Abstract
G-protein-coupled receptors (GPCRs), which are encoded by >300 genes in the human genome, are by far the largest class of targets for modern drugs. These macromolecules display inherent adaptability of function, which is partly due to the production of different forms of the receptor protein. These are commonly called ‘isoforms’ or ‘splice variants’ denoting the molecular process of their production/assembly. Not all GPCRs are expressed as splice variants, but certain subclasses of 5–HT receptors are for example, the 5–HT4 and 5–HT7 receptors. There are at least 11 human 5–HT4 and three h5–HT7 receptor splice variants. This review describestheir discoveries, nomenclature and structures. The discovery that particular splice variants are tissue specific (or prominent) has highlighted their potential as future drug targets. In particular, this review examines the functional relevance of different 5–HT4 and 5–HT7 receptor splice variants. Examples are given to illustrate that splice variants have differential modulatory influences on signalling processes. Differences in agonist potency and efficacies and also differences in desensitisation rates to 5–HT occur with both 5–HT4 and 5–HT7 receptor splice variants. The known and candidate signalling systems that allow for splice variant specific responses include GPCR interacting proteins (GIPs) and GPCR receptor kinases (GRKs) which are examined.Finally, the relevance of 5–HT receptor splice variants to clinical medicine and to the pharmaceutical industry is discussed.
Key Words: Serotonin receptors, GPCR receptor isoforms, GPCR receptor splice variants, GPCR interacting proteins, desensitisation, functional intestinal disorders, irritable bowel syndrome.
INTRODUCTION
G-protein-coupled receptors (GPCRs) are by far the largest class of targets for modern drugs. These macromolecules are encoded by >300 genes in the human genome. Once formed, they are transported and embedded in the cell-surface, where they take on their functions of detecting and responding to a diverse array of ligands. Numerous diseases and disorders have been linked to mutations and polymorphisms in GPCRs and in their natural states these receptors are the targets of an increasingly large number of therapeutic agents. It has been estimated that 50% of all modern drugs and almost one-quarter of the top 200 best-selling drugs in 2000 modulate GPCR activity (see [32] for review). Studies into GPCR splice variants or isoforms is a new research area that opens the possibilities to further refine safety margins of therapeutic drugs.
A general property of GPCRs is that they have inherent adaptability built into their function, which is partly due to the production of different forms of the receptor protein. Thus, different products can be generated from a single GPCR gene by the combination of alternative forms of particular exons. This process is referred to as ‘alternative splicing’ and translated products are called ‘splice variants’ or more commonly ‘isoforms’(see Fig. 1). Over 70% of multi-exon genes expressed in humans are alternatively spliced to form various splice variants and the proteins involved in cellular communication are common amongst examples [41]. The discovery that particular splice variants are tissue specific (or prominent) has highlighted their potential as future drug targets. Therefore, just as the discovery of different receptor subgroups opened up vast opportunities to develop new drug treatments, the discovery of splice variants promises to further expand and refine these opportunities. Examples are to be found with prostaglandin EP3 receptors, which are subject to splice variance at the C-terminus and, to date, 10 splice variants have been identified across species, six of these being expressed in man. In addition, there is evidence for a splice variant form of EP1 receptor that lacks the highly conserved seventh transmembrane domain (TM). Amongst adrenoceptors, four splice variants of the α1A-AR have been reported as well as variants of the β3-AR. Of the serotonin (5-hydroxytryptamine, 5–HT) receptors, the 5–HT4 and 5–HT7 receptors in particular are noteworthy for the production of several splice variants [40].
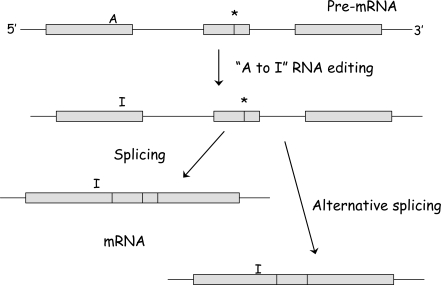
Fig. (1).
Methods of isoform or splice variant generation. RNA editing from adenosine deamination to form inosine which is read as guanosine and so codes for different amino acids. Alternatively if additional splice junctions are present (e.g. at * but also at the ends of exons depicted by boxes), exons can be incorporated or removed (drawn) resulting in different length mRNAs and so different proteins.
Several questions arise when considering the purpose of these GPCR splice variants. Are they expressed in specific tissues? Do they modulate signalling processes in different fashions? If so, could they act as potential therapeutic targets? Here we will discuss these questions in relation to serotonin receptor splice variants with an emphasis on the distribution of 5–HT4 and 5–HT7 receptor splice variants and their potential as therapeutic targets. We will emphasise the neuropharmacological aspects. However, 5–HT receptors are widely distributed, so it is important to note that substantial information has been acquired about their splice variants by studying peripheral tissues. Although 5–HT receptor splice variants are localised to nerve elements in many tissues, others are found in effector cells, such as smooth muscle. It is also important to note, that much of our knowledge about the function of splice variants comes from cell culture techniques. Although these can be criticised for being artificial and minimalist, they are the only practical way of studying individual splice variants, because most native cells express a mixture. In this review we first outline a brief commentary on the functions and classification system of 5–HT receptors. We will also refer to some excellent and extensive reviews on other aspects of the subject which are not covered in this paper. For instance, the structural and operational characteristics of 5–HT receptors are described [38] and the distribution and function of 5–HT7 receptors has been reviewed [48,73]. There are also extensive reviews of the medicinal chemistry and pharmacology of 5–HT4 agonists and antagonists [46] and of 5–HT7 receptor ligands [33]. In addition, the clinical relevance of 5–HT receptors in pathophysiological conditions and their targeting with therapeutic drugs is covered [29].
5–HT
5–HT has diverse physiological effects and broadly these encompass regulation of the cardiovascular, digestive and central nervous systems. Mammalian physiologists had known since the 1800s that a vasoconstrictor substance was formed when blood was allowed to clot. In 1948, Rapport named the serum vasoconstrictor ‘serotonin’and a year later he discovered that the active substance was 5-hydroxytryptamine. 5–HT is widely distributed in nature, occurring in both plants and animals. In mammals the largest amount of 5–HT is present along the length of the gastrointestinal tract (60-90%), mainly in enterochromaffin (EC) cells of the mucosal layer. The remainder occurs in the enteric nervous system with significant distribution in the brain and spinal cord, the heart and adrenocortical cells. 5–HT is synthesised from tryptophan in these sites, but the 5–HT content of platelets is acquired mainly from EC cells. It is now recognised that disturbances in the levels of 5–HT and/or the densities of its receptors contributes to the pathogenesis of many clinical conditions. Some examples are the carcinoid syndrome and gastrointestinal motility disorders in the periphery, and migraine, depression, anxiety, schizophrenia, obsessive compulsive disorders, eating disorders and the serotonin syndrome centrally.
5–HT RECEPTORS
The physiological and pathophysiological effects of 5–HT are mediated by at least 14 different receptor subtypes [2,37]. This relatively large number is attributed to the long evolutionary history of the 5–HT signalling system, which predates the separation of vertebrates and invertebrates, some 600 million years ago. Consequently, there has been abundant time for gene duplications, followed by mutations and sequence shifts to form the different genes encoding for the different subtypes. It has been speculated that the ancestral 5–HT receptors functioned to facilitate cell to cell connections and to promote growth and differentiation. This diversified to the level of complexity now apparent in the mammalian brain (for reviews, see [5,8,18,71]).
All mammalian 5–HT receptors are members of the GPCR superfamily of membrane-bound receptors (also called metabotropic receptors) with the exception of the 5–HT3 receptor which is an ion channel. These are classified into seven distinct classes, or families, according to their structure, pharmacological properties and preferred effector mechanisms according to the current IUPHAR appellation of 5–HT1, 5–HT2, 5–HT3, 5–HT4, 5–ht5, 5–HT6 and 5–HT7. Some of these classes include multiple receptors, which share similar structural and effector properties, but display very different operational profiles. The lower case character denotes that the class concerned has not been ascribed functional roles although structural and transduction information is known. The 5–HT1 receptor class comprises five different receptors; 5–HT1A, 5–HT1B, 5–HT1D, 5–ht1e and 5–HT1F which couple preferentially to Gi/Go to inhibit cAMP formation. The 5–HT2 receptor class comprises three receptors 5–HT2A, 5–HT2B and 5–HT2C that couple preferentially to Gq/G11 to increase the hydrolysis of inositol phosphates and elevate cytosolic [Ca2+]. Selective antagonists for each receptor are now becoming available. The 5–HT3 receptor is a pentameric ion channel that appears to be located exclusively in neuronal tissue where it mediates fast depolarization. 5–HT4, 5–HT6 and 5–HT7 receptors all couple preferentially to Gs and promote cAMP formation, while the 5–ht5, receptor is able to couple to several signalling pathways including Gi/Go [40]. Numerous selective 5–HT4 receptor agonists and antagonists are now available and selective antagonists for the 5–HT7 receptor and putative 5–HT6 receptor antagonists have also recently been reported. Many therapeutic drugs target 5–HT receptors, with notable examples being: the anxiolytic buspirone (5–HT1A agonist), the antimigraine drug sumatriptan (5–HT1B/D agonist), the antidepressant mianserine (5–HT2A/C antagonist), the antiemetic ondansetron (5–HT3 antagonist), and tegaserod (5–HT4 partial agonist), which has been used to treat the constipation-predominant form of irritable bowel syndrome (IBS).
5–HT RECEPTORS WITH ISOFORMS
The 5–HT1 receptor family (5–HT1A, 5–HT1B, 5–HT1D, 5–ht1e and 5–HT1F) are intronless so do not form splice variants [18,68]. Although the 5–HT2 receptor family (5–HT2A, 5–HT2B and 5–HT2C) contains introns, the splice variants formed by alternative splicing produce truncated non-functional proteins. However, the 5–HT2C receptor forms isoforms through RNA editing involving the enzyme family of adenosine deaminases that act on RNA (Fig. 1) where agonist potency, activation of phospholipase C and selectivity of G protein coupling are generally reduced (for a review see [67]). The 5–HT4, 5–ht6 and 5–HT7 receptors contain introns [18,68] and it has been demonstrated that the well characterised 5–HT4 and 5–HT7 receptors form multiple splice variants. These are the subject of this review.
Human 5–HT4 Receptor Splice Variants
There are at least 11 human 5–HT4 receptor splice variants. The major relevance of this recent knowledge is that differences in the tissue distribution and function of 5–HT4 splice variants could potentially be used as a basis for new drug development. For instance, discovery of heart-selective drugs is achievable if the heart is found to express a 5–HT4 splice variant as the therapeutic target that is unique to the heart or more prevalent to the heart than other organs.
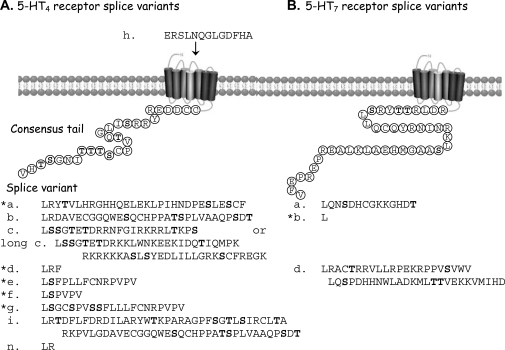
The 5–HT4 receptor present in the human atrium was the first to be cloned and characterised. Following its discovery in 1997 and naming as h5–HT4(a) [14]; several other splice variants have been cloned: h5–HT4(b) [15,72], two different h5–HT4(c) splice variants [13,15], h5–HT4(d) [15], h5–HT4(e) [13], h5–HT4(f) [13], h5–HT4(g) [13,24] (formerly called (e) [58] see [46]), h5–HT4(i) [20], h5–HT4(h) [13] the only example of an internal splice variant insert occurring in the 2nd extracellular loop, and h5–HT4(n) [74] (Fig. 2A). From the viewpoint of drug discovery, the tissue distribution of these human splice variants shows a degree of specificity (see [46] for review). In addition, cellular studies show that desensitisation rates of 5–HT4 receptor splice variants depends upon the GPCR kinases (GRK) present [10] and the splice variant [64].
Fig. (2).
Serotonin receptor splice variants. (A). The 11 human 5–HT4 receptor splice variants. Most of the splice variants occur in the C terminal tail following the splice site coding for the amino acid leucine 358 (shown as first amino acid in each splice variant). However, splice variant h5-HT4(h) occurs in the second extracellular domain and has been reported to contain the “b” splice variant tail. (B). The 3 human 5–HT7 receptor splice variants which occur in the C terminal tail following the splice site at coding for the amino acid leucine 432 (shown as first amino acid in each splice variant). Potential phosphorylation sites (S/T) are in bold; and * indicates a PDZ ligand.
The h5–HT4(b) is dominant in most tissues and the h5–HT4(a) is also common, so opportunities for drug discovery need to take advantage of other tissue-specific splice variants. Table 1 summarises the current state of knowledge. Focussing on the atrial tissue of the heart, it can be seen that it is relatively well endowed with splice variants compared to the kidney and bladder. Also, it expresses the (n) splice variant, as does the brain and oesophagus, but this is absent from the kidney, bladder, stomach, ileum and colon. The current state of knowledge shows the human small intestine and colon also express h5–HT4(d) and that this is not found in other tissues [13,15,56]. Many of the other splice variants are more widely distributed, so it is important to map both their distribution and quantity as their actual levels of expression may be low.
Table 1.
Distribution of Human 5–HT4 and 5–HT7 Receptor Splice Variants
| Tissue | 5–HT4 Splice Variant | 5–HT7 Splice Variant |
|---|---|---|
| Heart | atrium: ; a, b, c, g, n, i ventricle: a, b, g, i |
Total: a, b, d, d+5 |
| Brain | a, b, c, g, long c, n, e, f, i | a,b, d |
| Kidney | a, b | a, b, d |
| Bladder | a | |
| Spleen | a, b, d | |
| Lung | a, b, d | |
| Oesophagus | a, b, n | |
| Stomach | a, b, long c | |
| Ileum | a, b, c, d, g, e, f, i | a, b, d, d+5 |
| Colon | a, b, long c, d, g, e, f, i | a, b, d, d+5 |
Interestingly, 5–HT4 receptor splice variant expression has been shown to change dramatically in cancerous tissue. Normal adrenal tissue expressed both 5–HT4(a) and 5–HT4(b) and rarely 5–HT4(d) splice variants while adrenocortical aldosterone producing adenomas increased their expression of 5–HT4(d) and no longer expressed 5–HT4(a) or 5–HT4(b) receptor splice variants [22]. These results indicate the importance of knowing both receptor expression patterns in normal and diseased tissue as there is the potential to modulate receptor function with splice variant selective drugs.
Human 5–HT7 Receptor Splice Variants
The 5–HT7 receptor was first cloned from human tissues in 1993 [7]. Three human 5–HT7 splice variants were then discovered and named, h5–HT7(a), h5–HT7(b) and h5–HT7(d) that differ in their C terminal tails [36] (Fig.2B). It was found that the brain and spleen contained relatively small amounts of 5–HT7(d) mRNA. However, h5–HT7(d) was reported later to be predominantly expressed in the human small intestine and colon together with a certain amount of the h5–HT7(d+5) fragment [43]. The known distribution of 5–HT7 receptor (or more prevalent) is summarised in Table 1.
THE C TERMINAL TAILS
All of the splice variants of both the h5–HT4 and h5–HT7 receptors (except h5–HT4(h)) differ in the sequences of their intracellular (C-terminus) tails, but share an identical sequence up to Leu 358 for h5–HT4 and Leu 432 for h5–HT7 (see Fig.2). This major portion contains the 7 transmembrane loops and the recognition site for 5–HT.
Differences in Agonist Potency
The C-terminal tails of 5–HT4 receptor splice variants have been found to directly influence their functional properties and this is most dramatically seen in their transduction of agonist responses. A notable example is renzapride, which is nearly 20 times more potent at the h5–HT4(d) than at the (g) splice variants in inducing cyclic AMP formation in COS cells. Another difference is that renzapride behaves as a full agonist at the h5–HT4(d), but is a partial agonist at the (g) variant [59]. One interpretation for this phenomenon is that the C-terminus regions exert different torsion forces on the conserved transmembrane loops causing different steric presentations of the active site to its ligands. Some indirect support for this explanation comes from our observation that the different splice variants exhibit over 10-fold variations in their affinities for ligands in binding studies (Coupar, Tochon-Danguy, Irving unpublished observations). Another explanation for the functional differencesbetween 5–HT4 splice variants is that they can link to different G proteins. Experiments using human embryonic kidney (HEK) cells have shown that the potencies of 5–methoxytryptamine at 5–HT4(a) and 5–HT4(b) splice variants are different and that this is correlated to coupling to only Gs and to Gi/o plus Gs proteins, respectively [63]. More recent experiments using adenoviral expression of h5–HT4(b) and 5–HT4(d) splice variants in rodent cardiac myocytes that do not naturally express 5–HT4 receptors demonstrated that the 5–HT4(d) receptor was more efficiently coupled to adenylyl cyclase [23]. In addition, it was shown that pertussis toxin potentiated the stimulatory effect of 5–HT on L–type Ca2+ current in rat myocytes expressing the 5–HT4(b) splice variant but not the 5–HT4(d) [23] providing further support for the suggestion that the (b) splice variant couples to both Gs and Gi/o proteins.
5–HT7 receptors preferentially couple to adenylyl cyclase via Gsα [1] similar to 5–HT4 receptors. The screening of four agonists and a larger set of antagonists has so far failed to show any differences in binding affinities, potencies or efficacies at the three h5–HT7 splice variants [43,44]. However, with the discovery of an increasing number of 5–HT7 ligands (see [33] for review) this situation may change. A recent study, also using HEK 293 cells, has shown that the h5–HT7(d) splice variant exists in a greater internalised state in the absence of agonist (5–carboxamidotryptamine) compared to the other two variants; h5–HT7(a) and h5–HT7(b). Interestingly, the 5–HT7 antagonist, SB-269970, induced a partial translocation of the h5–HT7(d) variant from cytoplasm to plasma membrane. Another noted difference was that the h5–HT7(d) variant was associated with a lesser efficacy at stimulating adenylyl cyclase. As a result, it was suggested that the C terminal tail of the h5–HT7(d) splice variant, which is the longest of the three human splice variants, may contain a motif that interacts with cellular transport systems to limit the amount destined for the plasma membrane [34].
The ability of a ligand to provoke a GPCR-mediated response is measured in terms of ‘efficacy’ and the ligand is referred to as an ‘agonist’ in classical pharmacological terms. It is now apparent that a balance occurs between the molecular mechanisms controlling receptor signalling, desensitisation and resensitisation or down regulation. Hence, the selectivity of agonists may be influenced by differences in the individual rates at which their splice variants desensitise and/or interact with numerous intracellular GPCR interacting proteins (GIPs).
Differences in Intracellular Signalling Modulated by GIPs
The most common GIPs interact with the C terminal tails of GPCRs; and PDZ domain containing proteins are the most abundant members of this class. PDZ domains were first recognised as sequence repeats contained in three separate proteins: Post synaptic density (PSD) protein PSD-95, Discs large protein (the Drosophila homologue) and tight junction protein ZO-1. PDZ proteins are involved in scaffolding multi-protein complexes and have roles in protein trafficking (see [62] for a review). PDZ proteins bind to specific conserved consensus sequences that are found at the C-terminal end of proteins. These consensus ligands have been classified as class I PDZ ligand of S/TXǾ , class II of ǾXǾ and class III contains E/DXǾ where Ǿ represents a hydrophobic residue and X any amino acid [62]. Many GPCRs express a PDZ ligand at the extremity of their C terminus (usually the last 3-4 amino acids). Thus the C terminal tail of GPCRs can contain a PDZ ligand at its extremity while residues upstream of the last 3-4 are important in modulating the specificity of interactions with other proteins [17]. An example of how different splice variant tails can influence protein interactions is depicted in Fig. (3). This example is based on results of proteomic studies on mouse brain where two 5–HT4 receptor splice variants with different PDZ ligands in the extremity of their C terminal tails were used as a bait ligand to isolate the interacting proteins [42].
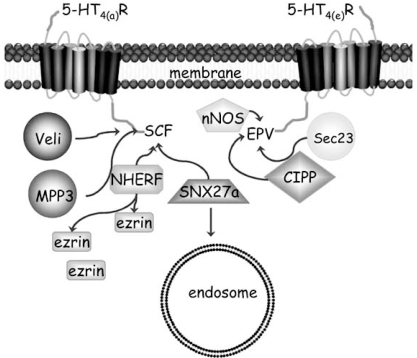
Fig. (3).
Example of PDZ proteins interacting with mouse 5–HT4 receptor splice variants (a) and (e). The PDZ proteins, Veli, MPP3, NHERF and SNX27a interact with the extreme C-tail of 5–HT4(a) which contains the amino acids SCF. NHERF interacts with the scaffold protein ezrin and is involved in localising the 5–HT4(a) splice variant to specific parts of the membrane and SNX27a is involved at a later stage in assisting the receptor to be targeted to endosomes for desensitisation. The 5 HT4(e) receptor splice variant associates with nNOS, Sec23 and CIPP (another scaffolding protein). Ar-rows indicate protein interactions. For further details see text.
Several serotonin receptors contain PDZ ligands at the extremity of their C-terminal tails and have been the focus of investigation. GIPs containing PDZ domains specifically regulate receptors in the serotonin receptor family. The 5–HT2A receptor contains a PDZ ligand at its extreme C terminus and directly binds to PSD-95 which augments signal transduction and inhibits agonist-induced receptor internalisation [75]. Moreover, recent proteomic experiments have demonstrated that the PDZ ligand of the 5–HT2A receptor interacts with a different set of PDZ proteins to that of the 5–HT2C receptor [12]. It is noteworthy that PSD-95 interacts with 5–HT2A and 5–HT2C but not 5–HT4(a) receptors indicating that the different tails containing PDZ ligands interact with specific sets of proteins [12,42]. These specific sets of different PDZ proteins probably contribute to the different signal transduction properties of these receptors. Recently, another example of a GIP involved in serotonin receptor function has been reported where a small protein, p11, is involved in specifically transporting 5–HT1B receptors to the plasma membrane [69]. These findings provide support to the tenet that “specific sets of GIPs interact with different sets of receptor splice variants and that this is also tissue dependent”.
There are five 5–HT4 and one 5–HT7 receptor splice variants with canonical PDZ C–terminal extremity ligand binding sites (Fig. 2). So far, there have been no reports on GIP interactions with any 5–HT4 or 5–HT7 receptor splice variants in human tissue. However, a recent study identified 13 GIPs (mainly PDZ domain proteins) that interact with either the mouse 5–HT4(a) or 5–HT4(e) receptors [42]. Of these proteins, 10 interact specifically with the mouse 5–HT4(a) receptor splice variant. One of these is sorting nexin (SNX27) which is enriched in the brain and is involved in escorting the 5–HT4(a) receptor splice variant to early endosomes for desensitisation. Another protein, NHERF appears to be involved in directing the 5–HT4(a) receptor to the microvilli region where the two proteins are co-localised with another protein called ezrin [42] that interacts with the membrane phosphatidylinositol-(4,5)-bisphosphate [9]. The 5–HT4(b) splice variant does not contain a PDZ domain (Fig. 2A) and did not concentrate in the microvilli area. While the 5–HT4(e) receptor splice variant co-localised with CIPP which is a scaffolding protein and importantly this was not seen with the 5–HT4(b) receptor splice variant which does not contain a PDZ ligand [42]. These exciting results do indicate that the PDZ ligand domains of 5–HT4 or 5–HT7 receptor splice variants contribute to the receptor localisation and also suggest that rates of receptor desensitisation may vary with the splice variant expressed.
Differences in Desensitisation Rate
The diversity in C-terminus sequences probably also contributes to the well known pharmacological fact that 5–HT induces desensitisation (tachyphylaxis) at different rates and magnitudes in different tissues. This natural phenomenon functions to limit the biological response to endogenous substances, such as 5–HT by uncoupling the GPCR from its signal transduction pathway (for reviews, see [30,57]). Desensitisation generally begins with agonist-induced phosphorylation of the GPCR by GPCR receptor kinases (GRKs). The cytosolic proteins, arrestins then bind the phosphorylated GPCRs and prevent further coupling of that GPCR with G proteins and so reduce second messenger synthesis. The arrestin-GPCR complex is internalised by endocytosis where it can be recycled back to the membrane or degraded [30,57] (Fig. 4). GRKs play a critical role in GPCR desensitisation. There are three GRK subfamilies: rhodopsin kinase containing GRK1 and 7; β adrenergic receptor kinase (GRK2 and 3); and GRK4 group (GRK4, 5 and 6). Both GRK2 and GRK5 can phosphorylate many GPCRs including 5–HT4 receptor splice variants [10] and GRK2 in particular is crucial for embryonic cardiac development [57]. The role of GRKs and receptor desensitisation has been the focus of many studies but their effects on serotonin receptors are less well documented.
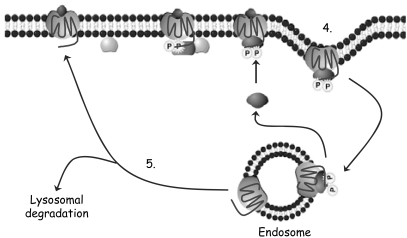
Fig. (4).
Process of GPCR desensitisation. (1). Activation and dissociation of G protein. (2). Gβγ recruits GPCR kinase (GRK) which phosphorylates the receptor. (3). Attachment of arrestin to GPCR (4). Internalisation of the arrestin – GPCR complex by endocytosis. (5). Dephosphorylated GPCR returns to the cell surface (resensitisation) or is degraded in the lysosome (down regulation).
Desensitisation also has the potential to lessen the beneficial effects of agonists when used as therapeutic treatments. Indeed, we have established that desensitisation of native 5–HT4 receptors occurs in vivo in the rat. We found that 5 day infusions of 5–HT (75 μg/kg per hour) induced rightward shifts of the 5–4 receptor-mediated concentration-effect curves to 5–HT and the partial 5–HT4 agonist, SC 53116, in the oesophagus [55]. Therefore, knowledge of the extent and mechanisms of desensitisation in human tissue is crucial to the success of drug discovery programs aimed at developing 5–HT receptor agonists. In spite of this, only two studies have investigated the desensitisation properties of h5–HT4 receptor splice variants (human c and d variants [60]; mouse a, b, e, and f variants [10]) in cell culture. On the other hand, the h5–HT7 receptor splice variants all mediate heterologous desensitisation, which seems to be induced by both the agonist 5–HT and some antagonists [45]. In addition, all h5–HT7 receptor splice variants mediate heterologous desensitisation of endogenous Gs-coupled receptors in HEK 293 cells through unknown mechanisms that are independent of cAMP dependent protein kinase activation [3,45].
CLINICAL RELEVANCE
5–HT4 Receptors
More than 50 patents have been lodged since 2000 covering potential clinical applications for 5–HT4 agonists (ISI Web of Knowledge, Derwent Innovations Index, (http://portal.isiknowledge.com/portal.cgi)). The claims are mainly for treatments of the digestive tract, notably irritable bowel syndrome (IBS), but also for gastroesophageal reflux disease (GORD), delayed gastric emptying and chronic constipation. The main rationale for these applications is that 5–HT4 receptors are distributed along the length of the digestive tract, where their localisation on cholinergic nerves functions to enhance the release of acetylcholine. Localisation to smooth muscle cells also affects muscle tone. In the oesophagus, 5–HT4 receptors are thought to be located in the presynaptic nerve terminals of cholinergic interneurons and motor neurons [16], as well as the muscularis mucosa of the rat oesophagus, where they mediate relaxation directly [11]. In the intestines, 5–HT4 receptor activation was originally shown to enhance acetylcholine released in isolated preparations of the guinea-pig ileum [25-27]. Patents also cover treatment of CNS disorders, such as Alzheimer's disease, anorexia, drug dependence, migraine and pain. As in the digestive tract, 5–HT4 receptor function in the brain is to enhance the release of acetylcholine, but they also modulate the release of dopamine, GABA and 5–HT [8], hence the interest in 5–HT4 agonists for treating cognitive disorders. Encouraging results have been obtained in rats, where the 5–HT4 agonist, RS 67333, improved associative memory and the 5–HT4 antagonist, RS 67532, decreased memory [52]. Their potential in the treatment of anorexia is suggested from experiments using obese mice which showed that the 5–HT4 agonist, mosepride, improved food intake [4]. None of the patents claim the novel 5–HT4 receptor agonists are splice variant-selective, however, there are patents covering the DNA of splice variants themselves. One suggests gene therapy using the nucleic acid sequence of the 5–HT4(h) splice variant for gastrointestinal diseases, while another the 5–HT4(d) splice variant for treating cardiac and bladder disorders. Reference to Table 1 shows there is potential for refining drug selectivity because the target organs for these conditions contain different combinations of splice variants. To date the digestive tract has received the greatest attention as an organ for treating various disorders using 5–HT4 agonists. Currently, these drugs are used to treat several types of motility disorders including gastro-oesophageal reflux disease (GORD). The most commonly used drugs were cisapride and metoclopramide, but cisapride has been virtually withdrawn (available on limited access) due to its ability to induce rare, but potentially fatal cardiac arrhythmias. Metoclopramide is a relatively old 5–HT4 agonist with significant affinity at other receptor types. The most selective 5–HT4 agonist to date is tegaserod, which is used to treat IBS. Consequently, the rational for using tegaserod for the treatment of IBS is outlined next.
Irritable Bowel Syndrome
It has been said that “A good set of bowels is worth more to a man than any quantity of brains” (Josh Billings, 1818-1885). Although this quotation was intended to be humorous there is some truth which is illustrated by the symptoms of the irritable bowel syndrome (IBS), which is a common functional bowel disorder, associated with abdominal pain, sensations of bloating and altered bowel habit. Much research effort has focused on the serotonin-modifying drugs to overcome dysfunction caused by perceived imbalance in either the amount of 5–HT released in the intestine or the expression of its receptors. This is because of the wealth of evidence showing that 5–HT alters the rate at which contents travel down the digestive tract and also the rate at which fluid is absorbed [31]. Consequently, 5–HT3 antagonists, 5–HT4 agonists and SSRI antidepressants have been the subject of intense investigation for the treatment of IBS [21,28]. For example, the 5–HT4 agonist, tegaserod, has been shown to reduce abdominal pain and give a degree of relief from other symptoms in patients with constipation-predominant IBS [61]. 5–HT4 receptors are present in several discrete tissue locations in the human colon. These include the mucosa where the response to 5–HT released by enterotoxins induces Cl- secretion resulting in diarrhoea [19]. The 5–HT4 receptor is also present in the circular smooth muscle cells of the human colon [39]. 5–HT4 agonists induce relaxation and inhibition of spontaneous contractions by activating adenylyl cyclase to increase intracellular levels of cAMP [53,54]. Paradoxically, 5–HT4 receptors expressed by cholinergic neurones in the human colon oppose the effect of this inhibitory postsynaptic 5–HT4 population by enhancing acetylcholine release [47]. The 5–HT receptors of cholinergic nerve endings also function to enhance transmitter release to the longitudinal muscle bands (taenia coli) [66]. Consequently, it has been suggested that the effects of 5–HT itself and 5–HT4 agonists to facilitate colonic propulsion are partly achieved by a coordinated combination of circular muscle relaxation and longitudinal muscle contraction [66]. Another less well established location of the 5–HT4 receptor in the human colon is on sensory nerve endings, where its function may be to increase sensory perceptions arising from the abdomen leading to altered motility patterns. A recent clinical study lends some support to this hypothesis, which showed that IBS patients have significantly lower perception and defecation thresholds to rectal thermal and pressure stimuli compared to age and gender matched control subjects [49]. The human small intestine and colon express various h5–HT4 splice variants (see Table 1, [13,15,56]). It is possible that different 5–HT4 receptor splice variants are expressed in the various locations and so contribute to the regulation of bowel functions in different ways. However, there is a lack of quantitative data for 5–HT4 receptor splice variant expression relative to the colon to substantiate such speculations.
5–HT7 Receptors
5–HT7 receptors have also been identified in the circular muscle of the human colon, but as yet not in nerves [39,65]. All 5–HT7 receptor splice variants are expressed in the human small intestine and colon including a certain amount of the h5–HT7(d+5) fragment [43].
The patent literature also covers the potential use of novel full and partial 5–HT7 receptor agonists. The proposed applications are for the treatments of depression, anxiety and eating disorders, correction of circadian rhythm and migraine. As with the 5–HT4 ligands, none are suggested to be splice variant-selective. These applications are well-founded given the widespread distribution of the 5–HT7 receptor in the CNS and accumulating evidence for its functional effects. This includes the findings that some antidepressants, such as amitriptyline, have relatively high affinities at the 5–HT7 receptor (see [48,50,73] for reviews). The 5–HT7 receptor was originally discovered in the CNS where particularly high expression was found in the thalamus and hypothalamus. Functional studies of these areas led to the suggestion that 5–HT7 receptors localised in the suprachiasmatic nuclei of the hypothalamus are involved in controlling circadian rhythm [51]. Although subsequent studies have generated some uncertainty, it has been established that the selective 5–HT7 receptor antagonist, SB-269970 reduces paradoxical sleep in rats [35,48]. It has also been suggested that the 5–HT7 receptor is involved in migraine (see [70] for review). Several lines of evidence support this, such as the findings that the receptor is expressed in cranial blood vessels of experimental animals and that 5–HT and 5–HT7-preferring agonists cause them to dilate. The transcript of the 5–HT7 receptor has been detected in human internal carotid and menigeal arteries, but functional evidence for its potential role in migraine is lacking. Other lines of evidence point to the involvement of the 5–HT7 receptor in hyperalgesic pain and neurogenic inflammation. The fact that prophylactic anti migraine 5–HT receptor antagonists have relatively high affinity for the 5–HT7 receptor further implicates this receptor in the abnormal vascular and neurogenic alterations that account for migraine headache.
CONCLUDING REMARKS
The intricate differences in structural forms of 5–HT4 and/or 5–HT7 splice variants suggest that they perform separate functions. This would have occurred over a long evolutionary period resulting in the refinement to the ancestral serotonin signalling system. Specific functions are also implied by the varying degrees of tissue-specific distribution of most splice variants and that such functions are important to the organs in which they are expressed (Table 1) and the disease state of the tissue. Refinement of function is conferred by the C terminal tails of the splice variants which allow them to interact with different GIPs, GRKs and even different G proteins. To date, the established net outcome of splice variant-specific interactions within the cell determine factors such as the rate, duration and intensity of the response to 5–HT.
Although it has been shown that some splice variants have different sensitivities to ligands, it has only been demonstrated with a small number of agonists. It remains a considerable challenge to identify and develop splice variant-selective drugs (no antagonists have yet been identified). However, the pressure to do so may increase as a result of the recent voluntary restriction of tegaserod to Special Access use only. This was necessary as IBS patients have been shown to experience a higher chance of cardiovascular events, such as heart attack, stroke, or severe heart-related chest pain. This predictable problem could be avoided if it were possible to discover an agonist with reasonable selectivity for a splice variant(s) present in the intestines, but not in the heart (e.g. 5–HT4(d or f)). Future drug discovery projects may also turn to the GIPs and GRKs in order to achieve tissue-specific effects.
REFERENCES
- 1.Adham N, Zgombick JM, Bard J, Branchek TA. Functional characterization of the recombinant human 5-hydroxytryptamine7(a) receptor isoform coupled to adenylate cyclase stimulation. J. Pharmacol. Exp. Ther. 1998;287:508–514. [PubMed] [Google Scholar]
- 2.Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels. Br. J. Pharmacol. (2nd edition) 2006;147 (Suppl. 3):S1–S180. doi: 10.1038/sj.bjp.0706651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andressen KW, Norum JH, Levy FO, Krobert KA. Activation of adenylyl cyclase by endogenous Gs-coupled receptors in human embryonic kidney 293 cells is attentuated by 5–HT7 receptor expression. Mol. Pharmacol. 2006;69:207–215. doi: 10.1124/mol.105.015396. [DOI] [PubMed] [Google Scholar]
- 4.Asakawa A, Ueno N, Katagi M, Ijuin Y, Morita Y, Mizuno S, Inui T, Sakamaki R, Shinfuku N, Uemoto M. Mosapride improves food intake, while not worsening glycemic control and obesity, in ob/ob obese mice with decreased gastric emptying. J. Diabetes Complicat. 2006;20:56–58. doi: 10.1016/j.jdiacomp.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Azmitia EC. Modern views on an ancient chemical: Serotonin effects on cell proliferation, maturation and apoptosis. Brain Res. Bull. 2001;56:413–424. doi: 10.1016/s0361-9230(01)00614-1. [DOI] [PubMed] [Google Scholar]
- 6.Bach T, Syversveen T, Kvingedal AM, Krobert KA, Brattelid T, Kaumann AJ, Levy FO. 5–HT4(a) and 5–HT4(b) receptors have nearly identical pharmacology and are both expressed in human atrium and ventricle. Naunyn Schmiedebergs Arch. Pharmacol. 2001;363:146–160. doi: 10.1007/s002100000299. [DOI] [PubMed] [Google Scholar]
- 7.Bard JA, Zgombick J, Adham N, Vaysse P, Branchek TA, Weinshank RL. Cloning of a novel human serotonin receptor (5–HT7) positively linked to adenylate cyclase. J. Biol. Chem. 1993;268:23422–23426. [PubMed] [Google Scholar]
- 8.Barnes NM, Sharpe T. A review of central 5–HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 9.Barret C, Roy C, Montcourrier P, Mangeat P, Niggli V. Mutagenesis of the phoshatidylinositol 4,5-bisphosphate (PIP2) binding site in the NH2-terminal domain of ezrin correlates with its altered cellular distribution. J. Cell Biol. 2000;151:1067–1080. doi: 10.1083/jcb.151.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barthet G, Gaven F, Framery B, Shinjo K, Nakamura T, Claeysen S, Bockaert J, Dumuis A. Uncoupling and endocytosis of 5-hydroxytryptamine 4 receptors Distinct molecular events with different GRK2 requirements. J. Biol. Chem. 2005;280:27924–27934. doi: 10.1074/jbc.M502272200. [DOI] [PubMed] [Google Scholar]
- 11.Baxter GS, Craig DA, Clarke DE. 5-Hydroxytryptamine4 receptors mediate relaxation of the rat oesophageal tunica muscularis mucosae. Naunyn Schmiedebergs Arch. Pharmacol. 1991;343:439–446. doi: 10.1007/BF00169544. [DOI] [PubMed] [Google Scholar]
- 12.Bécamel C, Gavarini S, Chanrion B, Alonso G, Galéotti N, Dumuis A, Bockaert J, Marin P. The serotonin 5–HT2A and 5–HT2C receptors interact with specific sets of PDZ proteins. J. Biol. Chem. 2004;279:20257–20266. doi: 10.1074/jbc.M312106200. [DOI] [PubMed] [Google Scholar]
- 13.Bender E, Pindon A, van Oers I, Zhang Y-B, Gommeren W, Verhasselt P, Jurzak M, Leysen J, Luyten W. Structure of the human serotonin 5–HT4 receptor gene and cloning of a novel 5–HT4 spice variant. J. Neurochem. 2000;74:478–489. doi: 10.1046/j.1471-4159.2000.740478.x. [DOI] [PubMed] [Google Scholar]
- 14.Blondel O, Vandecasteele G, Gastineau M, Leclerc S, Dahmoune Y, Langlois M, Fischmeister R. Molecular and functional characterisation of a 5–HT4 receptor cloned from human atrium. FEBS Lett. 1997;412:465–474. doi: 10.1016/s0014-5793(97)00820-x. [DOI] [PubMed] [Google Scholar]
- 15.Blondel O, Gastineau M, Dahmoune Y, Langlois M, Fischmeister R. Cloning, expression, and pharmacology of four human 5-hydroxytryptamine4 receptor isoforms produced by alternative splicing in the carboxyl terminus. J. Neurochem. 1998;70:2252–2261. doi: 10.1046/j.1471-4159.1998.70062252.x. [DOI] [PubMed] [Google Scholar]
- 16.Bockaert J, Fozard JR, Dumuis A, Clarke DE. The 5–HT4 receptor: a place in the sun. Trends Pharmacol. Sci. 1992;13:141–145. doi: 10.1016/0165-6147(92)90051-7. [DOI] [PubMed] [Google Scholar]
- 17.Bockaert J, Fagni L, Dumuis A, Marin P. GPCR interacting proteins (GIP) Pharmacol. Ther. 2004;102:203–211. doi: 10.1016/j.pharmthera.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Bockaert J, Claeysen S, Bécamel C, Dumuis A, Marin P. Neuronal 5–HT metabotrophic receptors fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 2006;326:553–572. doi: 10.1007/s00441-006-0286-1. [DOI] [PubMed] [Google Scholar]
- 19.Borman RA, Burleigh DE. Human colonic mucosa possesses a mixed population of 5–HT receptors. Eur. J. Pharmacol. 1996;309:271–274. doi: 10.1016/0014-2999(96)00466-9. [DOI] [PubMed] [Google Scholar]
- 20.Brattelid T, Kvingedal AM, Krobert KA, Andressen KW, Bach T, Hystad ME, Kaumann AJ, Levy FO. Cloning, pharmacological characterisation and tissue distribution of a novel 5–HT4 receptor splice variant, 5–HT4(i) Naunyn Schmiedebergs Arch. Pharmacol. 2004;369:616–628. doi: 10.1007/s00210-004-0919-4. [DOI] [PubMed] [Google Scholar]
- 21.Camilleri M. Treating irritable bowel syndrome: overview, perspective and future therapies. Br. J. Pharmacol. 2004;141:1237–1248. doi: 10.1038/sj.bjp.0705741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cartier D, Jégou S, Parmentier F, Lihrmann I, Louiset E, Kuhn J-M, Bastard C, Plouin P-F, Godin M, Vaudry H, Lefebvre H. Expression profile of serotonin4 (5–HT4) receptors in adrenocortical aldosteone-producing adenomas. Eur. J. Endocrinol. 2005;153:939–947. doi: 10.1530/eje.1.02051. [DOI] [PubMed] [Google Scholar]
- 23.Castro L, Mialet-Perez J, Guillemeau A, Stillitano F, Zolk O, Eschenhagen T, Lezoualc'h F, Bochet P, Fischmeister R. Differential functional effects of two 5–HT4 receptor isoforms in adult cardiomyocytes. J. Mol. Cell. Cardiol. 2005;39:335–344. doi: 10.1016/j.yjmcc.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Claeysen S, Sebben M, Becamel C, Bockaert J, Dumuis A. Novel brain-specific 5–HT4 receptor splice variants show marked constitutive activity: role of the C-terminal intracellular domain. Mol. Pharmacol. 1999;55:910–920. [PubMed] [Google Scholar]
- 25.Costall B, Naylor RJ, Tuladhar BR. 5–HT4 receptor mediated facilitation of the emptying phase of the peristaltic reflex in guinea-pig isolated ileum. Br. J. Pharmacol. 1993;110:1572–1578. doi: 10.1111/j.1476-5381.1993.tb14003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craig DA, Clarke D. EPharmacological characterisation for a neuronal receptor for 5-hydroxytryptamine in guinea-pig ileum with properties similar to the 5 HT4 receptor. J. Pharmacol. Exp. Ther. 1990;252:1378–1386. [PubMed] [Google Scholar]
- 27.Craig DA, Clarke DE. Peristalsis evoked by 5–HT and renzapride: evidence for a putative 5–HT4 receptor activation. Br. J. Pharmacol. 1991;102:563–564. doi: 10.1111/j.1476-5381.1991.tb12211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crowell DM. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br. J. Pharmacol. 2004;141:1285–1293. doi: 10.1038/sj.bjp.0705762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Ponti F. Pharmacology of serotonin what a clinician should know. Gut. 2004;53:1520–1535. doi: 10.1136/gut.2003.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferguson SSG. Evolving concepts in G protein-coupled receptor endocytosis the role in receptor desensitization and signalling. Pharmacol. Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 31.Gaginella TS, Galligan JJ. Serotonin and intestinal function. NewYork: CRC Press; 1995. [Google Scholar]
- 32.George SR, O'Dowd BF, Lee SP. G-protein-coupled receptor oligomerization and its potential for drug discovery. Nat. Rev. Drug Discov. 2002;1:808–820. doi: 10.1038/nrd913. [DOI] [PubMed] [Google Scholar]
- 33.Glennon RA. Higher-end serotonin receptors 5–HT5, 5–HT6 and 5–HT7. J. Med. Chem. 2003;46:2795–2812. doi: 10.1021/jm030030n. [DOI] [PubMed] [Google Scholar]
- 34.Guthrie CR, Murray AT, Franklin AA, Hamblin MW. Differential agonist-mediated internalization of the human 5-hydroxytryptamine 7 receptor isoforms. J. Pharmacol. Exp. Ther. 2005;313:1003–1010. doi: 10.1124/jpet.104.081919. [DOI] [PubMed] [Google Scholar]
- 35.Hagan JJ, Price GW, Jeffrey P, Deeks NJ, Stean T, Piper D, Smith MI, Upton N, Medhurst AD, Middlemiss DN, Riley GJ, Lovell PJ, Bromidge SM, Thomas DR. Characterization of SB-269970-A, a selective 5–HT(7) receptor antagonist. Br. J. Pharmacol. 2000;130:539–548. doi: 10.1038/sj.bjp.0703357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heidmann DE, Metcalf MA, Kohen R, Hamblin MW. Four 5-hydroxytryptamine7 (5–HT7) receptor isoforms in human and rat produced by alternative splicing species differences due to altered intron-exon organization. J. Neurochem. 1997;68:1372–1381. doi: 10.1046/j.1471-4159.1997.68041372.x. [DOI] [PubMed] [Google Scholar]
- 37.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol. Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 38.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5–HT receptors. Pharmacol. Biochem. Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 39.Irving HR, Tan YY, Tochon-Danguy N, Liu H, Chetty N, Desmond PV, Pouton CW, Coupar IM. Comparison of 5–HT4 and 5–HT7 receptor expression and function in the circular muscle of the human colon. Life Sci. 2007;80:1198–1205. doi: 10.1016/j.lfs.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 40.IUPHAR (2005) IUPHAR receptor database. http://www.iuphar-db.org/ GPCR/index.html . 2006 accessed November.
- 41.Johnson JM, Castle J, Garret-Engele P, Kan Z, Loerch PM, Armour CD, Santos R, Schadt EE, Stoughton R, Shoemaker DD. Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays. Science. 2003;302:2141–2144. doi: 10.1126/science.1090100. [DOI] [PubMed] [Google Scholar]
- 42.Joubert L, Hanson B, Barthet G, Sebben M, Claeysen S, Hong W, Marin P, Dumuis A, Bockaert J. New sorting nexin (SNX27) and NHERF specifically interact with the 5–HT4(a) receptor splice variant roles in receptor targeting. J. Cell Sci. 2004;117:5367–5379. doi: 10.1242/jcs.01379. [DOI] [PubMed] [Google Scholar]
- 43.Krobert KA, Bach T, Syversveen T, Kvingedal AM, Levy FO. The cloned human 5–HT7 receptor splice variants a comparative characterization of their pharmacology, function and distribution. Naunyn Schmiedebergs Arch. Pharmacol. 2001;363:620–632. doi: 10.1007/s002100000369. [DOI] [PubMed] [Google Scholar]
- 44.Krobert KA, Levy FO. The human 5HT7 serotonin receptor splice variants: constitutive activity and inverse agonist effects. Br. J. Pharmacol. 2002;135:1563–1571. doi: 10.1038/sj.bjp.0704588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krobert KA, Andressen KW, Levy FO. Heterologous desensitization is evoked by both agonist and antagonist stimulation of the human 5–HT7 serotonin receptor. Eur. J. Pharmacol. 2006;532:1–10. doi: 10.1016/j.ejphar.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 46.Langlois M, Fischmeister R. 5–HT4 receptor ligands: applications and new prospects. J. Med. Chem. 2003;46:319–344. doi: 10.1021/jm020099f. [DOI] [PubMed] [Google Scholar]
- 47.Leclere PG, Prins NH, Schuurkes JAJ, Lefebvre RA. 5–HT4 receptors located on cholinergic nerves in human colon circular muscle. Neurogastroenterol. Motil. 2005;17:366–375. doi: 10.1111/j.1365-2982.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 48.Leopoldo M. Serotonin7 receptors (5–HT7Rs) and their ligands. Curr. Med. Chem. 2004;11:629–661. doi: 10.2174/0929867043455828. [DOI] [PubMed] [Google Scholar]
- 49.Li Y, Wang Y, Zuo X, Guo Y, Zhang H, Lu X, Li J, Desmond PV. Visceral perception thresholds after rectal thermal and pressure stimuli in irritable bowel syndrome patients. J. Gastroenterol. Hepatol. 2004;19:187–191. doi: 10.1111/j.1440-1746.2004.03225.x. [DOI] [PubMed] [Google Scholar]
- 50.Liu H, Coupar IM, Irving HR. Effects of some antidepressant drugs on tryptaminergic responses of the rat jejunum. Pharmacology. 2003;69:88–92. doi: 10.1159/000072361. [DOI] [PubMed] [Google Scholar]
- 51.Lovenberg TW, Baron BM, de Lecea L, Miller JD, Prosser RA, Rea MA, Foye PE, Racke M, Slone AL, Siegel BW, Danielson PE, Sutcliffe JG, Erlander MG. A novel adenylyl cyclase-activating serotonin receptor (5–HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993;11:449–458. doi: 10.1016/0896-6273(93)90149-l. [DOI] [PubMed] [Google Scholar]
- 52.Marchetti E, Dumuis A, Bockaert J, Soumireu-Mourat B, Roman FS. Differential modulation of the 5–HT(4) receptor agonists and antagonist on rat learning and memory. Neuropharmacology. 2000;39:2017–2027. doi: 10.1016/s0028-3908(00)00038-1. [DOI] [PubMed] [Google Scholar]
- 53.McLean PG, Coupar IM, Molenaar P. A comparative study of functional 5–HT4 receptors in human colon, rat ileum and rat oesophagus. Br. J. Pharmacol. 1995;115:47–56. doi: 10.1111/j.1476-5381.1995.tb16318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McLean PG, Coupar IM. Further investigation into the signal transduction mechanism of the 5–HT4-like receptor in the smooth muscle of human colon. Br. J. Pharmacol. 1996; 118:1058–1064. doi: 10.1111/j.1476-5381.1996.tb15506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McLean PG, Coupar IM, Molenaar P. Changes in sensitivity of 5–HT receptor mediated functional responses in the rat oesophagus, fundus and jejunum following chronic infusion with 5-hydroxytrypamine. Naunyn Schmiedebergs Arch. Pharmacol. 1996;354:513–519. doi: 10.1007/BF00168444. [DOI] [PubMed] [Google Scholar]
- 56.Medhurst AD, Lezoulac'h F, Fischmeister R, Middlemiss DN, Sanger GJ. Quantitative mRNA analysis of five C-terminal splice variants of the human 5–HT4 receptor in the central nervous system by TaqMan real time RT-PCR. Mol. Brain Res. 2001;90:125–134. doi: 10.1016/s0169-328x(01)00095-x. [DOI] [PubMed] [Google Scholar]
- 57.Métayé T, Gibelin H, Perdrisot R, Kraimps J-L. Pathophysiological roles of G-protein-coupled receptor kinases. Cell Signal. 2005;17:917–928. doi: 10.1016/j.cellsig.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 58.Mialet J, Berque-Bestel I, Eftekhari P, Gastineau M, Giner M, Dahmoune Y, Donzeau-Gouge P, Hoebeke J, Langlois M, Sicsic S, Fischmeister R, Lezoualc'h F. Isolation of the serotoninergic 5–HT4(e) receptor from human heart and comparative analysis of its pharmacological profile in C6-glial and CHO cell lines. Br. J. Pharmacol. 2000;129:771–781. doi: 10.1038/sj.bjp.0703101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mialet J, Berque-Bestel I, Sicsic S, Langlois M, Fischmeister R, Lezoualc'h F. Pharmacological characterization of the human 5–HT4(d) receptor splice variant stably expressed in Chinese hamster ovary cells. Br. J. Pharmacol. 2000;131:827–835. doi: 10.1038/sj.bjp.0703641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mialet J, Fischmeister R, Lezoualc'h F. Characterisation of human 5–HT4(d) receptor desensitization in CHO cells. Br. J. Pharmacol. 2003;138:445–452. doi: 10.1038/sj.bjp.0705061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Müller-Lissner SA, Fumagalli I, Bardhan KD, Pace F, Pecher E, Nault B, Rüegg P. Tegaserod, a 5–HT4 receptor partial agonist, relieves symptoms in irritable bowel syndrome patients with abdominal pain, bloating and constipation. Aliment. Pharmacol. Ther. 2001;15:1655–1666. doi: 10.1046/j.1365-2036.2001.01094.x. [DOI] [PubMed] [Google Scholar]
- 62.Nourry C, Grant SGN, Borg J-P. PDZ domain proteins plug and play! . Sci.STKE. 2003;179 doi: 10.1126/stke.2003.179.re7. [DOI] [PubMed] [Google Scholar]
- 63.Pindon A, Van Hecke G, Van Gompel P, Lesage AS, Leysen JE, Jurzak M. Differences in signal transduction of two 5–HT4 receptor splice variants compound specificity and dual coupling with Gαs- and Gαi/o-proteins. Mol. Pharmacol. 2002;61:85–96. doi: 10.1124/mol.61.1.85. [DOI] [PubMed] [Google Scholar]
- 64.Pindon A, Van Hecke G, Josson K, Van Gompel P, Lesage A, Leysen JE, Jurzak M. Internalization of human 5–HT4a and 5–HT4b receptors is splice variant dependent. Biosci. Rep. 2004;24:215–223. doi: 10.1007/s10540-005-2582-5. [DOI] [PubMed] [Google Scholar]
- 65.Prins NH, Briejer MR, Van Bergen PJ, Akkermans LM, Schuurkes JA. Evidence for 5–HT7 receptors mediating relaxation of human colonic circular smooth muscle. Br. J. Pharmacol. 1999;128:849–852. doi: 10.1038/sj.bjp.0702762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prins NH, Akkermans LMA, Lefebvre RA, Schuurkes JAJ. 5–HT4 receptors on cholinergic nerves involved in contractility of canine and human large intestine longitudinal muscle. Br. J. Pharmacol. 2000;131:927–932. doi: 10.1038/sj.bjp.0703615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sanders-Bush E, Fentress H, Hazelwood L. Serotonin 5–HT2 receptors molecular and genomic diversity. Mol. Interv. 2003;3:319–330. doi: 10.1124/mi.3.6.319. [DOI] [PubMed] [Google Scholar]
- 68.Sanders-Bush E, Mayer SE. 5-Hydroxytryptamine (serotonin) receptor agonists and antagonists. In: Branton LL, Lazo JS, Parker KL, editors. The Pharmacological Basis of Therapeutics . Goodman &, Gilman's McGaw-Hill; 2006. pp. 297–315. [Google Scholar]
- 69.Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, Vaugeois J-M, Nomikos GG, Greengard P. Alterations in 5–HT1B receptor function by p11 in depression-like states. Science. 2006;311:77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- 70.Terrón JA. Is the 5–HT7 receptor involved in the pathogenesis and prophylactic treatment of migraine? Eur. J. Pharmacol. 2002;439:1–11. doi: 10.1016/s0014-2999(02)01436-x. [DOI] [PubMed] [Google Scholar]
- 71.Tierney AJ. Structure and function of invertebrate 5–HT receptors a review. Comp. Biochem. Physiol. Part A. 2001;128:791–804. doi: 10.1016/s1095-6433(00)00320-2. [DOI] [PubMed] [Google Scholar]
- 72.Van den Wyngaert I, Gommeren W, Verhasselt P, Jurzak M, Leysen J, Luyten W, Bender E. Cloning and expression of a human serotonin 5–HT4 receptor cDNA. J. Neurochem. 1997;69:1810–1819. doi: 10.1046/j.1471-4159.1997.69051810.x. [DOI] [PubMed] [Google Scholar]
- 73.Vanhoenacker P, Haegeman G, Leysen JE. 5–HT7 receptors: current knowledge and future prospects. Trends Pharmacol. Sci. 2000;21:70–77. doi: 10.1016/s0165-6147(99)01432-7. [DOI] [PubMed] [Google Scholar]
- 74.Vilaró MT, Doménech T, Palacios JM, Mengod G. Cloning and characterization of a novel human 5–HT4 receptor variant that lacks the alternatively spiced carboxy terminal exon. RT-PCR distribution in human brain and periphery of multiple 5–HT4 receptor variants. Neuropharmacology. 2002;42:60–73. doi: 10.1016/s0028-3908(01)00154-x. [DOI] [PubMed] [Google Scholar]
- 75.Xia Z, Gray JA, Compton-Toth BA, Roth BL. A direct interaction of PSD-95 with 5–HT2A serotonin receptors regulates receptor trafficking and signal transduction. J. Biol. Chem. 2003;278:21901–21908. doi: 10.1074/jbc.M301905200. [DOI] [PubMed] [Google Scholar]