Abstract
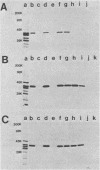
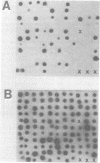
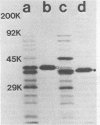
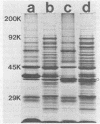
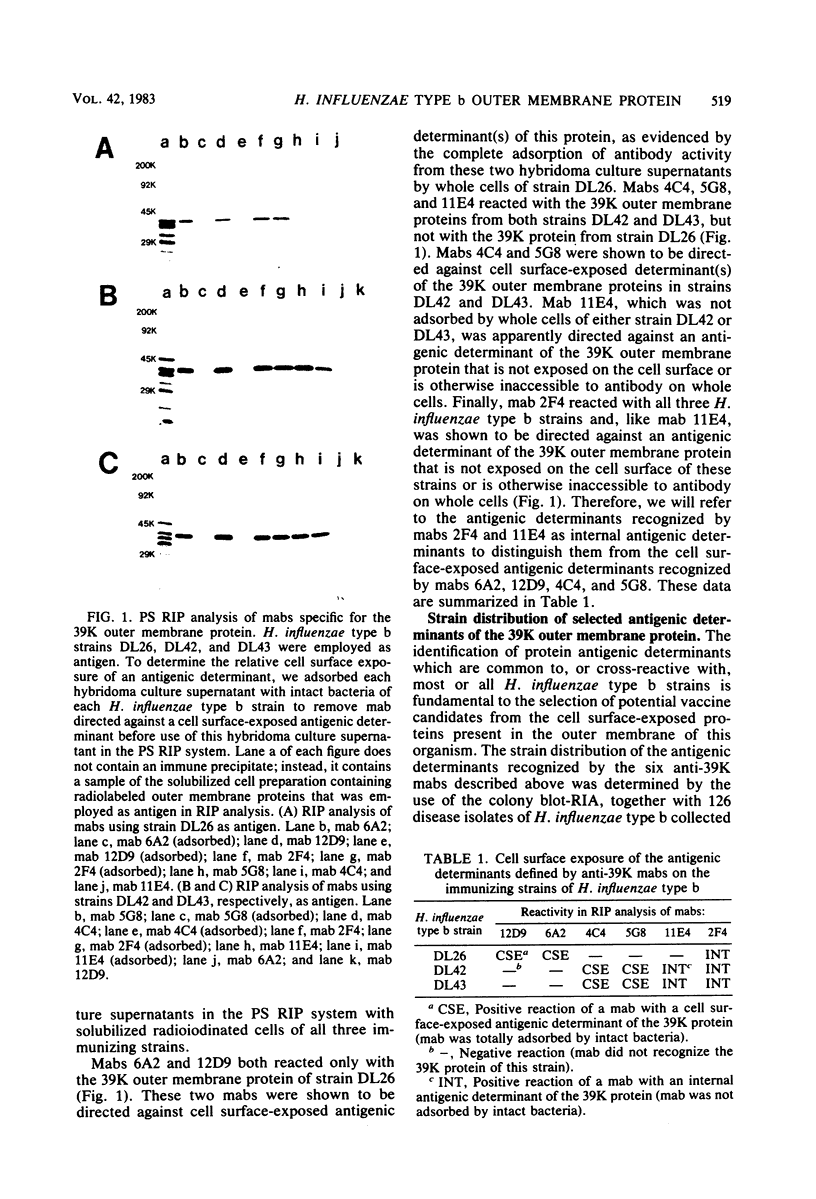
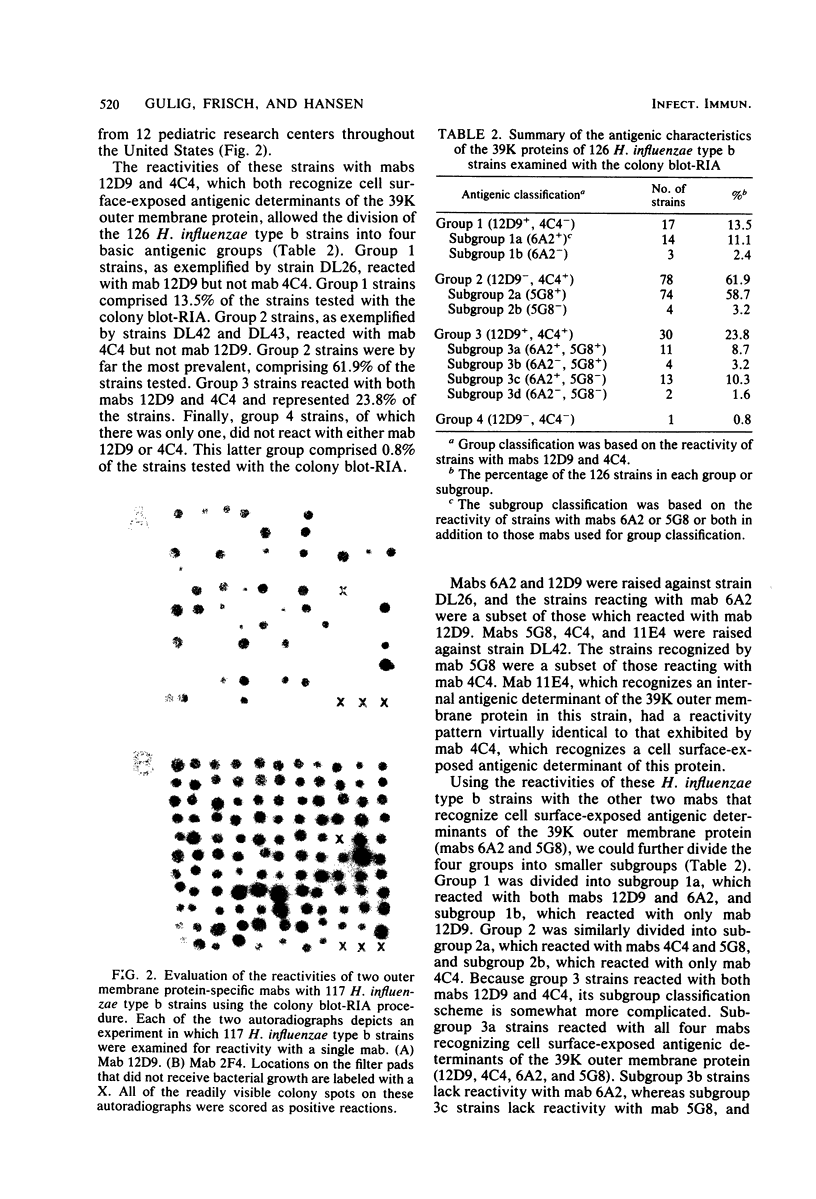
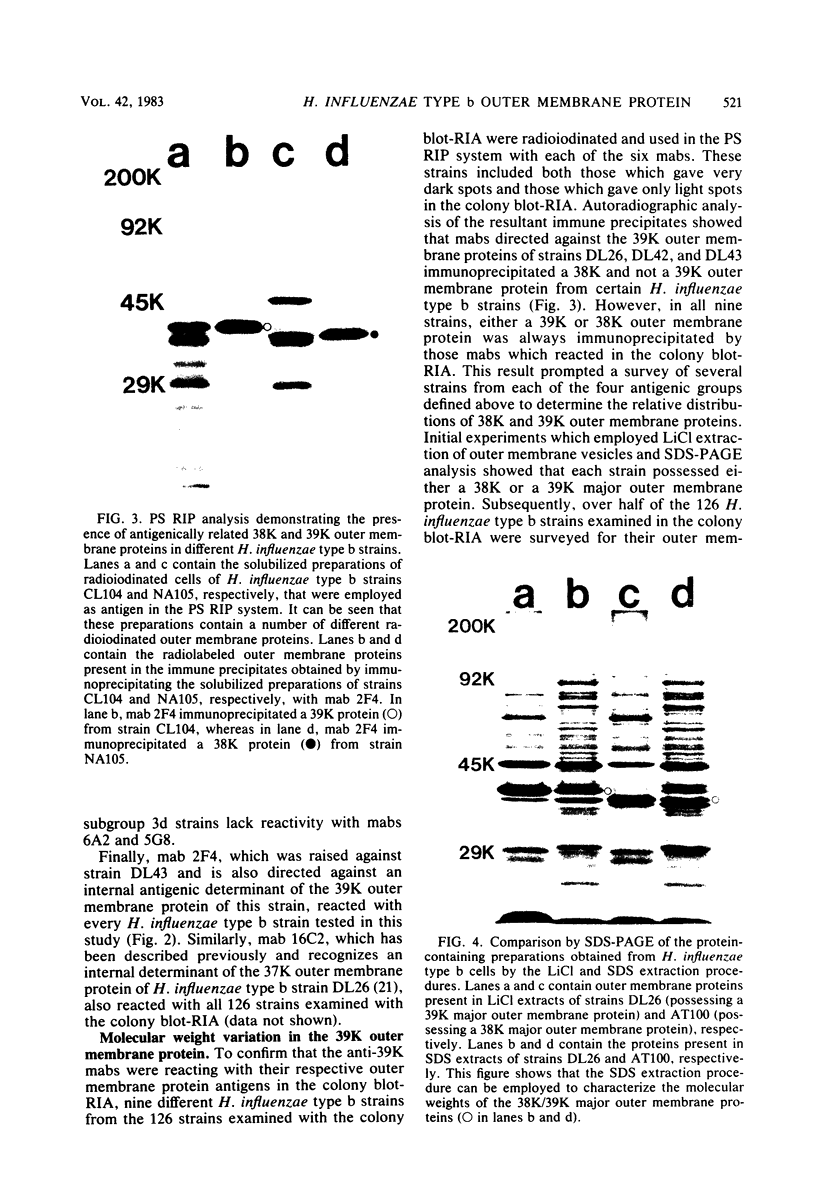
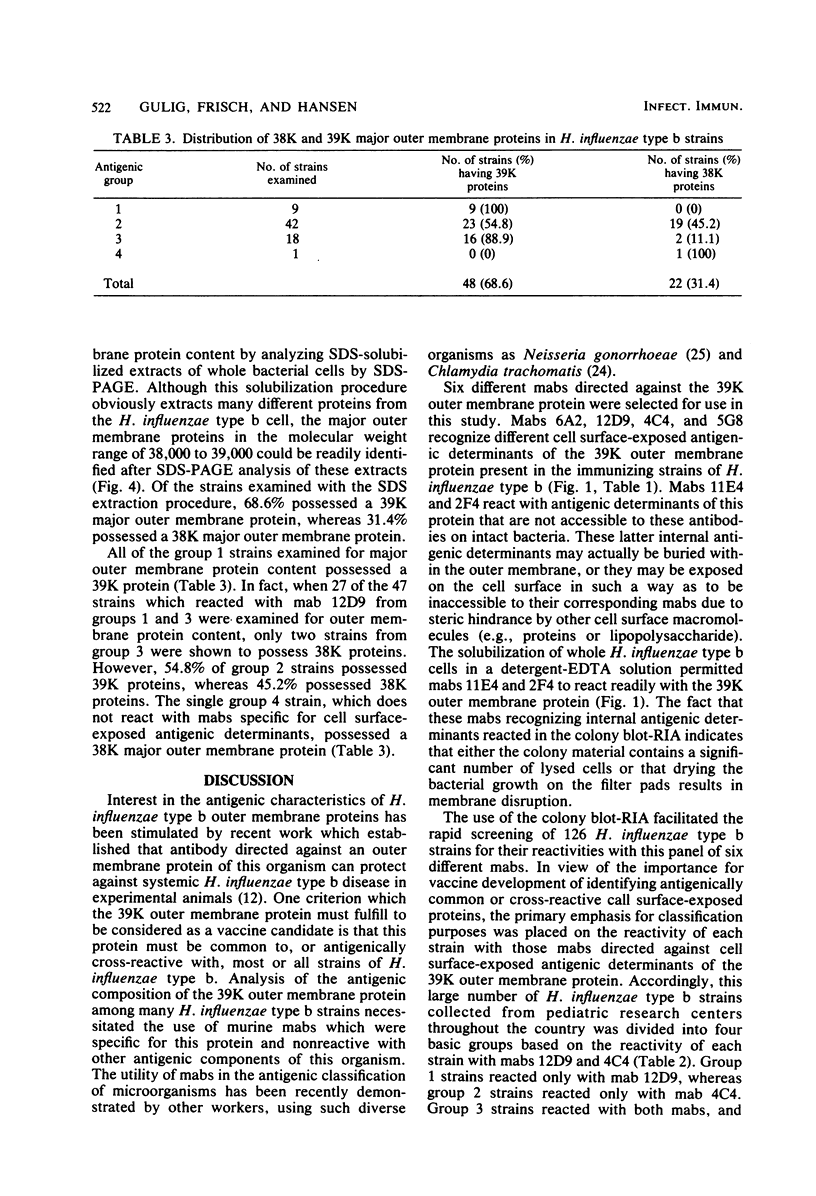
Six murine plasma cell hybridomas producing monoclonal antibodies (mabs) directed against the 39,000-molecular-weight (39K) major outer membrane protein of Haemophilus influenzae type b were employed in the antigenic analysis of the 39K protein. The initial characterization of the mabs by radioimmunoprecipitation analysis showed that four of these mabs reacted with antigenic determinants of the 39K protein that are exposed on the bacterial cell surface and accessible to antibody. The other two mabs reacted with antigenic determinants of the 39K protein that are either not exposed on the H. influenzae type b cell surface or not accessible to antibody (internal determinants). A total of 126 clinical isolates of H. influenzae type b obtained from pediatric research centers throughout the United States were examined for reactivity with the six mabs by using a solid-phase radioimmunoassay in which bacterial colony growth from agar plates was placed on filter paper and used as antigen. The reactivities of these strains with two of the mabs recognizing cell surface-exposed antigenic determinants of the 39K protein were used to divide the 126 strains into four different groups. Group 1 strains reacted with mab 12D9, group 2 strains reacted with mab 4C4, group 3 strains reacted with both mabs 12D9 and 4C4, and group 4 strains (only one was found) did not react with either mab. The reactivities of two other mabs recognizing cell surface-exposed antigenic determinants of the 39K protein were used to further divide the four groups into eight subgroups. A single mab recognizing an internal antigenic determinant of the 39K protein reacted with every H. influenzae type b strain examined in this study. These data indicate that only limited antigenic heterogeneity exists among the cell surface-exposed antigenic determinants of the 39K outer membrane proteins among H. influenzae type b strains and that at least one internal antigenic determinant of the 39K protein is universally present in all H. influenzae type b strains. Radioimmunoprecipitation analysis also demonstrated that H. influenzae type b strains which lacked a 39K major outer membrane protein possessed a 38K major outer membrane protein which reacted with the anti-39K mabs, indicating that the 38K and 39K outer membrane proteins of different H. influenzae type b strains are antigenically related.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander H. E., Heidelberger M., Leidy G. The Protective or Curative Element in Type B H. influenzae Rabbit Serum. Yale J Biol Med. 1944 May;16(5):425–434. [PMC free article] [PubMed] [Google Scholar]
- Anderson P. Antibody responses to Haemophilus influenzae type b and diphtheria toxin induced by conjugates of oligosaccharides of the type b capsule with the nontoxic protein CRM197. Infect Immun. 1983 Jan;39(1):233–238. doi: 10.1128/iai.39.1.233-238.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Subtyping isolates of Haemophilus influenzae type b by outer-membrane protein profiles. J Infect Dis. 1981 May;143(5):668–676. doi: 10.1093/infdis/143.5.668. [DOI] [PubMed] [Google Scholar]
- Chandler C. A., Fothergill L. D., Dingle J. H. STUDIES ON HAEMOPHILUS INFLUENZAE : II. A COMPARATIVE STUDY OF THE VIRULENCE OF SMOOTH, ROUGH, AND RESPIRATORY STRAINS OF HAEMOPHILUS INFLUENZAE AS DETERMINED BY INFECTION OF MICE WITH MUCIN SUSPENSIONS OF THE ORGANISM. J Exp Med. 1937 Nov 30;66(6):789–799. doi: 10.1084/jem.66.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigliotti F., Insel R. A. Protection from infection with Haemophilus influenzae type b by monoclonal antibody to the capsule. J Infect Dis. 1982 Aug;146(2):249–254. doi: 10.1093/infdis/146.2.249. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Rockwell R. Experimental Haemophilus influenzae type b meningitis: immunological investigation of the infant rat model. Infect Immun. 1978 Jun;20(3):705–713. doi: 10.1128/iai.20.3.705-713.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., McCracken G. H., Jr, Frisch C. F., Johnston K. H., Hansen E. J. Antibody response of infants to cell surface-exposed outer membrane proteins of Haemophilus influenzae type b after systemic Haemophilus disease. Infect Immun. 1982 Jul;37(1):82–88. doi: 10.1128/iai.37.1.82-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Haemophilus influenzae type b: disease and immunity in humans. Ann Intern Med. 1973 Feb;78(2):259–269. doi: 10.7326/0003-4819-78-2-259. [DOI] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., Johnston K. H. Detection of antibody-accessible proteins on the cell surface of Haemophilus influenzae type b. Infect Immun. 1981 Sep;33(3):950–953. doi: 10.1128/iai.33.3.950-953.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., McDade R. L., Jr, Johnston K. H. Identification of immunogenic outer membrane proteins of Haemophilus influenzae type b in the infant rat model system. Infect Immun. 1981 Jun;32(3):1084–1092. doi: 10.1128/iai.32.3.1084-1092.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Robertson S. M., Gulig P. A., Frisch C. F., Haanes E. J. Immunoprotection of rats against Haemophilus influenzae type B disease mediated by monoclonal antibody against a haemophilus outer-membrane protein. Lancet. 1982 Feb 13;1(8268):366–368. doi: 10.1016/s0140-6736(82)91394-0. [DOI] [PubMed] [Google Scholar]
- Heckels J. E. Structural comparison of Neisseria gonorrhoeae outer membrane proteins. J Bacteriol. 1981 Feb;145(2):736–742. doi: 10.1128/jb.145.2.736-742.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning U., Schwarz H., Chen R. Radioimmunological screening method for specific membrane proteins. Anal Biochem. 1979 Aug;97(1):153–157. doi: 10.1016/0003-2697(79)90339-7. [DOI] [PubMed] [Google Scholar]
- Hofstra H., Van Tol J. D., Dankert J. Cross-reactivity of major outer membrane proteins of Enterobacteriaceae, studied by crossed immunoelectrophoresis. J Bacteriol. 1980 Jul;143(1):328–337. doi: 10.1128/jb.143.1.328-337.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Human antibody response to individual outer membrane proteins of Haemophilus influenzae type b. Infect Immun. 1982 Sep;37(3):1032–1036. doi: 10.1128/iai.37.3.1032-1036.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Outer membrane protein composition in disease isolates of Haemophilus influenzae: pathogenic and epidemiological implications. Infect Immun. 1980 Dec;30(3):709–717. doi: 10.1128/iai.30.3.709-717.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuwissen J. H., Tauber I., Leeuwenberg A. D., Beckers P. J., Sieben M. Parasitologic and serologic observations of infection with Pneumocystis in humans. J Infect Dis. 1977 Jul;136(1):43–49. doi: 10.1093/infdis/136.1.43. [DOI] [PubMed] [Google Scholar]
- Moxon E. R., Vaughn K. A. The type b capsular polysaccharide as a virulence determinant of Haemophilus influenzae: studies using clinical isolates and laboratory transformants. J Infect Dis. 1981 Apr;143(4):517–524. doi: 10.1093/infdis/143.4.517. [DOI] [PubMed] [Google Scholar]
- Robertson S. M., Frisch C. F., Gulig P. A., Kettman J. R., Johnston K. H., Hansen E. J. Monoclonal antibodies directed against a cell surface-exposed outer membrane protein of Haemophilus influenzae type b. Infect Immun. 1982 Apr;36(1):80–88. doi: 10.1128/iai.36.1.80-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneerson R., Barrera O., Sutton A., Robbins J. B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980 Aug 1;152(2):361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Tam M. R., Kuo C. C., Nowinski R. C. Monoclonal antibodies to Chlamydia trachomatis: antibody specificities and antigen characterization. J Immunol. 1982 Mar;128(3):1083–1089. [PubMed] [Google Scholar]
- Tam M. R., Buchanan T. M., Sandström E. G., Holmes K. K., Knapp J. S., Siadak A. W., Nowinski R. C. Serological classification of Neisseria gonorrhoeae with monoclonal antibodies. Infect Immun. 1982 Jun;36(3):1042–1053. doi: 10.1128/iai.36.3.1042-1053.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E., Mocca L. F. Five structural classes of major outer membrane proteins in Neisseria meningitidis. J Bacteriol. 1981 Apr;146(1):69–78. doi: 10.1128/jb.146.1.69-78.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller P. F., Smith A. L., Anderson P., Smith D. H. The role of encapsulation and host age in the clearance of Haemophilus influenzae bacteremia. J Infect Dis. 1977 Jan;135(1):34–41. doi: 10.1093/infdis/135.1.34. [DOI] [PubMed] [Google Scholar]
- Weller P. F., Smith A. L., Smith D. H., Anderson P. Role of immunity in the clearance of bacteremia due to Haemophilus influenzae. J Infect Dis. 1978 Oct;138(4):427–436. doi: 10.1093/infdis/138.4.427. [DOI] [PubMed] [Google Scholar]