Abstract
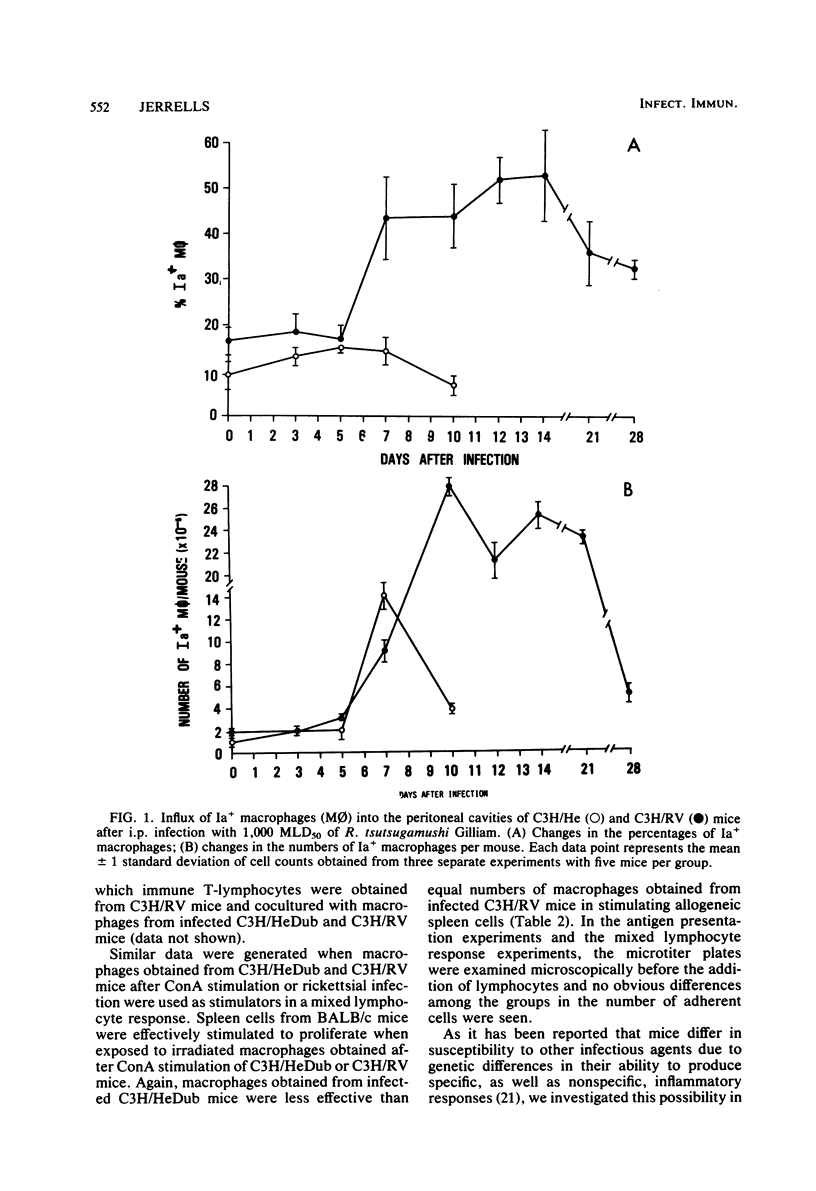
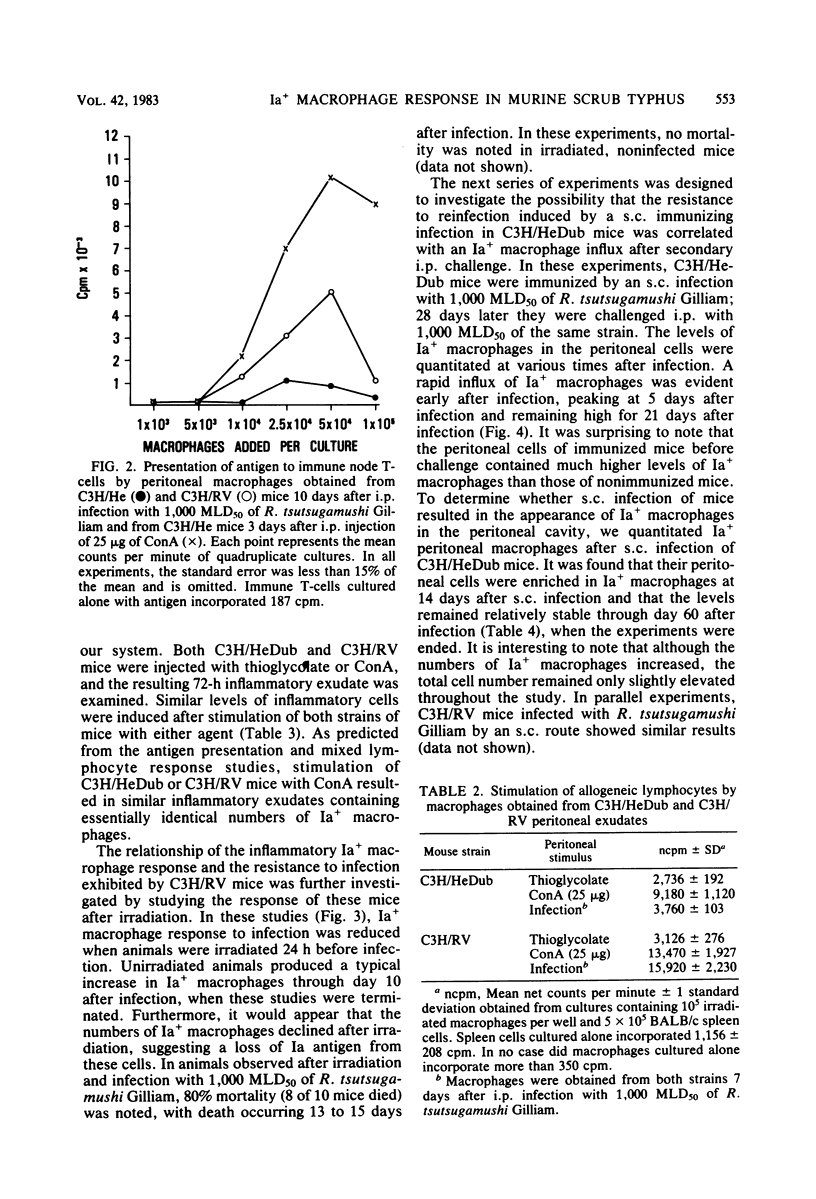
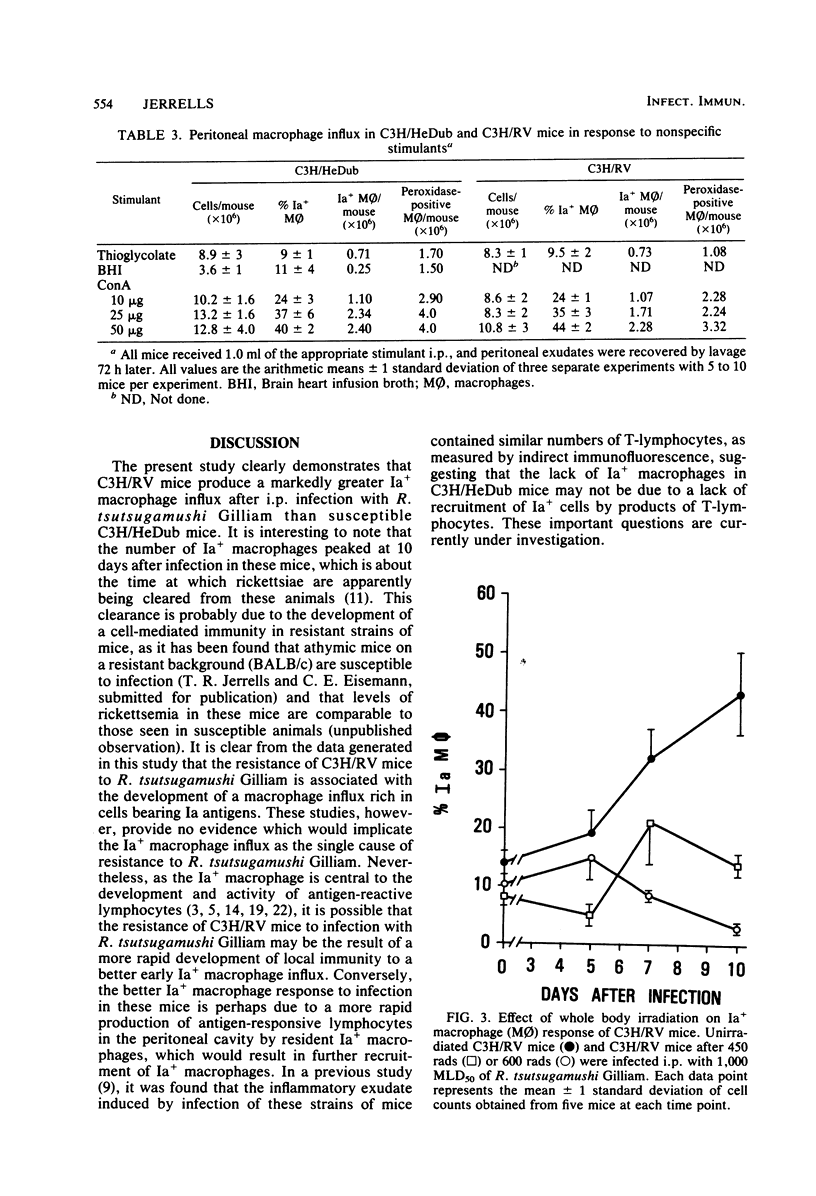
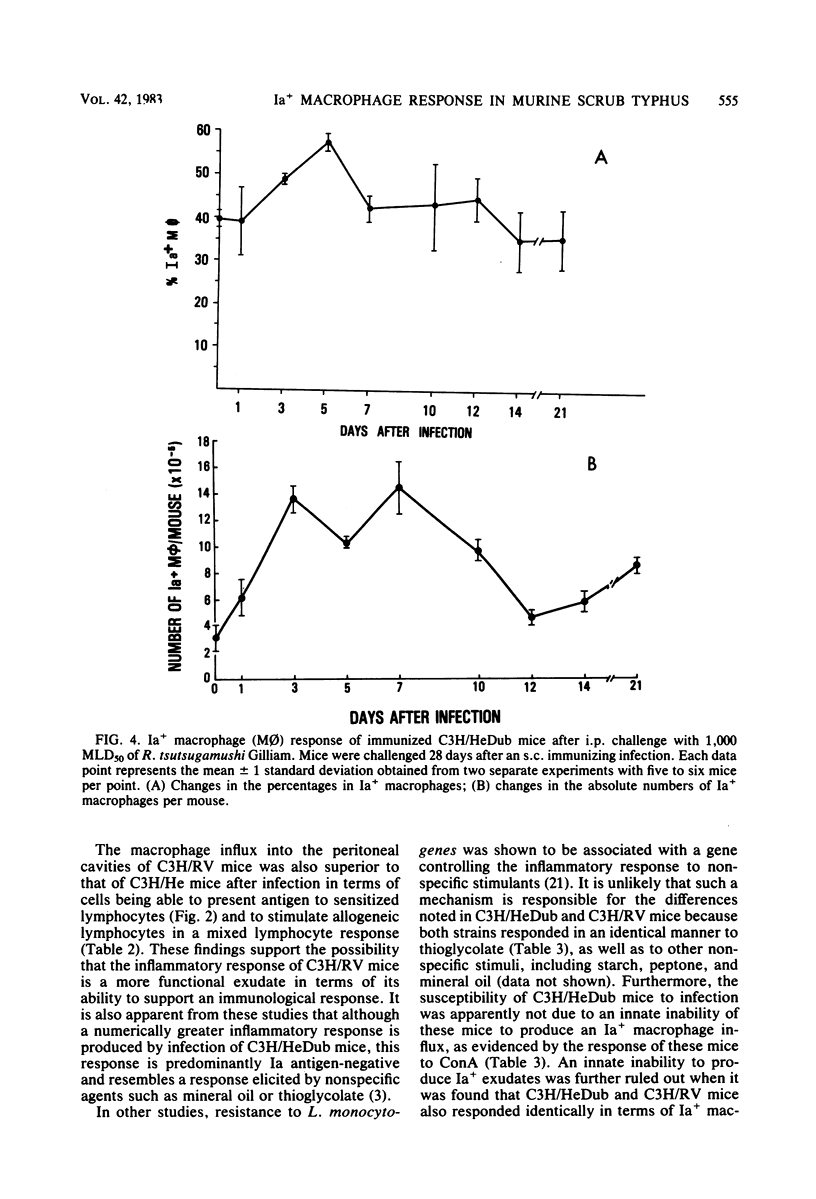
Strains of C3H mice differing in susceptibility to intraperitoneal infection with the Gilliam strain of Rickettsia tsutsugamushi were used to investigate the role of the I region-associated (Ia) antigen-bearing macrophage in the genetic resistance of mice to this organism. Resistant mice (C3H/RV) were found to produce a quantitatively greater Ia antigen-positive macrophage response after infection compared to mice (C3H/HeDub) which underwent a lethal infection. The macrophage influx produced in response to infection of the C3H/HeDub mice was deficient in Ia antigen-bearing cells, as evaluated by antigen presentation function and by the use of macrophages as stimulator cells in a mixed lymphocyte response. The resistance to infection, as well as the Ia-positive macrophage response in C3H/RV mice, was sensitive to 450 to 600 rads of irradiation. C3H/HeDub mice produced exudates rich in Ia-positive macrophages if stimulated with concanavalin A or after challenge with R. tsutsugamushi (if previously immunized), ruling out an innate inability of this strain of mice to produce Ia-positive macrophages exudates. Challenge of either strain of mice immunized by a prior subcutaneous infection resulted in a rapid (3 to 5 days) peak of Ia-positive macrophages responding to the peritoneal cavity. It also was noted that subcutaneous infection alone resulted in an increase in the proportion and number of "resident" macrophages which were Ia positive. These data suggest that the macrophage influx in terms of Ia-bearing cells is at least associated with the genetic resistance of C3H/RV mice to infection with this rickettsiae and may play a role in resistance. Furthermore, it would appear that the Ia-positive macrophage is a factor in acquired immunological resistance to reinfection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagasra O., Schell R. F., Le Frock J. L. Evidence for depletion of Ia+ macrophages and associated immunosuppression in African trypanosomiasis. Infect Immun. 1981 Apr;32(1):188–193. doi: 10.1128/iai.32.1.188-193.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behbehani K., Pan S. C., Unanue E. R. Marked increase in Ia-bearing macrophages during Trypanosoma cruzi infection. Clin Immunol Immunopathol. 1981 May;19(2):190–195. doi: 10.1016/0090-1229(81)90062-3. [DOI] [PubMed] [Google Scholar]
- Beller D. I., Kiely J. M., Unanue E. R. Regulation of macrophage populations. I. Preferential induction of Ia-rich peritoneal exudates by immunologic stimuli. J Immunol. 1980 Mar;124(3):1426–1432. [PubMed] [Google Scholar]
- Ewing E. P., Jr, Takeuchi A., Shirai A., Osterman J. V. Experimental infection of mouse peritoneal mesothelium with scrub typhus rickettsiae: an ultrastructural study. Infect Immun. 1978 Mar;19(3):1068–1075. doi: 10.1128/iai.19.3.1068-1075.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr A. G., Kiely J. M., Unanue E. R. Macrophage-T cell interactions involving Listeria monocytogenes--role of the H-2 gene complex. J Immunol. 1979 Jun;122(6):2395–2404. [PubMed] [Google Scholar]
- Groves M. G., Osterman J. V. Host defenses in experimental scrub typhus: genetics of natural resistance to infection. Infect Immun. 1978 Feb;19(2):583–588. doi: 10.1128/iai.19.2.583-588.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves M. G., Rosenstreich D. L., Taylor B. A., Osterman J. V. Host defenses in experimental scrub typhus: mapping the gene that controls natural resistance in mice. J Immunol. 1980 Sep;125(3):1395–1399. [PubMed] [Google Scholar]
- Jerrells T. R., Osterman J. V. Development of specific and cross-reactive lymphocyte proliferative responses during chronic immunizing infections with Rickettsia tsutsugamushi. Infect Immun. 1983 Apr;40(1):147–156. doi: 10.1128/iai.40.1.147-156.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Osterman J. V. Host defenses in experimental scrub typhus: inflammatory response of congenic C3H mice differing at the Ric gene. Infect Immun. 1981 Mar;31(3):1014–1022. doi: 10.1128/iai.31.3.1014-1022.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Osterman J. V. Role of macrophages in innate and acquired host resistance to experimental scrub typhus infection of inbred mice. Infect Immun. 1982 Sep;37(3):1066–1073. doi: 10.1128/iai.37.3.1066-1073.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongshavn P. A., Sadarangani C., Skamene E. Genetically determined differences in antibacterial activity of macrophages are expressed in the environment in which the macrophage precursors mature. Cell Immunol. 1980 Aug 1;53(2):341–349. doi: 10.1016/0008-8749(80)90334-2. [DOI] [PubMed] [Google Scholar]
- Lu C. Y., Peters E., Unanue E. R. Ia-bearing macrophages in athymic mice: antigen presentation and regulation. J Immunol. 1981 Jun;126(6):2496–2498. [PubMed] [Google Scholar]
- Nacy C. A., Groves M. G. Macrophages in resistance to rickettsial infections: early host defense mechanisms in experimental scrub typhus. Infect Immun. 1981 Mar;31(3):1239–1250. doi: 10.1128/iai.31.3.1239-1250.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadarangani C., Skamene E., Kongshavn P. A. Cellular basis for genetically determined enhanced resistance of certain mouse strains to listeriosis. Infect Immun. 1980 May;28(2):381–386. doi: 10.1128/iai.28.2.381-386.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher M. G., Beller D. I., Unanue E. R. Demonstration of a soluble mediator that induces exudates rich in Ia-positive macrophages. J Exp Med. 1980 Dec 1;152(6):1684–1698. doi: 10.1084/jem.152.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher M. G., Unanue E. R., Beller D. I. Regulation of macrophage populations. III. The immunologic induction of exudates rich in Ia-bearing macrophages is a radiosensitive process. J Immunol. 1982 Jan;128(1):447–450. [PubMed] [Google Scholar]
- Stadecker M. J., Wyler D. J., Wright J. A. Ia antigen expression and antigen-presenting function by macrophages isolated from hypersensitivity granulomas. J Immunol. 1982 Jun;128(6):2739–2744. [PubMed] [Google Scholar]
- Steinman R. M., Nogueira N., Witmer M. D., Tydings J. D., Mellman I. S. Lymphokine enhances the expression and synthesis of Ia antigens on cultured mouse peritoneal macrophages. J Exp Med. 1980 Nov 1;152(5):1248–1261. doi: 10.1084/jem.152.5.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M. M., Kongshavn P. A., Skamene E. Genetic linkage of resistance to Listeria monocytogenes with macrophage inflammatory responses. J Immunol. 1981 Aug;127(2):402–407. [PubMed] [Google Scholar]
- Weinberg D. S., Unanue E. R. Antigen-presenting function of alveolar macrophages: uptake and presentation of Listeria monocytogenes. J Immunol. 1981 Feb;126(2):794–799. [PubMed] [Google Scholar]


