Abstract
PTH(1-84) and PTH(7-84) are elevated in chronic kidney disease (CKD). These peptides, as their shorter analogs PTH(1-34) and PTH(7-34) both promote PTH receptor (PTH1R) internalization but only PTH(1-34) and PTH(1-84) activate the receptor. Here, we examined the effects of intermittent administration of PTH(1-34) and PTH(7-34) on mineral ion metabolism, bone architecture, and vascular calcification in rats with experimental CKD. CKD with or without parathyroidectomy (PTX) was established by 5/6 nephrectomy (NPX) in rats. Animals were divided into 4 groups: Sham PTX + sham NPX (Sham); PTX + sham NPX (PTX); Sham PTX+NPX (NPX); PTX+NPX (PTX/NPX). Rats were treated with single daily doses of 40 μg/kg PTH(1-34), PTH(7-34), or vehicle. Creatinine was higher in NPX and Ca lower in PTX and PTX/NPX groups than in Sham or NPX rats. Plasma phosphate was higher in PTX, NPX and PTX/NPX than in Sham rats. PTH(1-34) was more hypercalcemic than PTH(7-34) in PTX rats. Fractional bone volume in rats treated with PTH(1-34) increased significantly in all groups compared to that of vehicle treatment. In addition, trabecular number, thickness and volumetric bone density increased in rats treated with PTH(1-34). In contrast, PTH(1-34) diminished vascular calcification. Bone and renal PTH1R mRNA expression was reduced as much or more in PTX/NPX rats as in NPX alone, whereas PTH(7-34) had no effect on PTH1R expression. Renal but not bone PTH1R mRNA increased in response to PTH(1-34). These findings suggest that PTH(1-34) exerts greater hypercalcemic and anabolic effects in parathyroidectomized and/or nephrectomized rats than does PTH(7-34). There was no evidence for significant bone or vascular actions of PTH(7-34). We conclude that PTH(1-34) protects against vascular calcification and bone demineralization in experimental renal failure.
Keywords: nephrectomy, parathyroidectomy, PTH(1-34), PTH(7-34), bone, microarchitecture, PTH receptor
Introduction
Disturbances of bone and mineral metabolism are well recognized features of chronic kidney disease (CKD) [1, 2]. Vascular calcification and ectopic calcification of other soft tissues are commonly observed. These actions on mineral metabolism in patients with CKD are accompanied by increased synthesis, secretion and circulating levels of parathyroid hormone (PTH) and PTH fragments, which lead to the development of secondary hyperparathyroidism (SHPT) [3, 4]. The factors involved in the pathogenesis of SHPT in CKD, include phosphate retention, low 1,25(OH)2D3 and vitamin D receptors, decreased serum free calcium and parathyroid calcium-sensing receptors [5, 6], downregulation of PTH receptors [7-10], and the accumulation of uremic toxins and acidosis [9, 11] that contribute to skeletal resistance to the actions of PTH and a spectrum of bone diseases collectively called osteodystrophy. Although the mechanism of resistance is incompletely understood, uremic patients require PTH levels 2-3 times normal to maintain bone turnover [56]. Excessive suppression of PTH secretion, however, may lead to low bone turnover, so-called adynamic bone disease [12, 13].
The type I PTH receptor (PTH1R) is the predominant form expressed in bone and kidney and is a member of class B of the superfamily of G protein-coupled receptors (GPCRs) [14, 15]. It mediates the actions of PTH and of PTH fragments containing an intact amino terminus. In the kidney, the PTH1R mediates the regulation of PTH on the renal transport of phosphate and calcium [16]. In renal tubular epithelial cells, signal and expression of PTH1R appears to be modulated in a cell-specific manner. Mounting evidence supports the view that in renal failure serum PTH(7–84) accumulates to high levels [17, 18] that may approach those of PTH(1–84) [18-20]. It has been suggested that the competitive inhibition of PTH1R binding by amino-truncated PTH fragments contributes to the PTH resistance of uremia [21]. However, as indicated above, PTH1R downregulation has been described in the same pathological setting [7, 22], which is inconsistent with the view that PTH(7-34) acts exclusively as an antagonist.
Recent work shows that PTH(7–84) and its synthetic analog, PTH(7–34), internalize [23, 24] and downregulate [25] the PTH1R without antecedent or concomitant receptor activation, whereas PTH(1-34) promotes synchronized PTH1R activation and internalization. This phenomenon occurs in a cell-specific manner that depends on the expression of the scaffolding protein Na/H exchange regulatory factor (NHERF1) [23, 26], a cytoplasmic adaptor that interacts with the carboxy-terminus of the PTH1R and is implicated in protein targeting and in the assembly of protein complexes [26, 27]. These findings provide a plausible alternative explanation for PTH1R downregulation and resistance in renal failure. According to this hypothesis, PTH(7–84) may contribute to hormone resistance by inducing PTH1R internalization and downregulation without accompanying activation. Reducing the number of plasma membrane-delimited PTH receptors would diminish the action of full-length or amino-terminal PTH fragments.
Other studies addressed competitive interactions between amino-truncated PTH fragments and PTH(1-34) or PTH(1-84) [28, 29]. PTH(7-34) induces PTH1R internalization on its own and is only a competitive inhibitor at higher concentrations [23]. In the present study, we used exogenous PTH(1-34) and the amino-truncated peptide PTH(7-34) in animal models of CKD without secondary hyperparathyroidism to test the hypothesis that amino-truncated PTH fragments downregulate the PTH1R, thereby reducing bone demineralization and vascular calcification.
Material and methods
Animals and experimental protocol
All experiment protocols were approved by the University of Pittsburgh Institutional Animal Care and Use Committee (IACUC).
Nine-weeks old male Wistar rats weighing 250 - 300 grams, were purchased from Charles River Laboratories. Animals were fed standard diet (LabDiet, PMI Nutrition LLC, MO, US) containing 1.0% calcium, 0.75% phosphorus and 22.5% protein. The animals were allowed free access to food and water and housed in individual cages at constant room temperature with a 12-h light and dark cycle. Rats were randomly assigned to one of four experimental groups according to the type of surgery. Group 1: sham parathyroidectomy and sham nephrectomy (Sham), 23 rats; Group 2: parathyroidectomy and sham nephrectomy (PTX), 22 rats; Group 3: Sham parathyroidectomy and 5/6 Nephrectomy (NPX) (described below), 22 rats; Group 4: parathyroidectomy and 5/6 nephrectomy (PTX/NPX), 22 rats. The Animals were anesthetized by intraperitoneal injection of ketamine and xylazine before surgery and treated with subcutaneous buprenorphine for 3 days after the procedure to alleviate pain. During all surgical procedures, the vitals signs, electrolytes, and arterial blood gases were monitored and the animals were weighed twice per week.
Following an adaptation period of 48 h, the rats were parathyroidectomized under microscopic observation, dissecting and removing both parathyroid glands, while preserving the thyroid gland. The time course for the experimental protocol is shown in Fig. 1. Serum ionized calcium and serum PTH concentrations were measured before the surgical procedure, one week after surgery and at the end of the experiment. A decrease in serum calcium concentration <0.9 mM and undetectable serum PTH were taken as evidence of successful and complete parathyroidectomy. Ten days after PTX, the rats underwent right nephrectomy, and seven days later underwent 2/3 left nephrectomy using a suture placed and ligated around each pole of the kidney at 1/3 position. Each pole was then excised to produce total 5/6 nephrectomy. Sham operations (decapsulation) of the parathyroid glands and/or kidneys were performed at the same time intervals and served as controls. The day after nephrectomy (Day 21), animals from each surgical procedure were assigned to one of three treatment groups: vehicle (0.9% sodium chloride), PTH(1-34) (40 μg/Kg/day) (Bachem California, Torrance, CA), or PTH(7-34) (40 μg/Kg/day) (Molecular Medicine Institute, Peptide Synthesis Facility, University of Pittsburgh, PA). Vehicle or PTH was administered by subcutaneous injection once daily each morning for four weeks. At day 52 the rats were euthanized by intraperitoneal injection of pentobarbital. Blood samples were drawn and the femurs, tibia, kidneys and aorta were removed.
Fig. 1.

Experimental protocol for 5/6 nephrectomy and parathyroidectomy and time line for PTH administration. Sham = Sham operation; PTX = Parathyroidectomy; NPX 1st = First step of 5/6 nephrectomy; NPX 2nd = Second step of 5/6 nephrectomy.
Biochemistry
On days 3, 13, 20 and 52 the animals were anesthetized and a blood sample was obtained from the tail vein and blood collected into a heparinized tube. The blood samples were taken 24 hrs after the last PTH injection and 3 hrs after the last food access. Plasma was separated from whole blood by centrifugation at 10000 rpm for 10 min and an aliquot of plasma was used for ionized calcium and pH determination (Rapidlab 348, Bayer, East Walpole, MA). Plasma was stored at -80 C until assays were performed. Creatinine was determined spectrophotometrically (Beckman DU 530) using Infinity Creatinine (Thermo Fisher Scientific, Inc., Waltham, MA). Phosphorus was determined using the Inorganic Phosphorus Reagent Set (UV) (Pointe Scientific, Inc., Canton, MI). Serum levels of intact PTH(1-84) were measured with a rat PTH (ELISA) kit (Rat Bio Intact ELISA Kit, Immutopics, San Clemente, CA).
Micro-computed tomography
Trabecular bone volume and skeletal architecture of the distal femur of each rat was measured by micro-computed tomography using a μCT 40 (Scanco Medical AG, Bassersdorf, Switzerland) using the manufacturer’s software. In brief, cross-sectional images were obtained with a voxel size of 16 μm in all dimensions. Semi-automated contouring was used to select a region of interest (ROI) extending 3.2 mm proximal to the femoral growth plate, but excluding the cortex and subcortical bone, composed of 150 adjacent 16-micron slices. The three-dimensional volume and architecture of the distal femur was calculated by stacking the volume of each slice prior to applying an optimized Gaussian noise filter and grey scale threshold [30]. Fractional bone volume (bone volume per tissue volume, BV/TV), architectural parameters (trabecular thickness (Tb.Th), number (Tb.N) separation (Tb.Sp), and structural indices including connectivity density (Conn.D.) and structure model index (SMI) were calculated directly from the reconstructed trabecular structures as described elsewhere [31].
Vascular Calcification
At the time of euthanasia, the abdominal aorta was removed, flushed with phosphate-buffered saline through the intraluminal space and fixed in 10% buffered formalin. Four micrometer sections were stained for calcification by the Von Kossa method. Vascular calcification was analyzed histologically and examined by fluorescence microscopy (LEICA DMI600b, Center for Biological Imaging, University of Pittsburgh, PA). The extent of vascular calcification was determined by quantitative image analysis. Four independent samples for each experimental condition were digitized at 32-bit depth and uniformly thresholded and size analysis of particles 4-102 pixels was determined using Image J (http://rsb.info.nih.gov/ij).
RNA extraction and reverse transcription-polymerase chain reaction (RT-PCR) of bone and kidney
The dissected left tibia of each rat was flushed with phosphate-buffered saline (PBS) to remove the bone marrow and then frozen in liquid nitrogen. The proximal metaphysis and diaphysis were ground to a fine powder using a mortar and pestle under liquid nitrogen. The dissected kidney was stored in RNA stabilization solution (RNAlater, Ambion, Austin, TX, USA) until analysis.
RNA was extracted using Trizol (Invitrogen, CA, US) following the manufacturer’s instructions. Briefly, total RNA from bone or kidney cortex was used as the template for cDNA synthesis in a 10-μl volume using an RT-PCR kit (AccuScript High Fidelity Reverse Transcriptase, Stratagene, US) according to the manufacturer’s instructions. The reaction mixture contained RNase H-deficient Moloney murine leukemia virus reverse transcriptase, 0.5 M Tris-HCl (pH 8.3), 0.75 M KCl, 0.03 M MgCl2, 60 ng random primers, 12.5 mM dNTP, and 100 mM DTT (dithiothreitol). The mixture was incubated at 65 °C for 5 min, 42 °C for 30 min and stored at 4 °C. The PCR mixture (total 50 μl) contained 10X PCR buffer, 1 μl of cDNA, 1 μl of PfuUltra HF DNA polymerase (Stratagene, US), 12.5 mM dNTP and 1 μl (100 ng/μl) each of forward and reverse primers (Table 1). ß-Actin was used as an internal control. The thermal cycling protocol was 95 °C for 120 sec in Segment 1; 95 °C for 30 sec, 51 °C for 30 sec and 72 °C for 60 sec in Segment 2 for 30 cycles; and 72 °C for 10 min in Segment 3 (PCR Sprint, Hybaid Limited, UK). The amplification reaction products (50 μl) were analyzed using 1% agarose gel electrophoresis and visualized by ethidium bromide staining. Semiquantitative analysis was performed using NIH Image 1.62.
Table 1.
PCR primer sequences and their product sizes
| Primer | Forward, 5’-3’ | Reverse | Product size, bp |
|---|---|---|---|
| PTH1R | AGCGAGTGCCTCAAGTTCAT | ACAGCGTCCTTCACGAAGAT | 236 |
| ß-actin | TCATGAAGTGTGACGTTGACATCCGT | CCTAGAAGCATTTGCGGTGCACGATG | 285 |
Bone turnover markers
Osteocalcin (OC) and C-terminal fragments of type I collagen (CTX I) served as bone formation and turnover markers, respectively. OC and CTX I were measured in serum drawn at the time of euthanasia (day 52) and stored at -80 C until the time of assay. Serum intact osteocalcin was measured using Rat-MID™ Osteocalcin ELISA (Nordic Bioscience Diagnostics, Chesapeake, VA). CTX I was measured with RatLaps ELISA (Nordic Bioscience Diagnostics, Chesapeake, VA)
Statistical analysis
Data are expressed as the mean ± SD or ± SEM as indicated. Statistical significance was determined by analysis of variance (ANOVA) and post hoc comparison using the Bonferroni test (Prism, GraphPad). P values < 0.05 were considered significant.
Results
All animals gained weight from the time of entry in the protocol. Rats undergoing PTX gained as much weight as Sham animals. However, rats subjected to NPX or PTX/NPX gained significantly less weight (83% and 73% compared to Sham; P<0.05, P<0.01, respectively).
Renal and parathyroid function
Serum creatinine (Cr), calcium (Ca), and inorganic phosphorus (Pi) were measured to determine the effectiveness of PTX and 5/6 NPX. Table 2 summarizes the plasma biochemistry results. Pre-treatment levels of Cr, Ca and Pi were comparable among groups. At the time of euthanasia, Cr, Ca and Pi in the Sham groups were similar to their respective baseline values. PTX did not affect serum Cr but was associated with the expected increases of Pi (P<0.01) and decreases of Ca (P<0.01). Intermittent PTH(1-34) administration to PTX rats blunted the reduction of serum Ca but did not fully normalize these values. PTH(7-34) treatment did not ameliorate either Pi or Ca. NPX caused profound elevations in serum Cr and Pi that were not accompanied by changes in serum Ca. Serum calcium was also unaffected by PTH in NPX animals. Finally, combined PTX/NPX increased serum Cr and Pi, and caused a reduction of serum Ca comparable to that observed with PTX alone. Thus, the changes seen individually in PTX and NPX rats were not additive in the PTX/NPX animals. Treatment with PTH(1-34) or PTH(7-34) did not affect serum biochemistries except for an increase of Pi in PTX/NPX (P<0.05) compared to PTX alone. The calcium phosphate product (Ca × Pi, mg2/dl2) increased significantly following NPX but was unaffected by PTX alone or by the combination PTX/NPX. PTH(1-34) and PTH(7-34) increase the Ca × Pi product in the NPX rats (P<0.001).
Table 2.
Plasma biochemistry
| Maneuver | Treatment | n | Cr (mg/dl) | P (mmol/L) | Ca (mmol/L) | Ca ×P (mg2/dl2) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Euthanasia | Before treatment | Euthanasia | Before treatment | Euthanasia | Before treatment | Euthanasia | |||
| Vehicle | 10 | 0.54±0.08 | 0.56±0.11 | 1.96±0.05 | 1.85±0.21 | 1.26±0.03 | 1.25±0.04 | 30.75±1.31 | 30.27±1.85 | |
| Sham | PTH(1-34) | 7 | 0.54±0.09 | 0.57±0.13 | 2.15±0.10 | 2.02±0.11 | 1.22±0.04 | 1.23±0.06 | 33.19±2.46 | 31.89±3.12 |
| PTH(7-34) | 6 | 0.48±0.16 | 0.48±0.07 | 2.00±0.08 | 2.02±0.15 | 1.27±0.07 | 1.31±0.07 | 32.04±2.47 | 33.18±2.87 | |
| Vehicle | 10 | 0.59±0.11 | 0.59±0.15 | 2.62±0.34 | 2.70±0.37 | 0.84±0.11 | 0.74±0.08b | 27.5±5.53 | 24.65±6.3 | |
| PTX | PTH(1-34) | 6 | 0.52±0.07 | 0.61±0.08 | 2.70±0.14 | 2.65±0.17 | 0.84±0.13 | 0.89±0.11 | 26.99±5.51 | 27.22±1.59 |
| PTH(7-34) | 6 | 0.53±0.08 | 0.53±0.08 | 2.67±0.45 | 2.55±0.37 | 0.83±0.05 | 0.77±0.17 | 27.82±4.61 | 22.07±4.56 | |
| Vehicle | 10 | 0.49±0.08 | 1.20±0.12a | 2.02±0.09 | 2.82±0.67b | 1.24±0.13 | 1.27±0.05 | 32.15±3.03 | 38.74±6.14 | |
| NPX | PTH(1-34) | 6 | 0.52±0.09 | 1.27±0.18a | 2.1±0.10 | 2.84±0.20c | 1.24±0.04 | 1.28±0.07 | 32.39±1.23 | 46.23±3.77c |
| PTH(7-34) | 6 | 0.50±0.12 | 1.25±0.11a | 2.06±0.11 | 2.90±0.24c | 1.20±0.03 | 1.25±0.04 | 31.2±1.39 | 45.13±5.35c | |
| Vehicle | 8 | 0.49±0.18 | 1.13±0.11a | 2.80±0.15 | 2.97±0.40 | 0.79±0.15 | 0.77±0.21 | 25.04±4.52 | 24.10±8.59 | |
| PTX/ NPX | PTH (1-34) | 8 | 0.55±0.11 | 1.28±0.18a | 2.65±0.37 | 3.31±0.61d | 0.90±0.05 | 0.89±0.09 | 29.90±3.94 | 30.96±3.08 |
| PTH (7-34) | 6 | 0.53±0.08 | 1.18±0.07a | 2.42±0.42 | 3.15±0.82d | 0.83±0.12 | 0.84±0.14 | 24.21±8.02 | 26.55±6.40 | |
Sham = sham operation; PTX = parathyroidectomized; NPX = 5/6 nephrectomized; PTX/NPX = parathyroidectomized + 5/6 nephrectomized rats. Results are expressed as mean ± SD. Baseline values are those measured at Day 3 of the protocol (see Materials and Methods, Fig. 1); Before treatment are values determined at Day 20. Cr = Creatinine; Pi = Phosphorus; Ca = Calcium.
P<0.05 compared to Sham and PTX of the same group of injection in Euthanasia;
P<0.05 compared to Before treatment (Day 20) of the same group of injection;
P<0.001 compared to Before treatment (Day 20) of the same group;
P<0.005 vs pre-treatment of the same group.
Baseline plasma pH averaged 7.41-7.42 in Sham, NPX, and PTX/NPX groups but was significantly elevated (7.45) in PTX rats before treatment commencing on day 20. At the end of the 52-day protocol, pH had not changed in the Sham animals and had normalized in PTX rats. However, both NPX and PTX/NPX groups became significantly alkalotic (Fig. 2) (pH 7.48, 7.50, respectively).
Fig. 2.

Serum pH measurement. Serum pH was measured in the 4 experimental groups under baseline conditions (before surgery, day 3) and at euthanasia (day 52). Sham = Sham operation; PTX = parathyroidectomized; NPX = 5/6 nephrectomized; PTX/NPX = parathyroidectomized + 5/6 nephrectomized rats.
PTH levels were determined before surgery and four weeks after surgery. PTH levels were undetectable in the PTX and PTX/NPX groups. At the time of euthanasia, serum PTH was unaltered in Sham groups but increased significantly in NPX rats fro 69 ± 2 to 182 ± 24 pg/ml, P<0.01).
Bone Microarchitecture
The left femur of each rat was removed after euthanasia, preserved in 10% buffered formalin and analyzed by μCT. Representative cross-sectional images from Sham, PTX, NPX and PTX/NPX groups treated with vehicle (upper panel) or PTH(1-34) (lower panel) are shown in Fig. 3. The trabecular microarchitecture of animals treated with PTH(1-34) was denser than that of untreated animal in all samples.
Fig. 3.

MicroCT renderings of the left femur of the rat. PTH(1-34) increased bone density in all animals compared to vehicle group. Sham = Sham operation; PTX = parathyroidectomized; NPX = 5/6 nephrectomized; PTX/NPX = parathyroidectomized + 5/6 nephrectomized rats.
Trabecular bone volume and architectural parameters of the distal femur were quantified by μCT. These parameters are summarized in Fig.4 and Table 3. Fractional bone volume (BV/TV) increased with daily injection of PTH(1-34) by 39%, 20%, 54%, and 86% in Sham, PTX, NPX and PTX/NPX rats (P<0.05), respectively compared to vehicle treatment (Fig. 4). The BV/TV ratio was the same for Sham and NPX. PTH(1-34) also increased the trabecular number (Tb.N) in NPX and PTX/NPX animals (Table 3) (P<0.05). The trabecular thickness (Tb.Th.) increased by 27% and 64% upon injection of PTH(1-34) in Sham and PTX/NPX groups, respectively (P<0.05). There were no statistically significant changes in the Conn.D. index in or between groups. PTH(1-34) decreased the trabecular spacing (Tb.Sp.) in the PTX/NPX group using by 50% when compared to vehicle (P<0.05).
Fig 4.

PTH(1-34) increased BV/TV in all the groups. PTH(7-34) exerted no significant effects. Results are expressed as mean ± SEM. * P<0.05 vs. respective vehicle. Abbreviations as in Fig. 2.
Table 3.
Micro CT parameters
| n | Trabecular Number 1/mm | Trabecular Thickness μm | Trabecular Spacing μm | Connectivity Density 1/mm3 | ||
|---|---|---|---|---|---|---|
| Vehicle | 6 | 2.92 ± 0.5 | 0.11 ± 0.002 | 0.45 ± 0.11 | 58.33 ± 9.7 | |
| Sham | PTH(1-34) | 6 | 3.63 ± 0.2 | 0.14 ± 0.006a | 0.28 ± 0.02 | 56.72 ± 3.5 |
| PTH(7-34) | 6 | 3.21 ± 0.5 | 0.11 ± 0.003 | 0.39 ± 0.11 | 58.66 ± 8.5 | |
| Vehicle | 6 | 5.41 ± 0.6b | 0.14 ± 0.010b | 0.31 ± 0.09 | 89.31 ± 12.3 | |
| PTX | PTH (1-34) | 6 | 5.76 ± 0.7 | 0.17 ± 0.015 | 0.14 ± 0.02 | 97.60 ± 18.5 |
| PTH (7-34) | 6 | 5.10 ± 0.9 | 0.15 ± 0.020 | 0.23 ± 0.06 | 92.52 ± 16.6 | |
| Vehicle | 6 | 2.67 ± 0.6 | 0.11 ± 0.008 | 0.40 ± 0.13 | 50.05 ± 11.5 | |
| NPX | PTH(1-34) | 6 | 4.05 ± 0.2a | 0.13 ± 0.005 | 0.22 ± 0.02 | 66.96 ± 4.5 |
| PTH(7-34) | 6 | 2.30 ± 0.2 | 0.11 ± 0.004 | 0.51 ± 0.06 | 48.93 ± 6.3 | |
| Vehicle | 7 | 3.06 ± 0.5 | 0.11 ± 0.003 | 0.32 ± 0.06 | 71.51 ± 8.10 | |
| PTX/ NPX | PTH(1-34) | 8 | 5.41 ± 0.4a | 0.18 ± 0.010a | 0.16 ± 0.01a | 81.76 ± 15.4 |
| PTH(7-34) | 6 | 4.46 ± 0.9 | 0.13 ± 0.012 | 0.27 ± 0.01 | 88.93 ± 18.7 |
P<0.05 vs respective vehicle;
P<0.05 vs respective Sham vehicle
PTH1R in Bone and Kidney
PTH1R mRNA expression was examined in samples extracted from the proximal metaphysis and diaphysis of the tibia. PTX alone had no significant effect on PTH1R mRNA expression in bone (Fig. 5, top panel). Treatment with PTH(1-34) or PTH(7-34) did not change PTH1R mRNA levels in the PTX group. NPX and PTX/NPX caused highly significant decreases in receptor mRNA expression when compared to Sham (P<0.01). The daily injection of PTH(1-34) significantly increased bone PTH1R mRNA expression in NPX rats (P<0.01), whereas PTH(7-34) administration had no demonstrable effect.
Fig. 5.

Expression of PTH1R. PTH1R mRNA was measured by RT-PCR in the rat tibia (top) and kidney (bottom) and normalized to ß-actin determined simultaneously. * P<0.05, ** P<0.01 vs. respective Sham, c. a P<0.01 vs. NPX vehicle. Abbreviations as in Fig. 2.
PTH predominantly exerts its effects through the PTH1R [32]. PTH resistance in CKD may arise from receptor downregulation. The effect of CKD with or without PTX on PTH1R mRNA extracted from the kidney cortex was examined using RT-PCR (Fig. 5, bottom panel. The renal expression of PTH1R mRNA decreased significantly in PTX/NPX animals treated with vehicle (P<0.05). The PTX and PTX/NPX animals expressed higher levels of PTH1R mRNA when compared to Sham (P<0.05 and P<0.01, respectively) after administration of PTH(1-34).
Bone markers
The C-terminal telopeptide of type I collagen, (CTX I) is a degradation product of type 1 collagen, a component of the peptide-bound cross-link fraction and is a marker of bone resorption [33]. The level of serum CTX I was measured at the end of the experiment. The levels of CTX I were comparable in Sham and PTX animals, independent of the treatment (Fig. 6, top panel). CTX I significantly increased in the NPX and PTX/NPX animals (P<0.05 and P<0.01, respectively) compared to Sham. Administration of PTH(1-34) did not affect CTX I. However, PTH(7-34) reduced CTX I in PTX/NPX rats compared to vehicle or PTH(1-34)-treated rats.
Fig. 6.
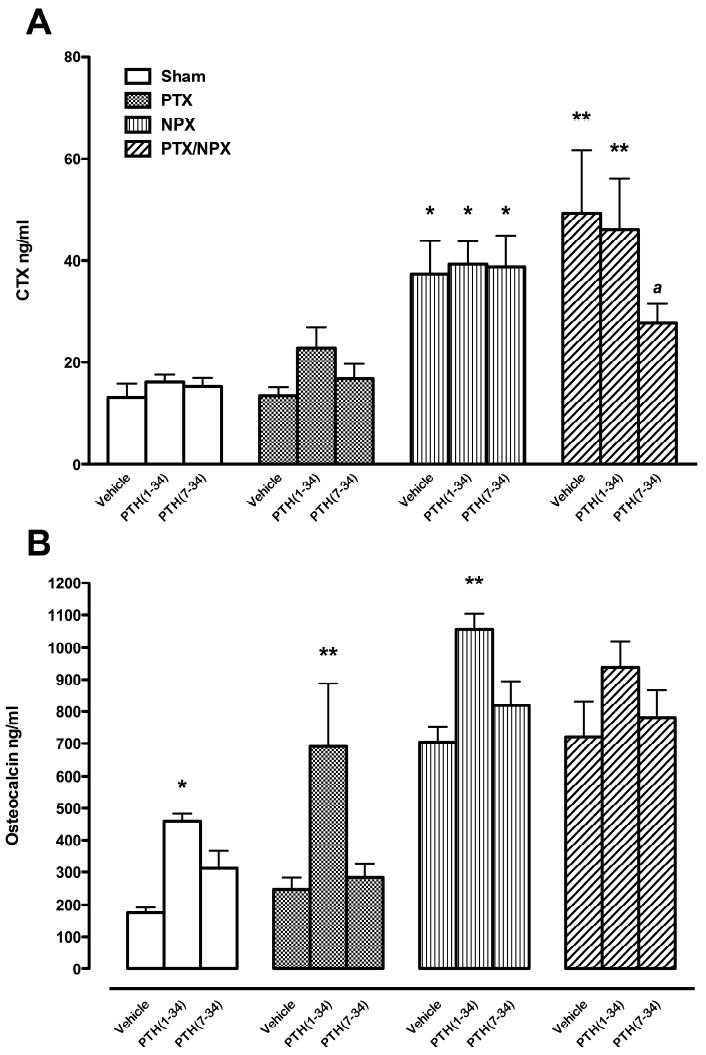
Bone turnover markers. C-terminal telopeptide of type I collagen, CTX I, (top) and, Osteocalcin, OC (bottom) were measured in serum collected at the time of euthanasia. Results are expressed as mean ± SEM. * P<0.05, ** P<0.01 vs. respective vehicle. Abbreviations as in Fig. 2.
Osteocalcin (OC) is a hydroxyapatite-binding protein synthesized by osteoblasts, odontoblasts, and hypertrophic chondrocytes that is expressed mainly during osteoid mineralization and serves as a marker of bone formation [34]. All animals exhibited high concentrations of serum OC after 4 weeks of treatment with PTH(1-34) (Fig. 6, bottom panel). NPX rats, with or without PTX, had higher OC concentrations compared to Sham animals. Consistent with the present results, Higher OC and CTX levels have been reported in chronic renal failure patients [60, 61]. However, OC also increased in Sham and PTX rats with PTH(1-34) treatment (P<0.05, P<0.01, respectively). The effect of PTH(1-34) also was associated with a significant increase of OC in NPX rats when compared to the effects of vehicle or PTH(7-34) treatment (P<0.01).
Vascular Calcification
Vascular calcification is a well recognized complication of CKD [35, 36]. The abdominal aortas of NPX and PTX/NPX rats and their corresponding Sham controls were examined by the von Kossa staining procedure to determine the extent of calcification. Representative results are illustrated in Fig. 7a. Vehicle-treated rats are shown in the upper panel, and animals receiving intermittent PTH(1-34) are displayed in the lower panel. There was conspicuous calcification in NPX rats receiving saline vehicle (arrows). PTH(1-34) decreased calcification in this group of rats. However, there was no difference in the vascular calcification after the administration of PTH(1-34) in PTX/ NPX rats. The results of quantitative analysis of calcification are displayed in Fig. 7b and bear out the visual results.
Fig. 7.


Reduction of vascular calcification by PTH(1-34). A) Histological sections of rat aorta. The arrows indicate calcification of the abdominal aorta staining by Von Kossa. PTH(1-34) decreased calcification in the aorta in NPX rats but there is not evident effect in the absence of parathyroid glands. * P<0.05, ** P<0.01 vs. respective Sham, c. a P<0.01 vs. NPX vehicle. B) Summary of 4 independent representative samples. * P<0.05, ** P<0.01 vs. vehicle-treated NPX. Abbreviations as in Fig.2
Discussion
The present studies were designed to analyze and compare the effects of intermittent PTH(1-34) or PTH(7-34) treatment on bone microarchitecture, vascular calcification, and PTH1R expression in an animal model of experimental renal failure. It has been reported that the administration of PTH prevents osteoporotic fractures in postmenopausal women, improves fracture healing and implant fixation, and osteogenesis in model animals [37-40]. We show here that in an animal model of experimental CKD, the intermittent administration of PTH(1-34) increases bone formation and expression of renal but not bone PTH1R mRNA, while decreasing vascular calcification.
To establish an experimental model of CKD without the effect of secondary hyperparathyroidism, we performed combined 5/6 nephrectomy and parathyroidectomy (PTX/NPX). The higher values of Cr, Pi and weight loss 4 weeks after NPX verified the success of the renal surgery, and the combination of lower levels of plasma Ca with undetectable circulating serum PTH confirm complete PTX. We performed PTX to eliminate PTH and other amino-truncated PTH derivatives. Considerable evidence demonstrates that CKD is accompanied by substantial increases in serum levels of amino-truncated PTH fragments [19, 41]. For instance PTH(7-84) [42], which has no effect on serum calcium or bone turnover in PTX/NPX rats, and blocked the action of PTH(1-84) on serum calcium and osteoblast activity [29]. Thus, in the present investigation, the effects of the administration of PTH peptides can be analyzed without the confounding influence of endogenous PTH1R ligands.
Under physiological conditions the kidneys excrete acid and regenerate bicarbonate to replenish the extracellular buffering capacity. In CKD, however, the ability to maintain acid-base balance is impaired because of the progressive reduction of functional renal mass with an increasing burden and inadequate capacity to eliminate the daily acid load [43]. Unexpectedly, the present findings show that plasma pH in both NPX and PTX/NPX rats became alkalotic (Fig.2). In PTX rats, plasma pH was significantly elevated (7.45) before treatment but had normalized by the time of euthanasia. Similar increases in the plasma pH of PTX rats have been noted [43]. In this case, the absence of PTH presumably increases phosphate absorption by proximal tubules. In PTX/NPX rats, PTH(1-34) and PTH(7-34) further decreased phosphate excretion resulting in greater increases of serum phosphate (Table 2). This finding suggests that the presence of amino-truncated PTH fragments may contribute to the hyperphosphatemia of CKD.
PTH was administered as single daily injections because intermittent PTH administration promotes bone formation, whereas continuous infusion causes bone resorption [44-46]. The mechanisms underlying these differences are not entirely clear. We elected to inject comparable amounts of PTH(1-34) and PTH(7-34). We used 40μg/Kg/day because the effect of PTH(1-34) treatment is dose-dependent, increasing mineral apposition rate and bone formation at this concentration [47], which in other studies was shown to be the lowest dose that elicited maximal effects when administered on an intermittent schedule [48]. It should be noted, however, that the Ka of the PTH1R for PTH(7-34) is an order of magnitude lower than that for PTH(1-34) [49, 50]. An effect of C-type PTH receptors, CPTHR, which would not be expected to bind or respond to PTH(7-34) but to PTH(7-84) cannot be excluded [51, 52]. It should be noted that PTH(1-34) and PTH(7-34) have different affinities for the PTH1R. Thus, it is possible that PTH(7-34) may exhibit effects when used at higher doses. The rapid clearance of PTH(1-34) leads to brief exposure (<3 h/d), which is clearly sufficient to increase bone mineral density [37]. The present findings show that several bone turnover and micro-architectural parameters were increased by intermittent treatment with PTH(1-34). In contrast, PTH(7-34) neither increased nor decreased bone micro-architecture.
In CKD, the changes in trabecular bone correlate with the degree of renal failure [53, 54]. The increase of PTH levels [53] and the downregulation of PTH1R are major contributing causes of renal osteodystrophy [44, 55]. In CKD rats, the use of pharmacological doses of intermittent PTH ameliorated the decreased bone formation in presence of both thyroparathyroidectomy and nephrectomy [63] by mechanisms not fully understood. Previous reports show decreased osteoblast activity and recruitment in PTX rats, decreased 1,25(OH)2D levels and osteoporosis [39, 56]. Measured standard bone microarchitectural parameters demonstrate that the anabolic effect of intermittent PTH(1-34) increased the fractional bone volume (BV/TV) and osteocalcin independent of NPX or PTX. OC and CTX I increase in CKD [60,61]. In PTX/NPX animals, the present findings show that intermittent administration of PTH(1-34) increased both trabecular number and thickness, and decreased trabecular separation. These findings suggest improvement in bone formation after PTH(1-34) but the results imply that different mechanisms are involved in PTX, NPX or PTX/NPX.
Here, we show that PTH1R mRNA expression in bone and kidney of untreated animals decreased after PTX, NPX, or PTX/NPX. Since PTH1R mRNA expression declined further in PTX/NPX animals compared with NPX rats, this finding confirms that the downregulation of PTH1R mRNA expression in CKD is not prevented by PTX [57]. These results suggest that the involvement of as yet undefined uremic factors may contribute to or mediate this effect [58]. PTH(1-34) blunted the decline of renal PTH1R mRNA expression in PTX and PTX/NPX rats, and increased PTH1R mRNA expression in bone in NPX animals when compared to vehicle. PTH(1-34) effects on PTX and PTX/NPX animals did not achieve statistical significance. Contrary to our prediction that amino-truncated PTH fragments would downregulate the PTH1R, PTH(7-34) had no effect on receptor expression. The failure of PTH(7-34) to promote PTH1R downregulation in the present setting may well be due to the relative short duration of treatment and the intermittent dosing protocol. With this caveat in mind, these data nonetheless predict that in bone, PTH1R mRNA reductions in NPX and PTX/NPX rats should result in PTH resistance in these animals if receptor protein is reduced proportionately, and if receptor abundance is rate-limiting to generating an anabolic response. Notably, intermittent daily PTH(1-34) administration effectively increased BV/TV under all conditions. This may be a consequence of the exquisite sensitivity of the rat skeleton to PTH or that the residual PTH1R expression was sufficient to mediate the anabolic actions of PTH(1-34).
Calcification of the intima and media of the aorta is common in CKD [59]. Vascular calcification is considered to be a regulated process that is influenced by tissue-specific cellular mechanisms and by discrete plasma components [60]. Treatment with vitamin D sterols may aggravate vascular calcification. The mechanism by which PTH causes vascular calcification remains uncertain. However, calcification of the rat aorta in vitro requires elevation of both Ca and Pi [61]. Although amino-truncated PTH fragments promote PTH1R downregulation in bone and kidney cells in vitro, no evidence was found that intermittent PTH(7-34) administration exerts biological actions that exacerbate or improve vascular calcification or skeletal deterioration in vivo. This may reflect a number of potential mechanistic considerations. We found that PTH(1-34) reduced vascular calcification in NPX rats. Rats with PTX/NPX showed similar decreased vascular calcification independent of the treatment. These observations suggest that the accumulation of antagonistic PTH fragments may contribute to the calcemic vasculopathy characteristic of end-stage renal disease. We suggest that PTH1R signaling plays important roles in promoting skeletal mineral accumulation, while simultaneously decreasing vascular calcification. We propose that PTH(1-34) continues to inhibit proximal tubule phosphate transport [23], while the reduced effect of PTH on the distal nephron likely represents a protective action to avoid concomitant hypercalcemia in the face of frank hyperphosphatemia. These effects would limit the rise of the Ca × Pi product, which is an important risk for vascular calcification and mortality [62].
Based on these findings, we conclude that intermittent administration of PTH(1-34) exerts a protective action that reduces bone demineralization and vascular calcification in short-term experimental renal failure. PTH(7-34) exhibited no detectable effects of its own in the absence of endogenous PTH. The lack of an effect of PTH(7-34) may be ascribed to pharmacokinetic effects, or to an action at the level of the PTH1R or CPTHR, or downstream signaling.
Acknowledgments
This work was supported by grant DK 54171 (PAF) and by a Minority Supplement (EMS) from the National Institutes of Health, an internal award from the Office of the Senior Vice Chancellor for the Health Sciences, University of Pittsburgh (EMS) and The Carl L. Nelson Chair of Orthopaedic Surgery (LJS). The authors thank Dr. Nathalie Taesch, who helped with vascular histology and staining.
Footnotes
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin KJ, Gonzalez EA. Metabolic Bone Disease in Chronic Kidney Disease. Journal of the American Society of Nephrology. 2007;18:875–885. doi: 10.1681/ASN.2006070771. [DOI] [PubMed] [Google Scholar]
- 2.Brown AJ. Vitamin D analogs for secondary hyperparathyroidism: What does the future hold? Journal of Steroid Biochemistry and Molecular Biology. 2007;103:578–583. doi: 10.1016/j.jsbmb.2006.12.089. [DOI] [PubMed] [Google Scholar]
- 3.Goodman WG. Calcium-sensing receptors. Seminars in Nephrology. 2004;24:17–24. doi: 10.1053/j.semnephrol.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Fukagawa M, Nakanishi S, Fujii H, Hamada Y, Abe T. Regulation of parathyroid function in chronic kidney disease (CKD) Clinical and Experimental Nephrology. 2006;10:175–179. doi: 10.1007/s10157-006-0432-9. [DOI] [PubMed] [Google Scholar]
- 5.Slatopolsky E, Brown A, Dusso A. Pathogenesis of secondary hyperparathyroidism. Kidney International Suppl. 1999;73:S14–S19. doi: 10.1046/j.1523-1755.1999.07304.x. [DOI] [PubMed] [Google Scholar]
- 6.Cozzolino M, Brancaccio D, Gallieni M, Galassi A, Slatopolsky E, Dusso A. Pathogenesis of parathyroid hyperplasia in renal failure. Journal of Nephrology. 2005;18:5–8. [PubMed] [Google Scholar]
- 7.Urena P, Kubrusly M, Mannstadt M, Hruby M, Trinh MM, Silve C, Lacour B, Abou-Samra AB, Segre GV, Drueke T. The renal PTH/PTHrP receptor is down-regulated in rats with chronic renal failure. Kidney International. 1994;45:605–611. doi: 10.1038/ki.1994.79. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Zhang J, Lin S. Down-regulation of PTH/PTHrP receptor in the kidney of patients with renal impairment. Chinese Medical Journal. 1998;111:24–27. [PubMed] [Google Scholar]
- 9.Picton ML, Moore PR, Mawer EB, Houghton D, Freemont AJ, Hutchison AJ, Gokal R, Hoyland JA. Down-regulation of human osteoblast PTH/PTHrP receptor mRNA in end-stage renal failure. Kidney International. 2000;58:1440–1449. doi: 10.1046/j.1523-1755.2000.00306.x. [DOI] [PubMed] [Google Scholar]
- 10.Edwards RM, Contino LC, Gellai M, Brooks DP. Parathyroid hormone-1 receptor down-regulation in kidneys from rats with chronic renal failure. Pharmacology. 2001;62:243–247. doi: 10.1159/000056102. [DOI] [PubMed] [Google Scholar]
- 11.Drüeke TB. Abnormal skeletal response to parathyroid hormone and the expression of its receptor in chronic uremia. Pediatric Nephrology. 1996;10:348–350. doi: 10.1007/BF00866780. [DOI] [PubMed] [Google Scholar]
- 12.Urena P. Use of calcimimetics in uremic patients with secondary hyperparathyroidism: review. Artificial Organs. 2003;27:759–764. doi: 10.1046/j.1525-1594.2003.07262.x. [DOI] [PubMed] [Google Scholar]
- 13.Brown AJ, Slatopolsky E. Drug Insight: vitamin D analogs in the treatment of secondary hyperparathyroidism in patients with chronic kidney disease. Nat Clin Pract Endocrinol Metab. 2007;3:134–44. doi: 10.1038/ncpendmet0394. [DOI] [PubMed] [Google Scholar]
- 14.Harmar AJ. Family-B G-protein-coupled receptors. Genome Biology. 2001;2:3013.1–3013.10. doi: 10.1186/gb-2001-2-12-reviews3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mannstadt M, Juppner H, Gardella TJ. Receptors for PTH and PTHrP: their biological importance and functional properties. American Journal of Physiology. 1999;277:F665–F675. doi: 10.1152/ajprenal.1999.277.5.F665. [DOI] [PubMed] [Google Scholar]
- 16.Yang TX, Hassan S, Huang YNG, Smart AM, Briggs JP, Schnermann JB. Expression of PTHrP, PTH/PTHrP receptor, and Ca2+-sensing receptor mRNAs along the rat nephron. American Journal of Physiology. 1997;272:F751–F758. doi: 10.1152/ajprenal.1997.272.6.F751. [DOI] [PubMed] [Google Scholar]
- 17.Monier-Faugere MC, Geng Z, Mawad H, Friedler RM, Gao P, Cantor TL, Malluche HH. Improved assessment of bone turnover by the PTH-(1-84)/large C-PTH fragments ratio in ESRD patients. Kidney International. 2001;60:1460–1468. doi: 10.1046/j.1523-1755.2001.00949.x. [DOI] [PubMed] [Google Scholar]
- 18.Coen G, Bonucci E, Ballanti P, Balducci A, Calabria S, Nicolai GA, Fischer MS, Lifrieri F, Manni M, Morosetti M, Moscaritolo E, Sardella D. PTH 1-84 and PTH “7-84” in the noninvasive diagnosis of renal bone disease. American Journal of Kidney Diseases. 2002;40:348–354. doi: 10.1053/ajkd.2002.34519. [DOI] [PubMed] [Google Scholar]
- 19.Brossard JH, Cloutier M, Roy L, Lepage R, Gascon-Barré N, D’Amour P. Accumulation of a non-(1-84) molecular form of parathyroid hormone (PTH) detected by intact PTH assay in renal failure: Importance in the interpretation of PTH values. Journal of Clinical Endocrinology and Metabolism. 1996;81:3923–3929. doi: 10.1210/jcem.81.11.8923839. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen-Yamamoto L, Rousseau L, Brossard JH, Lepage R, Gao P, Cantor T, D’Amour P. Origin of parathyroid hormone (PTH) fragments detected by intact-PTH assays. European Journal of Endocrinology. 2002;147:123–131. doi: 10.1530/eje.0.1470123. [DOI] [PubMed] [Google Scholar]
- 21.Slatopolsky E, Finch J, Clay P, Martin D, Sicard G, Singer G, Gao P, Cantor T, Dusso A. A novel mechanism for skeletal resistance in uremia. Kidney International. 2000;58:753–761. doi: 10.1046/j.1523-1755.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- 22.Tian J, Smogorzewski M, Kedes L, Massry SG. PTH-PTHrP receptor mRNA is downregulated in chronic renal failure. American Journal of Nephrology. 1994;14:41–46. doi: 10.1159/000168684. [DOI] [PubMed] [Google Scholar]
- 23.Sneddon WB, Syme CA, Bisello A, Magyar CE, Weinman EJ, Rochdi MD, Parent JL, Abou-Samra AB, Friedman PA. Activation-independent parathyroid hormone receptor internalization is regulated by NHERF1 (EBP50) Journal of Biological Chemistry. 2003;278:43787–43796. doi: 10.1074/jbc.M306019200. [DOI] [PubMed] [Google Scholar]
- 24.Sneddon WB, Bisello A, Magyar CE, Willick GE, Syme CA, Galbiati F, Friedman PA. Ligand-selective dissociation of activation and internalization of the parathyroid hormone receptor. Conditional efficacy of PTH peptide fragments. Endocrinology. 2004;145:2815–2823. doi: 10.1210/en.2003-1185. [DOI] [PubMed] [Google Scholar]
- 25.Magyar CE, Sneddon WB, Bisello A, Friedman PA. PTH(7-34) downregulates the PTH1 receptor by a ubiquitin-sensitive pathway. Journal of Bone and Mineral Research. 2004;19:174–175. [Google Scholar]
- 26.Weinman EJ, Hall RA, Friedman PA, Liu-Chen LY, Shenolikar S. The association of NHERF adaptor proteins with G protein-coupled receptors and receptor tyrosine kinases. Annual Review of Physiology. 2006;68:491–505. doi: 10.1146/annurev.physiol.68.040104.131050. [DOI] [PubMed] [Google Scholar]
- 27.Mahon MJ, Donowitz M, Yun CC, Segre GV. Na+/H+ exchanger regulatory factor 2 directs parathyroid hormone 1 receptor signalling. Nature. 2002;417:858–861. doi: 10.1038/nature00816. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen-Yamamoto L, Rousseau L, Brossard JH, Lepage R, D’Amour P. Synthetic carboxyl-terminal fragments of parathyroid hormone (PTH) decrease ionized calcium concentration in rats by acting on a receptor different from the PTH/PTH-related peptide receptor. Endocrinology. 2001;142:1386–1392. doi: 10.1210/endo.142.4.8093. [DOI] [PubMed] [Google Scholar]
- 29.Langub MC, Monier-Faugere MC, Wang G, Williams JP, Koszewski NJ, Malluche HH. Administration of PTH-(7-84) antagonizes the effects of PTH-(1-84) on bone in rats with moderate renal failure. Endocrinology. 2003;144:1135–1138. doi: 10.1210/en.2002-221026. [DOI] [PubMed] [Google Scholar]
- 30.Perrien DS, Akel NS, Dupont-Versteegden EE, Skinner RA, Siegel ER, Suva LJ, Gaddy D. Aging alters the skeletal response to disuse in the rat. American Journal of Physiology Regul Integr Comp Physiol. 2007;292:R988–R996. doi: 10.1152/ajpregu.00302.2006. [DOI] [PubMed] [Google Scholar]
- 31.Hildebrand T, Ruegsegger P. Quantification of Bone Microarchitecture with the Structure Model Index. Comput Methods Biomech Biomed Engin. 1997;1:15–23. doi: 10.1080/01495739708936692. [DOI] [PubMed] [Google Scholar]
- 32.Bergwitz C, Jusseaume SA, Luck MD, Jüppner H, Gardella TJ. Residues in the membrane-spanning and extracellular loop regions of the parathyroid hormone (PTH)-2 receptor determine signaling selectivity for PTH and PTH-related peptide. Journal of Biological Chemistry. 1997;272:28861–28868. doi: 10.1074/jbc.272.46.28861. [DOI] [PubMed] [Google Scholar]
- 33.Pagani F, Francucci CM, Moro L. Markers of bone turnover: biochemical and clinical perspectives. Journal of Endocrinological Investigation. 2005;28:8–13. [PubMed] [Google Scholar]
- 34.Delmas PD. Biochemical markers of bone turnover. Journal of Bone and Mineral Research. 1993;8(Suppl 2):S549–S555. doi: 10.1002/jbmr.5650081323. [DOI] [PubMed] [Google Scholar]
- 35.Cozzolino M, Brancaccio D, Gallieni M, Slatopolsky E. Pathogenesis of vascular calcification in chronic kidney disease. Kidney International. 2005;68:429–436. doi: 10.1111/j.1523-1755.2005.00421.x. [DOI] [PubMed] [Google Scholar]
- 36.Jono S, Shioi A, Ikari Y, Nishizawa Y. Vascular calcification in chronic kidney disease. Journal of Bone and Mineral Metabolism. 2006;24:176–181. doi: 10.1007/s00774-005-0668-6. [DOI] [PubMed] [Google Scholar]
- 37.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. New England Journal of Medicine. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 38.Friedl G, Turner RT, Evans GL, Dobnig H. Intermittent parathyroid hormone (PTH) treatment and age-dependent effects on rat cancellous bone and mineral metabolism. Journal of Orthopaedic Research. 2007;25:1454–1464. doi: 10.1002/jor.20433. [DOI] [PubMed] [Google Scholar]
- 39.Ahlgren O, Larsson SE. The role of the parathyroids for the adaptation to a low calcium intake. 3. The long-term effect of parathyroidectomy on the adaptation to a low calcium intake in adult rats with special reference to plasma calcium, bone tissue and adrenal glands. Acta Pathologica et Microbiologica Scandinavica. Section A, Pathology. 1975;83:590–602. [PubMed] [Google Scholar]
- 40.Skripitz R, Aspenberg P. Implant fixation enhanced by intermittent treatment with parathyroid hormone. Journal of Bone and Joint Surgery. 2001;83:437–440. doi: 10.1302/0301-620x.83b3.10256. British Volume. [DOI] [PubMed] [Google Scholar]
- 41.Brossard JH, Yamamoto LN, D’Amour P. Parathyroid hormone metabolites in renal failure: bioactivity and clinical implications. Seminars in Dialysis. 2002;15:196–201. doi: 10.1046/j.1525-139x.2002.00053.x. [DOI] [PubMed] [Google Scholar]
- 42.D’Amour P, Brossard JH, Rousseau L, Nguyen-Yamamoto L, Nassif E, Lazure C, Gauthier D, Lavigne JR, Zahradnik RJ. Structure of non-(1-84) PTH fragments secreted by parathyroid glands in primary and secondary hyperparathyroidism. Kidney International. 2005;68:998–1007. doi: 10.1111/j.1523-1755.2005.00493.x. [DOI] [PubMed] [Google Scholar]
- 43.Bank N, Su WS, Aynedjian HS. A micropuncture study of renal phosphate transport in rats with chronic renal failure and secondary hyperparathyroidism. Journal of Clinical Investigation. 1978;61:884–894. doi: 10.1172/JCI109014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Onyia JE, Helvering LM, Gelbert L, Wei T, Huang S, Chen P, Dow ER, Maran A, Zhang M, Lotinun S, Lin X, Halladay DL, Miles RR, Kulkarni NH, Ambrose EM, Ma YL, Frolik CA, Sato M, Bryant HU, Turner RT. Molecular profile of catabolic versus anabolic treatment regimens of parathyroid hormone (PTH) in rat bone: an analysis by DNA microarray. Journal of Cellular Biochemistry. 2005;95:403–418. doi: 10.1002/jcb.20438. [DOI] [PubMed] [Google Scholar]
- 45.Frolik CA, Black EC, Cain RL, Satterwhite JH, Brown-Augsburger PL, Sato M, Hock JM. Anabolic and catabolic bone effects of human parathyroid hormone (1-34) are predicted by duration of hormone exposure. Bone. 2003;33:372–379. doi: 10.1016/s8756-3282(03)00202-3. [DOI] [PubMed] [Google Scholar]
- 46.Rickard DJ, Wang FL, Rodriguez-Rojas AM, Wu Z, Trice WJ, Hoffman SJ, Votta B, Stroup GB, Kumar S, Nuttall ME. Intermittent treatment with parathyroid hormone (PTH) as well as a non-peptide small molecule agonist of the PTH1 receptor inhibits adipocyte differentiation in human bone marrow stromal cells. Bone. 2006;39:1361–1372. doi: 10.1016/j.bone.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 47.Simmons HA, Pirie CM, Thompson DD, Ke HZ. Parathyroid hormone (1-34) increased total body bone mass in aged female rats. Journal of Pharmacology and Experimental Therapeutics. 1998;286:341–344. [PubMed] [Google Scholar]
- 48.Iwasaki-Ishizuka Y, Yamato H, Nii-Kono T, Kurokawa K, Fukagawa M. Downregulation of parathyroid hormone receptor gene expression and osteoblastic dysfunction associated with skeletal resistance to parathyroid hormone in a rat model of renal failure with low turnover bone. Nephrology, Dialysis, and Transplantation. 2005;20:1904–1911. doi: 10.1093/ndt/gfh876. [DOI] [PubMed] [Google Scholar]
- 49.Goldman ME, McKee RL, Caulfield MP, Reagan JE, Levy JJ, Gay CT, DeHaven PA, Rosenblatt M, Chorev M. A new highly potent parathyroid hormone antagonist: [d-Trp12,Tyr34]bPTH-(7-34)NH2. Endocrinology. 1988;123:2597–2599. doi: 10.1210/endo-123-5-2597. [DOI] [PubMed] [Google Scholar]
- 50.Gardella TJ, Luck MD, Jensen GS, Schipani E, Potts JT, Jr, Jüppner H. Inverse agonism of amino-terminally truncated parathyroid hormone (PTH) and PTH-related peptide (PTHrP) analogs revealed with constitutively active mutant PTH/PTHrP receptors. Endocrinology. 1996;137:3936–3941. doi: 10.1210/endo.137.9.8756569. [DOI] [PubMed] [Google Scholar]
- 51.Murray TM, Rao LG, Divieti P, Bringhurst FR. Parathyroid hormone secretion and action: evidence for discrete receptors for the carboxyl-terminal region and related biological actions of carboxyl-terminal ligands. Endocrine Reviews. 2005;26:78–113. doi: 10.1210/er.2003-0024. [DOI] [PubMed] [Google Scholar]
- 52.Divieti P, Geller AI, Suliman G, Juppner H, Bringhurst FR. Receptors specific for the carboxyl-terminal region of parathyroid hormone on bone-derived cells: determinants of ligand binding and bioactivity. Endocrinology. 2005;146:1863–1870. doi: 10.1210/en.2004-1262. [DOI] [PubMed] [Google Scholar]
- 53.Miller MA, Chin J, Miller SC, Fox J. Disparate effects of mild, moderate, and severe secondary hyperparathyroidism on cancellous and cortical bone in rats with chronic renal insufficiency. Bone. 1998;23:257–266. doi: 10.1016/s8756-3282(98)00098-2. [DOI] [PubMed] [Google Scholar]
- 54.Magnusson P, Sharp CA, Magnusson M, Risteli J, Davie MW, Larsson L. Effect of chronic renal failure on bone turnover and bone alkaline phosphatase isoforms. Kidney International. 2001;60:257–265. doi: 10.1046/j.1523-1755.2001.00794.x. [DOI] [PubMed] [Google Scholar]
- 55.Fukagawa M, Kazama JJ, Shigematsu T. Skeletal resistance to PTH as a basic abnormality underlying uremic bone diseases. American Journal of Kidney Diseases. 2001;38:S152–S155. doi: 10.1053/ajkd.2001.27426. [DOI] [PubMed] [Google Scholar]
- 56.Epstein S, Dissanayake IR, Goodman GR, Bowman AR, Zhou H, Ma Y, Jee WS. Effect of the interaction of parathyroid hormone and cyclosporine a on bone mineral metabolism in the rat. Calcified Tissue International. 2001;68:240–247. doi: 10.1007/s002230001167. [DOI] [PubMed] [Google Scholar]
- 57.Ureña P, Mannstadt M, Hruby M, Ferreira A, Schmitt F, Silve C, Ardaillou R, Lacour B, Abou-Samra AB, Segre GV, Drüeke T. Parathyroidectomy does not prevent the renal PTH/PTHrP receptor down-regulation in uremic rats. Kidney International. 1995;47:1797–1805. doi: 10.1038/ki.1995.248. [DOI] [PubMed] [Google Scholar]
- 58.Disthabanchong S, Hassan H, McConkey CL, Martin KJ, Gonzalez EA. Regulation of PTH1 receptor expression by uremic ultrafiltrate in UMR 106-01 osteoblast-like cells. Kidney International. 2004;65:897–903. doi: 10.1111/j.1523-1755.2004.00472.x. [DOI] [PubMed] [Google Scholar]
- 59.London GM, Marchais SJ, Guerin AP, Metivier F. Arteriosclerosis, vascular calcifications and cardiovascular disease in uremia. Current Opinion in Nephrology and Hypertension. 2005;14:525–531. doi: 10.1097/01.mnh.0000168336.67499.c0. [DOI] [PubMed] [Google Scholar]
- 60.Goodman WG, London G, Amann K, Block GA, Giachelli C, Hruska KA, Ketteler M, Levin A, Massy Z, McCarron DA, Raggi P, Shanahan CM, Yorioka N. Vascular calcification in chronic kidney disease. American Journal of Kidney Diseases. 2004;43:572–579. doi: 10.1053/j.ajkd.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 61.Lomashvili K, Garg P, O’Neill WC. Chemical and hormonal determinants of vascular calcification in vitro. Kidney International. 2006;69:1464–1470. doi: 10.1038/sj.ki.5000297. [DOI] [PubMed] [Google Scholar]
- 62.Block GA, Port FK. Re-evaluation of risks associated with hyperphosphatemia and hyperparathyroidism in dialysis patients: recommendations for a change in management. American Journal of Kidney Diseases. 2000;35:1226–1237. doi: 10.1016/s0272-6386(00)70064-3. [DOI] [PubMed] [Google Scholar]


