Abstract
Exposure to cadmium poses a threat to human health, including increased susceptibility to developing the bone disease osteoporosis. Despite its recognized importance as an environmental toxin, little is known about how cadmium directly impacts bone-forming osteoblasts. We previously reported that cadmium induces apoptosis in human osteoblast-like Saos-2 cells. In this work, we hypothesize that cadmium exposure induces oxidative stress which leads to decreased RUNX2 mRNA expression and increased apoptotic death, and predict that the antioxidant NAC mitigates the damaging effects of cadmium. Oxidative stress is implicated in osteoporosis; furthermore the osteoblast transcriptional factor RUNX2 is reported to play a protective role against osteoporosis in postmenopausal women. Cells treated with 10 μM CdCl2 exhibited signs of oxidative damage including depletion in glutathione, increased reactive oxygen species formation, and enhanced lipid peroxidation. RUNX2 mRNA expression, by RT-PCR, was significantly reduced after exposure to 10 μM CdCl2. Pretreatment with the antioxidant NAC (1 mM) prevented cadmium-induced decrease in RUNX2 mRNA and protected cells from apoptotic death. This study provides insight into the mechanisms underlying cadmium-induced osteotoxicity. In addition, this study distinguishes itself by identifying RUNX2 as a target for heavy metal-induced osteotoxicity.
Keywords: Cadmium, RUNX2, Antioxidant, Osteoblast, Osteoporosis
1. Introduction
The heavy metal cadmium is a widespread environmental contaminant that recently has gained public attention due to the world-wide increase in discard of electronic-waste (e.g., cell phones and computers) containing this toxic metal (Järup, 2003; Rydh and Svärd, 2003; Wong et al., 2007). Human exposure primarily occurs via ingestion of contaminated food or water and by smoking. Upon entry into the body, bone is a critical target site for cadmium (Järup, 2003). The 1940s outbreak of Itai-Itai disease in Japan brought public awareness to the health risks of exposure to cadmium with reports of women living in a cadmium-polluted region suffering from advanced kidney and bone disease (Tsuchiya, 1978). Mounting world-wide epidemiological research indicates that chronic, low-level exposure to cadmium leads to increased risk of bone fractures and osteoporosis (Järup and Alfvén, 2004; Jin et al., 2004) and these findings are confirmed with experimental animals studies (Regunathan et al., 2003; Brzóska and Moniuszko-Jakoniuk, 2004, 2005). In 2005, a CDC report listed cadmium as a toxin that merits monitoring (Centers for Disease Control and Prevention, 2005). This report, combined with the escalating cost of healthcare for osteoporosis, emphasizes the importance of promoting research to decipher the underlying mechanisms of cadmium-induced osteotoxicity.
There are two proposed mechanisms to explain how cadmium influences bone function. One mechanism is indirect whereby cadmium damage to kidney or gastrointestinal organs produces a secondary effect on bone (Kjellström, 1992). Second, cadmium can act directly on bone by stimulating bone resorption by osteoclasts (Regunathan et al., 2003; Wang and Bhattacharyya, 1993) or inhibiting bone formation by osteoblasts (Iwami and Moriyama, 1993; Long, 1997a,b; Kaneki et al., 2000; Coonse et al., 2007). In either case, the end result leads to cadmium-induced bone loss and contributes to the pathogenesis of osteoporosis (Järup and Alfvén, 2004; Jin et al., 2004).
The mechanisms by which cadmium disrupts bone function are not well understood. In vitro osteoblastic studies indicate exposure to micromolar cadmium concentrations leads to decreased collagen synthesis and altered Ca2+ homeostasis in rat osteosarcoma cells (Long, 1997a,b), decreased alkaline phosphatase activity in MC3T3-E1 cells (Iwami and Moriyama, 1993) and reduced bone nodule formation in primary rat calvarial osteoblasts (Kaneki et al., 2000). In addition, we recently demonstrated that cadmium induces apoptosis, a strategic and organized mechanism of cell death, in the human osteoblast-like cell line Saos-2 via caspase-3 activation and this osteotoxicity is blocked by cadmium chelation (Coonse et al., 2007; Zhukalin et al., 2007). Since apoptosis is an integral component of bone remodeling, disruption of the apoptotic signaling cascades in osteoblasts may contribute to net bone loss leading to osteoporosis (Xing and Boyce, 2004).
One possible mechanism involved in cadmium-induced osteoblast apoptosis is oxidative stress (Ott et al., 2007). Oxidative stress is defined as disruption of the balance between antioxidant defense and accumulation of reactive oxygen species (ROS). Enhanced generation of intracellular ROS, such as superoxide anions, hydrogen peroxide, and hydroxyl radicals can overwhelm a cell’s intrinsic antioxidant defenses systems leading to excess oxidation of lipids, DNA, and proteins (Bertin and Averbeck, 2006). There is growing evidence that oxidative stress contributes to a number of age-related diseases, including osteoporosis, and that antioxidants function to mitigate the damaging effect of oxidative stress (Basu et al., 2001; Rao et al., 2007). Cadmium is not a Fenton metal and therefore cannot directly generate ROS, but it can indirectly induce oxidative stress through depletion of antioxidant molecules or inhibition of antioxidant enzymes. Treatment with micromolar cadmium concentrations leads to depletion of the antioxidant glutathione (GSH) in cultured C6 glioma, murine macrophage, human A549 lung, and rat neuronal cells (Wätjen and Beyersmann, 2004; Valko et al., 2006; Pathak and Khandelwal, 2006; López et al., 2006). There are also reports of increased lipid peroxidation and inhibition of key antioxidant enzymes in response to cadmium exposure (Waisberg et al., 2003), including a recent report of increased plasma lipid peroxidation and decreased superoxide dis-mutase levels in workers exposed to cadmium (Babu et al., 2006). The antioxidant N-acetylcysteine (NAC) is an important inducer of intracellular glutathione and has been shown to be a ROS scavenger in cadmium treated rat renal and liver tissue and human lung cells (Shaikh et al., 1999; Gaubin et al., 2000). In apoptotic studies, treatment with antioxidants such as NAC attenuates cadmium-induced apoptosis (Wätjen and Beyersmann, 2004; Valko et al., 2006; Pathak and Khandelwal, 2006; López et al., 2006); and it is significant to point out that none of these studies were in bone.
Oxidative stress, induced by hydrogen peroxide, has been reported to inhibit osteoblast differentiation and induce apoptosis in MC3T3-E1 osteoblastic cells (Bai et al., 2004; Arai et al., 2007). Furthermore, osteoblasts treated with hydrogen peroxide exhibit reduced expression of several osteoblastic markers, including RUNX2 (Arai et al., 2007). The runt-related transcriptional factor RUNX2 is a critical mediator of the osteoblast phenotype and plays a pivotal role in the process of osteoblast differentiation and function by regulating genes, such as osteocalcin, osteopontin, and bone sialoprotein, that are important in commiting cells to the osteoblast lineage (Ducy et al., 1997). Runx2 (−/−) null mice have few, if any, osteoblasts and die at birth due to the absence of mineralized bone (Komori et al., 1997). In humans, mutations in RUNX2 are found in patients with the skeletal disorder cleidocranial dysplasia (Komori, 2002) whom exhibit characteristics of disease related to impaired osteoblast function. After cells commit to the osteoblast lineage, RUNX2 also plays a role in osteoblast differentiation and mineralization of bone (Ducy et al., 1999). To date, changes in RUNX2 expression by cadmium, or any heavy metal, has not been investigated. Considering that RUNX2 also plays a protective role against osteoporosis in postmenopausal women by regulating bone mineral density (Vaughan et al., 2004) the effects of environmental toxins on RUNX2 expression is worthy of study.
This research builds upon our previous report that cadmium induces apoptosis in human osteoblast-like Saos-2 cells. The aim of this study is to test the hypothesis that cadmium exposure induces oxidative stress which leads to decreased RUNX2 mRNA expression and increased apoptotic death. We predict that the antioxidant NAC will mitigate the damaging effects of cadmium. Our goal is to advance the current understanding of how environmental toxins contribute to the pathogenesis of osteoporosis by studying the underlying mechanisms involved in cadmium-induced osteotoxicity.
2. Materials and methods
2.1. Cell culture
The human osteosarcoma cell line, Saos-2, was purchased from American Type Culture Collection (ATCC, Manassas, VA). Cells were cultured in Dulbecco’s modified Eagle medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (ATCC, Manassas, VA), 2 mM L-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin (Sigma–Aldrich, St. Louis, MO) at 37 °C in air containing 5% CO2. For routine maintenance, medium was changed every 3–4 days and cells were subcultured weekly.
2.2. Cell treatment
Cells were plated at different densities dependent on the assay. The culture medium was changed after 24 h and treatment was initiated with CdCl2 (Sigma–Aldrich, St. Louis, MO) for 3–48 h. Concentrations of CdCl2 used were within a range reported in the literature (Pulido and Parrish, 2003). Untreated control cells received culture medium only. Some experiments included pretreatment for 1 h with 1.0 mM N-acetylcysteine (NAC) (Sigma–Aldrich, St. Louis, MO). In order to determine NAC concentration, cells were treated with 0.1 mM, 1 mM, or 10 mM of NAC for 30 h. Treatment with 1 mM NAC resulted in recovery of RUNX2 and was not toxic to the cells (data not shown). After treatment, cultures were terminated and the adherent cells were collected for evaluation.
2.3. Cell viability assay
Cells were plated at a density of 1 × 105/well in a 96-well culture plate. After treatment, cells were washed with phosphate buffered saline (PBS) and incubated at 37 °C with 20 μg/ml MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium-bromide) for 4 h. The conversion of the tetrazolium salt MTT to a colored formazan by mitochondrial dehydrogenase was used to assess cell viability. After the supernatant was removed, 150 μl of DMSO was added to each well and absorbance was read at 540 nm.
2.4. Glutathione (GSH) determination
Cells were plated at 1 × 106 per flask. The concentration of GSH was determined using the ApoGSH™ Glutathione Detection Kit (Biovision, Mountain View, CA). After treatment, cells were trypsinized off the plate, collected and lysed using buffer provided by the kit. The lysate was transferred to a 96-well-plate and 2 μl of 25 mM monochlorobimane (MCB) dye and 2 μl of the 50 U/ml glutathione S-transferase (GST) reagent was added to each sample and incubated for 30 min at 37 °C. The GST catalyzes the binding of the MCB dye to available GSH generating a blue fluorescence (Ex./Em. = 360/460 nm). The fluorescence emission was analyzed using a BioTek Synergy HT. Samples were normalized for protein content using a Bradford assay (Bradford, 1976).
2.5. Intracellular ROS formation
The generation of ROS was determined using H2DCFDA (2′,7′-dichlorofluorescin diacetate) (Molecular Probes, Eugene, OR). Cells were plated at 2 × 104 cells/well in a 96-well culture plate. After treatment, cells were washed with PBS and incubated with 200 μM of H2DCFDA diluted in HEPES buffered saline with 0.3% bovine serum albumin for 30 min at 37 °C. The non-polar compound, H2DCFDA diffuses into the cell where intracellular esterases cut the diacetate. The newly formed polar compound, H2DCF, is trapped in the cell where it is oxidized by ROS to a compound, DCF, that fluoresces (Ex. = 485/20 nm; Em. = 460/40 nm). The fluorescence emission was analyzed using a BioTek Synergy HT. Samples were normalized for protein content using a Bradford assay (Bradford, 1976). Hydrogen peroxide (100 μM) was used as a positive control.
2.6. TBARS production
The thiobarbituric acid assay (TBARS) was used to detect lipid peroxidation (Draper and Hadley, 1990). Cells were plated at 2 × 106 cells per flask. After treatment, cells were washed with PBS, trypsinized off the plate, collected, and 106 cells were used for the assay. After washing with PBS, cells were lysed with lysis buffer from the ApoGSH™ Glutathione Detection Kit and the lysate was incubated for 15 min with ice cold 10% trichloroacetic acid (TCA) to precipitate protein, centrifuged for 15 min, and 200 μl of the supernatant was added to 200 μl of thiobarbituric acid (TBA). The samples were boiled for 17 min. and then cooled in ice for 20 min. After cooling, the absorbance of red pigment in the sample was measured at 532 nm. Malondialdehyde was used as a standard.
2.7. RT-PCR
Total RNA was extracted from cultured cells using TRIzol reagent (Invitrogen, Carlsbad, CA) and quantified at 260 nm. Using AffinityScript QPCR cDNA Synthesis Kit (Stratagene, La Jolla, CA), 500 ng of RNA from each sample was reverse transcribed, and cDNA was amplified by PCR using the Easy-A High Fidelity PCR cloning Enzyme protocol. The cDNA was substituted with RNase-free water in negative controls. Sequences for RUNX2 PCR primers were 5′-GCTGTTATGAAAAACCAAGT-3′and 5′-GGGAGGATTTGTGA AGAC-3′ (Integrated DNA Technologies Inc., Coralville, IA) (Bertaux et al., 2006). The amplification conditions for RUNX2 were 95 °C 3 min; 95 °C 30 s; 60 °C 30 s; 72 °C 30 s (35 cycles), and 72 °C 7 min. PCR products were visualized using 1.2% agarose FlashGels and quantified using Quantity One 1-D Analysis Software. The expression levels were normalized to the level of the housekeeping gene, GAPDH.
2.8. Apoptotic assay
After treatment, cells (2 × 104 cells/well) received fresh medium containing APOPercentage dye (Biocolor, Carrickfergus, UK) and incubated at 37 °C for 30 min. The APOPercentage dye is transported into an apoptotic cell during the translocation of phosphatidylserine from the inner leaflet to the outer leaflet of the cell membrane. Once the dye is taken up, cells are washed with PBS and APOPercentage dye releasing reagent is added to release the dye. Absorbance was read at 550 nm. Stained cells were visualized using a Nikon epifluorescence Eclipse E400 microscope. Digital images were captured using ImagePro software by Media Cybernetics (Silver Spring, MD).
2.9. Statistical analysis
Data represents the mean ± SEM for at least three separate experiments. Data were analyzed using a one-way analysis of variance followed by a Tukey test for multiple comparisons or by a Student’s t-test for comparison between two groups. A p-value < 0.05 was considered significant.
3. Results
3.1. Cadmium treatment decreases cell viability
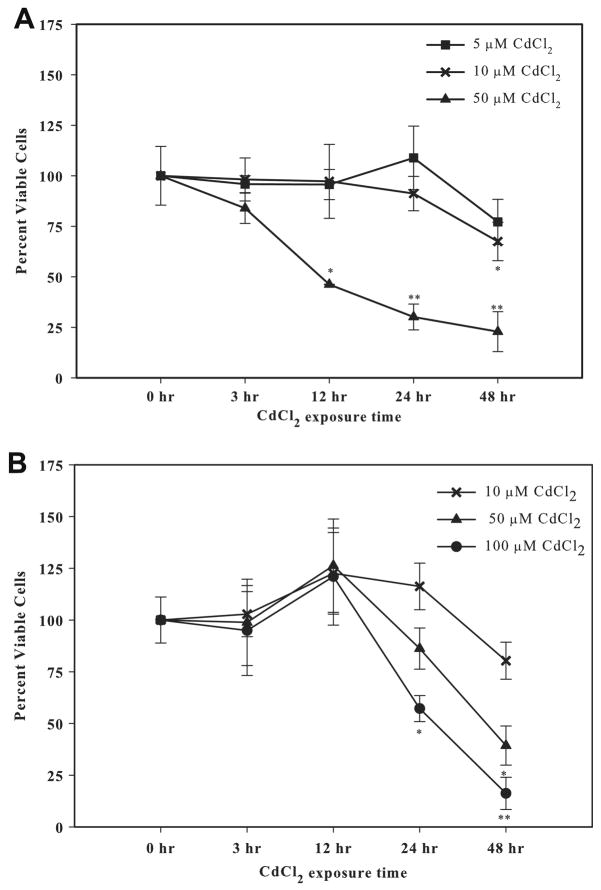
Saos-2 cells cultured in 1% FBS (Fig. 1A) were more sensitive to cadmium toxicity compared to cells cultured in 10% FBS (Fig. 1B), with an EC50 value at 24 h of 40 μM and 107 μM, respectively. While the use of reduced or serum-free culture medium lowers the CdCl2 concentration needed to elicit a response, serum deprivation can induce or enhance apoptosis (López et al., 2003) therefore subsequent experiments were conducted using 10% FBS containing culture medium. There was no detectable change in cell viability with 10 μM CdCl2 exposure over time. When the concentration was increased to 50 or 100 μM CdCl2 a significant decrease in cell viability was observed. A marked cytotoxic effect was observed with 100 μM CdCl2 treatment with only 16% viable cells remaining at 48 h. Subsequent experiments were conducted using 10 μM CdCl2 (Fig. 1B).
Fig. 1.
The effect of CdCl2 on cell viability using different FBS concentrations. (A) Cells were treated with 5 μM, 10 μM, or 50 μM CdCl2 for 0, 3, 12, 24, or 48 h in the presence of 1% FBS. (B) Cells were treated with 10 μM, 50 μM, or 100 μM CdCl2 for 0, 3, 12, 24, or 48 h in the presence of 10% FBS. Controls received culture medium only. Cell viability was determined using the MTT assay. Results are expressed as percent of viable cells. Each line represents the mean ± SEM of at least four independent experiments and 0 h represents untreated cells. *Denotes significant difference from control p < 0.05. **Denotes significant difference from control p < 0.01.
3.2. Cadmium exposure induces depletion in the intracellular antioxidant GSH
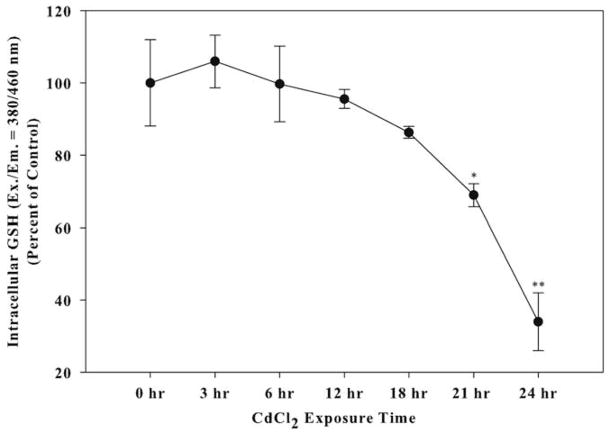
One feature of oxidative stress is depletion in the cellular antioxidant system, including the thiol-containing nonenzymatic antioxidant GSH. Treatment with 10 μM CdCl2 resulted in a significant decrease in the amount of GSH to 68% by 21 h and 34% by 24 h compared to untreated control levels (Fig. 2). Cadmium-induced depletion of GSH demonstrates a disruption in the antioxidant and pro-oxidant balance in Saos-2 cells.
Fig. 2.
The effect of CdCl2 on GSH depletion. Cells were treated with 10 μM CdCl2 for 0, 3, 6, 12, 18, 21, or 24 h. Control received culture medium only. Following treatment, cells were lysed and incubated with GST and MCB dye to detect GSH. Fluorescence was measured at Ex./Em. = 380/460 nm. Results were normalized for protein and expressed as percent of control. Each bar represents the mean ± SEM of at least five independent experiments and 0 h represents untreated cells. *Denotes significant difference from control p < 0.05. **Denotes significant difference from control p < 0.01.
3.3. Cadmium exposure leads to increased ROS formation
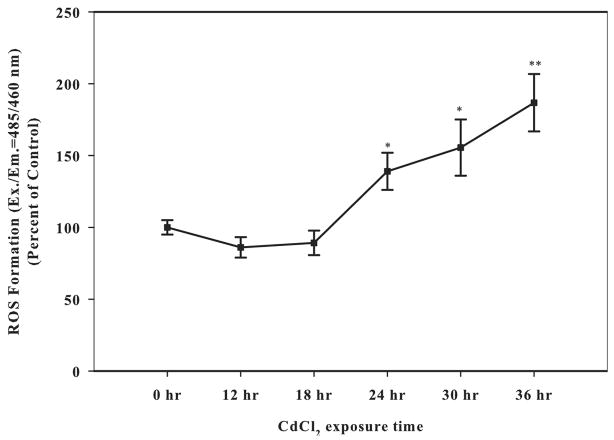
To further confirm that cadmium induces oxidative stress in Saos-2 cells, generation of ROS following cadmium exposure was determined with the fluorescent probe DCF. Treatment with 10 μM CdCl2 resulted in a significant induction of ROS at 24 h and was maintained over time (Fig. 3). Treatment with 100 μM hydrogen peroxide was used as a positive control (data not shown).
Fig. 3.
The effect of CdCl2 on intracellular ROS formation. Cells were treated with 10 μM CdCl2 for 0, 12, 18, 24, or 36 h. Control received culture medium only. Following treatment, cells were incubated with H2DCF/DA fluorescent dye to determine ROS formation. Fluorescence was read Ex./Em. = 485/460 nm. Results were normalized for protein and expressed as percent of control. Each bar represents the mean ± SEM of at least eight independent experiments. *Denotes significant difference from control p < 0.05. **Denotes significant difference from control p < 0.01.
3.4. Cadmium exposure induces lipid peroxidation
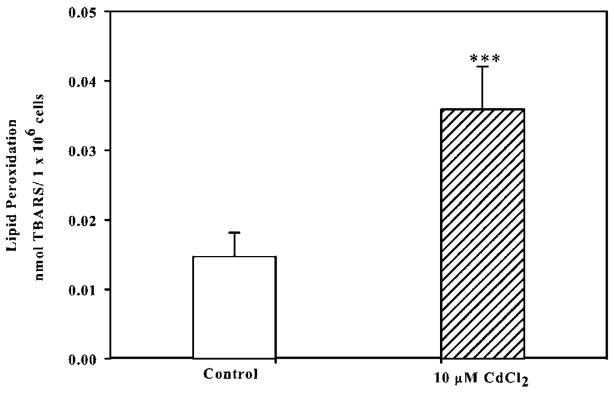
Enhanced ROS can overwhelm a cell’s intrinsic antioxidant defense systems leading to adverse oxidation of lipids, DNA, and proteins, therefore we examined whether cadmium treatment leads to increased lipid peroxidation in Saos-2 cells. Thiobarbituric acid reactive substances (TBARS) are formed during the breakdown of lipid hydroperoxides (Draper and Hadley, 1990). Exposure to 10 μM CdCl2 for 24 h significantly increased the formation of TBARS compared to untreated controls (Fig. 4). Collectively, these data demonstrate that cadmium exposure leads to oxidative stress through the depletion of GSH, increased ROS formation, and enhanced lipid peroxidation in Saos-2 cells.
Fig. 4.
The effect of CdCl2 on lipid peroxidation. Cells were treated with 10 μM CdCl2 for 24 h and controls received culture medium only. The thiobarbituric acid assay (TBARS) was used to detect lipid peroxidation. Results expressed as nmol TBARS per 106 cells. Each bar represents ± SEM of at least five independent experiments and control represents untreated cells. ***Denotes significant difference from control p < 0.001.
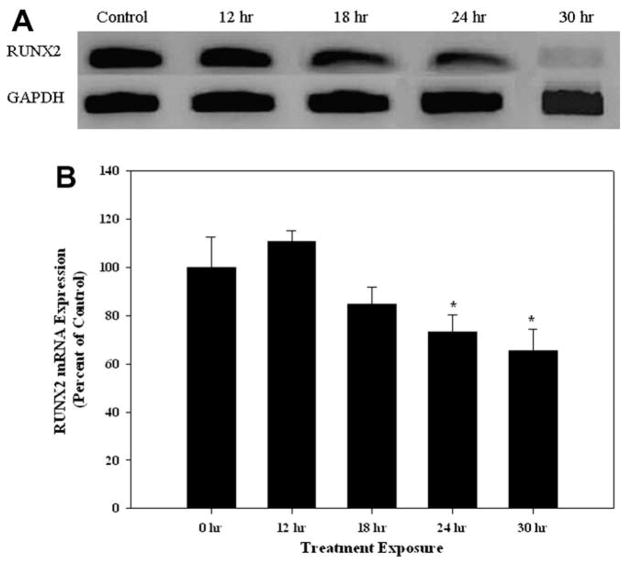
3.5. Cadmium exposure decreases RUNX2 mRNA expression
The expression of RUNX2 is essential for the development, maturation and maintenance of osteoblasts (Ducy et al., 1999), therefore we examined whether cadmium exposure alters the expression of this key transcriptional factor. Cells treated with 10 μM CdCl2 exhibited a time-dependent decrease in RUNX2 mRNA compared to untreated controls (Fig. 5A). The relative expression of RUNX2 in CdCl2 treated cells at 24 and 30 h were 73% and 65% compared to untreated controls, respectively (Fig. 5B).
Fig. 5.
The effect of CdCl2 on RUNX2 mRNA expression. (A) Cells were treated with 10 μM CdCl2 for 0, 12, 18, 24, or 30 h and RT-PCR was utilized for RUNX2 and GAPDH mRNA expression. Controls received culture medium only. (B) Band intensities were measured by densitometry and normalized to GAPDH and presented as percent of control. Each bar represents the mean ± SEM of at least five independent experiments. *Denotes significant difference from control p < 0.05.
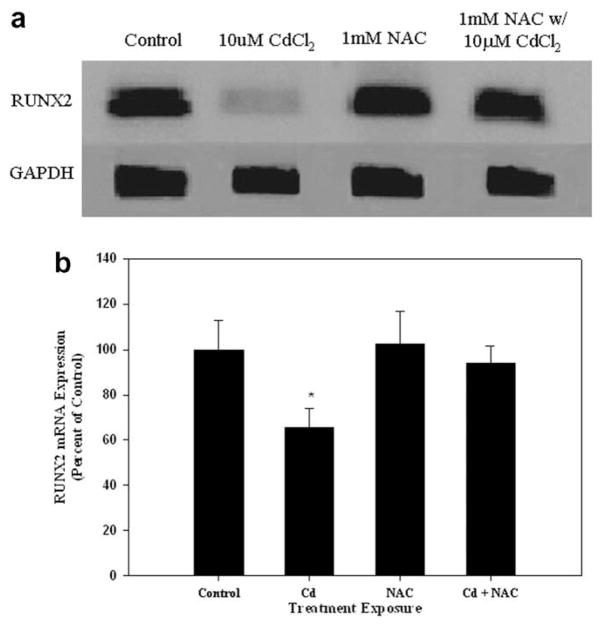
3.6. Pretreatment with the antioxidant NAC protects against cadmium-induced decrease in RUNX2 mRNA expression
In order to assess whether cadmium-induced oxidative damage preceded alterations in RUNX2, cells were pretreated with the antioxidant NAC, which is a precursor of glutathione. Pretreatment with 1 mM NAC blocked the decrease in RUNX2 mRNA expression induced by exposure to 10 μM CdCl2 for 30 h (Fig. 6).
Fig. 6.
The effect of pretreatment with NAC on RUNX2 mRNA expression in CdCl2 treated cells. (A) Cells were treated with 10 μM CdCl2 only, 1 mM NAC only, or pretreated with 1 mM NAC followed by 30 h treatment of 10 μM CdCl2. Controls received culture medium only. RT-PCR was utilized for RUNX2 and GAPDH mRNA expression. (B) Band intensities were measured by densitometry and normalized to GAPDH and presented as percent of control. Each bar represents the mean ± SEM of at least three independent experiments. *Denotes significant difference from control p < 0.05.
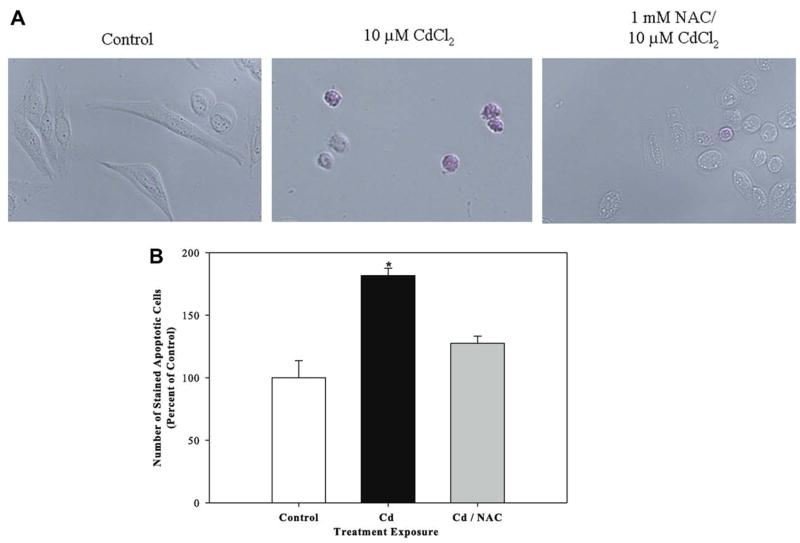
3.7. Pretreatment with the antioxidant NAC protects against cadmium-induced apoptosis
We previously reported that cadmium induces apoptosis in Saos-2 cells using several apoptotic indicators including detection of the translocation of phosphatidylserine to the outer leaflet of the cell membrane (Coonse et al., 2007). In this study, we examined whether pretreatment with NAC protects against cadmium-induced apoptosis. Treatment with 10 μM CdCl2 for 48 h resulted in a significant increase in apoptotic cells of about 181% compared to untreated controls (Fig. 7B) which was also evident when observing stained apoptotic cells (Fig. 7A). Treatment for 1 h with 1 mM NAC prior to 10 μM CdCl2 exposure protected cells against cadmium-induced apoptosis (Fig. 7). Cadmium-induced apoptosis was also attenuated in nontumor derived mouse MC3T3-E1 osteoblastic cells treated with 1 mM NAC prior to exposure to 10 μM CdCl2 (data not shown).
Fig. 7.
The effect of pretreatment with NAC on apoptosis in CdCl2 treated cells. Cells were treated with 10 μM CdCl2 only or pretreated with 1 mM NAC followed by 48 h exposure to 10 μM CdCl2. Controls received culture medium only. Apoptosis was determined using APOPercentage dye which is transported into an apoptotic cell during the translocation of phosphatidylserine from the inner leaflet to the outer leaflet of the cell membrane. (A) Images of pink stained (apoptotic) cells and (B) quantification of APOPercentage dye uptake. Each bar represents the mean ± SEM of at least three independent experiments. *Denotes significant difference from control p < 0.05.
4. Discussion
Exposure to the heavy metal cadmium poses a threat to human health, including increased susceptibility to developing the metabolic bone disease osteoporosis (Järup and Alfvén, 2004; Jin et al., 2004; Kazantzis, 2004; Nordberg et al., 2002). Despite its recognized importance as an environmental toxin, little is known about how cadmium directly impacts bone cells, in particular bone-forming osteoblasts. We hypothesize that cadmium exposure leads to oxidative damage in Saos-2 osteoblast-like cells. This hypothesis is supported by growing evidence that oxidative stress contributes to a number of age-related diseases, including osteoporosis, and that antioxidants function to mitigate the damaging effect of oxidative stress (Basu et al., 2001; Rao et al., 2007; Pari and Murugavel, 2007; Maggio et al., 2003). Our results demonstrate that cadmium exposure leads to oxidative damage through the depletion of GSH, increased intracellular ROS formation, and enhanced lipid peroxidation in cultured Saos-2 cells. Our results are consistent with other studies that report cadmium exposure can indirectly generate ROS formation through depletion of GSH in brain cells and murine thymocytes (Pathak and Khandelwal, 2006; López et al., 2006; Im et al., 2006; Pari and Murugavel, 2007) and adverse oxidation of lipids in other cell types (Wätjen and Beyersmann, 2004; López et al., 2006; Waisberg et al., 2003; Yiin et al., 2000).
Since cadmium is not a Fenton metal the production of ROS is indirect. For example, cadmium can diminish GSH levels and produce an imbalance between ROS and antioxidants. The marked decrease in GSH and rise of ROS may explain the increase in lipid peroxidation after cadmium treatment (Waisberg et al., 2003). Lipid peroxidation may be mediated by disturbances of natural antioxidant and/or metallothionein levels, which can result in ROS attacking and cleaving the double bonds in membrane lipids in the cell (Ercal et al., 2001). Depletion of antioxidants and production of ROS and lipid peroxidation after cadmium exposure may play an important role in the etiology of osteoporosis, as suggested in an in vivo study that demonstrates osteoporotic women have reduced levels of antioxidants and increased lipid peroxidation (Maggio et al., 2003).
To further explore the mechanisms involved in cadmium-induced oxidative damage in osteoblasts, we focused on RUNX2, a critical mediator of bone formation, function and differentiation (Ducy et al., 1997; Komori, 2002; Karsenty and Wagner, 2002). Studies suggest RUNX2 may play a protective role against osteoporosis in postmenopausal women (Vaughan et al., 2004; Doecke et al., 2006). These results led to our hypothesis that cadmium-induced oxidative stress decreases RUNX2 mRNA expression in Saos-2 cells. We report that cadmium decreases RUNX2 mRNA expression in Saos-2 cells, thus broadening the spectrum of known mechanisms by which cadmium can impair osteoblast function. It is well established that RUNX2 regulates the expression of key bone matrix proteins such as type I collagen, osteocalcin, and alkaline phosphatase and alterations to this transcriptional factor can result in osteopenia, a characteristic of osteoporosis (Ducy et al., 1997, 1999; Bertaux et al., 2006; Karsenty and Wagner, 2002; Alliston et al., 2001). Therefore, deciphering the mechanisms by which cadmium suppresses RUNX2 mRNA expression is imperative for the understanding of bone metabolic diseases such as osteoporosis.
To determine if the cadmium-induced decrease in RUNX2 mRNA is downstream of oxidative stress, we pretreated Saos-2 cells with the antioxidant NAC. Our results reveal that pretreatment with NAC mitigates cadmium’s suppression of RUNX2 mRNA. Although evidence linking oxidative stress to RUNX2 is limited, our work is consistent with other studies that report a decrease in RUNX2 mRNA and nuclear phosphorylated RUNX2 protein in hydrogen peroxide treated rabbit bone marrow stromal and MC3T3-E1 cells, respectively (Bai et al., 2004; Arai et al., 2007). Furthermore, inhibition of the ERK signaling pathway attenuates hydrogen peroxide-induced decrease in nuclear phosphorylated RUNX2 protein (Bai et al., 2004). It is documented that RUNX2 is regulated through phosphorylation via MAPKs, PKA, and PKC and thus these are signaling pathways to explore in cadmium-induced inhibition of RUNX2. The regulatory complexity of RUNX2 is emerging with a variety of signaling pathways and crosstalk among pathways being implied in RUNX2 transcription and protein-level regulation (Franceschi and Xiao, 2003; Jilka, 2007).
To build upon our previous report that cadmium increases apoptotic death in Saos-2 cells, in this study we demonstrate that pretreatment with NAC prevents cadmium-induced apoptosis. Previous studies have also reported that antioxidants, including NAC, mitigate the damaging effects of cadmium-mediated apoptosis in several cell types, however bone cells have not been examined to date (Wätjen and Beyersmann, 2004; Pathak and Khandelwal, 2006; López et al., 2006, 2003; Im et al., 2006).
This study demonstrates that cadmium-induced oxidative damage leads to a decrease in RUNX2 expression and ultimately osteoblast apoptosis, suggesting the RUNX2 may play an anti-apoptotic role in osteoblasts. For example, an anti-apoptotic role for RUNX2 is supported by Bellido et al. who reports the anti-apoptotic effect of parathyroid hormone (PTH) in osteoblastic cells is mediated by RUNX2 (Bellido et al., 2003). Over expression of RUNX2 mRNA or inhibition of RUNX2 proteasomal degradation in osteoblastic cells extends PTH inhibition of apoptosis, in part, by induction of a key anti-apoptotic protein Bcl-2 (Bellido et al., 2003). In addition, ectopically expressed RUNX2 leads to a lower apoptotic rate in lymphomas (Blyth et al., 2006). Contrary to the above studies, a recent report suggests that RUNX2 may play a proapoptotic role in BMP-2 induced osteoblast apoptosis (Eliseev et al., 2008). Thus, the complexity of RUNX2 regulation and its relationship to apoptotic signaling is unclear at this time but warrants further investigation.
This study provides insight into the mechanisms underlying cadmium-induced osteotoxicity. In addition, this study distinguishes itself by identifying RUNX2 as a target for heavy metal-induced osteotoxicity.
Acknowledgments
This work was supported by NIH Grant P20RR016454 from the INBRE Program of the National Center for Research Resources, NIH R15ES015866 Grant from the National Institute of Environmental Health Sciences, and Sigma Xi Scientific Research Society. The authors thank Kendra Coonse and Dr. Ann Koga for their helpful advice.
Footnotes
Conflict of interest statement
None declared.
References
- Alliston T, Choy L, Ducy P, Karsenty G, Derynck R. TGF-β-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. The EMBO Journal. 2001;20:2254–2272. doi: 10.1093/emboj/20.9.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai M, Shibata Y, Pugdee K, Abiko Y, Ogata Y. Effects of reactive oxygen species (ROS) on antioxidant system and osteoblastic differentiation in MC3T3-E1 cells. IUBMB Life. 2007;59:27–33. doi: 10.1080/15216540601156188. [DOI] [PubMed] [Google Scholar]
- Babu KR, Rajmohan HR, Rajan BK, Kumar KM. Plasma lipid peroxidation and erythrocyte antioxidant enzymes status in workers exposed to cadmium. Toxicology and Industrial Health. 2006;22:329–335. doi: 10.1177/0748233706071736. [DOI] [PubMed] [Google Scholar]
- Bai X, Lu D, Bai J, Zheng H, Ke Z, Li X, Luo SQ. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-κB. Biochemical and Biophysical Research Communications. 2004;314:197–207. doi: 10.1016/j.bbrc.2003.12.073. [DOI] [PubMed] [Google Scholar]
- Basu S, Michaelsson K, Olofsson H, Johansson S, Melhus H. Association between oxidative stress and bone mineral density. Biochemical and Biophysical Research Communications. 2001;288:275–279. doi: 10.1006/bbrc.2001.5747. [DOI] [PubMed] [Google Scholar]
- Bellido T, Ali A, Plotkin L, Fu Q, Gubrij I, Roberson P, Weinstein RS, O’Brien CA, Manolagas SC, Jilka RL. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. The Journal of Biological Chemistry. 2003;278:50259–50272. doi: 10.1074/jbc.M307444200. [DOI] [PubMed] [Google Scholar]
- Bertaux K, Brouz O, Chauveau C, Hardouin P, Jeanfils J, Devedjian JC. Runx2 regulates the expression of GNAS on SaOs-2 cells. Bone. 2006;38:943–950. doi: 10.1016/j.bone.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Bertin G, Averbeck D. Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review) Biochimie. 2006;88:1549–1559. doi: 10.1016/j.biochi.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Blyth K, Vaillant F, Hanlon L, Mackay N, Bell M, Jenkins A, Neil JC, Cameron ER. Runx2 and MYC collaborate in lymphoma development by suppressing apoptotic and growth arrest pathways in vivo. Advances in Cancer Research. 2006;66:2195–2201. doi: 10.1158/0008-5472.CAN-05-3558. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein–dye-binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brzóska MM, Moniuszko-Jakoniuk J. Low-level lifetime exposure to cadmium decreases skeletal mineralization and enhances bone loss in aged rats. Bone. 2004;35:1180–1191. doi: 10.1016/j.bone.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Brzóska MM, Moniuszko-Jakoniuk J. Disorders in bone metabolism of female rats chronically exposed to cadmium. Toxicology and Applied Pharmacology. 2005;202:68–83. doi: 10.1016/j.taap.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Third National Report on Human Exposure to Environmental Chemicals (NCEH Publication No. 05-0570) National Center for Environmental Health, Division of Laboratory Sciences; Atlanta, Georgia: 2005. pp. 30341–3724. [Google Scholar]
- Coonse KG, Coonts AJ, Morrison EV, Heggland SJ. Cadmium induces apoptosis in the osteoblast-like cell line, Saos-2. Journal of Toxicology and Environmental Health, Part A. 2007;70:575–581. doi: 10.1080/15287390600882663. [DOI] [PubMed] [Google Scholar]
- Doecke JD, Day CJ, Stephens A, Carter S, van Daal A, Kotowicz M, Nicholson GC, Morrison NA. Association of functionally different RUNX2 P2 promoter alleles with BMD. Journal of Bone Mineral Research. 2006;21:265–273. doi: 10.1359/JBMR.051013. [DOI] [PubMed] [Google Scholar]
- Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods in Enzymology. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Ducy P, Starbuck M, Priemel M, Shen J, Pinero G, Geoffroy V, Amling M, Karsenty G. A Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes and Development. 1999;13:1025–1036. doi: 10.1101/gad.13.8.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliseev RA, Dong YF, Sampson E, Zuscik MJ, Schwarz EM, O’Keefe RJ, Rosier RN, Drissi MH. Runx2-mediated activation of the Bax gene increases osteosarcoma cell sensitivity to apoptosis. Oncogene. 2008;27:3605–3614. doi: 10.1038/sj.onc.1211020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic metals and oxidative stress. Part I: mechanisms involved in metal induced oxidative damage. Current Topics in Medicinal Chemistry. 2001;1:529–539. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- Franceschi RT, Xiao G. Regulation of the osteoblast-specific transcription factor, RUNX2: responsiveness to multiple signal transduction pathways. Journal of Cellular Biochemistry. 2003;88:446–454. doi: 10.1002/jcb.10369. [DOI] [PubMed] [Google Scholar]
- Gaubin Y, Vaissade F, Croute F, Beau B, Soleilhavoup J, Murat J. Implication of free radicals and glutathione in the mechanism of cadmium-induced expression of stress proteins in the A549 human lung cell-line. Biochimica et Biophysica Acta. 2000;1495:4–13. doi: 10.1016/s0167-4889(99)00149-4. [DOI] [PubMed] [Google Scholar]
- Im JY, Paik SG, Han PL. Cadmium-induced astroglial death proceeds via glutathione depletion. Journal of Neuroscience Research. 2006;83:301–308. doi: 10.1002/jnr.20722. [DOI] [PubMed] [Google Scholar]
- Iwami K, Moriyama T. Comparative effect of cadmium on osteoblastic cells and osteoclastic cells. Archives of Toxicology. 1993;67:352–357. doi: 10.1007/BF01973707. [DOI] [PubMed] [Google Scholar]
- Järup L. Hazards of heavy metal contamination. British Medical Bulletin. 2003;68:167–182. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- Järup L, Alfvén T. Low level cadmium exposure, renal and bone effects – the OSCAR study. Biometals. 2004;17:505–509. doi: 10.1023/b:biom.0000045729.68774.a1. [DOI] [PubMed] [Google Scholar]
- Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–1446. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Nordberg G, Ye T, Bo M, Wang H, Zhu G, Kong Q, Bernard A. Osteoporosis and renal dysfunction in a general population exposed to cadmium in China. Environmental Research. 2004;96:353–359. doi: 10.1016/j.envres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Kaneki H, Hayamizu N, Fujieda M, Kiriu M, Mizuochi S, Ide H. Age-dependent changes in the effect of zinc and cadmium on bone nodule formation in rat calvarial osteoblasts. Journal of Health Science. 2000;46:480–488. [Google Scholar]
- Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Developmental Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Kazantzis G. Cadmium, osteoporosis, and calcium metabolism. Biometals. 2004;17:493–498. doi: 10.1023/b:biom.0000045727.76054.f3. [DOI] [PubMed] [Google Scholar]
- Kjellström T. Mechanism and epidemiology of bone effects of cadmium. Vol. 18. IARC Scientific Publications; 1992. pp. 301–310. [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Komori T. Runx2, a multifunctional transcription factor in skeletal development. Journal of Cellular Biochemistry. 2002;87:1–8. doi: 10.1002/jcb.10276. [DOI] [PubMed] [Google Scholar]
- Long GJ. Cadmium perturbs calcium homeostasis in rat osteosarcoma (ROS 17/2.8) cells; a possible role for protein kinase C. Toxicology Letters. 1997a;91:91–97. doi: 10.1016/s0378-4274(97)03880-0. [DOI] [PubMed] [Google Scholar]
- Long GJ. The effect of cadmium on cytosolic free calcium, protein kinase C, and collagen synthesis in rat osteosarcoma (ROS 17/2.8) cells. Toxicology and Applied Pharmacology. 1997b;143:189–195. doi: 10.1006/taap.1996.8060. [DOI] [PubMed] [Google Scholar]
- López E, Figueroa S, Oset-Gasque MJ, González MP. Apoptosis and necrosis: two distinct events induced by cadmium in cortical neurons in culture. British Journal of Pharmacology. 2003;138:901–911. doi: 10.1038/sj.bjp.0705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López E, Arce C, Oset-Gasque MJ, Canadas S, González MP. Cadmium induces reactive oxygen species generation and lipid peroxidation in cortical neurons in culture. Free Radical Biology and Medicine. 2006;40:940–951. doi: 10.1016/j.freeradbiomed.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Maggio D, Barabani M, Pierandrei M, Polidori MC, Catani M, Mecocci P, Senin U, Pacifici R, Cherubini A. Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. The Journal of Clinical Endocrinology and Metabolism. 2003;88:1523–1527. doi: 10.1210/jc.2002-021496. [DOI] [PubMed] [Google Scholar]
- Nordberg G, Jin T, Bernard A, Fierens S, Buchet JP, Ye T, Kong Q, Wang H. Low bone density and renal dysfunction following environmental cadmium exposure in China. Ambio. 2002;31:478–481. doi: 10.1579/0044-7447-31.6.478. [DOI] [PubMed] [Google Scholar]
- Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress, and cell death. Apoptosis. 2007;12:913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- Pari L, Murugavel P. Diallyl tetrasulfide improves cadmium induced alterations of acetylcholinesterase, ATPases and oxidative stress in brain of rats. Toxicology. 2007;234:44–50. doi: 10.1016/j.tox.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Pathak N, Khandelwal S. Influence of cadmium on murine thymocytes: potentiation of apoptosis and oxidative stress. Toxicology Letters. 2006;165:121–132. doi: 10.1016/j.toxlet.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Pulido M, Parrish A. Metal-induced apoptosis: mechanisms. Mutation Research. 2003;533:227–241. doi: 10.1016/j.mrfmmm.2003.07.015. [DOI] [PubMed] [Google Scholar]
- Rao LG, Mackinnon ES, Josse RG, Murray TM, Strauss A, Rao AV. Lycopene consumption decreases oxidative stress and bone resorption markers in postmenopausal women. Osteoporosis International. 2007;18:109–115. doi: 10.1007/s00198-006-0205-z. [DOI] [PubMed] [Google Scholar]
- Regunathan A, Glesne DA, Wilson AK, Song J, Nicolae D, Flores T, Bhattacharyya MH. Microarray analysis of changes in bone cell gene expression early after cadmium gavage in mice. Toxicology and Applied Pharmacology. 2003;191:272–293. doi: 10.1016/s0041-008x(03)00163-7. [DOI] [PubMed] [Google Scholar]
- Rydh CJ, Svärd B. Impact on global metal flows arising from the use of portable rechargeable batteries. The Science of the Total Environment. 2003;302:167–184. doi: 10.1016/s0048-9697(02)00293-0. [DOI] [PubMed] [Google Scholar]
- Shaikh ZA, Vu TT, Zaman K. Oxidative stress as a mechanism of chronic cadmium-induced hepatotoxicity and renal toxicity and protection by antioxidants. Toxicology and Applied Pharmacology. 1999;154:256–263. doi: 10.1006/taap.1998.8586. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K. Cadmium Studies in Japan: A Review. Elsevier North Holland Biomedical Press; 1978. Etiology of itai itai disease; pp. 269–300. [Google Scholar]
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals, and antioxidants in oxidative stress-induced cancer. Chemico-biological Interactions. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Vaughan T, Reid D, Morrison N, Ralston S. RUNX2 alleles associated with BMD in Scottish women; interaction of RUNX2 alleles with menopausal status and body mass index. Bone. 2004;34:1029–1036. doi: 10.1016/j.bone.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Waisberg M, Joseph P, Hale B, Beyersmann D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology. 2003;192:95–117. doi: 10.1016/s0300-483x(03)00305-6. [DOI] [PubMed] [Google Scholar]
- Wang C, Bhattacharyya MH. Effect of cadmium on bone calcium and 45Ca in nonpregnant mice on a calcium-deficient diet: evidence of direct effect of cadmium on bone. Toxicology and Applied Pharmacology. 1993;120:228–239. doi: 10.1006/taap.1993.1107. [DOI] [PubMed] [Google Scholar]
- Wätjen W, Beyersmann D. Cadmium-induced apoptosis in C6 glioma cells: influence of oxidative stress. Biometals. 2004;17:65–78. doi: 10.1023/a:1024405119018. [DOI] [PubMed] [Google Scholar]
- Wong CSC, Duzgoren-Aydin NS, Aydin A, Wong MH. Evidence of excessive releases of metals from primitive e-waste processing in Guiyu, China. Environmental Pollution. 2007;148:62–72. doi: 10.1016/j.envpol.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Xing L, Boyce BF. Regulation of apoptosis in osteoclasts and osteoblastic cells. Biochemical and Biophysical Research Communications. 2004;328:709–720. doi: 10.1016/j.bbrc.2004.11.072. [DOI] [PubMed] [Google Scholar]
- Yiin SJ, Chern CL, Sheu JY, Lin TH. Cadmium-induced liver, heart, and spleen peroxidation in rats and protection by selenium. Biological Trace Element Research. 2000;62:47–56. doi: 10.1385/BTER:78:1-3:219. [DOI] [PubMed] [Google Scholar]
- Zhukalin M, Blanksma MK, Silva TD, Suyehira SW, Harvey WA, Heggland SJ, Craig PR. Characterization and in vitro cytotoxicity testing of ethanolamine-derived cadmium chelating agents. Biometals. 2007;20:61–72. doi: 10.1007/s10534-006-9015-1. [DOI] [PubMed] [Google Scholar]