Abstract
The extracellular signal-regulated kinases 1 and 2 (ERK) cascade, member of the mitogen-activated protein kinases superfamily of signalling pathways, is one of the best characterized pathways as many protein interactions and phosphorylation events have been systematically studied. Traditionally, ERK are associated with the regulation of proliferation and differentiation as well as survival of various cell types. Their activity is controlled by phosphorylation on specific aminoacidic residues, which is induced by a variety of external cues, including growth-promoting factors.
In the nervous system, ERK phosphorylation is induced by binding of neurotrophins to their specific tyrosine kinase receptors or by neuronal activity leading to glutamate release and binding to its ionotropic and metabotropic receptors. Some studies have provided evidence of its importance in neuroplastic events. In particular, ERK phosphorylation in the spinal cord was shown to be nociceptive-specific and its upregulation, occurring in cases of chronic inflammatory and neuropathic pain, seems to be of the utmost importance to behavioural changes observed in those conditions. In fact, experiments using specific inhibitors of ERK phosphorylation have proved that ERK directly contributes to allodynia and hyperalgesia caused by spinal cord injury or chronic pain. Additionally, spinal ERK phosphorylation regulates the micturition reflex in experimental models of bladder inflammation and chronic spinal cord transection.
In this review we will address the main findings that suggest that ERK might be a future therapeutic target to treat pain and other complications arising from chronic pain or neuronal injury.
Key Words: ERK, MAPK, somatic pain, visceral pain, visceral reflex activity, ERK inhibition, pain.
INTRODUCTION
In the past few years, the Mitogen-Activated Protein Kinase (MAPK) family of signalling cascades has received a great deal of attention, especially the ERK 1 and 2 pathway. This signalling cascade is involved in the regulation of a variety of cellular functions, ranging from the government of cell fate and survival to several plastic changes. Its importance in proliferative phenomena has attracted immense attention as it seems to be extremely important for some types of cancers, an issue that outbounds the present review.
In the nervous system, activation the ERK pathway occurs in a variety of locations and situations, some of which contribute to painful conditions. Pain is a complex multi-dimensional experience related to actual or potential tissue damage, according to the International Association for the Study of Pain [72]. It can occur as a symptom of a disease as in the case of chronic inflammation or a disorder in its own right as in case of painful neuropathies. In both cases, several changes occur in the nervous system, which depend on the activation of the ERK pathway at the different neuronal levels, including dorsal root ganglia (DRG), spinal cord and supraspinal centres. This issue will constitute a main topic of the present review.
In viscera, conditions leading to visceral pain are also commonly associated with changes in the reflex control of the affected organs. The events that occur in the neuronal circuitry responsible for the modulation and integration of noxious input arising from viscera and the respective reflex control are still poorly understood. However, there is now strong evidence that the ERK signalling pathway may be extremely relevant. Therefore, this issue will also be thoroughly discussed in this review.
Finally, given the importance of the ERK pathway in a variety of painful disorders and altered visceral reflex control, attention will also be paid to the use of ERK inhibitors to provide analgesia and regulate visceral reflex activity.
THE MAPK FAMILY OF SIGNALLING PATHWAYS
Eukaryotic cells are constantly submitted to various forms of physical and chemical stress, including contact with neighbouring cells, hormones, growth factors and cytokines. After identification of the external stimulus by membrane receptors, a cellular response programme is set in motion, involving the activation of intracellular signalling pathways composed of sequentially activated enzymes. The final response may be the expression of new genes, modulation of structural elements or alterations in cell cycle progression.
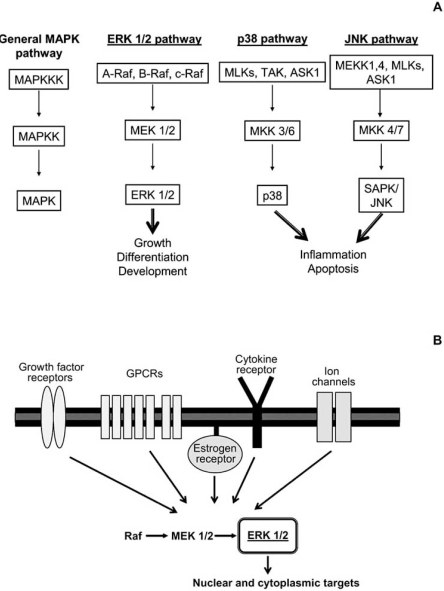
One of the families of signalling pathways that has attracted more attention in the past few years is the MAPK family. As many of the known signalling pathways, such as the Protein Kinase C (PKC) [80], Protein Kinase A (PKA) [75] or the Protein Kinase G (PKG) pathways [107], MAPKs are regulated by phosphorylation, which consists in the covalent binding of phosphate groups to specific aminoacid residues. This process is catalyzed by enzymes designated as kinases [51]. The first members of the MAPK family were identified in the 1980s, when different research groups demonstrated the existence, in several non-neuronal cell types, of a 42 kDa protein, phosphorylated in tyrosine residues upon stimulation with growth factors and phorbol esters [21, 55]. At the same time, two serine/threonine kinases activated by the insulin receptor were characterized [3, 89]. These kinases were named according to their substrate, thus termed myelin basic kinase (MBP) and microtubule-associated kinase 2 (MAP-2). With time, it became evident that these kinases were the 42 kDa protein phosphorylated at the tyrosine residues. They were named MAPK [19] and later Extracellular-signal Regulated Kinase (ERK), to reflect the variety of stimuli that can lead to its activation [8]. Since then, other kinases sharing a high degree of homology with ERK were identified and the term MAPK was used to identify the whole family (Fig. 1) which includes now 5 distinct branches: ERK 1 and 2; c-Jun N-terminal kinases (JNKs) 1, 2 and 3; p38 (isoforms α, β, γ, δ); ERK 3, 4 and ERK 5; e, more recently ERK 6, 7 and 8 [7]. Interestingly, each branch seems to have a preferential activating stimulus: trophic factors for ERK, UV radiations for p38 and extracellular osmolarity for JNK [14].
Fig. (1).
The MAPK family with its classical cascades, the ERK 1 and 2,p38 and JNK. (A) The MAPK family of signalling pathways is characterized by a central motif of three kinases, which activate each other in a sequential order by phosphorylation of specific residues. Classically, 3 distinct pathways are included in the MAPK family (the ERK 1 and 2, p38 and JNK),the activation of which leads to different cellular outcomes. (B) The activation of the ERK pathway depends on a variety of membrane bound receptors,including growth factor receptors, G protein coupled receptors (GPCRs), the estrogen receptor and ionic channels. In some cases, binding of certain cytokines to their specific receptor may also lead to the activation of this pathway. Once active (that is, phosphorylated) ERK can target a variety of nuclear and cytoplasmic elements.
Although each MAPK branch has distinctive characteristics, there are common features among the MAPK pathways. The most striking aspect of these signalling cascades is a central core of three serially linked kinases in each pathway (Fig. 1) and the existence of especial aminoacidic domains in their structure, important for a correct running of MAPK signalling. In what concerns the specificity, duration and magnitude of MAPK signalling, those are assured by several mechanisms that require the participation of structural and accessory proteins. Although important, these aspects of MAPK signalling are not the focus of the present review. More information on these issues can be found elsewhere [47].
The ERK 1 and 2 Signalling Pathway
The first MAPK to be cloned and properly characterized was ERK 1 [10], quickly followed by ERK 2 [9]. ERK 1 and 2 share an 83% homology in their sequences and are expressed in all tissues [15, 90]. Upstream of ERK, there is the small GTPase Ras, the Ras-activated kinase (Raf) and the MAP kinase kinases (MEK) 1 and 2. Raf, from which 3 isoforms are known, is the first to become active and phosphorylates MEK 1 and 2 at two serine or threonine residues. In turn, MEK phosphorylates ERK at two tyrosine and threonine residues. Dual phosphorylation is crucial for full activation of MEK and ERK [9, 87].
Once active, ERK can phosphorylate various elements located in different sub-cellular compartments. Some of the substrates include membrane proteins (CD120a, Syk and calnexin), nuclear proteins (SRC-1, Pax6, MEF2 and STAT3), cytoskeletal elements (neurofilaments and paxilin) [14, 15, 84, 90] and cytoplasmic kinases, [90], including members of its own pathway [13, 58, 68]. It is this extensive interaction with different types of cellular elements that enable ERK to interfere with a vast array of cellular events [22, 66, 104].
Interruption of ERK signalling may occur by (1) hyperphosphorylation of Raf and MEK 1 and 2, (2) dephosphorylation of aminoacidic residues of ERK and (3) sequestration of ERK in the nucleus. In regard to the levels of phosphorylation of Raf and MEK, it is possible that ERK, apart from phosphorylating downstream substrates, could also phosphorylate upstream targets, such as those two kinases [13, 58, 68] generating a negative feedback dependent on hyperphosphorylation [15, 45].
The removal of phosphate groups depends on specific enzymes known as MAPK phosphatases (MKPs) which dephosphorylate tyrosine, serine/threonine residues or both groups of residues. Although most of these phosphatases are cytosolic, some may be found in the nucleus where they are responsible for ERK sequestration [15, 59, 60, 86, 105, 106].
ERK REGULATES NEURONAL CELL FATE AND PLASTICITY
In the nervous system, the first descriptions of ERK expression and activation date from the early 1990’s and relate to the role of ERK in proliferation and differentiation of PC12 cells, derived from the rat adrenal phaechromocytoma [41]. Nerve growth factor (NGF) triggers cell cycle exit and differentiation of PC12 cells into sympathetic neurons, whereas treatment with epidermal growth factor (EGF) induces PC12 cell proliferation. As part of the response to those trophic factors, a robust activation of the ERK cascade was demonstrated. Interestingly, NGF induced a more long-lasting phosphorylation, with translocation of the active forms into the nucleus and activation of the expression of specific neuronal genes. On the other hand, EGF triggered a brief ERK activation, insufficient to induce nuclear translocation and consequent neuronal differentiation [22, 41, 44, 114].
It is well known that for PC12 cells and neurons, including sympathetic and cerebellar neurons [111] kept in culture, removal of NGF from the culture medium constitutes a strong pro-apoptotic stimulus. However, in these cases, apoptosis can be prevented if cells are transfected with hyper-active forms of kinases from the ERK pathway [69, 112]. Activation of the anti-apoptotic gene bcl-2 might be the mechanism by which neuronal survival is regulated by the ERK cascade [73].
Although crucial for neuronal survival, one should take in consideration that extremely prolonged activation of the ERK pathway can be deleterious. As a matter of fact, excessively long-lasting neuronal ERK activation has already been demonstrated in neurodegenerative diseases such as Parkinson`s and Alzheimer`s Diseases [32, 46, 57, 91].
In what concerns neuronal plasticity, ERK intervene in long-term potentiation (LTP), the basis of the learning process, acquisition and maintenance of long-term memory in mammals [67]. ERK participation in LTP was demonstrated for the first time in 1996. In vitro studies showed the occurrence of ERK activation in the CA1 area of the hippocampus after stimulation of the glutamate N-methyl-D-aspartate (NMDA) ionotropic receptor [33, 34]. In latter studies it was further demonstrated that ERK also participate in NMDA-independent forms of LTP [20] and their activation can also occur in other areas of the hippocampus, including the dentate gyrus. Today it has been accepted that, besides the hippocampus, ERK also play a part in LTP occurring in the amygdala, insula and in the synapses between thalamic and amygdalar neurons [52, 93]. In a general way, ERK activation seems to contribute to LTP by controlling the expression of genes such as zif268 and arc [88, 113].
Given that ERK 1 and 2 intracellular signalling pathway plays an important role in the acquisition and consolidation of long-term memory, it would be reasonable to expect that suppression of one of the genes that code for elements of this cascade would alter those functions. Thus, it was shown that knockdown of a Ras accessory protein (involved in the initial steps of activation of this signalling pathway) affects the normal function of the amygdala and the consolidation of long-term memory [11]. Nonetheless, surprisingly, knockdown of the gene coding for ERK1 did not alter the establishment and consolidation of long-term memory [70, 99], although no satisfying explanation for this has been forwarded.
ERK ACTIVATION BY ACUTE NOXIOUS STIMULI
The first indication of ERK involvement in the processing of noxious stimuli was provided in 1999 by Ji and collaborators. They demonstrated the occurrence of ERK phosphorylation in cells located in laminae I and IIo following electrical stimulation of nociceptive afferents or peripheral stimulation with capsaicin. Spinal ERK phosphorylation was ipsilateral to stimulation, NMDA-depen-dent and necessary for pain behaviour during the 2nd phase of the formalin test. Based on these results, it was proposed that spinal ERK phosphorylation was involved in the generation of pain hypersensitivity [48].
Subsequent studies further confirmed ERK activation occurred in response to acute noxious stimulation of primary afferent neurons, [37, 53, 54], the predominant location being the superficial laminae of the ipsilateral dorsal horn [53, 54]. ERK phosphorylation was strictly restricted to neurons [24, 27] and short-lived, basal levels being reached 1 to 2 hours after stimulation [37, 48, 53]. Similarly to what had been observed in the spinal cord, ERK activation was also observed in the trigeminal nucleus, following perioral injection of formalin [43].
In the DRG of non-stimulated animals, levels of ERK activation are very low and mostly restricted to small diameter cells, presumably nociceptive neurons. ERK phosphorylation in response to acute noxious stimulation has also been described in these cells. ERK activation was observed in the perycaria of small primary afferent neurons [29, 30, 83, 94] (Fig. 2) but it also occurred in the peripheral processes of these cells [4, 6, 29, 30]. Curiously, phosphoERK levels were found increased in primary afferents following exposure to NGF and seem to be important for the retrograde transport of that neurotrophin [6].
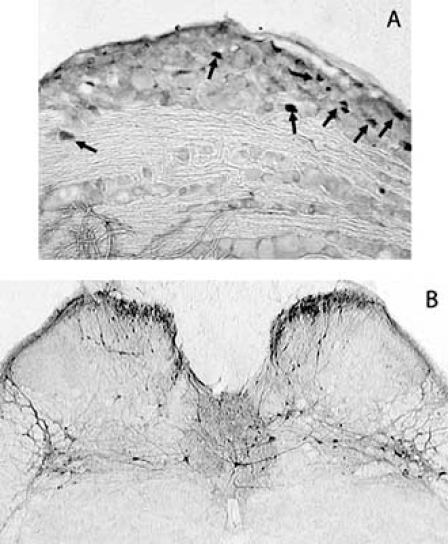
Fig. (2).
PhosphoERK immunoreactive neurons in sections from the L6 dorsal root ganglion (A) and spinal cord (B). Animals were submitted to acute noxious bladder distension. ERK activation occurred mostly in smallto medium diameter neurons (A, arrows), most likely nociceptive neurons.In the spinal cord (B), immunoreactive neurons were located bilaterally in the superficial laminae of the cord, in the dorsal commissure and in the intermediolateral grey matter, areas known to receive bladder sensory input (Cruz et al., unpublished observations).
In supraspinal nuclei, activation of the ERK pathway in response to acute noxious stimulation is still poorly documented. The few studies available refer the occurrence of immunoreactive cells for the phosphorylated forms of ERK in the brainstem and in several structures of the tele- and diencephalon following noxious stimulation, including the hypothalamus, the paraventricular nucleus of the thalamus, the parabrachial nucleus, the dorsal raphe nucleus [16, 38, 39, 40]. ERK activation has also been shown to occur in the hippocampus (dentate gyrus and CA3 zone) and hypothalamic paraventricular nucleus following intrathecal injection of Substance P [16].
ERK ACTIVATION BY CHRONIC NOXIOUS STIMULI
ERK activation by chronic noxious stimulation is not particularly different from that seen after acute noxious stimulation. In the vast majority of situation, ERK activation occurs in the same areas. The differences rest on the more intense levels of ERK phosphorylation and longer duration [2, 6, 24, 27, 36, 49, 83, 96]. In addition, as an additional distinction from acute noxious stimulation, in some models of neuropathic pain ERK activation was also found in non-neuronal cells (see below).
In animals with chronic inflammation of the hindpaw or joint, spinal ERK activation was upregulated and became persistent, with levels remaining elevated up to a maximum of 3 days [27, 49]. Furthermore, in the case of chronic joint inflammation, ERK phosphorylation was not only upregulated but also occurred in deep laminae of the cord [27], probably reflecting changes occurring in neuronal circuitry associated with chronic joint pain [79, 92].
In what concerns spinal ERK phosphorylation after visceral inflammation, the scarce studies available show that noxious stimulation of the chronic inflamed colon and bladder induces prolonged and intense ERK activation in the lumbosacral spinal cord [24, 36] where most of the primary afferents innervating these organs terminate. Thus, in the case of chronic cystitis, ERK phosphorylation occurred bilaterally in neurons located in the superficial dorsal horns, intermediolateral grey matter areas and dorsal commissure [24] (Fig. 2).
Spinal ERK phosphorylation has also been addressed in models of neuropathic pain. In this case, ERK phosphorylation was found in the cytoplasm and nuclei of spinal neurons [31, 62, 102, 117] but also in glial cells, including microglia and astrocytes [31, 63, 117]. Curiously, in animals with spinal nerve ligation, ERK phosphorylation followed a specific pattern. It was firstly observed in neurons, then in microglia (between days 1 and 3 after surgery), in astrocytes and microglia (day 10) and, finally, appeared restricted to astrocytes (day 21) [117]. In the DRG, in contrast with acute noxious stimulation or inflammation, ERK phosphorylation occurred in medium-to-large neurons [81, 82, 83], as well as in satellite cells [82, 83], reflecting the involvement of A-fibres and glial cells in neuropathic pain.
WHY NOXIOUS STIMULI LEAD TO ERK ACTIVATION
The reasons why ERK phosphorylation is upregulated by acute and chronic noxious conditions are various but a common denominator can be found. In all cases studied increases in the spinal levels of excitatory aminoacids and neurotrophins have been reported. It is widely accepted that during inflammation peripheral levels of neurotrophins, ATP, protons, bradykinin among others, are upregulated, leading to sensitization of peripheral sensory fibres [109, 110]. Thus, they are more likely activated by stimulation and will release increased amounts of neurotrophins, retrogradely transported from peripheral tissue or produced in the soma [56], and glutamate in the spinal cord. As the main focus of the present review is not pain, further detail on this subject can be found elsewhere [74].
The identification of membrane receptors that lead to downstream ERK phosphorylation has been widely addressed. Available data points to the involvement in the spinal cord of both ionotropic [48, 54, 61], or metabotropic 1 and 5 glutamate receptors [53, 54, 61, 96]. Binding of brain-derived neurotrophic factor (BDNF) to its specific TrkB receptor is also important for spinal activation of the ERK pathway [85, 100] (Fig. 3). The importance of the spinal substance P (SP) receptor (the NK1 receptor) is, at present less clear. In fact, while some studies demonstrated that SP induces spinal ERK activation [54, 108], other have provided opposing data [61]. Finally, the contribution of other signalling pathways should not be ruled out as it has been verified that activation of the PKC and PKA pathways may lead to ERK phosphorylation [54, 108].
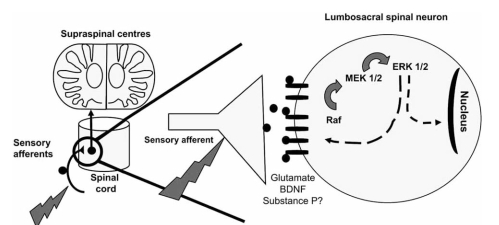
Fig. (3).
Mechanisms of ERK activation in the spinal cord. Upon noxious peripheral stimulation, glutamate and BDNF are released onto the spinal cord. Upon binding to their respective receptors, activation of the pathway occurs in the cytoplasm of spinal neurons. Once activated, ERK can modulate the activity of membrane receptors by phosphorylating specific subunits. Activated ERK can also translocate to the nucleus and induce gene transcription by phosphorylation of transcription factors. The importance of substance P is still in debate.
Traditionally, spinal ERK phosphorylation has been assumed to depend exclusively on sensory afferent input. Current data, however, shows that supraspinal serotonergic input may also contribute to ERK activation in the spinal cord. Spinal 5-HT3 receptors seem to be involved as intrathecal administration of a selective antagonist of this receptor attenuates ERK activation induced by formalin injection in the paw [103]. On the other hand, supraspinal input may also inhibit spinal ERK phosphorylation. As such, in animals with chronic spinal cord transection ERK phosphorylation was shown to be upregulated [see below; 26]. However, the nature of this inhibitory supraspinal input is still to be identified.
In the peripheral nervous system, the ATP receptor, P2X3, seems to play an important role in regulating ERK phosphorylation in DRG neurons, especially in models of inflammatory joint pain [6, 83]. Lastly, inter-connections with other signalling pathways in DRG neurons, namely the phosphatidylinositol 3-kinase (PI3K) pathway [118], appear to also conduct to ERK phosphorylation.
In what concerns ERK phosphorylation increase in glia cells in cases of neuropathic pain, a growing body of evidence indicates they play a pivotal role in this form of chronic pain [for review see 71]. ERK activation might be important for the expression of pro-nociceptive enzymes and cytokines including inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), interleukin-1beta (IL-1β), tumour necrosis factor alpha (TNF-α) and interleukin-6 (IL-6), making the ERK pathway a key signalling pathway in glia cells. Nonetheless, there are currently no direct data supporting these deductions. Further studies are necessary to clarify the significance of ERK phosphorylation in glia cells as well as to define the precise stimuli that produce ERK activation in this type of cells.
ERK ACTIVATION FOLLOWING SPINAL CORD INJURY
Very few studies have concentrated on the effects of spinal cord lesions on the levels of ERK phosphorylation. Nevertheless, available data indicates that spinal injuries such as contusion, excitotoxic injury or chronic complete transection lead to upregulation of spinal levels of ERK phosphorylation [23, 26, 115]. In the cases of contusion and excitotoxicity, ERK phosphorylation was particularly upregulated in the proximal areas (caudal and rostral in relation to the injury site) [23, 115]. Furthermore, these high levels of ERK activation were correlated with increased expression of the receptor NK1, NMDA subunits NR1, NR2A [115] and the transcription factor cAMP response element binding protein (CREB) [23]. Such changes were only observed in animals that displayed injury-induced pain behaviour.
Interestingly, in cases of chronic spinal cord transection at high thoracic segments, ERK phosphorylation was upregulated in L6 segment, that is, in a distal segment to the injury site. This is the segment that receives the majority of bladder-generated sensory input [76, 77]. In cases of spinal cord injury such sensory input is upregulated and its source switched from Aδ to C-fibres [28].
In models of cord injury, as in pain models, ERK phosphorylation seems to rely on excitatory aminoacids and neurotrophins. In fact, it has been well documented that spinal cord lesions produce excessive glutamate release at the lesion site and in its vicinity [78]. Furthermore, in paradigms of spinal injury-induced bladder hyperactivity, the importance of neurothrophins is quite established with high amounts of BDNF and NGF being observed in both the urinary bladder and spinal cord in animals with chronic spinal transection [97, 98, 119].
CONSEQUENCES OF ERK ACTIVATION
The reasons why ERK activation in the nociceptive system may contribute to altered pain states is presently under active investigation. So far, it seems clear that ERK contribution is related to control of gene expression and interaction with membrane receptors. These events may occur in the peripheral and central nervous system either independently or in a concurrent fashion.
In the DRG, ERK activation has been shown to be responsible for the upregulation of BDNF levels in the cell bodies of DRG neurons in models of peripheral inflammation and neuropathic pain [81, 82, 83]. Furthermore, in a model of neuropathic pain induced by chronic constriction of the sciatic nerve, ERK phosphorylation in the DRG was shown to lead to increases in the levels of neuropeptide Y (NPY) in damaged neurons [81]. In all cases, blockade of ERK phosphorylation lead to downregulation of the contents of BDNF and NPY, correlating with improvement in pain hypersensitivity.
ERK activation in DRG neurons may also contribute to altered heat sensitivity by interacting with the transient receptor potential vanilloid receptor-1 (TRPV1), as demonstrated by Zhuang et al. (2004) [118]. Accordingly, inhibition of ERK activation almost completely inhibited the facilitation of heat-induced currents in DRG neurons [35]. Furthermore, ERK inhibition has been shown to partially reduce the upregulation of TRPV1 expression induced by NGF. However, in this process the participation of other Ras-dependent signalling pathways should also be taken into consideration [12].
Finally, ERK phosphorylation may also be accounted to participate in the development and establishment of opioid tolerance which is thought to derive, at least in part, from increased activity of nociceptive pathways. In fact, in vitro studies have shown upregulation in the levels of substance P and calcitonin gene related peptide (CGRP) in DRG neurons chronically exposed to morphine [64]. Such upregulation was shown to be mediated by ERK [65].
In the spinal cord, ERK phosphorylation is important to modulate the activity of the NMDA receptor and the potassium channel Kv 4.2. Upon noxious stimulation and subsequent release of BDNF onto the spinal cord, activated ERK phosphorylates the NR1 subunit of the NMDA receptor [101]. This phosphorylation increases its opening probability, leading to increased neuronal excitability. In what concerns the Kv 4.2 channel, recent studies demonstrate that ERK can phosphorylate the pore-forming subunit of this channel and contribute to pain plasticity at the spinal cord level [1, 42].
Regarding the contribution to the regulation of neuronal gene expression, ERK phosphorylation was shown to be crucial for the upregulation of the spinal levels of NK1 and prodynorphin seen in animals with hindpaw inflammation [49]. Another spinal gene whose expression is believed to be regulated by ERK is c-fos, a pain-evoked immediate early gene, in spite of the evidence that in non-spinal neuronal cells c-fos expression may occur without ERK participation [50]. In the spinal cord neurons, upon ERK blockade with specific inhibitors delivered intrathecally, a reduction was observed in spinal c-fos expression induced by noxious somatic [54] and visceral stimulation [25].
BLOCKING ERK: A NEW THERAPEUTIC TARGET?
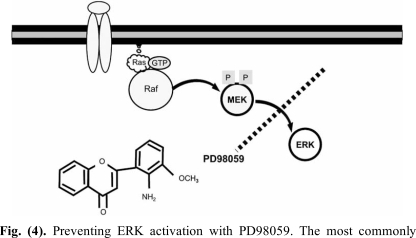
Because in most studies ERK phosphorylation was found in neurons involved in nociception at both the peripheral and central nervous system and upregulated by chronic inflammation, it was hypothesized that it should constitute an important mechanism for pain perception. Furthermore, because high levels of ERK activation correlated with the occurrence of both allodynia and hyperalgesia in several pain models, it is likely that this mechanism should contribute to plastic neuronal changes associated with chronic pain. On this ground, inhibitors of ERK phosphorylation have been used in order to reverse those altered pain states. The most commonly used strategy to prevent ERK activation is by blocking the activation of ERK by its upstream kinase MEK (Fig. 4). The classical molecules used to fulfil this purpose were the MEK inhibitors U0126 and PD98059. This last compound proved to be useful to reduce the second phase of the formalin test when given as an intrathecal infusion [17, 48]. Although several routes of administration have been tested, including intravenous [36], intradermal [30], joint [95], intrathecal [24, 27, 48] and intracerebroventricular injections [17], they should be used with care as the site in which ERK inactivation occurs may be difficult to ascertain (such as in the case of intravenous injection) and the role of ERK phosphorylation in peripheral fibres is still poorly understood.
Fig. (4).
Preventing ERK activation with PD98059. The most commonly used approach to avert ERK phosphorylation consists in blocking the interaction between phosphorylated MEK and inactive ERK. Several inhibitors have been developed to accomplish this task, the most frequently used of which is PD98059. This inhibitor is a non-competitive cyclic molecule with an amino moiety.
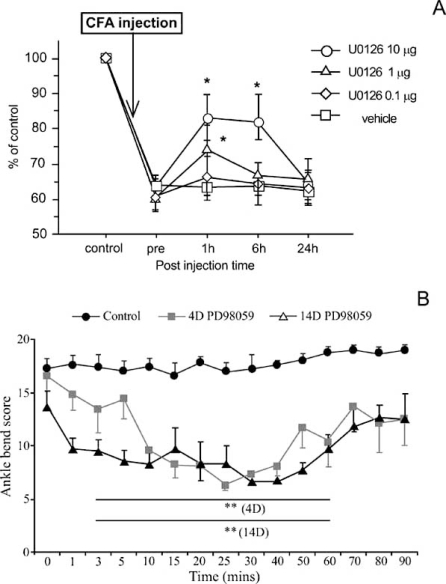
Nevertheless, available results show that intrathecal administration of U0126 or PD98059 successfully attenuated mechanical allodynia in cases of inflammatory [17, 36, 54, 82, 83, 96] and neuropathic pain [81, 82, 102, 117], heat hypersensitivity [2, 5, 17, 29, 30] and mechanical hyperalgesia [30, 31]. In the particular case of chronic hindpaw inflammation, ERK blockade also prevented the upregulation in the spinal levels of prodynorphin and NK1 receptor observed in such animals [49]. Furthermore, ERK inactivation also lead to reduction of neuropathic static allodynia [18], suppression of autotomy [83], decreased referred hyperalgesia following colon inflammation [36] and reduction in nociceptive behaviour derived from joint inflammation [27, 96]. Interestingly, it should be noted that the use of ERK inhibitors in animals with chronic joint inflammation to minimise allodynia allowed the evaluation of the effects of ERK activation at different sites. Intra-articular administration of U0126 in animals with knee inflammation clearly improved the struggle threshold (Fig. 5A), [96]. This serves as evidence of the importance of ERK phosphorylation in the peripheral processes of sensory neurons. Also in animals with chronic joint inflammation, intrathecal administration has been tested and a reduction in the levels of allodynia was also found (Fig. 5B), [27]. This indicates that ERK phosphorylation in spinal neurons or at the central processes of joint primary afferents is vital for the decreased mechanical threshold observed in those animals.
Fig. (5).
Reduction of allodynia in animals with chronic joint inflammation following administration of ERK inhibitor. In (A), U0126 (another ERK inhibitor) was delivered an intra-articular injection. As a consequence, the angle of knee movement, which was less than 70% of control, was improved with the administration of 1 and 10µg of intra-articular inhibitor. Adapted from [95]. In (B), ankle-bend scores for saline- and intrathecal injected PD98059 in monoarthritic (MA) rats at different time points. The anklebend test for MA rats was performed immediately before the intrathecal injection (time 0) of either saline (control; black circles) or 1 µg (grey squares) and 2 µg (white triangles) of PD98059 in MA rats with 4 and 14 days of evolution, respectively. The high struggle scores induced by ankle bending observed in control MA animals was increased thought-out the all experimental period. On the contrary, ankle-bend scores for the PD98059 injected groups were significantly decreased. Adapted from [27]. CD Cruz,Pain (2005) 116:411-419. Used with permission. D Seino, Pain (2006)123:193-203. Used with permission.
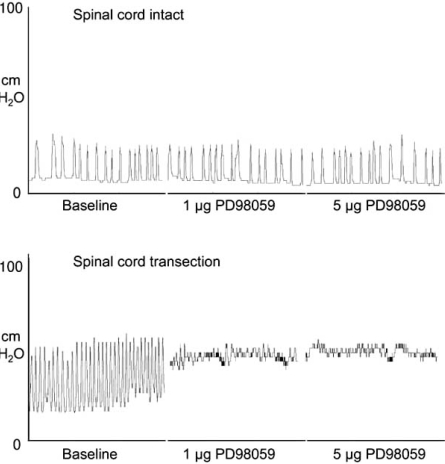
Additionally, available data also showed correlation between high levels of ERK phosphorylation in lumbosacral spinal cord and bladder hyperactivity caused by chronic bladder inflammation and spinal cord transection. In both cases, intrathecal injection of PD98059 strongly reduced the frequency [24, 26] and amplitude of bladder contractions [26] (Fig. 6), indicating that spinal ERK phosphorylation is important to regulate micturition in pathological conditions. Also relevant to the eventual therapeutic application of ERK inhibitors was the finding that administration of the same doses of PD98059 in intact animals did not produce any effect whatsoever on bladder reflex activity, including the frequency and amplitude of bladder contractions [24, 26]. This finding may indicate that ERK inhibition might prove to be a good therapeutic strategy, at least for the treatment of bladder dysfunction.
Fig. (6).
Effect of intrathecal administration of PD98059 on bladder reflex activity in animals with intact and chronically transected spinal cord. In animals with intact cords, PD98059 had no effect in bladder contractions,despite the amount of inhibitor injected. In animals that underwent chronic spinal cord transection at high thoracic levels, bladder reflex activity is clearly altered. These animals display high frequency of bladder contractions with strong micturition pressures. PD98059 visibly reduced both the frequency and amplitude of bladder contractions (Cruz et al., unpublished results).
CONCLUDING REMARKS AND FUTURE PERSPECTIVES
From the present review it becomes clear that the activation of the ERK signalling pathway might be a key mechanism in the development and maintenance of altered pain states. ERK activation occurs in both glia cells and neurons located in the peripheral and central nervous system, and is strongly upregulated in conditions associated with increased sensory input. Phosphorylated ERK can exert its effects by controlling gene regulation and/or modulating membrane receptors and ionic channels. By preventing ERK phosphorylation, the levels of allodynia and hyperalgesia are reduced. Such results clearly indicate that this signalling pathway might constitute an attractive target for pain relief. As seen above, the classical inhibitors PD98059 and U0126 have been used in several models of pain. Intense efforts have, however, been made in order to develop other inhibitors with increased efficiency, specificity and less prone to cause side-effects [for review see 95]. Studies have focused in 3 proteins: Ras, Raf and MEK. Different types of inhibitors have been developed, ranging from modified nucleotides to small-molecule inhibitors. Interestingly, some of these inhibitors may be administered orally and are already available in the market. Nevertheless, in the available literature, no data was found regarding the use of these new inhibitors to treat pain, a gap which hopefully will be corrected in a near future.
Furthermore, the activation of this cascade seems also to be highly important in the regulation of visceral reflex activity in animal models of chronic cystitis and spinal cord transection. In patients with conditions mimicked by these models, bladder reflex activity is hugely increased, with patients often complaining about intense urinary frequency and incontinence. The ability of ERK inhibitors to decrease the frequency and amplitude of bladder contractions in inflamed or cord transected animals without affecting bladder reflex activity in intact animals forwards an exceptional therapeutic window for this type of drugs, which should be actively pursued in the very next future.
Finally, when considering using ERK inhibitors as therapeutic tools, it should be taken into consideration the ubiquitous and vital role played by this pathway for cell survival. Adjusting the characteristics and dosage of ERK inhibitors will be of capital importance. Also, given the organization of the ERK pathway and the interaction with other signalling cascades, the development of new and more powerful inhibitors will necessary aim to target protein-protein interactions, rather than just interfering with enzymatic activity. Although such goal seems difficult to achieve, overcoming this problem may have enormous impact in pain therapeutics and management of bladder dysfunction.
ACKNOWLEDGEMENTS
We thank Dr. Jorge Ferreira for critical reading of the manuscript.Supported by POCI/SAU-NEU/55983/2004, FCT, Portugal.
ABBREVIATIONS
- BDNF
= Brain derived neurotrophic factor
- CGRP
= Calcitonin gene related peptide
- COX-2
= Cyclooxygenase-2
- CREB
= cAMP response element binding protein
- DRG
= Dorsal root ganglia
- EGF
= Epidermal growth factor
- ERK
= Extracelular signal regulated kinase
- IL-1β
= Interleukin-1beta
- IL-6
= Interleukin-6
- iNOS
= Inducible nitric oxide synthase
- JNKs
= Jun kinases
- LTP
= Long term potentiation
- MAP-2
= Microtubule associated kinase 2
- MAPK
= Mitogen activated protein kinase
- MBP
= Mielin basic protein kinase
- MEK
= Mitogen-activated protein kinase kinase
- MKPs
= MAPK phosphatases
- NGF
= Nerve growth factor
- NK1
= Neurokinin 1
- NMDA
= N-methyl-D-aspartate
- NPY
= Neuropeptide Y
- PD98059
= [2-(2’-amino-3’-methoxyphenyl)-oxanaphthalen-4-one]
- PKA
= Protein kinase A
- PKC
= Protein kinase C
- PKG
= Protein kinase G
- Raf
= Ras effector
- SP
= Substance P
- TNF-α
= Tumour necrosis factor alpha
- TRPV1
= Transient receptor potential vanilloid receptor-1
- U0126
= 1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenlythio]butadiene
REFERENCES
- 1.Adams J.P, Anderson A.E, Varga A.W, Dineley K.T, Cook .RG, Pfaffinger P.J, Sweatt J.D. The A-type potassium channel Kv4. is a substrate for the mitogen-activated protein kinase ERK. J. Neurochem. 2000;75:2277–2287. doi: 10.1046/j.1471-4159.2000.0752277.x. [DOI] [PubMed] [Google Scholar]
- 2.Adwanikar H, Karim F, Gereau IV R.W. Inflammation persistently enhances nocifensive behaviors mediated by spinal group I mGluRs through sustained ERK activation. Pain. 2004;111:125–135. doi: 10.1016/j.pain.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Ahn N.G, Krebs E.G. Evidence of an epidermal growth factor-stimulated kinase cascade in Swiss 3T3 cells.Activation of serine peptide kinase activity by mielin basic protein kinases. J. Biol. Chem. 1990;265:11495–11501. [PubMed] [Google Scholar]
- 4.Aley K.O, Martin A, McMahon T, Mok J, Levine J.D, Messing R.O. Nociceptor sensitization by extracellular signal-regulated kinases. J. Neurosci. 2001;21:6933–6939. doi: 10.1523/JNEUROSCI.21-17-06933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambrosini S.S, Coderre T.J. Intracellular messengers involved in spontaneous pain, heat hyperalgesia, and mechanical allodynia induced by intrathecal dihydroxyphenylglycine. Neurosci. Lett. 2006;409:224–229. doi: 10.1016/j.neulet.2006.09.051. [DOI] [PubMed] [Google Scholar]
- 6.Averill S, Delcroix J.D, Michael G.J, Tomlinson D.R, Fernyhough P, Priestley J.V. Nerve growth factor modulates the activation status and fast axonal transport of ERK 1/2 in adult nociceptive neurones. Mol. Cell. Neurosci. 2001;18:183–96. doi: 10.1006/mcne.2001.1015. [DOI] [PubMed] [Google Scholar]
- 7.Bogoyevitch M.A, Court N.W. Counting on mitogen- activated protein kinases-ERK 3, 4, 5, 6, 7 and 8. Cell. Signal. 2004;16:1345–1354. doi: 10.1016/j.cellsig.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Boulton T.G, Gregory J.S, Cobb M.H. Purification and properties of extracellular signal-regulated kinase 1, an insulin-stimuleted microtubule-associated protein 2 kinase. Biochemistry. 1990a;30:278–286. doi: 10.1021/bi00215a038. [DOI] [PubMed] [Google Scholar]
- 9.Boulton T.G, Nye S.H, Robbins D.J, Ip N.Y, Radziejewska E, Morgenbesser S.D, DePinho RA, Panayotatos N, Cobb M.H, Yancopoulos G.D. ERK a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991;65:663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- 10.Boulton T.G, Yancopoulos G.D, Gregory J.S, Slaughter C, Moomaw C, Hsu J, Cobb M.H. An insulin-stimulated protein kinase similar to yeast kinases involved in cell cycle control. Science. 1990b;249:64–67. doi: 10.1126/science.2164259. [DOI] [PubMed] [Google Scholar]
- 11.Brambilla R, Gnesutta N, Minichiello L, White G, Roylance A.J, Herron C.E, Ramsey M, Wolfer D.P, Cestari V, Rossi-Arnaud C, Grant SG, Chapman P.F, Lipp H.P, Sturani E, Klein R. A role for the Ras signalling pathway in synaptic transmission and long-term memory. Nature. 1997;390:281–286. doi: 10.1038/36849. [DOI] [PubMed] [Google Scholar]
- 12.Bron R, Klesse L.J, Shah K, Parada L.F, Winter J. Activation of Ras is necessary and sufficient for upregulation of vanilloid receptor type 1 in sensory neurons by neurotrophic factors. Mol. Cell Neurosci. 2003;22:118–132. doi: 10.1016/s1044-7431(02)00022-2. [DOI] [PubMed] [Google Scholar]
- 13.Brunet A, Pages G, Pouysségur J. Growth factor-stimulated MAP kinase induces rapid retrophosphorylation and inhibition of MAP kinase kinase (MEK1) FEBS Lett. 1994;346:299–303. doi: 10.1016/0014-5793(94)00475-7. [DOI] [PubMed] [Google Scholar]
- 14.Chang L, Karim M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z, Gibson T.B, Robinson F, Silvestro L, Pearson G, Xu B, Wright A, Vanderbilt C, Cobb M.H. MAP kinases. Chem. Rev. 2001;101:2449–2476. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- 16.Choi S-S, Seo Y-J, Kwon M-S, Shim E-J, Lee J-Y, Ham Y-O, Park S-H, Suh H-W. Involvement of phosphorylated extracellular signal-regulated kinase in the mouse substance P pain model. Mol. Brain Res. 2005;137:152–158. doi: 10.1016/j.molbrainres.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Choi S.S, Seo Y.J, Shim E.J, Kwon M.S, Lee J.Y, Ham Y.O, Suh H.W. Involvement of phosphorylated Ca2+/calmodulin-dependent protein kinase II and phosphorylated extracellular signal-regulated protein in the mouse formalin pain model. Brain Res. 2006;1108:28–38. doi: 10.1016/j.brainres.2006.06.048. [DOI] [PubMed] [Google Scholar]
- 18.Ciruela A, Dixon A.K, Bramwell S, Gonzalez M.I, Pinnock R.D, Lee K. Identification of MEK1 as a novel target for the treatment of neuropathic pain. Br. J. Pharmacol. 2003;138:751–756. doi: 10.1038/sj.bjp.0705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cobb M.H, Robbins D.J, Boulton T.G. ERK extracellular signal-regulated MAP-2 kinases. Curr. Opin. Cell Biol. 1991;3:1025–1032. doi: 10.1016/0955-0674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 20.Coogan A.N, O'Leary D.M, O'Connor J.J. P42/44 MAP kinase inhibitor PD98059 attenuates multiple forms of synaptic plasticity in rat dentate gyrus. J. Neurophysiol. 1999;81:103–110. doi: 10.1152/jn.1999.81.1.103. [DOI] [PubMed] [Google Scholar]
- 21.Cooper J.A, Bowen-Pope D.F, Raines E, Ross R, Hunter T. Similar effects of platelet-derived growth factor and epidermal growth factor on the phosphorylation of tyrosine in cellular proteins. Cell. 1982;31:263–273. doi: 10.1016/0092-8674(82)90426-3. [DOI] [PubMed] [Google Scholar]
- 22.Cowley S, Paterson H, Kemp P, Marshall C.J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 23.Crown E.D, Ye Z, Johnson K.M, Xu G.Y, McAdoo D.J, Hulsebosch C.E. Increases in the activated forms of ERK 1/2, p38 MAPK, and CREB are correlated with the expression of at-level mechanical allodynia following spinal cord injury. Exp. Neurol. 2006;199:97–407. doi: 10.1016/j.expneurol.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Cruz C.D, Avelino A, McMahon S.B, Cruz F. Increased spinal cord phosphorylation of extracellular signal-regulated kinases mediates micturition overactivity in rats with chronic bladder inflammation. Eur. J. Neurosci. 2005a;21:773–81. doi: 10.1111/j.1460-9568.2005.03893.x. [DOI] [PubMed] [Google Scholar]
- 25.Cruz C.D, Ferreira D, McMahon S.B, Cruz F. The activation of the ERK pathway contributes to the spinal c-fos observed after noxious bladder stimulation. Somatosens. Mot. Res. 2007;24:15–20. doi: 10.1080/08990220601143265. [DOI] [PubMed] [Google Scholar]
- 26.Cruz C.D, McMahon S.B, Cruz F. Spinal ERK activation contributes to the regulation of bladder function in spinal cord injured rats. Exp. Neurol. 2006;200:66–73. doi: 10.1016/j.expneurol.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Cruz C.D, Neto F.L, Castro-Lopes J, McMahon S.B, Cruz F. Inhibition of ERK phosphorylation decreases nociceptive behaviour in monoarthritic rats. Pain. 2005b;116:411–419. doi: 10.1016/j.pain.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 28.Cruz F. Mechanisms involved in new therapies for overactive bladder. Urology. 2004;63:65–73. doi: 10.1016/j.urology.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Dai Y, Fukuoka T, Wang H, Yamanaka H, Obata K, Tokunaga A, Noguchi K. Contribution of sensitized P2X receptors in inflamed tissue to the mechanical hypersensitivity revealed by phosphorylated ERK in DRG neurons. Pain. 2004;108:258–266. doi: 10.1016/j.pain.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 30.Dai Y, Iwata K, Fukuoka T, Kondon E, Tokunaga A, Yamanaka H, Tachibana T, Liu Y, Noguchi K. Phosphorylation of extracellular signal-regulated kinase in primary afferent neurons by noxious stimulation and its involvement in peripheral sensitization. J. Neurosci. 2002;22:7737–7745. doi: 10.1523/JNEUROSCI.22-17-07737.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daulhac L, Mallet C, Courteix C, Etienne M, Duroux E, Privat A.M, Eschalier A, Fialip J. Diabetes-induced mechanical hyperalgesia involves spinal mitogen-activated protein kinase activation in neurons and microglia N-methyl-D-aspartate-dependent mechanisms. Mol. Pharmacol. 2006;70:1246–54. doi: 10.1124/mol.106.025478. [DOI] [PubMed] [Google Scholar]
- 32.Dineley K.T, Westerman M, Bui D, Bell K, Ashe K.H, Sweatt J.D. Beta-amyloid activates the mitogen-activated protein kinase cascade hippocampal alpha7 nicotinic acetylcholine receptors: and mechanisms related to Alzheimer's disease. J. Neurosci. 2001;21:4125–4133. doi: 10.1523/JNEUROSCI.21-12-04125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.English J.D, Sweatt J.D. Activation of p42 mitogen-activated protein kinase in hippocampal long term potentiation. J. Biol. Chem. 1996;271:24329–24332. doi: 10.1074/jbc.271.40.24329. [DOI] [PubMed] [Google Scholar]
- 34.English J.D, Sweatt J.D. A requirement for the mitogen-activated protein kinase cascade in hippocampal long term potentiation. J. Biol. Chem. 1997;272:19103–19106. doi: 10.1074/jbc.272.31.19103. [DOI] [PubMed] [Google Scholar]
- 35.Firner M, Greffrath W, Treede R.D. Phosphorylation of extracellular signal-related protein kinase is required for rapid facilitation of heat-induced currents in rat dorsal root ganglion neurons. Neuroscience. 2006;143:253–263. doi: 10.1016/j.neuroscience.2006.07.047. [DOI] [PubMed] [Google Scholar]
- 36.Gálan A, Cervero F, Laird J.M. Extracellular signalling-regulated (ERK 1/2) mediate referred hyperalgesia in a murine model of visceral pain. Mol. Brain Res. 2003;116:126–134. doi: 10.1016/s0169-328x(03)00284-5. [DOI] [PubMed] [Google Scholar]
- 37.Gálan A, Lopez-Garcia J.A, Cervero F, Laird J.M. Activation of spinal extracellular signalling-regulated kinase-1 and -2 by intraplantar carrageenan in rodents. Neurosci. Lett. 2002;322:37–40. doi: 10.1016/s0304-3940(02)00078-2. [DOI] [PubMed] [Google Scholar]
- 38.Gioia M, Galbiati S, Rigamonti L, Moscheni C, Gagliano N. Extracellular signal-regulated kinases 1 and 2 phosphorylated neurones in the tele- and diencephalon of rat after visceral pain stimulation: an immunocytochemical study. Neurosci. Lett. 2001;308:177–180. doi: 10.1016/s0304-3940(01)02008-0. [DOI] [PubMed] [Google Scholar]
- 39.Gioia M, Moscheni C, Gagliano N. Distribution of extracellular signal-regulated kinase 1- and 2-activated neurones in the rat periaqueductal gray matter after noxious stimulation. Anat. Rec. Part A. 2005;284A:460–465. doi: 10.1002/ar.a.20188. [DOI] [PubMed] [Google Scholar]
- 40.Gioia M, Moscheni C, Galbiati S, Gagliano N. Immunocytochemical localization of extracellular signal-regulated kinases 1 and 2 phosphorylated neurones in the brainstem of rats following visceral noxious stimulation. Neurosci. Lett. 2003;349:167–170. doi: 10.1016/s0304-3940(03)00821-8. [DOI] [PubMed] [Google Scholar]
- 41.Greene L.A, Tischler A.S. Establishment of a noradrenergic clonal line of rat adrenal phaechromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu H.J, Carrasquillo Y, Karim F, Jung W.E, Nerbonne J.M, Schwarz T.L, Gereau R.W. 4th The kv4.potassium channel subunit is required for pain plasticity. Neuron. 2006;50:89–100. doi: 10.1016/j.neuron.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Huang W.J, Wang B.R, Yao L.B, Huang C.S, Wang X, Zhang P, Jiao X.Y, Duan X.L, Chen B.F, Ju G. Activity of p44/p42 MAP kinase in the caudal subnucleus of trigeminal spinal nucleus is increased following perioral noxious stimulation in the mouse. Brain Res. 2000;861:181–185. doi: 10.1016/s0006-8993(00)02015-1. [DOI] [PubMed] [Google Scholar]
- 44.Huff K, End D, Guroff G. Nerve growth factor-induced alteration in the response of PC12 pheochromocytoma cells to epidermal growth factor. J. Cell Biol. 1981;88:189–198. doi: 10.1083/jcb.88.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signalling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- 46.Hyman B.T, Elvhage T.E, Reiter J. Extracellular signal regulated kinases.Localization of protein and mRNA in the human hippocampal formation in Alzheimer's disease. Am. J. Pathol. 1994;144:565–572. [PMC free article] [PubMed] [Google Scholar]
- 47.Imajo M, Tsuchiya Y, Nishida E. Regulatory mechanisms and functions of MAP Kinase signalling pathways. IUBMB Life. 2006;58:312–317. doi: 10.1080/15216540600746393. [DOI] [PubMed] [Google Scholar]
- 48.Ji R.R, Baba H, Brenner G.J, Woolf C.J. Nociceptive-specific activation of ERK in spinal neurones contributes to pain hypersensitivity. Nat. Neurosci. 1999;2:1114–1119. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- 49.Ji R.R, Befort K, Brenner G.J, Woolf C.J. ERK MAP kinase activation in superficial spinal cord neurones induces prodynorphin and NK-1 upregulation and contributes to persistent inflammatory pain hypersensitivity. J. Neurosci. 2002;22:478–485. doi: 10.1523/JNEUROSCI.22-02-00478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson C.M, Hill C.S, Chawla S, Treisman R, Bading H. Calcium controls gene expression three distinct pathways that can function independently of the Ras/mitogen-activated protein kinases (ERKs) signaling cascade. J. Neurosci. 1997;17:6189–6202. doi: 10.1523/JNEUROSCI.17-16-06189.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson G.L, Lapdat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK and p38 protein kinases. Scienc. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 52.Jones M.W, French P.J, Bliss T.V, Rosenblum K. Molecular mechanisms of long-term potentiation in the insular cortex. J. Neurosci. 1999;19:RC36 –(1-8). doi: 10.1523/JNEUROSCI.19-21-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karim F, Wang C-C, Gereau IV R.W. Metabotropic glutamate receptor subtypes 1 and 5 are activators of the extracellular signal-regulated kinase signalling required for inflammatory pain in mice. J. Neurosci. 2001;21:3771–3779. doi: 10.1523/JNEUROSCI.21-11-03771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawasaki Y, Kohno T, Zhuang Z-Y, Brenner G.J, Wang H, Van Der Meer C, Befort K, Woolf C.J, Ji R-R. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C and Src contribute to c-fiber-induced ERK activation and cAMP Response Element-Binding protein phosphorylation in dorsal horn neurones, leading to central sensitization. J. Neurosci. 2004;24:8310–8321. doi: 10.1523/JNEUROSCI.2396-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kazlauskas A, Cooper J.A. Protein kinase C mediates platelet-derived growth factor-induced tyrosine phosphorylation of p42. J. Cell Biol. 1988;106:1395–1402. doi: 10.1083/jcb.106.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kerr B.J, Bradbury E.J, Bennett D.L, Trivedi P.M, Dassan P, French J, Shelton D.B, McMahon S.B, Thompson S.W. Brain-derived neurotrophic factor modulates nociceptive sensory inputs and NMDA-evoked responses in the rat spinal cord. J. Neurosci. 1999;19:5138–5148. doi: 10.1523/JNEUROSCI.19-12-05138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kulich S.M, Chu C.T. Sustained extracellular signal-regulated kinase activation by 6-hydroxydopamine implications for Parkinson's disease. J. Neurochem. 2001;77:1058–1066. doi: 10.1046/j.1471-4159.2001.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee R.M, Cobb M.H, Blackshear P.J. Evidence that extracellular signal-regulated kinases are the insulin-activated Raf-1 kinase kinases. J. Biol. Chem. 1992;267:1088–1092. [PubMed] [Google Scholar]
- 59.Lenormand P, Brondello J.M, Brunet A, Pouysségur J. Growth factor-induced p42/p44 MAPK nuclear translocation and retention requires both MAPK activation and neosynthesis of nuclear anchoring proteins. J. Cell Biol. 1998;142:625–633. doi: 10.1083/jcb.142.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lenormand P, Sardet C, Pages G, L'Allemain G, Brunet A, Pouysségur J. Growth factors induce nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45mapkk) in fibroblasts. J. Cell Biol. 1993;122:1079–88. doi: 10.1083/jcb.122.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lever I.J, Pezet S, McMahon S.B, Malcangio M. The signalling components of sensory fiber transmission involved in the activation of ERK MAP kinase in the mouse dorsal horn. Mol. Cell Neurosci. 2003;24:259–270. doi: 10.1016/s1044-7431(03)00200-8. [DOI] [PubMed] [Google Scholar]
- 62.Liu Y, Obata K, Yamanaka H, Dai Y, Fukuoka T, Tokunaga A, Noguchi K. Activation of extracellular signal-regulated protein kinase in dorsal horn neurons in the rat neuropathic intermittent claudication model. Pain. 2004;109:64–72. doi: 10.1016/j.pain.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 63.Ma W, Quirion R. Partial sciatic nerve ligation induces increase in the phosphorylation of extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) in astrocytes in the lumbar spinal dorsal horn and the gracile nucleus. Pain. 2002;99:175–84. doi: 10.1016/s0304-3959(02)00097-0. [DOI] [PubMed] [Google Scholar]
- 64.Ma W, Zheng W.H, Kar S, Quirion R. Morphine treatment induced calcitonin gene-related peptide and substance P increases in cultured dorsal root ganglion neurons. Neuroscience. 2000;99:529–539. doi: 10.1016/s0306-4522(00)00226-8. [DOI] [PubMed] [Google Scholar]
- 65.Ma W, Zheng W.H, Powell K, Jhamandas K, Quirion R. Chronic morphine exposure increases the phosphorylation of MAP kinases and the transcription factor CREB in dorsal root ganglion neurons an and study. Eur. J. Neurosci. 2001;14:1091–1104. doi: 10.1046/j.0953-816x.2001.01731.x. [DOI] [PubMed] [Google Scholar]
- 66.Marshall C.J. Specificity of receptor tyrosine kinase signalling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 67.Martin S.J, Grimwood P.D, Morris R.G. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu. Rev. Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 68.Matsuda S, Gotoh Y, Nishida E. Phosphorylation of Xenopus mitogen-activated protein (MAP) kinase kinase by MAP kinase kinase kinase and MAP kinase. J. Biol. Chem. 1993;268:3277–3281. [PubMed] [Google Scholar]
- 69.Mazzoni I.E, Said F.A, Aloyz R, Miller F.D, Kaplan D. Ras regulates sympathetic neuron survival by suppressing the p53-mediated cell death pathway. J. Neurosci. 1999;19:9716–9727. doi: 10.1523/JNEUROSCI.19-22-09716.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mazzuchelli C, Vantaggiato C, Ciamei A, Fasano S, Pakhotin P, Krezel W, Welzl H, Wolfer D.P, Pages G, Valverde O, Marowsky A, Porrazzo A, Orban P.C, Maldonado R, Ehrengruber M.U, Cestari V, Lipp H.P, Chapman P.F, Poysségur J, Brambilla R. Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron. 2002;34:807–820. doi: 10.1016/s0896-6273(02)00716-x. [DOI] [PubMed] [Google Scholar]
- 71.McMahon S.B, Cafferty W.B, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp. Neurol. 2005;192:444–462. doi: 10.1016/j.expneurol.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 72.Mersky H, Bogduk N, editors. Classification of chronic pain. 2nd edition. Seatle.: IASP Press ; 1994. [Google Scholar]
- 73.Michaelidis T.M, Sendtner M, Cooper J.D, Airaksinen M.S, Holtmann B, Meyer M, Thoene H. Inactivation of bcl-2 results in progressive degeneration of motoneurones, sympathetic and sensory neurones during early postnatal development. Neuron. 1996;17:75–89. doi: 10.1016/s0896-6273(00)80282-2. [DOI] [PubMed] [Google Scholar]
- 74.Millan M.J. The induction of pain: an integrative review. Prog. Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 75.Montminy M. Transcriptional regulation by cyclic AMP. Annu. Rev. Biochem. 1997;66:807–822. doi: 10.1146/annurev.biochem.66.1.807. [DOI] [PubMed] [Google Scholar]
- 76.Morgan C, Nadelhaft I, de Groat W.C. The distribution of visceral primary afferents from the pelvic nerve to Lissauer's tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. J. Comp. Neurol. 1981;201:415–440. doi: 10.1002/cne.902010308. [DOI] [PubMed] [Google Scholar]
- 77.Nadelhaft I, Booth A.M. The location and morphology of preganglionic neurons and the distribution of visceral afferents from the rat pelvic nerve: a horseradish peroxidase study. J. Comp. Neurol. 1984;226:238–245. doi: 10.1002/cne.902260207. [DOI] [PubMed] [Google Scholar]
- 78.Nesic O, Svrakic N.M, Xu G.Y, McAdoo D, Westlund K.N, Hulsebosch C.E, Ye Z, Galante A, Soteropoulos P, Tolias P, Young W, Hart R.P, Perez-Polo J.R. DNA microarray analysis of the contused spinal cord effect of NMDA receptor inhibition. J. Neurosci. Res. 2002;68:406–423. doi: 10.1002/jnr.10171. [DOI] [PubMed] [Google Scholar]
- 79.Neto F.L, Schadrack J, Ableitner A, Castro-Lopes J.M, Bartenstein P, Zieglgansberger W, Tolle T.R. Supraspinal metabolic activity changes in the rat during adjuvant monoarthritis. Neuroscience. 1999;94:607–621. doi: 10.1016/s0306-4522(99)00185-2. [DOI] [PubMed] [Google Scholar]
- 80.Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem. J. 2003;370:361–371. doi: 10.1042/BJ20021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Obata K, Yamanaka H, Dai Y, Mizushima T, Fukuoka T, Tokunaga A, Noguchi K. Differential activation of MAPK in injured and uninjured DRG neurons following chronic constriction injury of the sciatic nerve in rats. Eur. J. Neurosci. 2004;20:2881–2895. doi: 10.1111/j.1460-9568.2004.03754.x. [DOI] [PubMed] [Google Scholar]
- 82.Obata K, Yamanaka H, Dai Y, Mizushima T, Fukuoka T, Tokunaga A, Noguchi K. Activation of extracellular signal-regulated protein kinase in the dorsal root ganglion following inflammation near the nerve cell body. Neuroscience. 2004;126:1011–1021. doi: 10.1016/j.neuroscience.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 83.Obata K, Yamanaka H, Dai Y, Tachibana T, Fukuoka T, Tokunaga A, Yoshikawa H, Noguchi K. Differential activation of extracellular signal-regulated protein kinase in primary afferent neurones regulates brain-derived neurotrophic factor expression after peripheral inflammation and nerve injury. J. Neurosci. 2003;23:4117–4126. doi: 10.1523/JNEUROSCI.23-10-04117.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peyssonnaux C, Eychène A. The Raf/MEK/ERK pathway: new concepts of activation. Biol. Cell. 2001;93:53–62. doi: 10.1016/s0248-4900(01)01125-x. [DOI] [PubMed] [Google Scholar]
- 85.Pezet S, Malcangio M, Lever I.J, Perkinton M.S, Thompson S.W, Williams R.J, McMahon S.B. Noxious stimulation induces Trk receptor and downstream ERK phosphorylation in spinal dorsal horn. Mol. Cell. Neurosci. 2002;21:684–695. doi: 10.1006/mcne.2002.1205. [DOI] [PubMed] [Google Scholar]
- 86.Pouysségur J, Volmat V, Lenormand P. Fidelity and spatiotemporal control in MAP kinase (ERK) signalling. Biochem. Pharmacol. 2002;64:755–763. doi: 10.1016/s0006-2952(02)01135-8. [DOI] [PubMed] [Google Scholar]
- 87.Robbins D.J, Zhen E, Owaki H, Vanderbilt C.A, Ebert D, Geppert T.D, Cobb M.H. Regulation and properties of extracellular signal-regulated protein kinases 1 and 2. J. Biol. Chem. 1993;268:5097–5106. [PubMed] [Google Scholar]
- 88.Rosenblum K, Futter M, Voss K, Erent M, Skehel P.A, French P, Obosi L, Jones M.W, Bliss T.V. The role of extracellular regulated kinases I/II in late-phase long-term potentiation. J. Neurosci. 2002;22:5432–5441. doi: 10.1523/JNEUROSCI.22-13-05432.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rossomando A.J, Payne D.M, Weber M.J, Sturgill T.W. Evidence that pp42, a major tyrosine kinase target protein, is a mitogen-activated serine/threonine protein kinase. Proc. Natl. Acad. Sci. USA. 1989;86:6940–6943. doi: 10.1073/pnas.86.18.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roux P.P, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Russo C, Dolcini V, Salis S, Venezia V, Zambrano N, Russo T, Schettini G. Signal transduction through tyrosine-phosphorylated C-terminal fragments of amyloid precursor protein an enhanced interaction with Shc/Grb2 adaptor proteins in reactive astrocytes of Alzheimer's disease brain. J. Biol. Chem. 2002;277:35282–35288. doi: 10.1074/jbc.M110785200. [DOI] [PubMed] [Google Scholar]
- 92.Schadrack J, Neto F.L, Ableitner A, Castro-Lope J.M, Willoch F, Bartenstein P, Zieglgansberger W, Tolle T.R. Metabolic activity changes in the rat spinal cord during adjuvant monoarthritis. Neuroscience. 1999;94:595–605. doi: 10.1016/s0306-4522(99)00186-4. [DOI] [PubMed] [Google Scholar]
- 93.Schafe G.E, Atkins C.M, Swank M.W, Bauer E.P, Sweatt J.D, LeDoux J.E. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J. Neurosci. 2000;20:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schicho R, Liebmann I, Lippe I.T. Extracellular signal-regulated kinase-1 and -2 are activated gastric luminal injury in dorsal root ganglion neurones N-methyl-D-aspartate receptors. Neuroscience. 2005;134:505–514. doi: 10.1016/j.neuroscience.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 95.Sebolt-Leopold J.S, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat. Rev. Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 96.Seino D, Tokunaga A, Tachibana T, Yoshiya S, Dai Y, Obata K, Yamanaka H, Kobayashi K, Noguchi K. The role of ERK signalling and the P2X receptor on mechanical pain evoked by movement of inflamed knee joint. Pain. 2006;123:193–203. doi: 10.1016/j.pain.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 97.Seki S, Sasaki K, Fraser M.O, Igawa Y, Nishizawa O, Chancellor M.B, de Groat W.C, Yoshimura N. Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats. J. Urol. 2002;168:2269–2274. doi: 10.1016/S0022-5347(05)64369-8. [DOI] [PubMed] [Google Scholar]
- 98.Seki S, Sasaki K, Igawa Y, Nishizawa O, Chancellor M.B, De Groat W.C, Yoshimura N. Suppression of detrusor-sphincter dyssynergia by immunoneutralization of nerve growth factor in lumbosacral spinal cord in spinal cord injured rats. J. Urol. 2004;171:478–482. doi: 10.1097/01.ju.0000088340.26588.74. [DOI] [PubMed] [Google Scholar]
- 99.Selcher J.C, Nekrasova T, Paylor R, Landreth G.E, Sweatt J.D. Mice lacking the ERK1 isoform of MAP kinase are unimpaired in emotional learning. Learn. Mem. 2001;8:11–19. doi: 10.1101/lm.37001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Slack S.E, Grist J, Mac Q, McMahon S.B, Pezet S. TrkB expression and phospho-ERK activation by brain-derived neurotrophic factor in rat spinothalamic tract neurones. J. Comp. Neurol. 2005;489:59–68. doi: 10.1002/cne.20606. [DOI] [PubMed] [Google Scholar]
- 101.Slack S.E, Pezet S, McMahon S.B, Thompson S.W, Malcangio M. Brain-derived neurotrophic factor induces NMDA receptor subunit one phosphorylation ERK and PKC in the rat spinal cord. Eur. J. Neurosci. 2004;20:1769–1778. doi: 10.1111/j.1460-9568.2004.03656.x. [DOI] [PubMed] [Google Scholar]
- 102.Song X.S, Cao J.L, Xu Y.B, He J.H, Zhang L.C, Zeng Y.M. Activation of ERK/CREB pathway in spinal cord contributes to chronic constrictive injury-induced neuropathic pain in rats. Acta Pharmacol.Sin. 2005;26:789–798. doi: 10.1111/j.1745-7254.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- 103.Svensson C.I, Tran T.K, Fitzsimmons B, Yaksh T.L, Hua X.Y. Descending serotonergic facilitation of spinal ERK activation and pain behavior. FEBS Lett. 2006;580:6629–6634. doi: 10.1016/j.febslet.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tombes R.M, Auer K.L, Mikkelsen R, Valerir K, Wymann M.P, Marshall C.J, McMahon M, Dent P. The mitogen-activated protein (MAP) kinase cascade can either stimulate or inhibit DNA synthesis in primary cultures of rat hepatocytes depending upon whether its activation is acute/phasic or chronic. Biochem. J. 1998;330:1451–1460. doi: 10.1042/bj3301451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Volmat V, Camps M, Arkinstall S, Poysségur J, Lenormand P. The nucleus, a site for signal termination by sequestration and inactivation of p42/p44 MAP kinases. J. Cell Sci. 2001;114:3433–3343. doi: 10.1242/jcs.114.19.3433. [DOI] [PubMed] [Google Scholar]
- 106.Volmat V, Pouysségur J. Spatiotemporal regulation of the p42/p44 MAPK pathway. Biol. Cell. 2001;93:71–79. doi: 10.1016/s0248-4900(01)01129-7. [DOI] [PubMed] [Google Scholar]
- 107.Wang X, Robinson P.J. Cyclic GMP-dependent protein kinase and cellular signalling in the nervous system. J. Neurochem. 1997;68:443–456. doi: 10.1046/j.1471-4159.1997.68020443.x. [DOI] [PubMed] [Google Scholar]
- 108.Wei F, Vadakkan K.I, Toyoda H, Wu L.J, Zhao M.G, Xu H, Shum F.W, Jia Y.H, Zhuo M. Calcium calmodulin-stimulated adenylyl cyclases contribute to activation of extracellular signal-regulated kinase in spinal dorsal horn neurons in adult rats and mice. J. Neurosci. 2006;26:851–861. doi: 10.1523/JNEUROSCI.3292-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Woolf C.J, Costigan M. Transcriptional and posttranslational plasticity and the generation of inflammatory pain. Proc. Natl. Acad. Sci. USA. 1999;96:7723–7730. doi: 10.1073/pnas.96.14.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Woolf C.J, Salter M.W. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 111.Xia Z, Dickens M, Raingeaud J, Davis R.J, Greenberg M.E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 112.Xue L, Murray J.H, Tolkovsky A.M. The Ras/phosphatidylinositol 3-kinase and Ras/ERK pathways function as independent survival modules each of which inhibits a distinct apoptotic signalling pathway in sympathetic neurones. J. Biol. Chem. 2000;275:8817–8824. doi: 10.1074/jbc.275.12.8817. [DOI] [PubMed] [Google Scholar]
- 113.Ying S.W, Futter M, Rosenblum K, Webber M.J, Hunt S.P, Bliss T.V, Bramham C.R. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J. Neurosci. 2002;22:1532–1540. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.York R.D, Yao H, Dillon T, Ellig C.L, Eckert S.P, McClesky E.W, Stork P.J. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature. 1998;392:622–626. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]
- 115.Yu C.G, Yezierski R.P. Activation of the ERK1/2 signaling cascade by excitotoxic spinal cord injury. Mol. Brain Res. 2005;138:244–255. doi: 10.1016/j.molbrainres.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 116.Yu Y.Q, Chen J. Activation of spinal extracellular signaling-regulated kinases by intraplantar melittin injection. Neurosci. Lett. 2005;381:194–198. doi: 10.1016/j.neulet.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 117.Zhuang Z.Y, Gerner P, Woolf C.J, Ji R.R. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 118.Zhuang Z.Y, Xu H, Clapham D.E, Ji R.R. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J. Neurosci. 2004;24:8300–8309. doi: 10.1523/JNEUROSCI.2893-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zvarova K, Murray E, Vizzard M.A. Changes in galanin immunoreactivity in rat lumbosacral spinal cord and dorsal root ganglia after spinal cord injury. J. Comp. Neurol. 2004;475:590–603. doi: 10.1002/cne.20195. [DOI] [PubMed] [Google Scholar]