoshimura H. Metabolism in vitro of sulindac. Sulfoxide-reducing enzyme systems in guinea pig liver. J. Pharmacobiodyn. 1980;3:290–298. doi: 10.1248/bpb1978.3.290. [DOI] [PubMed] [Google Scholar]
- Leite S, Martins NM, Dorta DJ, Curti C, Uyemura SA, Cardozo Dos Santos A. Mitochondrial uncoupling by the sulindac metabolite, sulindac sulfide. Basic Clin. Pharmacol. Toxicol. 2006;99:294–299. doi: 10.1111/j.1742-7843.2006.pto_490.x. [DOI] [PubMed] [Google Scholar]
- Lemasters JJ, Ji S, Thurman RG. Centrilobular injury following hypoxia in isolated, perfused rat liver. Science. 1981;213:661–663. doi: 10.1126/science.7256265. [DOI] [PubMed] [Google Scholar]
- Luster MI, Germolec DR, Yoshida T, Kayama F, Thompson M. Endotoxin-induced cytokine gene expression and excretion in the liver. Hepatology. 1994;19:480–488. [PubMed] [Google Scholar]
- Luyendyk JP, Maddox JF, Cosma GN, Ganey PE, Cockerell GL, Roth RA. Ranitidine treatment during a modest inflammatory response precipitates idiosyncrasy-like liver injury in rats. J. Pharmacol. Exp. Ther. 2003;307:9–16. doi: 10.1124/jpet.103.054288. [DOI] [PubMed] [Google Scholar]
- Luyendyk JP, Shaw PJ, Green CD, Maddox JF, Ganey PE, Roth RA. Coagulation-mediated hypoxia and neutrophil-dependent hepatic injury in rats given lipopolysaccharide and ranitidine. J. Pharmacol. Exp. Ther. 2005;314:1023–1031. doi: 10.1124/jpet.105.087981. [DOI] [PubMed] [Google Scholar]
- Morgan ET. Suppression of constitutive cytochrome P-450 gene expression in livers of rats undergoing an acute phase response to endotoxin. Mol. Pharmacol. 1989;36:699–707. [PubMed] [Google Scholar]
- O'Connor N, Dargan PI, Jones AL. Hepatocellular damage from non-steroidal anti-inflammatory drugs. Q. J. M. 2003;96:787–791. doi: 10.1093/qjmed/hcg138. [DOI] [PubMed] [Google Scholar]
- Roth RA, Luyendyk JP, Maddox JF, Ganey PE. Inflammation and drug idiosyncrasy—Is there a connection? J. Pharmacol. Exp. Ther. 2003;307:1–8. doi: 10.1124/jpet.102.041624. [DOI] [PubMed] [Google Scholar]
- Saheki T, Komorizono K, Miura T, Ichiki H, Yagi Y, Hashimoto S. Clearance of argininosuccinate synthetase from the circulation in acute liver disease. Clin. Biochem. 1990;23:139–141. doi: 10.1016/0009-9120(90)80026-f. [DOI] [PubMed] [Google Scholar]
- Salgado A, Boveda JL, Monasterio J, Segura RM, Mourelle M, Gomez-Jimenez J, Peracaula R. Inflammatory mediators and their influence on haemostasis. Haemostasis. 1994;24:132–138. doi: 10.1159/000217093. [DOI] [PubMed] [Google Scholar]
- Savage RL, Moller PW, Ballantyne CL, Wells JE. Variation in the risk of peptic ulcer complications with nonsteroidal antiinflammatory drug therapy. Arthritis Rheum. 1993;36:84–90. doi: 10.1002/art.1780360114. [DOI] [PubMed] [Google Scholar]
- Schleef RR, Bevilacqua MP, Sawdey M, Gimbrone MA, Jr, Loskutoff DJ. Cytokine activation of vascular endothelium. Effects on tissue-type plasminogen activator and type 1 plasminogen activator inhibitor. J. Biol. Chem. 1988;263:5797–5803. [PubMed] [Google Scholar]
- Shaw PJ, Hopfensperger MJ, Ganey PE, Roth RA. Lipopolysaccharide and trovafloxacin coexposure in mice causes idiosyncrasy-like liver injury dependent on tumor necrosis factor-alpha. Toxicol. Sci. 2007;100:259–266. doi: 10.1093/toxsci/kfm218. [DOI] [PubMed] [Google Scholar]
- Shen ES, Garry VF, Anders MW. Effect of hypoxia on carbon tetrachloride hepatotoxicity. Biochem. Pharmacol. 1982;31:3787–3793. doi: 10.1016/0006-2952(82)90294-5. [DOI] [PubMed] [Google Scholar]
- Su GL. Lipopolysaccharides in liver injury: Molecular mechanisms of Kupffer cell activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283:G256–265. doi: 10.1152/ajpgi.00550.2001. [DOI] [PubMed] [Google Scholar]
- Tarazi EM, Harter JG, Zimmerman HJ, Ishak KG, Eaton RA. Sulindac-associated hepatic injury: Analysis of 91 cases reported to the Food and Drug Administration. Gastroenterology. 1993;104:569–574. doi: 10.1016/0016-5085(93)90428-f. [DOI] [PubMed] [Google Scholar]
- Teoh NC, Farrell GC. Hepatotoxicity associated with non-steroidal anti-inflammatory drugs. Clin. Liver Dis. 2003;7:401–413. doi: 10.1016/s1089-3261(03)00022-9. [DOI] [PubMed] [Google Scholar]
- Tukov FF, Luyendyk JP, Ganey PE, Roth RA. The role of tumor necrosis factor alpha in lipopolysaccharide/ranitidine-induced inflammatory liver injury. Toxicol. Sci. 2007;100:267–280. doi: 10.1093/toxsci/kfm209. [DOI] [PubMed] [Google Scholar]
- Tukov FF, Maddox JF, Amacher DE, Bobrowski WF, Roth RA, Ganey PE. Modeling inflammation-drug interactions in vitro: A rat Kupffer cell-hepatocyte coculture system. Toxicol In Vitro. 2006;20:1488–1499. doi: 10.1016/j.tiv.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Walker AM. Quantitative studies of the risk of serious hepatic injury in persons using nonsteroidal antiinflammatory drugs. Arthritis Rheum. 1997;40:201–208. doi: 10.1002/art.1780400204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.