Abstract
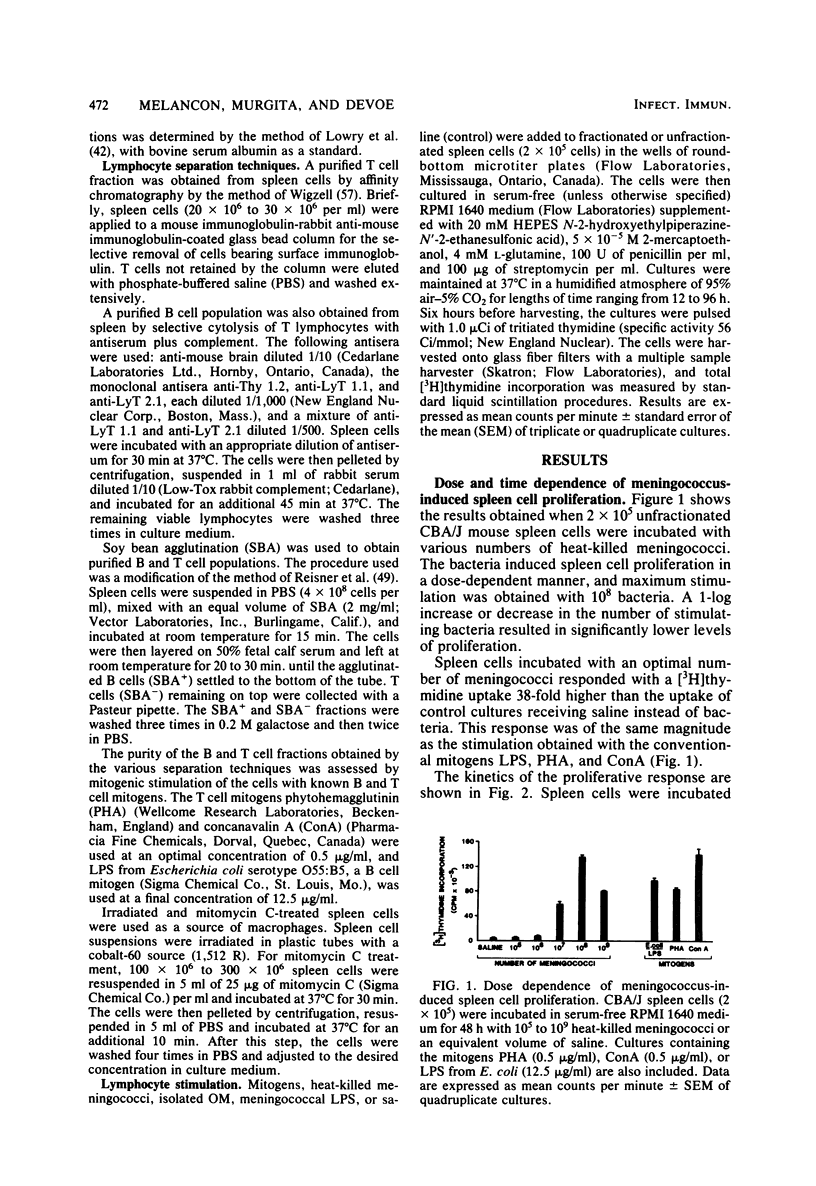
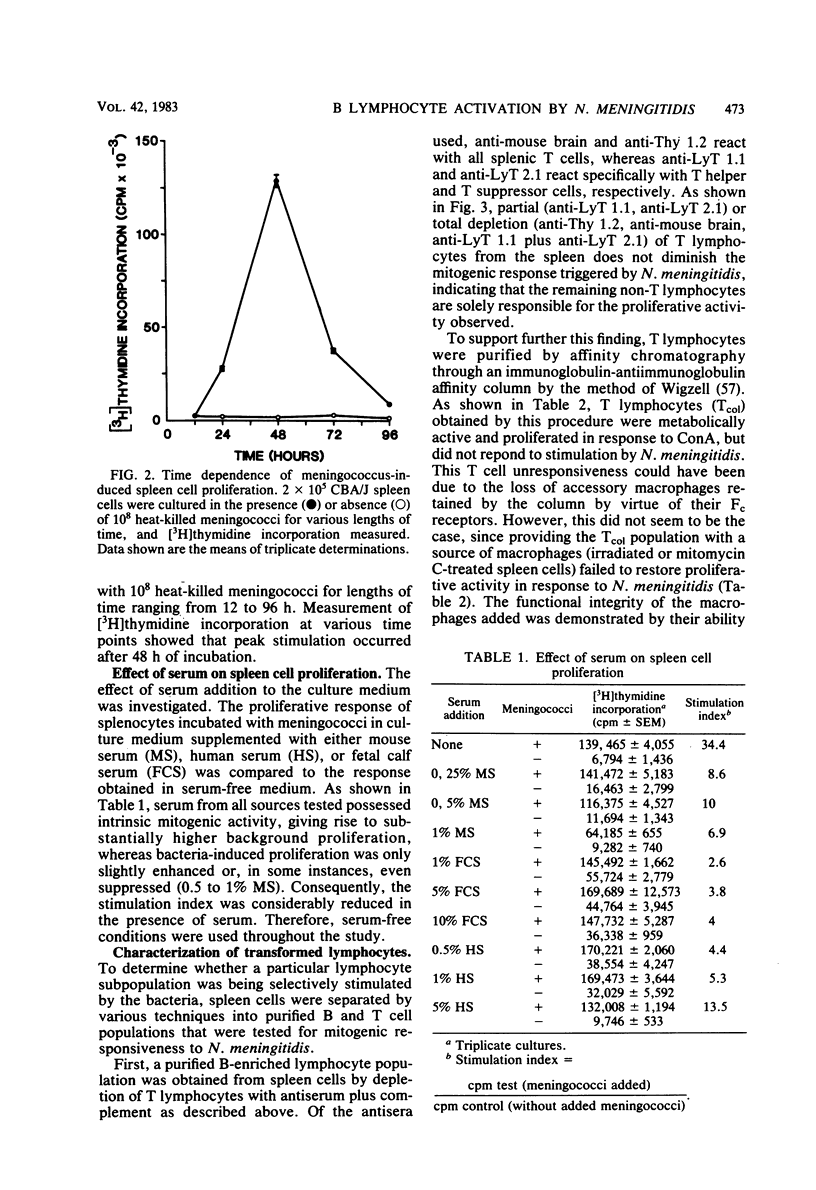
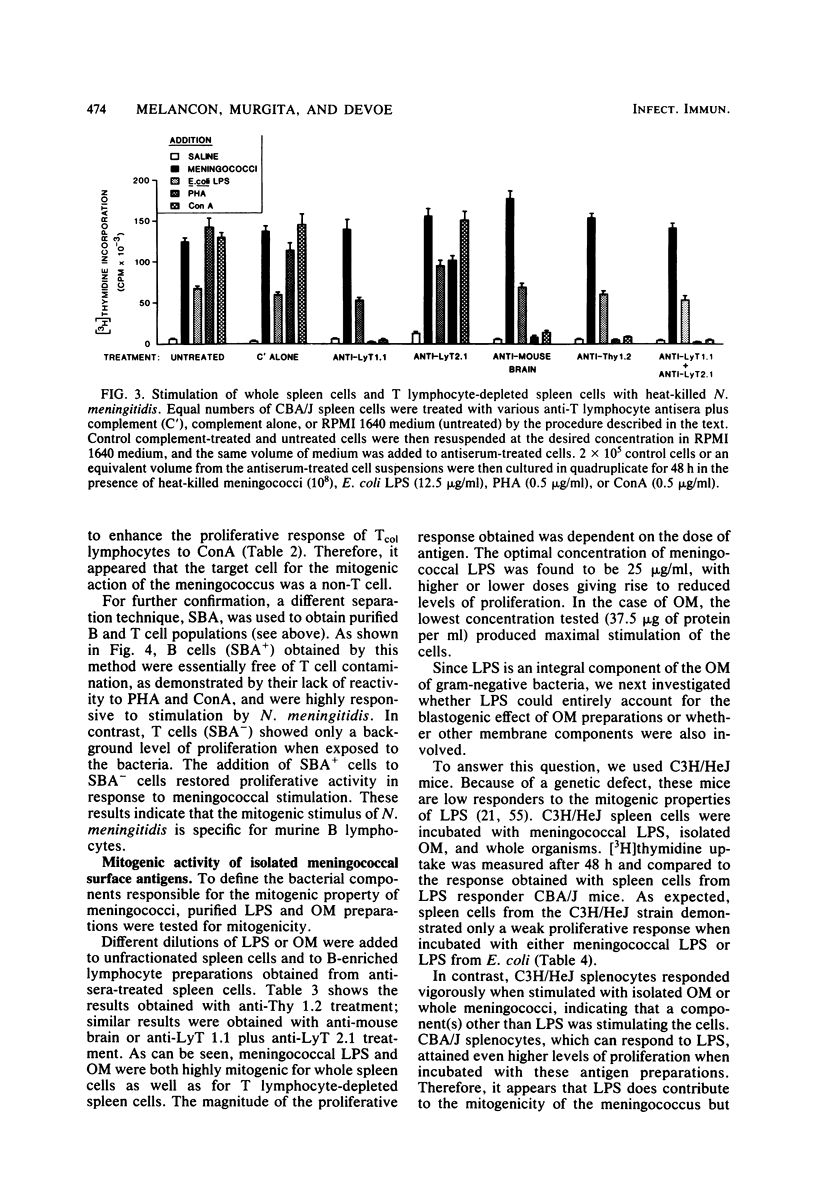
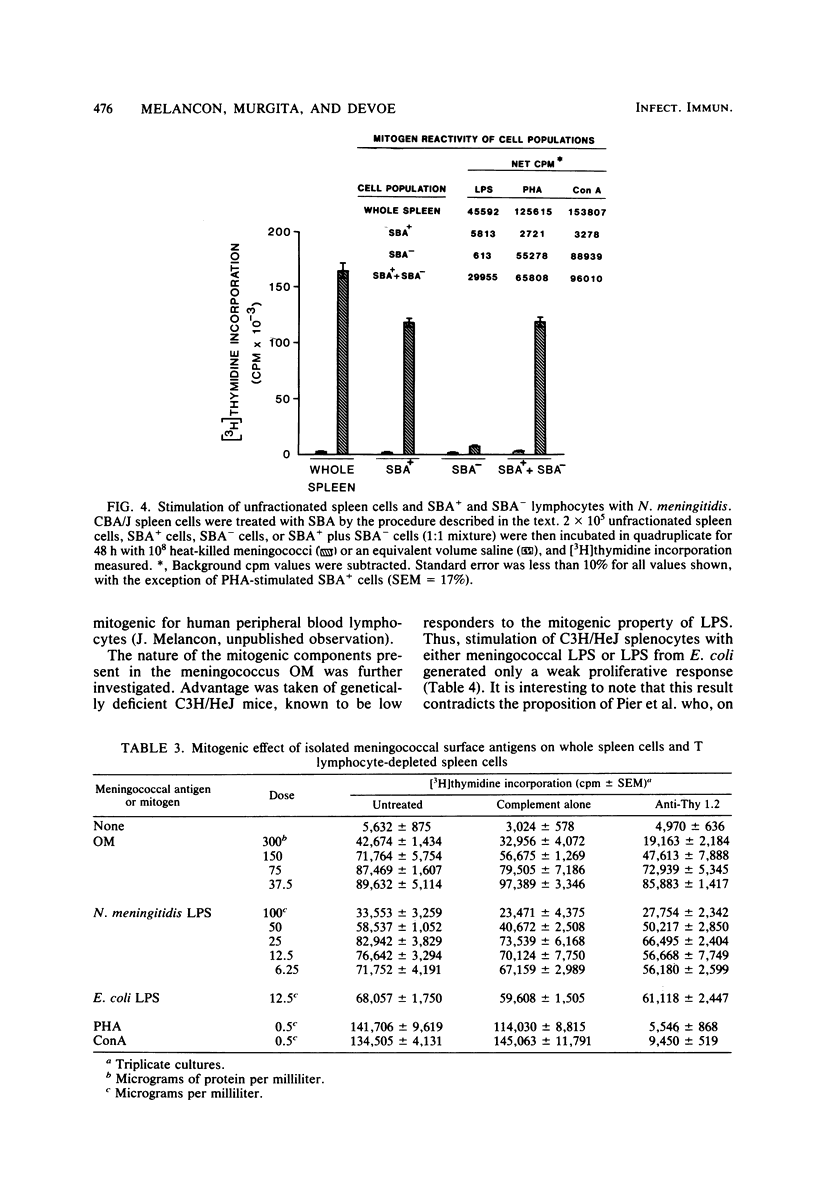
Heat-killed Neisseria meningitidis was found to be a potent mitogen for mouse splenic lymphocytes. Results obtained with different cell separation techniques indicated that the bacteria acted to selectively induce proliferation of B lymphocytes. First, partial or total depletion of T lymphocytes by treatment with various anti-T-cell antisera plus complement did not affect the ability of the remaining spleen cells to proliferate in response to N. meningitidis. Second, T lymphocytes purified by affinity chromatography through an immunoglobulin-antiimmunoglobulin-coated glass bead column were unresponsive to meningococcal stimulation, even when provided with a source of macrophages (irradiated or mitomycin C-treated spleen cells). Finally, treatment of spleen cells with soy bean agglutinin showed that, whereas the soy bean agglutinin-positive population (B-enriched lymphocytes) was highly responsive to stimulation by N. meningitidis, the soy bean agglutinin-negative population (T-enriched lymphocytes) displayed only a background level of proliferation when exposed to the bacteria. Isolated meningococcal surface antigens such as lipopolysaccharide (LPS) and outer membranes also possessed mitogenic activity and induced proliferation of B lymphocytes in a dose-dependent manner. Both LPS and non-LPS components contributed to the mitogenicity of outer membranes since the addition of outer membrane preparations to spleen cells from the low LPS responder C3H/HeJ mouse strain gave rise to a high level of proliferative activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen V., Hansen N. E., Karle H., Lind I., Hoiby N., Weeke B. Sequential studies of lymphocyte responsiveness and antibody formation in acute bacterial meningitis. Clin Exp Immunol. 1976 Dec;26(3):469–477. [PMC free article] [PubMed] [Google Scholar]
- Artenstein M. S., Branche W. C., Jr, Zimmerly J. G., Cohen R. L., Tramont E. C., Kasper D. L., Harkins C. Meningococcal infections. 3. Studies of group A polysaccharide vaccines. Bull World Health Organ. 1971;45(3):283–286. [PMC free article] [PubMed] [Google Scholar]
- Artenstein M. S., Gold R., Zimmerly J. G., Wyle F. A., Schneider H., Harkins C. Prevention of meningococcal disease by group C polysaccharide vaccine. N Engl J Med. 1970 Feb 19;282(8):417–420. doi: 10.1056/NEJM197002192820803. [DOI] [PubMed] [Google Scholar]
- Artenstein M. S. Meningococcal infections. 5. Duration of polysaccharide-vaccine-induced antibody. Bull World Health Organ. 1971;45(3):291–293. [PMC free article] [PubMed] [Google Scholar]
- BENOIT F. L. CHRONIC MENINGOCOCCEMIA. CASE REPORT AND REVIEW OF THE LITERATURE. Am J Med. 1963 Jul;35:103–112. doi: 10.1016/0002-9343(63)90167-0. [DOI] [PubMed] [Google Scholar]
- Beuvery E. C., Leussink A. B., Van Delft R. W., Tiesjema R. H., Nagel J. Immunoglobulin M and G antibody responses and persistence of these antibodies in adults after vaccination with a combined meningococcal group A and group C polysaccharide vaccine. Infect Immun. 1982 Aug;37(2):579–585. doi: 10.1128/iai.37.2.579-585.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuvery E. C., van Rossum F., Nagel J. Comparison of the induction of immunoglobulin M and G antibodies in mice with purified pneumococcal type 3 and meningococcal group C polysaccharides and their protein conjugates. Infect Immun. 1982 Jul;37(1):15–22. doi: 10.1128/iai.37.1.15-22.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaillon J. G., McSween G., Portelance V., Kluepfel D., Micusan V. V. Evaluation of the immunogenicity of a CaCl2 extract from Neisseria meningitidis group Y. Can J Microbiol. 1982 Sep;28(9):1022–1031. doi: 10.1139/m82-153. [DOI] [PubMed] [Google Scholar]
- Brandt B. L., Artenstein M. S. Duration of antibody responses after vaccination with group C Neisseria meningitidis polysaccharide. J Infect Dis. 1975 May;131 (Suppl):S69–S72. doi: 10.1093/infdis/131.supplement.s69. [DOI] [PubMed] [Google Scholar]
- Brandt B. L., Artenstein M. S., Smith C. D. Antibody responses to meningococcal polysaccharide vaccines. Infect Immun. 1973 Oct;8(4):590–596. doi: 10.1128/iai.8.4.590-596.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur B. R., Johnson W. M., Diena B. B., Visentin L. P., Johnson K. G. Mitogenic activity of Neisseria gonorrhoeae surface antigens in mouse splenic lymphocyte culture. Can J Microbiol. 1977 Sep;23(9):1154–1160. doi: 10.1139/m77-173. [DOI] [PubMed] [Google Scholar]
- Craven D. E., Frasch C. E. Protection against group B meningococcal disease: evaluation of serotype 2 protein vaccines in a mouse bacteremia model. Infect Immun. 1979 Oct;26(1):110–117. doi: 10.1128/iai.26.1.110-117.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. A., Peters N., Mohammed I., Major G. A., Holborow E. J. Circulating immune complexes in a patient with meningococcal disease. Br Med J. 1976 Jun 12;1(6023):1445–1446. doi: 10.1136/bmj.1.6023.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoe I. W., Gilchrist J. E. Release of endotoxin in the form of cell wall blebs during in vitro growth of Neisseria meningitidis. J Exp Med. 1973 Nov 1;138(5):1156–1167. doi: 10.1084/jem.138.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziarski R. Preferential induction of autoantibody secretion in polyclonal activation by peptidoglycan and lipopolysaccharide. II. In vivo studies. J Immunol. 1982 Mar;128(3):1026–1030. [PubMed] [Google Scholar]
- Farquhar J. D., Hankins W. A., DeSanctis A. N., DeMeio J. L., Metzgar D. P. Clinical and serological evaluation of purified polysaccharide vaccines prepared from Neisseria meningitidis group Y. Proc Soc Exp Biol Med. 1977 Sep;155(4):453–455. doi: 10.3181/00379727-155-39828. [DOI] [PubMed] [Google Scholar]
- Frasch C. E., Peppler M. S. Protection against group B Neisseria meningitidis disease: preparation of soluble protein and protein-polysaccharide immunogens. Infect Immun. 1982 Jul;37(1):271–280. doi: 10.1128/iai.37.1.271-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Robbins J. D. Protection against group B meningococcal disease. III. Immunogenicity of serotype 2 vaccines and specificity of protection in a guinea pig model. J Exp Med. 1978 Mar 1;147(3):629–644. doi: 10.1084/jem.147.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E. Role of protein serotype antigens in protection against disease due to Neisseria meningitidis. J Infect Dis. 1977 Aug;136 (Suppl):S84–S90. doi: 10.1093/infdis/136.supplement.s84. [DOI] [PubMed] [Google Scholar]
- Garzelli C., Colizzi V., Campa M., Bozzi L., Falcone G. Depression of the antibody response in Pseudomonas aeruginosa-injected mice. Infect Immun. 1979 Apr;24(1):32–38. doi: 10.1128/iai.24.1.32-38.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glode L. M., Rosenstreich D. L. Genetic control of B cell activation by bacterial lipopolysaccharide is mediated by multiple distinct genes or alleles. J Immunol. 1976 Dec;117(6):2061–2066. [PubMed] [Google Scholar]
- Gold R., Artenstein M. S. Meningococcal infections. 2. Field trial of group C meningococcal polysaccharide vaccine in 1969-70. Bull World Health Organ. 1971;45(3):279–282. [PMC free article] [PubMed] [Google Scholar]
- Gold R., Lepow M. L., Goldschneider I., Draper T. F., Gotschlich E. C. Antibody responses of human infants to three doses of group A Neisseria meningitidis polysaccharide vaccine administered at two, four, and six months of age. J Infect Dis. 1978 Dec;138(6):731–735. doi: 10.1093/infdis/138.6.731. [DOI] [PubMed] [Google Scholar]
- Gold R., Lepow M. L., Goldschneider I., Gotschlich E. C. Immune Response of human infants of polysaccharide vaccines of group A and C Neisseria meningitidis. J Infect Dis. 1977 Aug;136 (Suppl):S31–S35. doi: 10.1093/infdis/136.supplement.s31. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969 Jun 1;129(6):1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman G. W., Sultzer B. M. Endotoxin protein is a mitogen and polyclonal activator of human B lymphocytes. J Exp Med. 1979 Mar 1;149(3):713–723. doi: 10.1084/jem.149.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Goldschneider I., Artenstein M. S. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J Exp Med. 1969 Jun 1;129(6):1367–1384. doi: 10.1084/jem.129.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Goldschneider I., Artenstein M. S. Human immunity to the meningococcus. V. The effect of immunization with meningococcal group C polysaccharide on the carrier state. J Exp Med. 1969 Jun 1;129(6):1385–1395. doi: 10.1084/jem.129.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood B. M., Oduloju A. J., Ade-Serrano M. A. Cellular immunity in patients with meningococcal disease and in vaccinated subjects. Clin Exp Immunol. 1979 Oct;38(1):9–15. [PMC free article] [PubMed] [Google Scholar]
- Greenwood B. M., Onyewotu I. I., Whittle H. C. Complement and meningococcal infection. Br Med J. 1976 Apr 3;1(6013):797–799. doi: 10.1136/bmj.1.6013.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood B. M., Whittle H. C., Bryceson A. D. Allergic complications of meningococcal disease. II. Immunological investigations. Br Med J. 1973 Jun 30;2(5869):737–740. doi: 10.1136/bmj.2.5869.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiss J. M. Bactericidal activity of meningococcal antisera. Blocking by IgA of lytic antibody in human convalescent sera. J Immunol. 1975 Jun;114(6):1779–1784. [PubMed] [Google Scholar]
- Griffiss J. M., Bertram M. A. Immunoepidemiology of meningococcal disease in military recruits. II. Blocking of serum bactericidal activity by circulating IgA early in the course of invasive disease. J Infect Dis. 1977 Dec;136(6):733–739. doi: 10.1093/infdis/136.6.733. [DOI] [PubMed] [Google Scholar]
- Griffiss J. M., Brandt B. L., Broud D. D. Human immune response to various doses of group Y and W135 meningococcal polysaccharide vaccines. Infect Immun. 1982 Jul;37(1):205–208. doi: 10.1128/iai.37.1.205-208.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. C., Weiss E. Protein fraction with immunogenic potential and low toxicity isolated from the cell wall of Neisseria meningitidis group B. Infect Immun. 1974 Sep;10(3):605–615. doi: 10.1128/iai.10.3.605-615.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs J. R., Milner R. D., Watt P. J. Gamma-M deficiency predisposing to meningococcal septicaemia. Br Med J. 1967 Dec 9;4(5579):583–586. doi: 10.1136/bmj.4.5579.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izui S., Zaldivar N. M., Scher I., Lambert P. H. Mechanism for induction of anti-DNA antibodies by bacterial lipopolysaccharides in mice. I. Anti-DNA induction by LPS without significant release of DNA in circulating blood. J Immunol. 1977 Dec;119(6):2151–2156. [PubMed] [Google Scholar]
- Kasper D. L., Winkelhake J. L., Zollinger W. D., Brandt B. L., Artenstein M. S. Immunochemical similarity between polysaccharide antigens of Escherichia coli 07: K1(L):NM and group B Neisseria meningitidis. J Immunol. 1973 Jan;110(1):262–268. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larson H. E., Nicholson K. G., Loewi G., Tyrrell D. A., Posner J. Arthritis after meningococcal meningitis. Br Med J. 1977 Mar 5;1(6061):618–618. doi: 10.1136/bmj.1.6061.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell G. H., Smith L. F., Artenstein M. S., Nash G. S., MacDermott R. P., Jr Antibody-dependent cell-mediated antibacterial activity of human mononuclear cells. I. K lymphocytes and monocytes are effective against meningococi in cooperation with human imune sera. J Exp Med. 1979 Jul 1;150(1):127–137. doi: 10.1084/jem.150.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell G. H., Smith L. F., Griffiss J. M., Brandt B. L., MacDermott R. P. Antibody-dependent mononuclear cell-mediated antimeningococcal activity. Comparison of the effects of convalescent and postimmunization immunoglobulins G, M, and A. J Clin Invest. 1980 Aug;66(2):260–267. doi: 10.1172/JCI109852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers F., Braun V., Galanos C. The lipoprotein of the outer membrane of Escherichia coli: a B-lymphocyte mitogen. J Exp Med. 1975 Aug 1;142(2):473–482. doi: 10.1084/jem.142.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä P. H., Peltola H., Käyhty H., Jousimies H., Pettay O., Ruoslahti E., Sivonen A., Renkonen O. V. Polysaccharide vaccines of group A Neisseria meningtitidis and Haemophilus influenzae type b: a field trial in Finland. J Infect Dis. 1977 Aug;136 (Suppl):S43–S50. doi: 10.1093/infdis/136.supplement.s43. [DOI] [PubMed] [Google Scholar]
- Otokunefor T. V., Galsworthy S. B. Immunosuppression, nonspecific B-cell activation, and mitogenic activity associated with a high molecular weight component from Listeria monocytogenes. Can J Microbiol. 1982 Dec;28(12):1373–1381. doi: 10.1139/m82-204. [DOI] [PubMed] [Google Scholar]
- Peltola H., Mäkelä H., Käyhty H., Jousimies H., Herva E., Hällström K., Sivonen A., Renkonen O. V., Pettay O., Karanko V. Clinical efficacy of meningococcus group A capsular polysaccharide vaccine in children three months to five years of age. N Engl J Med. 1977 Sep 29;297(13):686–691. doi: 10.1056/NEJM197709292971302. [DOI] [PubMed] [Google Scholar]
- Peppler M. S., Frasch C. E. Protection against group B Neisseria meningitidis disease: effect of serogroup B polysaccharide and polymyxin B on immunogenicity of serotype protein preparations. Infect Immun. 1982 Jul;37(1):264–270. doi: 10.1128/iai.37.1.264-270.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Markham R. B., Eardley D. Correlation of the biologic responses of C3H/HEJ mice to endotoxin with the chemical and structural properties of the lipopolysaccharides from Pseudomonas aeruginosa and Escherichia coli. J Immunol. 1981 Jul;127(1):184–191. [PubMed] [Google Scholar]
- Reisner Y., Ravid A., Sharon N. Use of soybean agglutinin for the separation of mouse B and T lymphocytes. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1585–1591. doi: 10.1016/s0006-291x(76)80195-7. [DOI] [PubMed] [Google Scholar]
- Rosenthal L., Möller E. Specific and non-specific activation of human and mouse lymphocytes in vitro by pathogenic and apathogenic Neisseria strains. Scand J Immunol. 1978 Apr;7(4):321–329. doi: 10.1111/j.1365-3083.1978.tb00460.x. [DOI] [PubMed] [Google Scholar]
- Schaad U. B. Arthritis in disease due to Neisseria meningitidis. Rev Infect Dis. 1980 Nov-Dec;2(6):880–888. doi: 10.1093/clinids/2.6.880. [DOI] [PubMed] [Google Scholar]
- Simonson C., Trivett T., DeVoe I. W. Energy-independent uptake of iron from citrate by isolated outer membranes of Neisseria meningitidis. Infect Immun. 1981 Feb;31(2):547–553. doi: 10.1128/iai.31.2.547-553.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M. Infection with Bacillus Calmette-Guérin activates murine thymus-independent (B) lymphocytes. J Immunol. 1978 Jan;120(1):254–261. [PubMed] [Google Scholar]
- Watson J., Kelly K., Largen M., Taylor B. A. The genetic mapping of a defective LPS response gene in C3H/HeJ mice. J Immunol. 1978 Feb;120(2):422–424. [PubMed] [Google Scholar]
- Whittle H. C., Abdullahi M. T., Fakunle F. A., Greenwood B. M., Bryceson A. D., Parry E. H., Turk J. L. Allertic complications of meningococcal disease. I. Clinical aspects. Br Med J. 1973 Jun 30;2(5869):733–737. doi: 10.1136/bmj.2.5869.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigzell H. Specific affinity fractionation of lymphocytes using glass or plastic bead columns. Scand J Immunol. 1976 Jun;Suppl 5:23–30. doi: 10.1111/j.1365-3083.1976.tb03853.x. [DOI] [PubMed] [Google Scholar]
- Wyle F. A., Artenstein M. S., Brandt B. L., Tramont E. C., Kasper D. L., Altieri P. L., Berman S. L., Lowenthal J. P. Immunologic response of man to group B meningococcal polysaccharide vaccines. J Infect Dis. 1972 Nov;126(5):514–521. doi: 10.1093/infdis/126.5.514. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E., Altieri P., Berman S., Lowenthal J., Artenstein M. S. Safety and immunogenicity of a Neisseria meningitidis type 2 protein vaccine in animals and humans. J Infect Dis. 1978 Jun;137(6):728–739. doi: 10.1093/infdis/137.6.728. [DOI] [PubMed] [Google Scholar]



