Abstract
Previous research has documented several PAHs that interact synergistically, causing severe teratogenicity in developing fish embryos. The coexposure of CYP1A inhibitors (e.g. FL or ANF) with AHR agonists (e.g. BaP or BNF) results in a synergistic increase in toxicity. As with chemical CYP1A inhibitors, it has also been shown that CYP1A morpholinos exacerbate BNF-induced embryotoxicity. We hypothesized that a hypoxia-induced reduction in CYP1A activity in BNF or BaP-exposed zebrafish embryos would similarly enhance pericardial effusion and other developmental abnormalities. BaP, BNF, ANF, and FL exposures, both individually and as BaP+FL or BNF+ANF combinations, were performed under hypoxia and normoxia. CYP1A activity in the BaP+hypoxia and BNF+hypoxia embryos was reduced by approximately 60% relative to normoxia embryos. Although CYP1A activity was significantly reduced, we did not observe any increase in pericardial effusion in either group. An unexpected yet particularly interesting result of these experiments was the observed interaction of both FL and ANF with hypoxia. Relatively high, yet environmentally relevant concentrations of FL (100–500 µg L−1) interact with moderate hypoxia (7.3% DO) through an unknown mechanism, resulting in pericardial effusion and severe lordosis. Additionally, ANF exposures (100 µg L−1) which are not normally teratogenic caused dramatic pericardial effusion, but not lordosis, when embryos were coexposed to hypoxia. These results suggest that reduced CYP1A activity may not exclusively underlie observed developmental toxicity, and that hypoxia may exacerbate the developmental toxicity of some PAH mixtures.
Keywords: Polycyclic Aromatic Hydrocarbons, Low Oxygen, Developmental Toxicity, Multiple Stressors, Aryl Hydrocarbon Receptor, Cytochrome P450, Risk Assessment
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are common environmental contaminants which originate primarily from the incomplete combustion of wood or petroleum products, with the majority coming from anthropogenic activities (Douben, 2003). This class of persistent organic pollutants (POPs) is made up of hundreds of individual substances. Individual PAHs are known to be clastogenic, genotoxic, carcinogenic, teratogenic, and mutagenic. Concentrations of PAHs are highest in estuaries and coastal environments near urban areas, derived largely from wastewater effluents, industrial outfalls, atmospheric deposition, and fossil fuel spills or leaks (Latimer and Zheng, 2003). The toxicity of PAHs to developing fish embryos is well established (Billiard et al., 1999; Carls et al., 1999; Wassenberg et al., 2002; Wassenberg and Di Giulio, 2004; Incardona et al., 2005; Billiard et al., 2006; Incardona et al., 2006; Billiard et al., 2007). While individual PAHs can be quite toxic, they are always found as complex mixtures in the environment. Mixtures of PAHs can interact in a variety of ways, and it has proven to be extremely difficult to accurately predict the toxicity of complex mixtures (Reeves et al., 2001).
Zebrafish (Danio rerio) and Atlantic killifish (Fundulus heteroclitus) studies have shown that exposure to the model PAH β-naphthoflavone (BNF) or the environmentally relevant PAH benzo[a]pyrene (BaP) substantially increases cytochrome P4501A CYP1A) expression and activity, while co-exposure to the CYP1A inhibitors α-naphthoflavone (ANF) or fluoranthene (FL) blocks the BNF or BaP-mediated induction of CYP1A activity (Wassenberg and Di Giulio, 2004; Billiard et al., 2006). This reduction in CYP1A activity is thought to account for the synergistic developmental toxicity (e.g. pericardial effusion and jaw deformities) observed in BNF+ANF and BaP+FL coexposed embryos, effects not observed in embryos exposed to comparable doses of these compounds independently. This synergistic embryotoxicity seems to be a generalized response to the coexposure of an aryl hydrocarbon receptor (AHR) agonist and CYP1A inhibitor (Wassenberg and Di Giulio, 2004). The mechanisms through which this synergistic interaction occurs has been investigated (Wassenberg and Di Giulio, 2004; Billiard et al., 2006; Timme-Laragy et al., 2007). Billiard et al. (2006) employed a morpholino approach in zebrafish and confirmed that this interaction is AHR-mediated. Timme-Laragy et al. (2007) went on to show that this synergistic interaction with CYP1A inhibition, at least with BNF+ANF, is governed at the protein level, and not by alterations in mRNA expression. CYP1A mRNA was actually much higher in the coexposed fish, which contrasts sharply with BNF-induced CYP1A protein activity which is reduced by ANF. A plausible explanation for this interaction is that reduced metabolism, resulting from CYP1A activity inhibition, leads to prolonged activation of the AHR, which appears to underlie the developmental toxicities of recalcitrant AHR agonists, such as dioxins and co-planar PCBs (Prasch et al., 2003; Carney et al., 2004; Antkiewicz et al., 2005; Goldstone and Stegeman, 2006).
There is significant concern over potential interactions between PAHs and environmental hypoxia. Environmental hypoxia is a significant problem in many estuaries and marine coastal regions around the world, and global warming may exacerbate the problem through a variety of mechanisms (Diaz and Rosenberg, 1995; Schiedek et al., 2007). For obvious reasons, hypoxia can have dramatic effects on fish and other marine organisms. PAH contamination is ubiquitous in estuaries and marine coastal environments (Latimer and Zheng, 2003). PAHs come from multiple sources, are persistent, and significant reductions in PAH contamination are unlikely in the near future. Understanding the interactions between environmental hypoxia and PAHs will improve our understanding of ecological risks in these critical habitats.
Significant interaction between PAHs and environmental hypoxia is not unexpected given the overlap between the protein pathways that regulate the molecular response to these two stressors in vertebrates. The AHR pathway responds to a wide variety of exogenous chemicals, including some PAHs, and initiates both Phase I and Phase II metabolism, which facilitate the biotransformation and elimination of the chemicals. The hypoxia inducible factor 1α (HIF-1α) pathway initiates a cascade of events in response to low oxygen concentrations in cells, including vasculogenesis and angiogenesis (Ton et al., 2003). A previous study investigated the potential interactions of the AHR agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) with hypoxia in zebrafish (Prasch et al., 2004). Prasch et al. (2004) documented directional cross-talk between the hypoxia and AHR pathways in zebrafish. Hypoxia exposure in zebrafish embryos resulted in a reduced AHR response, as measured by CYP1A mRNA, to TCDD. However, this TCDD exposure did not reduce the embryos’ molecular response to hypoxia. Additionally, Hendon et al. (2008) failed to find any evidence that pyrene altered a hypoxia-responsive gene, vascular endothelial growth factor (VEGF), in Cyprinodon variegatus. However, this study only examined the effect of pyrene on endogenously-controlled transcription of VEGF, not addressing the issue of hypoxia-induced VEGF transcription. The mechanism by which hypoxia interferes with the AHR pathway remains unknown. There are several proteins that are shared between these two pathways, with aryl hydrocarbon receptor nuclear translocator (ARNT) being the most well known. There are also several transcriptional coactivators which have been shown to alter AHR and/or hypoxia pathway responses (Kobayashi et al., 1997; Kasper et al., 2005; Ruas et al., 2005; Harper et al., 2006). This extensive overlap between these two signaling pathways suggests a potential for reciprocal cross-talk, likely through sequestration of one of these shared proteins. While the possible role of ARNT in hypoxia/AHR cross-talk continues to be explored (Prasch et al., 2004), previous research suggests that ARNT is not a limiting factor (Pollenz et al., 1999). It is also possible that a simple reduction of available oxygen reduces cellular function and thus alters the AHR response.
The first goal of this study was to test the hypothesis that reducing BaP or BNF-mediated CYP1A activity via hypoxia exposure in zebrafish embryos would elicit the same teratogenic response that is elicited by reducing CYP1A activity with chemical inhibitors or morpholinos. The second goal of this study was to look for evidence of unexpected interactions between environmental hypoxia and the PAHs used in these experiments.
The PAHs, BaP and FL were selected for this study because their effects on fish development have been demonstrated (Wassenberg and Di Giulio, 2004), and because they are often among the most prevalent PAHs at contaminated sites (Latimer and Zheng, 2003). BaP and FL are also identified by the US Environmental Protection Agency as being among the 16 priority pollutant PAHs (EPA, 1987). Fluoranthene has also been shown to cause increased mortality and growth inhibition in long-term (32 days) early-life-stage studies with the fathead minnow, Pimephales promelas (Spehar et al., 1999). BNF and ANF were used to confirm the generalized response to an AHR agonist and CYP1A inhibitors. The effects of, and response to, this combination of model PAHs has been well documented in fish embryos (Billiard et al., 2006; Timme-Laragy et al., 2007). ANF has also been shown to exacerbate the embryotoxicity of another PAH, retene (Hodson et al., 2007).
2. Materials and Methods
2.1 Fish care
Adult zebrafish were fed a mixture of TetraMin® Tropical Flakes (Tetra, Blacksburg, VA) and live Artemia (Brine Shrimp Direct, Ogden, UT), and maintained at 26°C on a 14h light, 10h dark cycle. Males and females were housed together and eggs were collected via an egg collection box. Eggs were collected from the collection boxes, rinsed, and placed in 1X Danieau water (Nasevicius and Ekker, 2000). Eggs were then screened to ensure that they were fertilized and developing normally. Embryos were maintained at 28.5°C under the same photoperiod as the adults for the duration of the experiments. All fish care and experimental techniques were reviewed and approved by the Duke University Institutional Animal Care & Use Committee (A326-05-11).
2.2. Chemicals and dosing
Benzo[a]pyrene (BaP), fluoranthene (FL), β-naphthoflavone (BNF), α-naphthoflavone (ANF), dimethyl sulfoxide (DMSO), and ethoxyresorufin were purchased from Sigma-Aldrich (St. Louis, MO). BaP, FL, BNF, ANF, and ethoxyresorufin were dissolved in DMSO and stored in amber glass vials at −20°C. All chemicals were thawed and vortexed prior to administration. Dosing concentrations were based on previous experiments investigating the teratogenicity of PAH mixtures of killifish (Fundulus heteroclitus) and zebrafish (Wassenberg and Di Giulio, 2004; Billiard et al., 2006). BaP exposures were conducted at nominal concentrations of 10 µg L−1. FL exposures were performed using nominal concentrations of 100 and 500 µg L−1. For BaP+FL mixtures, concentrations of 10 and 500 µg L−1, respectively, were used. The model PAHs BNF and ANF were also used to test for similar responses to the environmentally relevant PAHs, BaP and FL. BNF and ANF exposures were conducted at 1 and 100 µg L−1, respectively. Exposures conducted with mixtures of BNF+ANF were performed at the same concentrations. Normally developing zebrafish embryos were dosed at 24 hours post fertilization (hpf). Embryos remained in the original dosing solution for the duration of the experiments, which were ended at 96 hpf. Embryos were dosed in groups of five in 20-mL glass scintillation vials with 7.5 mL of 1X Danieau water. DMSO was used as the carrier control in all experiments; a final DMSO concentration of 0.03% was used in all exposures. At least three replicate groups of each exposure regime were dosed for each experiment. For experiments that included in vivo EROD analysis, 21 µg L−1 of ethoxyresorufin was also added at 24 hpf. At 96 hpf, following 72 h exposures, embryos were removed from the dosing solution and rinsed in 1X Danieau water. Dosing experiments were replicated at least three times.
2.3 Hypoxia Exposure
For the purposes of this experiment, hypoxia exposures were all conducted at 7.3 % DO (2.7 mg L−1), or approximately 35% of normal oxygen levels. Normoxia exposures were conducted at normal oxygen saturation or approximately 20.8% DO (7.8 mg L−1). Hypoxia exposures were conducted identically to normoxia exposures with respect to temperature, light cycle, and dosing methods. However, for hypoxia exposures, vials were placed in a secondary chamber within the same incubator for the duration of the experiment (24 hpf – 96 hpf). The oxygen level was regulated in the acrylic secondary chamber using a Proox Model 110 (BioSpherix, Ltd., Redfield, NY) oxygen controller and nitrogen gas. Since atmospheric oxygen was regulated and not DO directly, it took several hours for DO levels to reach 7.3% DO. As a result, the actual time-period at which the full hypoxia exposures took place was approximately 32 hpf – 96 hpf.
2.4 In vivo EROD assay
CYP1A activity was measured via an in vivo ethoxyresorufin-O-deethylase (EROD) activity assay (Billiard et al., 2006), which was modified from in ovo EROD methods described by Nacci et al. (1998; 2005). Briefly, CYP1A metabolism converts ethoxyresorufin to resorufin, which was then quantified in the gastrointestinal tract of 96 hpf zebrafish larvae via fluorescence microscopy. Ethoxyresorufin was added to dosing solutions at 24 hpf, and at 96 hpf larvae were rinsed and anesthetized with MS-222 (Sigma-Aldrich). Larvae were then immobilized in 3% methylcellulose in a left lateral orientation for imaging. A detailed description of this method is provided in Billiard et al. (2006). EROD estimates are presented as % normoxia DMSO control, thus all estimates represent their deviation from a normal non-induced state.
2.5 Deformity Screening
All deformity screening was performed on 96 hpf larvae. Larvae were anesthetized, immobilized, and brightfield images captured at 50X were analyzed using IPLabs Software (BD Biosciences, Rockville, MD). The software calculated the 2-dimensional (2D) area of the pericardium after the area was manually traced. As there is no reliable method to convert the 2D pericardium area measurements to a 3D volume estimate, the 2D measurements used in this study likely underestimated the magnitude of pericardial effusion. Data are presented as the raw 2D pixel area of the pericardium.
Larvae were also screened for lordosis, a severe ventral curvature of the spine. Only larvae with obvious, beyond normal flexure of the spine, lordosis were categorized as having lordosis. Data were pooled by experiment for statistical analysis.
2.6 Statistical Analysis
All data were analyzed using SPSS ver. 15 (Chicago, IL). All data were analyzed using ANOVA to test for significant differences among treatments. Bonferroni-corrected post hoc multiple comparisons were conducted to determine which pairwise comparisons were statistically significant. EROD and pericardial effusion data were analyzed using data pooled by exposure vial (generally 3 vials/treatment/experiment); however, lordosis data were analyzed by experiment due to the discrete nature of the data. Statistical significance was accepted at p ≤ 0.05 for all tests.
3. Results
3.1 CYP1A protein activity as measured via in vivo EROD assay
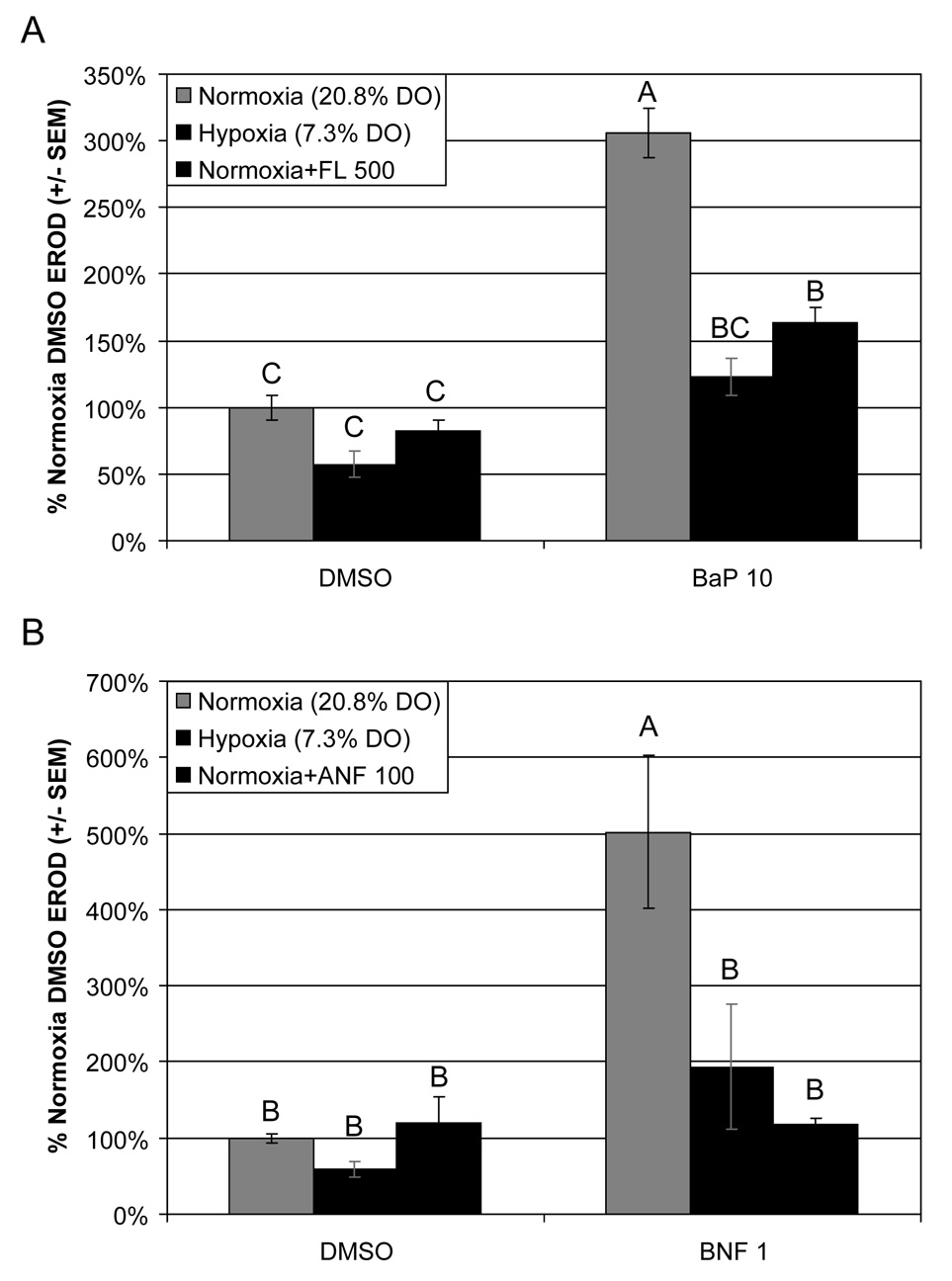
Larvae that had been dosed with BaP (10 µg L−1) showed expectedly high induction of CYP1A activity, 306% of non-induced DMSO, as measured via in vivo EROD assay (Fig. 1A). Fluoranthene coexposure reduced BaP-induced CYP1A activity by 46%. Hypoxia-induced reduction of BaP-mediated CYP1A induction was even more effective, reducing CYP1A activity by 60%, relative to BaP exposure under normoxic conditions. These data confirm that environmentally relevant hypoxia conditions (2.7 mg L−1) are capable of significantly reducing AHR function, and that the reduction in BaP-induced CYP1A activity is comparable to levels that have been shown to result in synergistic teratogenicity with these and other PAHs (Wassenberg and Di Giulio, 2004; Billiard et al., 2006; Matson et al., 2008). BNF exposures revealed a similar pattern, with BNF-induced EROD activity 502% of DMSO controls. ANF coexposure reduced BNF-mediated CYP1A activity by 76%, whereas hypoxia reduced BNF-induced CYP1A activity by 61% relative to normoxia BNF exposure (Fig.1B). While hypoxia did not reduce EROD activity as much as ANF in these BNF exposures, the difference was not statistically significant and both had less than 50% of the CYP1A activity of the normoxia BNF group.
Fig. 1.
CYP1A activity as measured via in vivo EROD assay. EROD data are presented as percent activity relative to DMSO control. EROD activity was measured in 96 hpf larvae. Embryos were dosed at 24 hpf with either (A) 10 µg L−1 BaP, and/or 500 µg L−1 FL; or (B) 1 µg L−1 BNF and/or 100 µg L−1 ANF. Embryos were then exposed to either normoxia (20.8% DO) or hypoxia (7.3% DO). DMSO was used as the carrier control in all experiments. These data confirm that hypoxia is capable of significantly reducing CYP1A activity to a similar degree as the CYP1A chemical inhibitors, FL and ANF. Groups not sharing a common letter are significantly different (p ≤ 0.05), based on Bonferroni-corrected post hoc multiple comparisons following ANOVA.
3.2 Deformity Screening
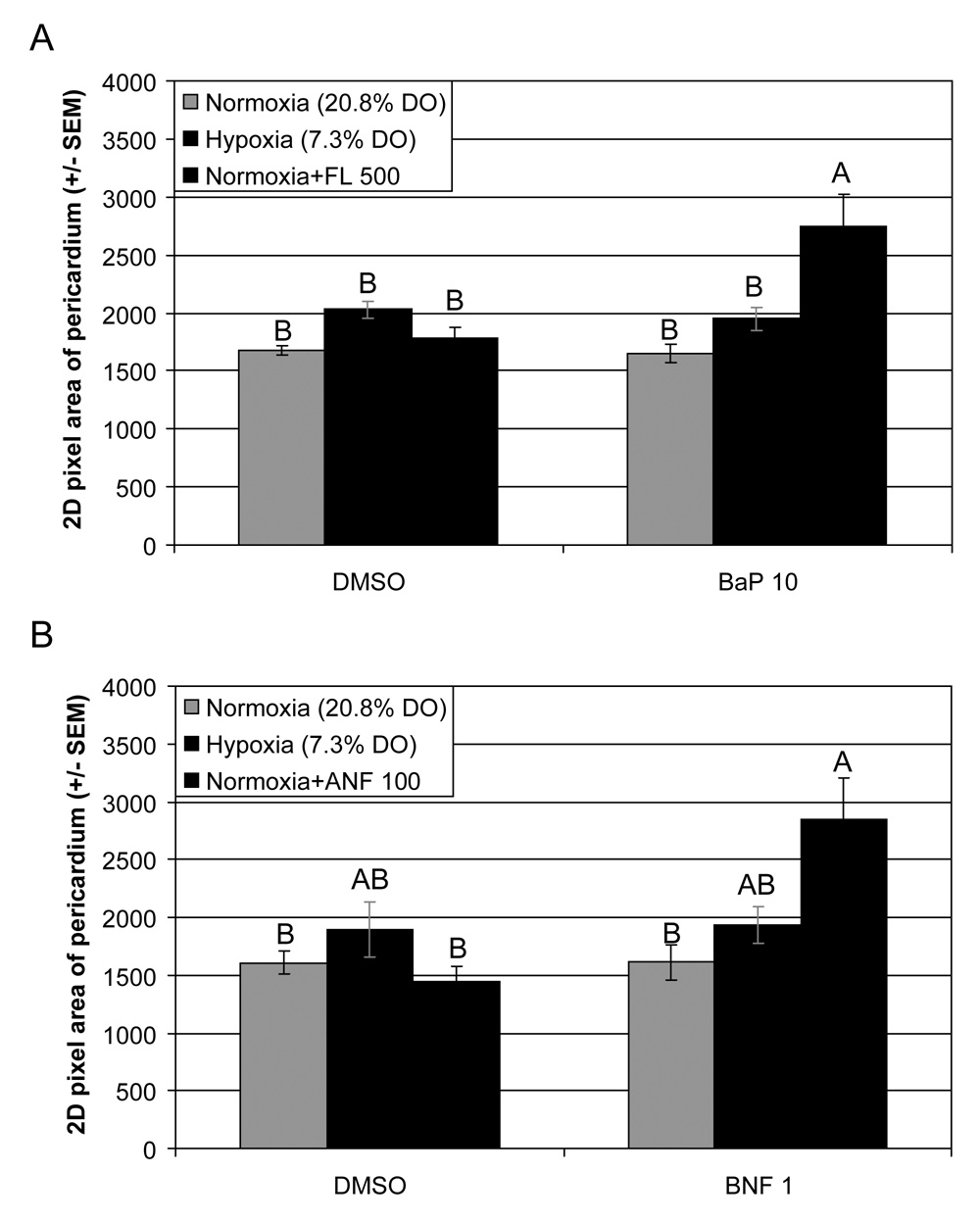
The area of the pericardium was quantified in 96 hpf larvae and is presented as mean 2D pixel area of the pericardium (Fig. 2). DMSO controls, FL, hypoxia, BaP, and BaP+hypoxia exposure groups did not show any evidence of pericardial effusion. The BaP+FL-exposed larvae were the only fish in this experiment with significant pericardial effusion (64% larger than DMSO controls; Fig. 2A). The BNF/ANF experiment was quite similar to the BaP/FL data. DMSO controls, ANF, hypoxia, BNF, and BNF+hypoxia exposure groups did not have any significant pericardial effusion (Fig. 2B). However, there was a significant interaction between BNF and ANF resulting in a 77% larger pericardium, relative to DMSO controls.
Fig. 2.
Mean pericardium size, based on 2D pixel area, was used to investigate pericardial effusion in 96 hpf zebrafish embryos. Embryos were dosed at 24 hpf with either (A) 10 µg L−1 BaP, and/or 500 µg L−1 FL; or (B) 1 µg L−1 BNF and/or 100 µg L−1 ANF. Embryos were then exposed to either normoxia (20.8% DO) or hypoxia (7.3% DO). DMSO was used as the carrier control in all experiments. These data show that while hypoxia is capable of significantly reducing BaP-induced CYP1A induction (Fig. 1), this reduction does not lead to an increase in pericardial effusion as seen with FL or ANF. Groups not sharing a common letter are significantly different (p ≤ 0.05), based on Bonferroni-corrected post hoc multiple comparisons following ANOVA.
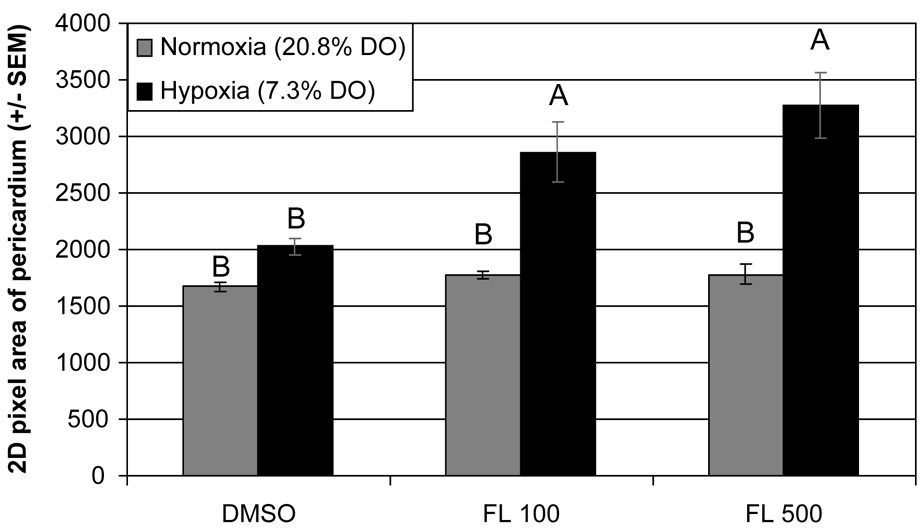
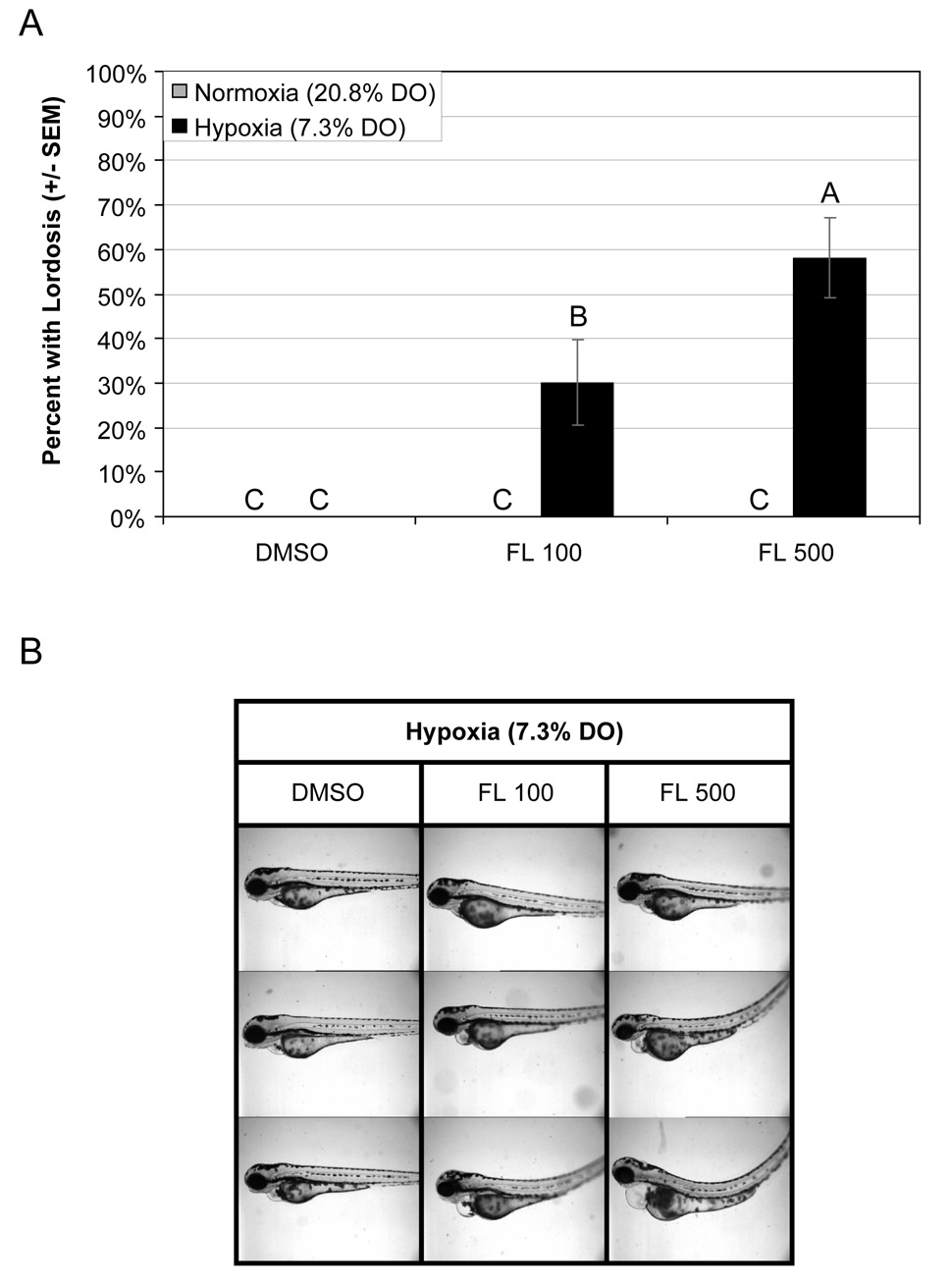
The most striking interaction in this study was that of FL and hypoxia. While not a part of the original experiment, when FL+hypoxia coexposures were performed we discovered that unexpected, yet significant interactions occurred. Fluoranthene concentrations of both 100 and 500 µg L−1 resulted in significant pericardial effusion (Fig. 3) and lordosis (Fig. 4) when larvae were coexposed to environmental hypoxia. There was a significant dose-dependant response to FL under hypoxic conditions, with relative increases of 71% and 95% over controls in 2D area of the pericardium, with 100 and 500 µg L−1 respectively (p = 0.001, 2-way ANOVA, interaction of hypoxia and FL). There was also an interaction between ANF and hypoxia; however, the interaction seemed to cause severe cardiovascular toxicity and pericardial effusion, but not lordosis (Fig. 5). Quantification of pericardial effusion was not possible as the majority of ANF+hypoxia coexposed embryos were unusable, resulting from pericardial or yolk-sac ruptures during orientation in 3% methylcellulose prior to imaging. However, the experiment has been replicated and the results were consistent. There also seems to be an increase in mortality associated with the ANF+hypoxia phenotype, likely as a direct result of the extreme pericardial effusion. This response is similar to, but more extreme than, that of BNF+ANF coexposures.
Fig. 3.
Estimates of pericardial effusion showing a dose response to 100 or 500 µg L−1 fluoranthene (FL) exposure under hypoxic conditions (7.3% DO), and no change in mean pericardium area in FL-exposed embryos under normoxic conditions (20.8% DO). There is a significant interaction between hypoxia and FL (p = 0.001). Groups not sharing a common letter are significantly different (p ≤ 0.05), based on Bonferroni-corrected post hoc multiple comparisons following ANOVA.
Fig. 4.
(A) Occurrence of lordosis in developing zebrafish larvae exposed to hypoxia (7.3% DO) and 0, 100, or 500 µg L−1 fluoranthene (FL). Groups not sharing a common letter are significantly different (p ≤ 0.05), based on Bonferroni-corrected post hoc multiple comparisons following ANOVA. (B) Images of 96 hpf zebrafish larvae showing the range of trunk abnormalities observed for coexposed embryos. While embryos exposed only to hypoxia and carrier control (DMSO) showed no evidence of trunk abnormalities, a significant proportion of embryos coexposed to hypoxia and FL developed lordosis.
Fig. 5.
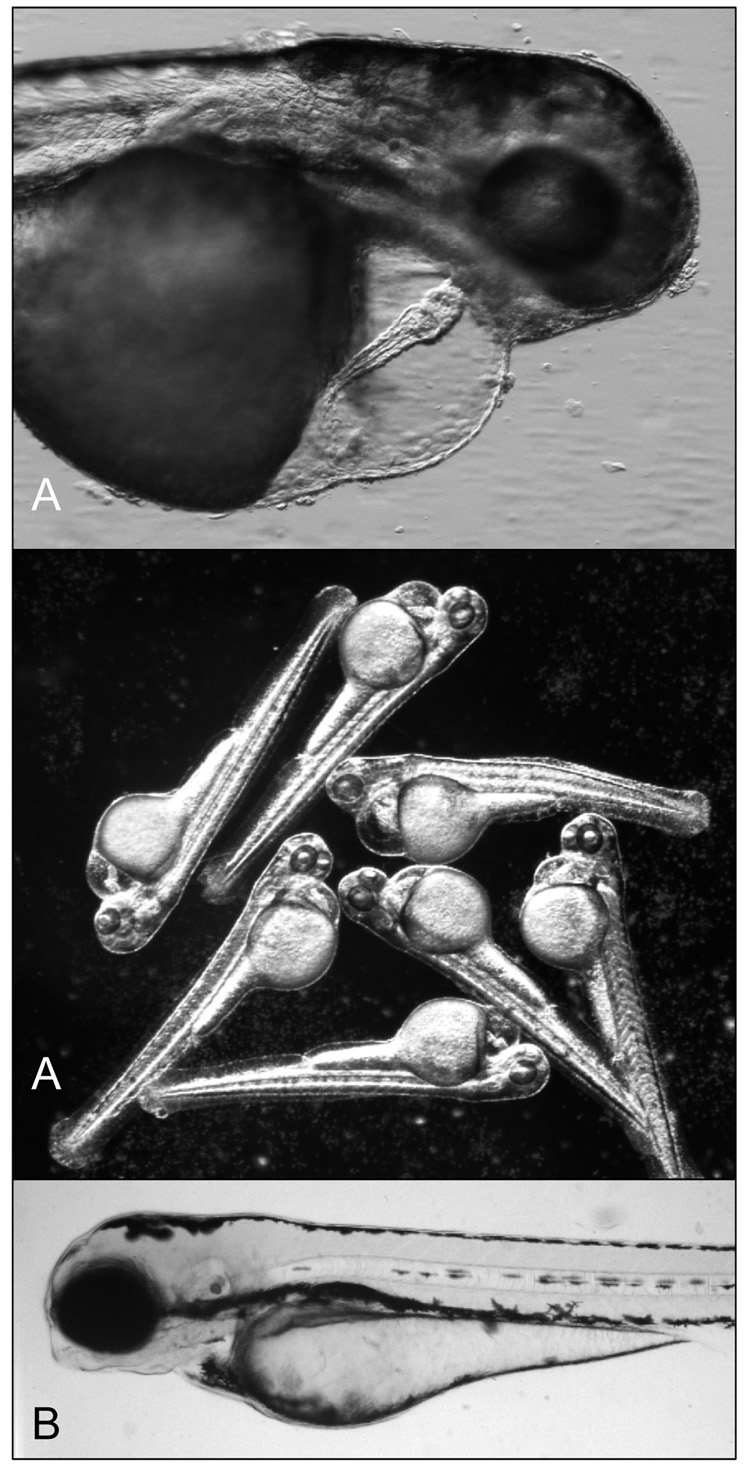
(A) Representative images of zebrafish embryos coexposed to hypoxia (7.3% DO) and 100 µg L−1 ANF. Note the consistent and substantial pericardial effusion in all of the 96 hpf larvae, relative to (B) a control zebrafish larvae. There is also a lack of lordosis which was previously described in the fluoranthene/hypoxia coexposed larvae.
4. Discussion and conclusions
There have been many mechanisms proposed to explain PAH toxicity and non-additive interactions (Billiard et al., 2007). These mechanisms have been incorporated into two hypotheses that attempt to explain the synergistic interaction of PAH-type AHR agonists and CYP1A inhibition. The first is that CYP1A inhibition significantly slows down metabolism of the AHR agonists, thus extending the half-life of the parent compound. The substantial increase in toxicity of the AHR agonists BaP and BNF via chemical or morpholino inhibition of CYP1A activity could support this hypothesis (Wassenberg and Di Giulio, 2004; Billiard et al., 2006; Matson et al., 2008). Whichever method of CYP1A knockdown is used, the result is the same. However, these studies only support this hypothesis if a reduction in CYP1A activity actually slows down Phase I metabolism. In support of this hypothesis, Hodson et al. (2007) were able to show that high concentrations of ANF significantly reduced the metabolism and excretion of retene in rainbow trout, Oncorhynchus mykiss. Our results with hypoxia appear to contradict the extended half-life hypothesis. Hypoxia may be interfering with the AHR pathway via cross-talk between the two molecular pathways or an overall reduction in cellular function. Either way, one would expect all of the AHR inducible genes to be downregulated by hypoxia. We expected to be able to address whether hypoxia was acting in a similar fashion to an AHR2 or a CYP1A morpholino. However, the complicating factor of the FL and ANF interactions with hypoxia prevented us from being able to adequately address whether hypoxia acts similarly to an AHR2 morpholino (i.e. rescues BAP+FL and BNF+ANF deformities). We have conclusively shown that hypoxia does not interact with BaP or BNF causing synergistic embryotoxicity, as is the case with CYP1A chemical inhibitors and morpholinos. The exact manner in which hypoxia interacts with PAHs and the AHR pathway is yet to be determined. It is possible that blocking the initiation of an AHR response (e.g. AHR2 morpholino) results in an entirely different response than blocking the response before it results in transcription (e.g. hypoxia), translation (CYP1A morpholino), or CYP1 activity (e.g. ANF or FL). This is clearly an area of research that needs to be pursued more fully.
A second hypothesis suggests that CYP1A inhibitors shift PAH metabolism towards more toxic metabolites. For example, Hodson et al. (2007) were able to document clear evidence of an ANF-induced shift in retene metabolism in rainbow trout dosed with low to intermediate concentrations of ANF. Work with CYP1A1 null mice also suggests that the metabolism of BaP by CYP1s other than CYP1A1 (i.e., CYP1B1) leads to increased production of BaP adducts, clearly suggesting that other CYP1s may be more likely to produce adduct forming BaP metabolites than CYP1A1 (Uno et al., 2001). While our hypoxia data do not directly support this hypothesis, it does suggest that simply slowing down metabolism is not sufficient to elicit teratogenicity. Since a hypoxia-mediated reduction in CYP1A activity does not elicit teratogenicity, perhaps the metabolic shift hypothesis might better explain observed patterns of teratogenicity. When CYP1A, CYP1B1, and CYP1C1 proteins are inhibited via an AhR knockdown or presumably hypoxia, no increase in teratogenicity is observed. A plausible hypothesis for the synergistic interaction of BaP and FL is that by reducing CYP1A activity, BaP metabolism is shifted to other enzymes, which may be less efficient, and/or more likely to produce toxic metabolites.
The lack of reciprocal cross-talk between the AHR and HIF-1α pathways is in agreement with previous research focusing on the potential for TCDD to interact with hypoxia (Prasch et al., 2004). This lack of cross-talk does not seem to protect fish from interactions between environmental hypoxia and at least some PAHs. While interactions between AHR agonist type PAHs and hypoxia were hypothesized because of the significant overlap of proteins on the AHR and HIF-1α pathways, and the potential for hypoxia to slow down PAH metabolism, the only significant interactions we found were with either partial AHR agonists or CYP1A inhibitors. While the mechanism through which FL and ANF interact with hypoxia to produce teratogenicity remains unknown, preliminary results from zebrafish AHR2 morpholino experiments suggest that this effect is independent of the AHR pathway (data not shown). Future work will evaluate the nature of these PAH/hypoxia interactions in a mechanistic framework. Regardless of the mechanisms responsible for this interaction, the fact that there is a significant increase in teratogenicity when embryos are coexposed to environmentally-relevant fluoranthene and moderate hypoxia poses a significant challenge for current environmental risk assessments of estuarine environments. Fluoranthene is one of the most abundant PAHs in contaminated marine environments (Latimer and Zheng, 2003). The prevalence of fluoranthene in the same estuaries where seasonal hypoxia occurs provides the potential for significant interactions, which could have profound effects on developing fish embryos. Also, it is currently unknown if this interaction may apply to other PAHs. Current risk assessment methods do not consider this previously unknown interaction.
Acknowledgements
We would like to thank Carrie R. Fleming, Kathleen P. Davis, Lauren P. Battle, Dawoon Jung, Lindsey A. Van Tiem, and Bryan W. Clark for their laboratory advice and assistance. We would also like to thank Dr. Margaret L. Kirby and Dr. Elwood Linney for providing zebrafish embryos. This work was funded in part by the National Institute of Environmental Health Sciences through the Duke Superfund Basic Research Center (P42ES010356), and the Duke Integrated Toxicology and Environmental Health Program (ES-T32-0007031).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antkiewicz DS, Burns CG, Carney SA, Peterson RE, Heideman W. Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicol. Sci. 2005;84:368–377. doi: 10.1093/toxsci/kfi073. [DOI] [PubMed] [Google Scholar]
- Billiard SM, Meyer JN, Wassenberg DM, Hodson PV, Di Giulio RT. Non-additive effects of PAHs on early vertebrate development: mechanisms and implications for risk assessment. Toxicol. Sci. 2007 doi: 10.1093/toxsci/kfm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiard SM, Querbach K, Hodson PV. Toxicity of retene to early life stages of two freshwater fish species. Environ. Toxicol. Chem. 1999;18:2070–2077. [Google Scholar]
- Billiard SM, Timme-Laragy AR, Wassenberg DM, Cockman C, Di Giulio RT. The role of the aryl hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons to zebrafish. Toxicol. Sci. 2006;92:526–536. doi: 10.1093/toxsci/kfl011. [DOI] [PubMed] [Google Scholar]
- Carls MG, Rice SD, Hose JE. Sensitivity of fish embryos to weathered crude oil: Part I. Low-level exposure during incubation causes malformations, genetic damage, and mortality in larval Pacific herring (Clupea pallasi) Environ. Toxicol. Chem. 1999;18:481–493. [Google Scholar]
- Carney SA, Peterson RE, Heideman W. 2,3,7,8-tetrachlorodibenzo-p-dioxin activation of the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator pathway causes developmental toxicity through a CYP1A-independent mechanism in zebrafish. Mol. Pharmacol. 2004;66:512–521. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- Diaz RJ, Rosenberg R. Marine benthic hypoxia: A review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanogr. Mar. Biol.-Annu. Rev. 1995;33:245–303. [Google Scholar]
- Douben PET. Introduction. In: Douben PET, editor. PAHs: An Ecotoxicological Perspective. West Sussex, England: John Wiley & Sons; 2003. pp. 3–6. [Google Scholar]
- EPA. Quality Criteria for Water 1986. EPA 440/5-86-001. Washington, DC: US Environmental Protection Agency; 1987. [Google Scholar]
- Goldstone HMH, Stegeman JJ. Molecular mechanisms of 2,3,7,8-tetrachlorodibenzo-p-dioxin cardiovascular embryotoxicity. Drug Metab. Rev. 2006;38:261–289. doi: 10.1080/03602530600570099. [DOI] [PubMed] [Google Scholar]
- Harper PA, Riddick DS, Okey AB. Regulating the regulator: Factors that control levels and activity of the aryl hydrocarbon receptor. Biochem. Pharmacol. 2006;72:267–279. doi: 10.1016/j.bcp.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Hendon LA, Carlson EA, Manning S, Brouwer M. Molecular and developmental effects of exposure to pyrene in the early life-stages of Cyprinodon variegatus. Comp. Biochem Physiol. C-Toxicol. Pharmacol. 2008;147:205–215. doi: 10.1016/j.cbpc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Hodson PV, Qureshi K, Noble CAJ, Akhtar P, Brown RS. Inhibition of CYP1A enzymes by α-naphthoflavone causes both synergism and antagonism of retene toxicity to rainbow trout (Oncorhynchus mykiss) Aquat. Toxicol. 2007;81:275–285. doi: 10.1016/j.aquatox.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Carls MG, Teraoka H, Sloan CA, Collier TK, Scholz NL. Aryl hydrocarbon receptor-independent toxicity of weathered crude oil during fish development. Environ. Health Perspect. 2005;113:1755–1762. doi: 10.1289/ehp.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona JP, Day HL, Collier TK, Scholz NL. Developmental toxicity of 4-ring polycyclic aromatic hydrocarbons in zebrafish is differentially dependent on AH receptor isoforms and hepatic cytochrome P4501A metabolism. Toxicol. Appl. Pharmacol. 2006;217:308–321. doi: 10.1016/j.taap.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Kasper LH, Boussouar F, Boyd K, Xu W, Biesen M, Rehg J, Baudino TA, Cleveland JL, Brindle PK. Two transactivation mechanisms cooperate for the bulk of HIF-1-responsive gene expression. EMBO J. 2005;24:3846–3858. doi: 10.1038/sj.emboj.7600846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, NumayamaTsuruta K, Sogawa K, FujiiKuriyama Y. CBP/p300 functions as a possible transcriptional coactivator of Ah receptor nuclear translocator (Arnt) J. Biochem. 1997;122:703–710. doi: 10.1093/oxfordjournals.jbchem.a021812. [DOI] [PubMed] [Google Scholar]
- Latimer JS, Zheng J. The sources, transport, and fate of PAHs in the marine environment. In: Douben PET, editor. PAHs: An Ecotoxicological Perspective. West Sussex, England: John Wiley & Sons; 2003. pp. 9–33. [Google Scholar]
- Matson CW, Clark BW, Jenny MJ, Fleming CR, Hahn ME, Di Giulio RT. Development of the morpholino gene knockdown technique in Fundulus heteroclitus: A tool for studying molecular mechanisms in an established environmental model. Aquat. Toxicol. 2008;87:289–295. doi: 10.1016/j.aquatox.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacci D, Coiro L, Kuhn A, Champlin D, Munns W, Jr, Specker J, Cooper K. Nondestructive indicator of ethoxyresorufin-O-deethylase activity in embryonic fish. Environ. Toxicol. Chem. 1998;17:2481–2486. [Google Scholar]
- Nacci D, Coiro L, Wassenberg DM, Di Giulio RT. A non-destructive technique to measure cytochrome P4501A enzyme activity in living embryos of the estuarine fish Fundulus heteroclitus. In: Ostrander GK, editor. Techniques in Aquatic Toxicology. Vol 2. Boca Raton, FL: CRC Press; 2005. pp. 209–225. [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene 'knockdown' in zebrafish. Nat. Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Pollenz RS, Davarinos NA, Shearer TP. Analysis of aryl hydrocarbon receptor-mediated signaling during physiological hypoxia reveals lack of competition for the aryl hydrocarbon nuclear translocator transcription factor. Mol. Pharmacol. 1999;56:1127–1137. doi: 10.1124/mol.56.6.1127. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Andreasen EA, Peterson RE, Heideman W. Interactions between 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and hypoxia signaling pathways in zebrafish: hypoxia decreases responses to TCDD in zebrafish embryos. Toxicol. Sci. 2004;78:68–77. doi: 10.1093/toxsci/kfh053. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Teraoka H, Carney SA, Dong W, Hiraga T, Stegeman JJ, Heideman W, Peterson RE. Aryl hydrocarbon receptor 2 mediates 2,3,7,8-tetrachlorodibenzo-p-dioxin developmental toxicity in zebrafish. Toxicol. Sci. 2003;76:138–150. doi: 10.1093/toxsci/kfg202. [DOI] [PubMed] [Google Scholar]
- Reeves WR, Barhoumi R, Burghardt RC, Lemke SL, Mayura K, McDonald TJ, Phillips TD, Donnelly KC. Evaluation of methods for predicting the toxicity of polycyclic aromatic hydrocarbon mixtures. Environ. Sci. Technol. 2001;35:1630–1636. doi: 10.1021/es001689a. [DOI] [PubMed] [Google Scholar]
- Ruas JL, Poellinger L, Pereira T. Role of CBP in regulating HIF-1-mediated activation of transcription. J. Cell Sci. 2005;118:301–311. doi: 10.1242/jcs.01617. [DOI] [PubMed] [Google Scholar]
- Schiedek D, Sundelin B, Readman JW, Macdonald RW. Interactions between climate change and contaminants. Mar. Pollut. Bull. 2007;54:1845–1856. doi: 10.1016/j.marpolbul.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Spehar RL, Poucher S, Brooke LT, Hansen DJ, Champlin D, Cox DA. Comparative toxicity of fluoranthene to freshwater and saltwater species under fluorescent and ultraviolet light. Arch. Environ. Contam. Toxicol. 1999;37:496–502. doi: 10.1007/s002449900544. [DOI] [PubMed] [Google Scholar]
- Timme-Laragy AR, Cockman CJ, Matson CW, Di Giulio RT. Synergistic induction of AHR regulated genes in developmental toxicity from co-exposure to two model PAHs in zebrafish. Aquat. Toxicol. 2007;85:241–250. doi: 10.1016/j.aquatox.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton C, Stamatiou D, Liew C-C. Gene expression profile of zebrafish exposed to hypoxia during development. Physiol. Genomics. 2003;13:97–106. doi: 10.1152/physiolgenomics.00128.2002. [DOI] [PubMed] [Google Scholar]
- Uno S, Dalton TP, Shertzer HG, Genter MB, Warshawsky D, Talaska G, Nebert DW. Benzo[a]pyrene-induced toxicity: paradoxical protection in cyp1a1(−/−) knockout mice having increased hepatic BaP-DNA adduct levels. Biochem. Biophys. Res. Commun. 2001;289:1049–1056. doi: 10.1006/bbrc.2001.6110. [DOI] [PubMed] [Google Scholar]
- Wassenberg DM, Di Giulio RT. Synergistic embryotoxicity of polycyclic aromatic hydrocarbon aryl hydrocarbon receptor agonists with cytochrome P4501A inhibitors in Fundulus heteroclitus. Environ. Health Perspect. 2004;112:1658–1664. doi: 10.1289/ehp.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenberg DM, Swails EE, Di Giulio RT. Effects of single and combined exposures to benzo(a)pyrene and 3,3′4,4′5-pentachlorobiphenyl on EROD activity and development in Fundulus heteroclitus. Mar. Environ. Res. 2002;54:279–283. doi: 10.1016/s0141-1136(02)00182-4. [DOI] [PubMed] [Google Scholar]