Abstract
Mice null for the gene encoding protein kinase Cα (Prkca), or mice treated with pharmacologic inhibitors of the PKCα/β/γ isoforms, show an augmentation in cardiac contractility that appears to be cardioprotective. However, it remains uncertain if PKCα itself functions in a myocyte autonomous manner to effect cardioprotection in vivo. Here we generated cardiac myocyte-specific transgenic mice using a tetracycline-inducible system to permit controlled expression of dominant negative (dn) PKCα in the heart. Consistent with the proposed function of PKCα, induction of dnPKCα expression in the adult heart enhanced baseline cardiac contractility. This increase in cardiac contractility was associated with a partial protection from long-term decompensation and secondary dilated cardiomyopathy following myocardial infarction (MI) injury. Similarly, Prkca null mice were also partially protected from infarction-induced heart failure, although the area of infarction injury was identical to controls. Thus, myocyte autonomous inhibition of PKCα protects the adult heart from decompensation and dilated cardiomyopathy following infarction injury, in association with a primary enhancement in contractility.
INTRODUCTION
The protein kinase C (PKC) family of Ca2+ and/or lipid-activated serine-threonine kinases function downstream of many membrane-associated signal transduction pathways (4,12). Approximately 10 different isozymes comprise the PKC family, which are broadly classified by their activation characteristics. The conventional PKC isozymes (α, ßI, ßII, and γ) are Ca2+- and lipid-activated, while the novel isozymes (ε, θ, η, and δ) and atypical isozymes (ζ, and λ) are Ca2+-independent but activated by distinct lipids (4,12). PKCα is the predominant PKC isoform expressed in the mouse, human, and rabbit heart, while PKCβ and PKCγ are detectable and may have partially overlapping functions (7,9,10). With respect to the heart, a number of reports have associated PKC activation or an increase in PKCα expression with hypertrophy, dilated cardiomyopathy, ischemic injury, or mitogen stimulation (4,12).
We and others have shown that PKCα functions as a novel regulator of cardiac contractility through effects on Ca2+ handling and myofilament proteins (1,2,5,6). For example, Prkca (PKCα−/−) gene-deleted mice are hypercontractile, while transgenic mice overexpressing PKCα in the heart are hypocontractile. Enhancement in cardiac contractility associated with Prkca deletion protected against pressure overload-induced heart failure and dilated cardiomyopathy associated with deletion of the muscle lim protein (MLP) gene (Csrp3) in the mouse (2). More recently, we extended these results to include an analysis of pharmacologic inhibitors that are generally specific for the PKCα/β/γ isoform subclass. Ro-32−0432 or Ro-31−8220 each significantly augmented cardiac contractility in vivo and in an isolated work performing heart preparation in wildtype (Wt) mice, but not in Prkca deficient mice (7). While inhibition of PKCα would appear to be an attractive therapeutic approach for affecting heart disease, some areas of uncertainty remain. We addressed these uncertainties by 1) using a myocyte autonomous expression system for in vivo functional assessment, 2) employing yet another model of heart failure associated with infarction injury, and 3) bypassing developmental compensatory issues by using an inducible system.
MATERIALS AND METHODS
Animals
The Prkca null mice have been described previously (2). The rabbit dnPKCα cDNA (−4 – +2647) with an L368A mutation was described previously (2). dnPKCα transgenic mice (FVBN strain) were generated by fusing dnPKCα cDNA, which was isolated and purified from the previously described rabbit adenoviral dnPKCα, to the cardiac-specific, inducible and attenuated α-MHC promoter (“responder” line) previously described (11). dnPKCα transgenic mice were bred with transcriptional transactivator (tTA) (“driver” line) mice (11), which in the presense of doxycycline (Dox) administration inhibits all expression. Dox was removed at 4 weeks of age, producing expression within another 8 weeks (although expression was not immediate), at which time experiments were performed. Dox was administered in the food with a special diet formulated by Purina (625 mg/kg in pellets). Animal experiments were approved by the Institutional Animal Care and Use Committee.
Experimental Design
Western blotting was performed as described previously with primary antibodies against PKCα (Santa Cruz Biotechnology) (2). Chemifluorescent detection was performed with the Vistra ECF reagent (Amersham Pharmacia Biotech) and scanned with a phosphorImager. For invasive hemodynamics in the closed-chest mouse, a 1.4 F Millar catheter was placed into the left ventricle through the right carotid artery to monitor real time heart rate, arterial and left ventricular pressures, and +dP/dt (dP/dtmax) and -dP/dt (dP/dtmin), using a PowerLab system and Chart software (AD Instruments, Colorado Springs, CO), as described previously (2). In this preparation, dobutamine was given at 32 μg/kg/min. The ischemia-reperfusion (IR) model was described previously (8), while the myocardial infarction (MI) injury model involved a similar procedure except that the left coronary artery (LCA) was permanently ligated in 12−14 week old mice (or a sham procedure for controls). For IR, a suture with a slipknot was tied around the LCA, and mice were revived for a 60-min ischemia period after which the knot was released and reperfusion in the heart occurred for 24 hours. After reperfusion, mice were sacrificed, the slipknot retied, and hearts were analyzed as previously described using 2% triphenyltetrazolium chloride (TTC) in saline. Myocardial area not at risk, area at risk (AAR), and infarcted area (IA) were quantified using ImageJ software (Scion, Frederick, MD). For echocardiography, all mice were anesthetized with isoflurane, and a Hewlett Packard 5500 instrument with a 15-MHz microprobe was used. Measurements were taken on M-mode in triplicate for the numbers indicated for each group. Mice were sacrificed at 2 weeks and 16 weeks for analysis. Heart weight to body weight (HW/BW) ratios were recorded and then hearts were fixed in 10% formalin/PBS, embedded in paraffin and 8-μm heart sections were analyzed (8).
Statistical Analysis
All data are shown as means +/− SEM. Data were tested for significance with a one-way analysis of variance (ANOVA) followed by a Newman-Keuls post-hoc test. For multiple groups a two-way ANOVA was used followed by a pair-wise multi-group comparison by the Holm-Sidak method at each time point shown in Figures 2A, 2B and 3A.
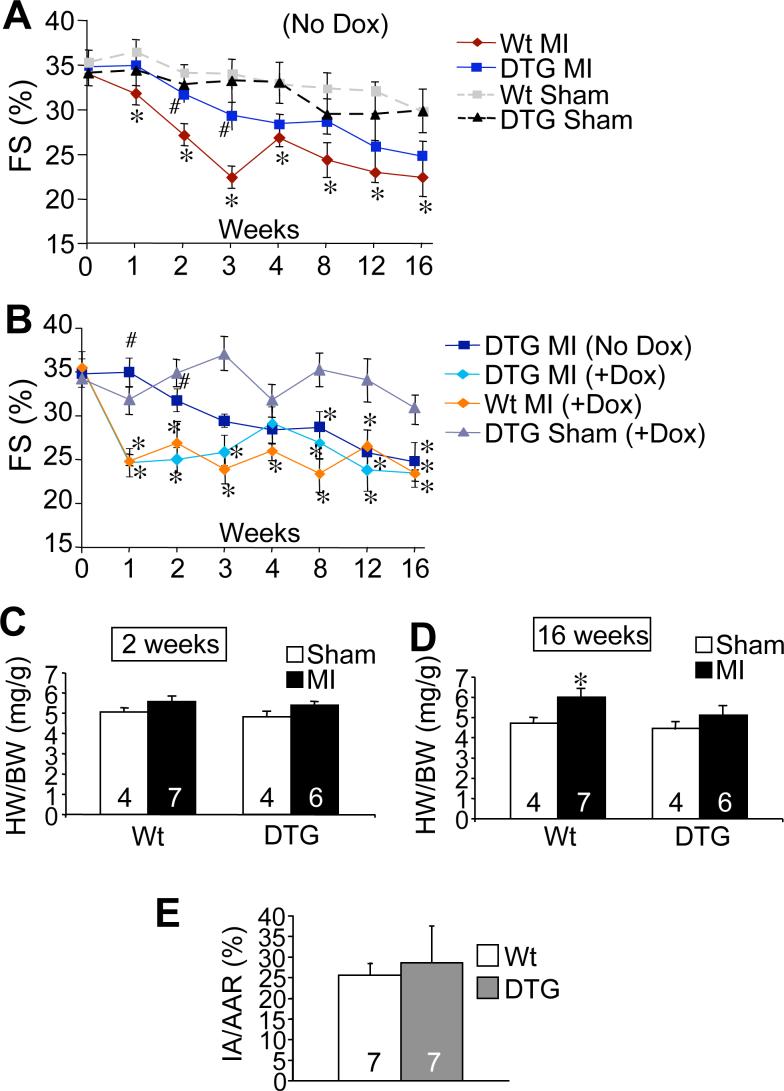
Figure 2.
dnPKCα expression partially protects from MI-induced decompensation and remodeling. (A) Assessment of fractional shortening (FS=(LVED-LVES)/LVED*100) by echocardiography following MI or sham surgical procedure in DTG (*N=19−14;8) following MI compared to Wt mice after MI (*N=19−14;7), along with Wt (*N=12−11;4) and DTG (*N=10−9;4) sham groups. (B) Function in DTG (No Dox) mice compared to control groups on Dox following MI (no expression). The control groups were DTG MI (Dox) (*N=14−8;4−3), Wt MI (Dox) (*N=8−4;4), and DTG (Dox) Sham (*N=6;5−4). (C) HW/BW ratios of Wt and DTG (off Dox) 2 or (D) 16 weeks following MI or Sham. (E) Quantification of infarct area (IA) versus area at risk (AAR) after ischemia-reperfusion injury in Wt and DTG mice. (*Note: Some mice were sacrificed at 2 and 16 weeks. The first set of numbers represents the N from weeks 0−2, while the second set represents the N following week 2 through week 16.) *p≤0.05 versus WT Sham; #p≤0.05 versus Wt MI.
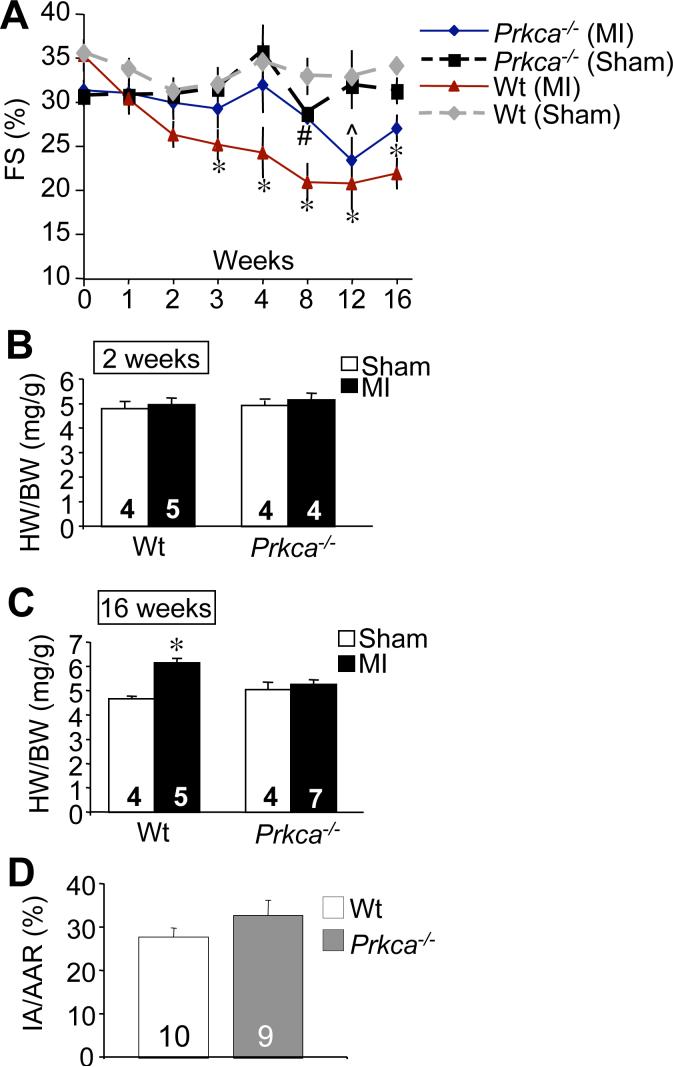
Figure 3.
Prkca−/− mice are protected from MI-induced heart failure. (A) Assessment of fractional shortening in Prkca−/− mice (*N=18−14;8−7) following MI compared to Wt mice (*N=8−6;5), along with Wt (*N=8−6;5) and Prkca−/− (*N=8;4) sham groups. (B) HW/BW ratios of Wt and Prkca−/− mice 2 and (C) 16 weeks following MI or Sham. (D) Quantification of infarct area (IA) versus area at risk (AAR) after ischemia-reperfusion injury in Wt and Prkca−/− mice. N is represented in the figure for B, C, and D. (*Note: Some mice were sacrificed at weeks 2 and 16. The first set of numbers represents the N from weeks 0−2, while the second set represents the N following week 2 through week 16.) *p≤0.05 versus Wt Sham; ^p≤0.05 versus Prkca−/− Sham; #p≤0.05 versus Wt MI.
RESULTS
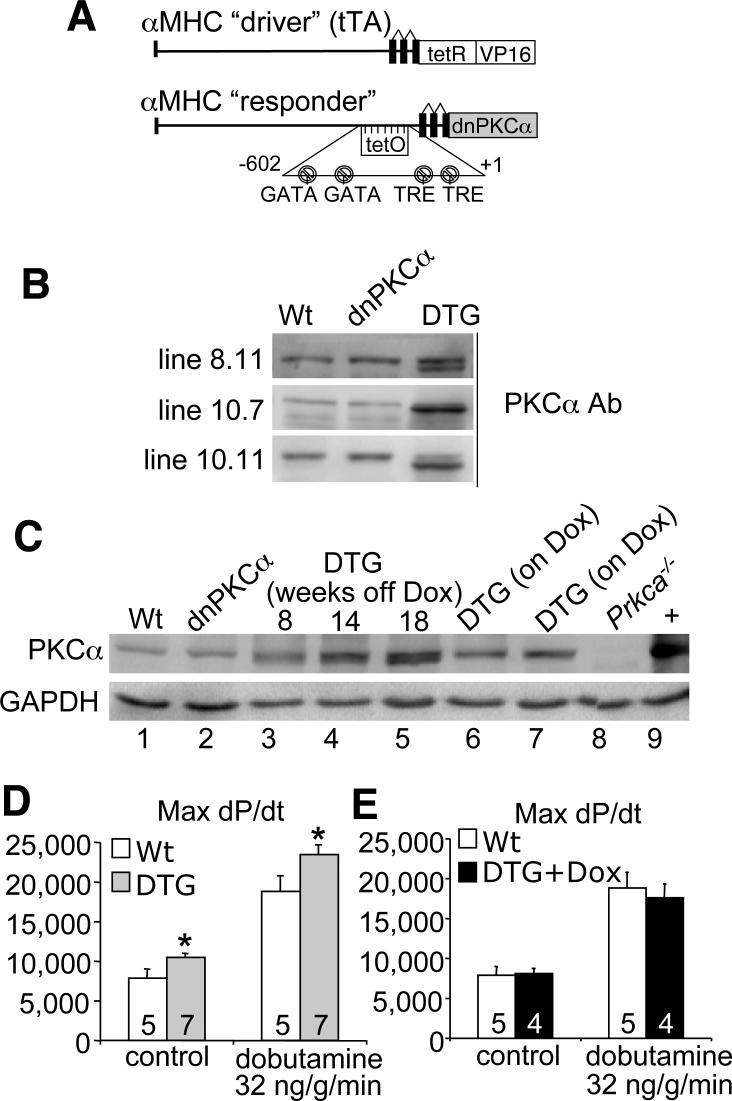
To examine more precisely the role of PKCα in regulating cardiac contractility and heart failure propensity we generated myocyte-specific transgenic mice with inducible expression of a cDNA encoding dnPKCα. We used the newly re-engineered bi-transgenic tetracycline-regulated system that permits robust expression only when both transgenes are present in the absence of Dox (Figure 1A) (11). Six dnPKCα transgenic lines were originally generated, from which three lines were selected based on a lack of basal expression when crossed with the tTA “driver” transgene (making double transgenic mice; DTG) in the presence of Dox, but high levels of expression when Dox was removed (Figure 1B). The dnPKCα protein migrates slightly faster on a western blot because it is devoid of autophosphorylation. Line 10.11 was used for all subsequent analysis, as it appeared essentially the same as the other 2 lines. More careful characterization of this line showed no protein expression from the single transgene (Figure 1C, lane 2), but good induction of dnPKCα in DTG mice in the absence of Dox (lanes 3, 4, and 5). Expression became gradually stronger over time as the Dox completely cleared the animal's system (Figure 1C). Importantly, when placed on Dox from birth or only for 3 days, DTG mice showed no dnPKCα expression (Figure 1C, lanes 7 and 8). Consistent with Prkca−/− mice, adult DTG mice had increased contractility at baseline and with dobutamine infusion compared to Wt, while DTG mice on Dox (no expression) did not have increased contractility compared to Wt (Figures 1D and 1E). Dobutamine was used as a means of assessing the upper range of contractile responsiveness, which remained significantly greater in DTG mice.
Figure 1.
Induced expression of dnPKCα in the adult heart augments contractility. (A) Diagram of promoter constructs used for the dual transgene tetracycline-inducible system based on the cardiac-specific α-MHC promoter. (B) Western blot analysis of dnPKCα inducible expression in three transgenic lines that are double transgenic (DTG) with the tTA transgene in the absence of Dox. Single dnPKCα transgenic mice show no expression, similar to Wt. (C) Western blot analysis examining PKCα expression in Wt (lane 1), in dnPKCα single transgenic (lane 2), in DTG following Dox removal (lanes 3−5), in DTG born on Dox (lane 6), in DTG following Dox addition back to the diet (lane 7), and in negative and positive controls, respectively (lanes 8−9). (D,E) Invasive hemodynamics to assess cardiac function at baseline or after dobutamine ß-agonist infusion in Wt and DTG mice off (D) and on (E) Dox. N is represented in the figure. *p<0.05 versus WT.
To further examine the concept that subtle, albeit significant increases in cardiac contractile performance can benefit the heart following pathologic stimulation, we performed MI injury in DTG mice. Following MI, ventricular function assessed by echocardiography decreased within one week in Wt mice, but was maintained in DTG mice. Fractional shortening (FS) % in DTG MI mice was significantly greater than in Wt MI mice up to 3 weeks following MI (Figure 2A). As a control, DTG mice without Dox (induced) were compared to DTG mice on Dox (no expression), along with Wt mice on Dox. DTG mice on Dox showed a significant reduction in functional performance following MI, similar to Wt mice on Dox, yet DTG mice off Dox (induced) were partially protected at 1 and 2 weeks following MI (Figure 2B). However, by 12 and 16 weeks after MI, even DTG mice off Dox (induced) showed signs of reduced ventricular performance that was similar to Wt mice (see Discussion).
Two weeks after MI injury all groups showed no significant increase in HW/BW (Figure 2C). However, by 16 weeks Wt mice had developed a significant increase in HW/BW, while the DTG mice did not (Figure 2D). This partial protection from loss of ventricular performance and secondary increases in HW/BW in DTG mice after MI was not due to less acute injury associated with PKCα inhibition, as Wt mice had an equivalent IR injury response to DTG mice, and scar sizes were also equivalent at the end of the different experimental protocols (Figure 2E, and data not shown). Finally, we have previously shown that PKCα does not directly regulate cardiac hypertrophy in mice (2), hence we interpret the lack of hypertrophy at 16 weeks in DTG mice to be associated with augmented function (see Discussion).
The results presented with dnPKCα inducible transgenic mice were compared against Prkca−/− mice. As with DTG mice, ventricular function was maintained in Prkca−/− mice after MI compared to Wt (Figure 3A). Perhaps in an even more dramatic manner than the DTG mice, the FS (%) only decreased in the Prkca−/− mice at 2 and 12 weeks after MI, while all other time points showed better function compared with Wt MI mice (Figure 3A). As with DTG mice, HW/BW ratios were not significantly altered at 2 weeks after MI (Figure 3B), but at 16 weeks after MI, HW/BW ratios were increased in Wt but not Prkca−/− mice (Figure 3C). As with the DTG mice, Prkca−/− mice showed nearly identical infarction injuries as Wt mice, as assessed by histological analysis and Masson's trichrome staining (data not shown). Similar to DTG mice, we also determined that loss of Prkca−/− did not alter the area of myocardial death within 24 h or IR injury compared with Wt mice (Figure 3D). These observations suggest that loss or inhibition of PKCα protects the heart in association with augmented contractile function.
DISCUSSION
The data presented here further strengthen the case for inhibiting PKCα as a therapeutic strategy for treating select forms of heart disease associated with reduced ventricular performance. We had previously shown that Prkca−/− mice were hypercontractile at baseline and partially protected from heart failure induced by long-term pressure overload or associated with loss of the Csrp3 gene (MLP) (2). However, a significant concern with our previous data in Prkca−/− mice is that of developmental compensation, which often plagues gene-targeting experiments in the mouse. Also, loss of Prkca was not myocyte-specific so effects in non-myocytes (and tissues outside the heart) could have secondarily impacted the contractile response in vivo. Despite these potential issues, acute inhibition of PKCα with the myocyte-specific inducible dominant-negative transgene produced a very similar phenotype to the Prkca−/− mouse. We also previously employed two distinct PKC inhibitory compounds in mice, resulting in an acute increase in cardiac contractility and a restoration of cardiac function in Crsp3 null mice (7). However, neither compound was completely selective for PKCα, so it was unclear as to which isoform was most important for inhibition. The use of dnPKCα affords greater specificity and was expressed at a more precise temporal moment, bypassing any potential compensatory effects by other genes. Another novel aspect of the current study was the examination of heart failure secondary to MI, which had not been previously analyzed.
Inhibition of PKCα with the dnPKCα only imparted a temporary increase in cardiac function within the first 1−3 weeks after MI, while at later time-points function deteriorated to levels that were more reminiscent of Wt mice. However, Prkca−/− mice, which are maximally inhibited compared with only partial inhibition in dnPKCα mice, showed a better improvement in function after MI at nearly every time point up to 16 weeks. This dramatic protection observed in the Prkca−/− mice reduced secondary hypertrophy by 16 weeks of age, although even the partial protection observed in dnPKCα mice was sufficient to reduce the secondary hypertrophy response at 16 weeks. The augmentation in ventricular performance after MI observed in either dnPKCα or Prkca−/− mice was not due to differences in the size of the initial infarction injury within the heart, suggesting that secondary remodeling was directly affected, potentially due to an intrinsic increase in contractility.
That augmentation in cardiac contractility can benefit an injured heart remains controversial. While traditional inotropes shorten life span in heart failure patients, and putatively negative inotropic agents (β-adrenergic receptor blockers) extend life span, recent evidence in animal models of heart failure suggests that inotropic support, if properly targeted, can be of therapeutic value (3). The hypothesis put forth is that by selectively augmenting cardiac contractility in heart failure, function is restored above a threshold that abates neuroendocrine drive and the associated ventricular remodeling and progressive loss of myocytes from the heart (3). Inhibition of PKCα may be an ideal choice as a novel inotropic strategy, as it more directly targets calcium handling and possibly myofilament function without engaging upstream signaling pathways that typifies other inotropic strategies (1,2).
While there is a clear need for novel inotropes to support late-stage heart failure patients in acute crisis, there may also be a therapeutic niche in earlier stages of heart failure if the inotrope is safe and not overly potent. Indeed, inhibition of PKCα may provide such an opportunity, as it appears to be a mild inotrope that is not subject to desensitization, and its mechanism of action at the level of the sarcoplasmic reticulum and myofilament proteins suggest that it could be safer than cAMP elevating agents. However, it remains possible that inhibition of PKCα may also benefit the heart for reasons other than alterations in contractile performance, such as positively affecting ventricular remodeling and the activity of other stress signaling pathways. Either way, the data that has emerged in animal models suggest a number of therapeutic opportunities for focusing on PKCα as a heart failure target.
ACKNOWLEDGMENTS
None
GRANTS
This work was supported by grants from the National Institutes of Health (J.R., J.D.M.), an American Heart Association Established Investigator Grant (J.D.M.), and by the Fondation Leducq (Heart failure network grant to J.D.M).
REFERENCES
- 1.Belin RJ, Sumandea MP, Allen EJ, Schoenfelt K, Wang H, Solaro RJ, de Tombe PP. Augmented protein kinase C-alpha-induced myofilament protein phosphorylation contributes to myofilament dysfunction in experimental congestive heart failure. Circ Res. 2007;101:195–204. doi: 10.1161/CIRCRESAHA.107.148288. [DOI] [PubMed] [Google Scholar]
- 2.Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB, Iodi B, Wang S, Schwartz A, Lakatta EG, DePaoli-Roach AA, Robbins J, Hewett TE, Bibb JA, Westfall MV, Kranias EG, Molkentin JD. PKCα regulates cardiac contractility and propensity towards heart failure. Nat Med. 2004;10:248–254. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 3.Dorn GW, 2nd, Molkentin JD. Manipulating cardiac contractility in heart failure: data from mice and men. Circulation. 2004;109:150–158. doi: 10.1161/01.CIR.0000111581.15521.F5. [DOI] [PubMed] [Google Scholar]
- 4.Dorn GWII, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest. 2005;115:527–537. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Armouche A, Singh J, Naito H, Wittkopper K, Didie M, Laatsch A, Zimmermann WH, Eschenhagen T. Adenovirus-delivered short hairpin RNA targeting PKCalpha improves contractile function in reconstituted heart tissue. J Mol Cell Cardiol. 2007;43:371–376. doi: 10.1016/j.yjmcc.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Hahn HS, Marreez Y, Odley A, Sterbling A, Yussman MG, Hilty KC, Bodi I, Liggett SB, Schwartz A, Dorn GW., 2nd Protein kinase Calpha negatively regulates systolic and diastolic function in pathological hypertrophy. Circ Res. 2003;93:1111–1119. doi: 10.1161/01.RES.0000105087.79373.17. [DOI] [PubMed] [Google Scholar]
- 7.Hambleton M, Hahn H, Pleger ST, Kuhn MC, Klevitsky R, Carr AN, Kimball TF, Hewett TE, Dorn GW, 2nd, Koch WJ, Molkentin JD. Pharmacological- and gene therapy-based inhibition of protein kinase Calpha/beta enhances cardiac contractility and attenuates heart failure. Circulation. 2006;114:574–582. doi: 10.1161/CIRCULATIONAHA.105.592550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaiser RA, Liang Q, Bueno O, Huang Y, Lackey T, Klevitsky R, Hewett TE, Molkentin JD. Genetic inhibition or activation of JNK1/2 protects the myocardium from ischemia-reperfusion-induced cell death in vivo. J Biol Chem. 2005;280:32602–32608. doi: 10.1074/jbc.M500684200. [DOI] [PubMed] [Google Scholar]
- 9.Pass JM, Gao J, Jones WK, Wead WB, Wu X, Zhang J, Baines CP, Bolli R, Zheng YT, Joshua IG, Ping P. Enhanced PKC beta II translocation and PKC beta II-RACK1 interactions in PKC epsilon-induced heart failure: a role for RACK1. Am J Physiol Heart Circ Physiol. 2001;281:H2500–H2510. doi: 10.1152/ajpheart.2001.281.6.H2500. [DOI] [PubMed] [Google Scholar]
- 10.Ping P, Zhang J, Qiu Y, Tang XL, Manchikalapudi S, Cao X, Bolli R. Ischemic preconditioning induces selective translocation of protein kinase C isoforms epsilon and eta in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity. Circ Res. 1997;81:404–414. doi: 10.1161/01.res.81.3.404. [DOI] [PubMed] [Google Scholar]
- 11.Sanbe A, Gulick J, Hanks MC, Liang Q, Osinska H, Robbins J. Reengineering inducible cardiac-specific transgenesis with an attenuated myosin heavy chain promoter. Circ Res. 2003;92:609–616. doi: 10.1161/01.RES.0000065442.64694.9F. [DOI] [PubMed] [Google Scholar]
- 12.Vlahos CJ, McDowell SA, Clerk A. Kinases as therapeutic targets for heart failure. Nat Rev Drug Discov. 2003;2:99–113. doi: 10.1038/nrd1009. [DOI] [PubMed] [Google Scholar]