Abstract
Background. The effect of cinacalcet on the structural pattern of hyperplastic parathyroid glands was evaluated, using high-resolution colour Doppler (CD) sonography, in haemodialysis patients with severe, inadequately controlled, secondary hyperparathyroidism (sHPT).
Methods. Nine patients (6 males, 3 females; mean age ± SD, 55.5 ± 12.6 years) received cinacalcet, with adaptation of existing concomitant therapies. Biochemical parameters and the morphology and vascular pattern of hyperplastic parathyroid glands were measured at baseline and every 6 months thereafter, for a follow-up period of 24–30 months.
Results. At baseline, 28 hyperplastic glands were identified. Cinacalcet led to a reduction in glandular volume during the course of the study: 68% in glands with a baseline volume <500 mm3 and 54% in glands with a baseline volume ≥500 mm3. The mean volume ± SD of glands <500 mm3 changed significantly from the baseline (233 ± 115 mm3) to the end of follow-up (102 ± 132 mm3, P = 0.007). Levels of mean serum phosphorus, calcium and calcium–phosphorus product decreased, but not significantly, whereas there were significant decreases in mean parathyroid hormone ± SD levels (1196 ± 381 pg/ml versus 256 ± 160 pg/ml; P < 0.0001) and alkaline phosphatase ± SD levels (428 ± 294 versus 223 ± 88 IU/l; P = 0.04), accompanied by an improvement in a subjective clinical score.
Conclusions. Cinacalcet, in combination with conventional treatments, led to an improvement in biochemical and clinical parameters of sHPT and reduced glandular volume in patients with severe sHPT. Volume reduction was more evident in smaller glands. Longer term, larger, randomized clinical trials are needed to confirm these preliminary findings and to further define a more systematic approach in the treatment of sHPT.
Keywords: chronic kidney disease, cinacalcet, high-resolution sonography, parathyroid hyperplasia, secondary hyperparathyroidism
Introduction
Secondary hyperparathyroidism (sHPT) develops progressively during the course of chronic kidney disease (CKD). Phosphorus retention and reduced synthesis of 1–25 vitamin D result in low serum calcium levels, which causes a feedback secretion of intact parathyroid hormone (iPTH). Long-term hyperstimulation of parathyroids enhances cell proliferation resulting first in diffuse polyclonal hyperplasia and finally in monoclonal nodular hyperplasia [1–3]. Since nodular hyperplasia does not involve all parathyroid glands, or all patients with CKD, to the same degree, it is logical to suppose that genetic mechanisms are involved [4–7].
Glandular volume is a key marker for the severity of sHPT and increased secretion of iPTH [3,8]. However, only indirect measurements of parathyroid volumes are possible due to their location, small size and the difficulty in obtaining accurate measurements of glandular volume with common non-invasive techniques. In healthy patients, the identification of parathyroid glands is particularly difficult [9]. In sHPT, hyperplastic glands become distinguishable from the thyroid parenchyma due to their increased cellularity. High-resolution colour Doppler (CD) sonography is the only tomographic technique able to measure accurately volumetric variations in parathyroid glands. There is no defined size limit over which a parathyroid gland can be considered pathological, although a hypoechoic gland with two diameters >5 mm is generally considered to be hyperplastic [10]. In addition, CD sonography simultaneously provides semi-quantitative measurements of glandular perfusion [11–13].
Patients are considered to have severe sHPT when serum phosphorus (P), calcium (Ca), the Ca–P product (Ca × P) and iPTH levels can no longer be adequately controlled by conventional therapies [14], and when clinical symptoms are associated with a significantly increased risk of cardiovascular morbidity and mortality [14,15]. Following international guidelines, parathyroidectomy (PTX) becomes mandatory when one or more parathyroid glands are enlarged (volume >500 mm3), iPTH values are >700 pg/ml and the response to conventional therapy is poor [13,16–19].
The introduction of the calcimimetic cinacalcet (Mimpara®/Sensipar®) into clinical practice has led to its use in patients with various degrees of severity of sHPT, including those who are not candidates for PTX. Cinacalcet is an allosteric modulator of the calcium receptor (CaR). It increases the sensitivity of the CaR to activation by extracellular calcium and thus suppresses PTH release, while simultaneously lowering levels of serum P and Ca, and Ca × P [13,20–23]. However, no studies to date have been published evaluating morphological variations in the parathyroid glands of maintenance haemodialysis (MHD) patients treated with cinacalcet.
The aim of this study was to evaluate the effect of cinacalcet on the morphology (volume, structure and vascular pattern) of parathyroid glands in MHD patients with severe sHPT, in relation to biochemical parameters and the duration of therapy, using high-resolution CD sonography.
Subjects and methods
Patients
Nine consecutive MHD patients awaiting PTX at our nephrology and dialysis unit (6 males, 3 females; mean age ± SD, 56 ± 13 years) were recruited and prospectively studied. All patients had severe sHPT, and none, due to age, or cardiovascular or general conditions, was an ideal candidate for PTX. In our centre, patients undergo subtotal PTX when one or more glands have a volume >500 mm3 by CD sonography and iPTH values are >700 pg/ml, to avoid the risk of nodular degeneration of the remnant glands. During the period of the study, one additional young patient awaiting transplantation stopped treatment with cinacalcet after 1 month due to drug intolerance (epigastralgia). She was excluded from the study and underwent PTX.
As comparison, we have considered a historical control group that included 11 consecutive patients with clinical, biochemical and morphological signs of severe and refractory sHPT treated with conventional PTX between 2003 and 2006.
Study design
The treatment protocol integrated cinacalcet with patients’ existing pharmacological sHPT therapies (calcium supplements, phosphate binders or vitamin D sterols). Treatment with cinacalcet did not require a pharmacological wash-out period. The starting dose of cinacalcet was 30 mg/day. This was increased by 30 mg every 21–30 days, until the patient reached the targets indicated in the KDOQI™ guidelines. The maximum dose of cinacalcet administered was 150 mg/day. As cinacalcet treatment was used for compassionate use before commercialization in Italy, all patients signed an informed consent form.
Conventional therapy was adjusted during follow-up according to the OPTIMA algorithm [23]. When indicated, the dose of a vitamin D sterol was reduced by ∼50% in sequential steps until a minimum administered dose was reached: intravenous calcitriol, 0.5 μg three times per week; intravenous alfacalcidol, 1 μg three times per week; intravenous paricalcitol, 2 μg three times per week; oral calcitriol, 0.25 μg three times per week; oral alfacalcidol, 0.25 μg/day. The dose of cinacalcet was reduced if a patient was not receiving vitamin D. Phosphate binder dosages were modulated depending on serum Ca and P levels in an attempt to maintain Ca × P within the international guidelines range [23,13].
Biochemical parameters [P, Ca, Ca × P, iPTH and alkaline phosphatase (ALP)] and the morphology and vascular pattern of the parathyroid glands were measured at baseline and every 6 months thereafter, for a follow-up period of 24–30 months. Changes in cinacalcet dosing and the onset of any side effects were recorded throughout this period. iPTH was measured using the Nichols IRMA method.
To prove the effect of cinacalcet on the functional behaviour of parathyroid glands, an additional experiment was performed to see whether or not serum iPTH increased immediately after an interruption of cinacalcet. In the absence of any change in other treatments, the drug was stopped for 2 weeks in three patients who had experienced the highest reductions in iPTH and gland volume and in two patients who had experienced the highest reduction in iPTH, but only mild reductions in gland volume and vascularization. iPTH was measured twice, under exactly the same conditions (i.e. at morning, before the dialysis, at the start and at the end of the test and finally 2 weeks after restarting the therapy).
Imaging techniques
High-resolution CD sonography was carried out in our unit by an expert nephrologist using digital equipment (Logiq 9TM, General Electric Medical Systems, USA) and a small parts linear array (matrix 1.5D). Transmission frequency (9–14 MHz) provided an axial and a lateral resolution of 0.2 mm. Images were acquired using harmonic imaging and spatial compounding to reduce speckle.
Patients were examined with the neck hyperextended in a supine decubitus. The anatomical location of the parathyroid glands [23–25] was determined by a sequence of transverse, longitudinal and oblique scans in the laterocervical region from the corner of the jaw to the superior mediastinum. The mediocervical transverse scan was essential to identify the thyroid lobes and isthmus. The same transverse scan along the laterocervical line was used to identify the usual landmarks (sternocleidomastoid muscle, pre-thyroid muscles, epiaortic vessels, oesophagus, pre-vertebral muscles). Longitudinal and oblique scans were used to define the anatomical relationships with the homolateral thyroid lobe, the oesophagus, the long muscles of the neck, the laterocervical vessels and the thyroid and cricoid cartilages. Additional oblique scans were carried out on the jugular fossa to identify the inferior parathyroid glands, sometimes located in the superior mediastinum or in the horn of the thymus. The inferior thyroid artery in the mid-cervical tract was also located to define vascularization of parathyroid glands.
Normal parathyroid glands are rich in adipose cells so they are indistinguishable from the thyroid parenchyma even using high-resolution probes. Glandular hyperplasia changes the ultrasound pattern: the gland becomes diffusely hypoechoic and is outlined from the thyroid parenchyma by a thin hyperechoic edge. Using the ellipsoid formula (4/3 π × 1/2 anteroposterior diameter × 1/2 latero-lateral diameter × 1/2 craniocaudal diameter), it is possible to calculate the gland volume in mm3. Glands with nodular hyperplasia appear as hypoechoic nodules that are well outlined from the thyroid lobes in common locations. Glands with at least two diameters >5 mm and a volume <500 mm3 are considered definitively to be pathological (diffuse or polyclonal hyperplasia), whereas glands with a diameter >1 cm and overall volume ≥500 mm3 are considered to have monoclonal nodular degeneration [16,17,25,26]. Parameters used to evaluate morphological changes in the glands were (1) gland diameter and volume; (2) presence of anechoic areas (suggesting cystic degeneration) [27] and absence of vascularized areas; (3) echogenicity.
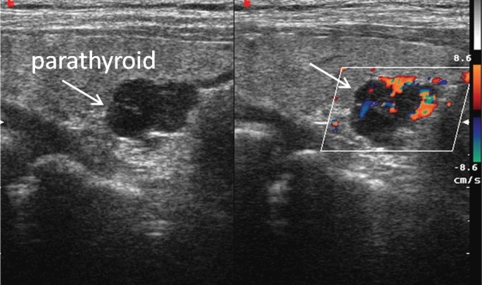
B-Mode sonography was combined with CD sampling. Nodular flow was characterized using a pulse repetition frequency (PRF) <800 kHz, CD frequency 3.8 MHz and mid-high colour gain (set to 45–50, with a range of 0–60), and a steering angle of 30°. Vascular pattern is a semi-quantitative parameter, dependent on both the settings of the sonolayer and subjective evaluation by the operator. Three different vascularization patterns were identified: type 1, glands with no Doppler signal; type 2, hypovascularized glands with a poor or weak Doppler signal, represented by occasional colour spots in the hilar/endonodular region; type 3, hypervascularized glands having an enlarged feeding artery at the hilum, a peripheral arc of vascularity and/or ray-like endonodular vessels [27–29] (Figure 1). All sonographic examinations were recorded and reviewed by two other skilled operators who used the same parameters. An intra-class correlation coefficient (ICC) was used to assess inter-observer reliability. Intra-observer ICC was 0.95 whereas inter-observer ICC was 0.91 for measurements of glandular volume.
Fig. 1.
Longitudinal scan of right lobe of thyroid. The image shows a hypervascularized parathyroid gland, characterized by an enlarged feeding artery at the hilum, a peripheral arc of vascularity and ray-like endonodular vessels.
Statistical analysis
Data were analysed using Student's t-test for paired and non-paired data and were considered significant for values of P < 0.05. The conformity of our data set was measured using the ICC (an ICC >0.70 was considered valid). The correlation between two variables was assessed using the Pearson product–moment correlation coefficient.
Results
Baseline clinical and biochemical parameters
Causes of CKD were hereditary and/or congenital (n = 4), glomerulonephritis (n = 3), pyelonephritis (n = 1) and chronic ischaemic nephropathy (n = 1). Causes of CKD in the control group were hereditary and/or congenital (n = 3), glomerulonephritis (n = 6) and pyelonephritis (n = 2). Baseline biochemical parameters of mineral metabolism were consistent with severe sHPT and were similar in the two groups. Mean values of serum P and Ca, Ca × P, iPTH, ALP, mean dialytic age and mean duration of pre-dialysis CKD duration are presented in Table 1.
Table 1.
Baseline clinical, biochemical and sonographic parameters
| Historical control group (n = 11) | Cinacalcet group (n = 9) | P-value | |
|---|---|---|---|
| Mean age ± SD (years) | 46 ± 14 | 56 ± 13 | NS |
| Sex | 5 M/6 F | 6 M/3 F | |
| Mean ± SD duration of dialysis uraemia (months) | 62 ± 48 | 111 ± 75 | NS |
| Mean ± SD duration of pre-dialysis uraemia (months) | 74 ± 53 | 136 ± 67 | 0.01 |
| Phosphorus ± SD (mg/dl) | 6.3 ± 0.8 | 5.4 ± 1.3 | NS |
| Calcium ± SD (mg/dl) | 10.1 ± 0.7 | 9.5 ± 0.7 | 0.03 |
| Ca × P ± SD (mg2/dl2) | 64 ± 11 | 50 ± 11 | 0.01 |
| iPTH ± SD (pg/ml) | 1405 ± 590 | 1196 ± 381 | NS |
| ALP ± SD (IU/l) | 265 ± 113 | 428 ± 294 | NS |
| Parathyroid glands (n) | 36 | 28 | |
| <500 mm3 | 19 | 15 | |
| ≥500 mm3 | 17 | 13 | |
| Mean ± SD glandular volume (mm3) | 798 ± 740 | 731 ± 897 | NS |
| B-mode structural pattern | Hypoechoic | Hypoechoic | |
| Vascular pattern, n (%) | |||
| Type 1 | 0 | 0 | |
| Type 2 | 7 (19) | 3 (11) | |
| Type 3 | 29 (81) | 25 (89) | |
| Involutive cystic areas | 0 | 0 |
ALP, alkaline phosphatase; Ca × P, calcium–phosphorus product; iPTH, intact parathyroid hormone; NS, not significant.
iPTH: 1 pmol/l = 1 pg/ml × 0.105; calcium: 1 mmol/l = 1 mg/dl × 0.25; phosphorus: 1 mmol/l = 1 mg/dl × 0.323.
Baseline morphological parameters
High-resolution sonography identified two or more hyperplastic parathyroid glands in each patient (two glands in two patients, three glands in four patients and four glands in three patients), giving a total of 28 hyperplastic glands. Twenty-five glands were in common locations, and three were in the jugular fossa. None of the glands showed signs of cystic degeneration. The mean volume ± SD was 731 ± 897 mm3 (range 42–4180 mm3): 15 glands had a volume <500 mm3, whereas 13 glands had a volume ≥500 mm3; CD sampling revealed a hypervascular pattern in the hyperplastic glands. Pre-surgical sonography in the historical control group revealed 36 glands (two glands in two patients, three glands in four patients and four glands in five patients). The mean volume ± SD was 798 ± 740 mm3 (range 55–2257 mm3): 19 glands had a volume <500 mm3 and 17 glands a volume ≥500 mm3. None of the glands showed cystic degeneration. Also in this group, CD sampling revealed a hypervascular pattern in the hyperplastic glands. The clinical, biochemical and sonographic profiles of the two groups are outlined in Table 1. Forty glands were excised. At histological examination, 18 glands showed a nodular pattern, while 22 glands revealed a diffused hyperplasia. Glands with a nodular pattern showed a sonographic volume ≥500 mm3 in 84% of cases.
Baseline pharmacological sHPT therapies and clinical scores
Table 2 shows baseline pharmacological sHPT therapies. The historical control group received similar conventional therapies [vitamin D derivatives or phosphate binders (sevelamer or calcium-based phosphate binders) at various doses and in various combinations] to the cinacalcet group. In the study group, the severity of sHPT-related clinical symptoms (diffuse bone pain, articular tenderness, walking difficulties, muscular pain and itching) was subjectively evaluated and scored by two clinicians, from 0 to 4: 0 = no symptoms; 1 = mild osteoarticular tenderness, with no adverse effect on daily life; 2 = diffuse osteoarticular tenderness, limiting normal activities; 3 = diffuse osteoarticular tenderness, with total limitation of daily activities and/or itching on administration of drugs; 4 = unbearable itching accompanied by skin injury due to scratching, severe deficiency in limb functionality, and limited ability to walk. Clinical scores were similar between the two groups.
Table 2.
Pharmacological treatment at baseline
| Historical control group (n = 11) | Cinacalcet group (n = 9) | |
|---|---|---|
| Vitamin D | ||
| Received vitamin D derivative, n (%) | 9 (81) | 8 (89) |
| Mean ± SD paricalcitol dose equivalentsa (μg/week) | 3.2 ± 1.2 | 3.6 ± 1.9 |
| Phosphate binders | ||
| Sevelamer | ||
| Received sevelamer, n (%) | 8 (72) | 5 (56) |
| Mean ± SD sevelamer dose (mg/day) | 4220 ± 580 | 3360 ± 876 |
| Calcium-based phosphate binder | ||
| Received calcium-based phosphate binder, n (%) | 6 (54) | 3 (33) |
| Mean ± SD total elemental calcium intake (mg/day) | 3600 ± 980 | 3100 ± 1087 |
a2 μg paricalcitol = 1 μg doxercalciferol = 0.5 μg calcitriol.
Follow-up periods and dose adjustments
Four patients remained in the study for a 30-month follow-up period. Five patients had a follow-up of 24 months, two of whom died due to major cardiovascular events. Three patients received a final cinacalcet dose of 30 mg/day, four patients received 60 mg/day and two patients received 120 mg/day.
Effect of cinacalcet on biochemical parameters
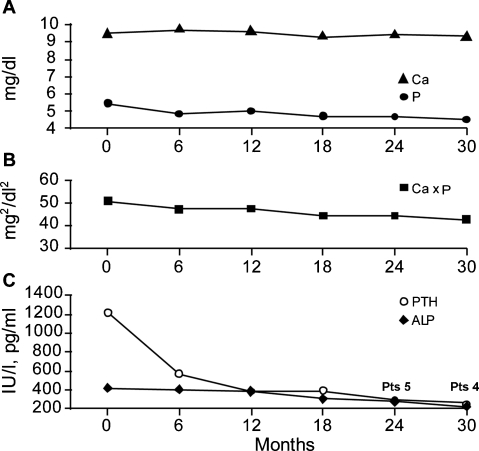
Mean serum P and Ca, and Ca × P decreased throughout the course of the study, but not significantly. However, there was a significant change in mean levels of iPTH ± SD (1196 ± 381 pg/ml versus 256 ± 160 pg/ml; P < 0.0001) and mean serum ALP ± SD values (428 ± 294 versus 223 ± 88 IU/l; P = 0.04). Variations in mean serum P and Ca, Ca × P, iPTH and ALP are shown in Figure 2. Values for biochemical parameters at baseline and at the end of the follow-up period are shown in Table 3.
Fig. 2.
(A–C). (A) Variations in mean serum phosphorus (P) and calcium (Ca), (B) the Ca–P product (Ca × P), and (C) intact parathyroid hormone (iPTH) and alkaline phosphatase (ALP) following treatment with cinacalcet. The mean serum P and Ca, and Ca × P did not change significantly with cinacalcet treatment. Mean ± SD iPTH decreased from 1196 ± 381 pg/ml at baseline to 256 ± 160 pg/ml at the end of follow-up (P < 0.0001). Mean ± SD serum ALP decreased from 428 ± 294 IU/l at baseline to 223 ± 88 IU/l at the end of follow-up (P = 0.04).
Table 3.
Biochemical parameters at baseline and following treatment with cinacalcet
| Baseline | End of follow-up (24–30 months) | P-value | |
|---|---|---|---|
| Phosphorus ± SD (mg/dl) | 5.4 ± 1.3 | 4.5 ± 0.8 | NS |
| Calcium ± SD (mg/dl) | 9.5 ± 0.7 | 9.3 ± 0.4 | NS |
| Ca × P ± SD (mg2/dl2) | 50 ± 11 | 42 ± 8 | NS |
| iPTH ± SD (pg/ml) | 1196 ± 381 | 256 ± 160 | <0.0001 |
| ALP ± SD (IU/l) | 428 ± 294 | 223 ± 88 | 0.04 |
ALP, alkaline phosphatase; Ca × P, calcium–phosphorus product; iPTH, intact parathyroid hormone; NS, not significant.
iPTH: 1 pmol/l = 1 pg/ml × 0.105; calcium: 1 mmol/l = 1 mg/dl × 0.25; phosphorus: 1 mmol/l = 1 mg/dl × 0.323.
Effect of cinacalcet on clinical scores
Clinical scores, measuring subjective symptoms, showed substantial improvement during the course of the study in all patients. In patients with severe symptomatology (baseline score of 4), the score improved by two or three points, with an improvement in pain, osteoarticular tenderness and itching.
Effect of cinacalcet on morphological ultrasound parameters
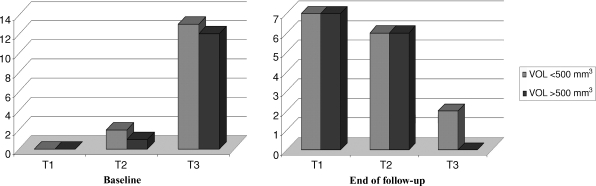
Twenty-eight glands of various shapes and volumes were examined at baseline and at 6 monthly intervals until the end of the follow-up period. Overall, the mean volume ± SD of the glands did not change significantly during the course of the study (from 731 ± 897 mm3 at baseline to 539 ± 1017 mm3; P = 0.5), but there was a correlation of 0.91 between baseline glandular volume and glandular volume following treatment with cinacalcet. There was no statistically significant association between serum iPTH and parathyroid gland size at baseline or at the end of the study. A summary of the change in sonographic patterns before and after treatment is reported in Table 4.
Table 4.
Sonographic variations of parathyroid glands after cinacalcet treatment
| Baseline | End of follow-up | P-value | |
|---|---|---|---|
| Glands (N) | 28 | 23a | |
| <500 mm3 | 15 | 17a | |
| ≥500 mm3 | 13 | 6 | |
| Total mean ± SD volume (mm3) | 731 ± 897 | 539 ± 1017 | NS |
| <500 mm3 | 233 ± 115 | 102 ± 132b | 0.007 |
| ≥500 mm3 | 1036 ± 1062 | 837 ± 1290b | NS |
| Reduction in volume (%) | 60 | ||
| <500 mm3 | 68 | ||
| ≥500 mm3 | 54 | ||
| Cystic areas (N) | 0 | 13 | |
| <500 mm3 | 5 | ||
| ≥500 mm3 | 8 |
NS, not significant.
aFive glands with baseline volume <500 mm3 became indistinguishable.
bOne gland in each group increased in volume due to involutive cystic degeneration.
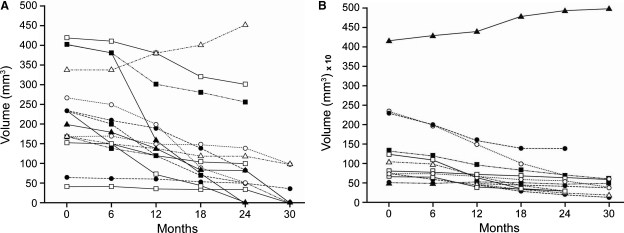
Fifteen glands had a baseline volume <500 mm3. The mean volume ± SD of these glands changed significantly during the course of the study (from 233 ± 115 mm3 at baseline to 102 ± 132 mm3; P = 0.007), with a mean decrease in volume of 68% (Figures 3A and 4 and Table 4). At the end of the follow-up period, five glands had become indistinguishable from the thyroid parenchyma, nine glands had diminished in volume and one gland was enlarged, due to the appearance of an extended anechoic area within the nodule. Moreover, eight glands acquired a hyperechoic pattern, six became hypovascularized and seven became avascularized. Anechoic areas of cystic degeneration appeared in five glands, which had a baseline volume >350 mm3.
Fig. 3.
(A) Volume changes in glands with baseline volume <500 mm3 following treatment with cinacalcet. Fifteen glands had a baseline volume <500 mm3. The mean volume ± SD changed from f ± 115 mm3 at baseline to 102 ± 132 mm3 at the end of follow-up (P = 0.007). (B) Volume changes in glands with baseline volume ≥500 mm3 following treatment with cinacalcet. Thirteen glands had a baseline volume ≥500 mm3. The mean volume ± SD changed from 1036 ± 1062 mm3 at baseline to 837 ± 1290 mm3 at the end of follow-up (P = 0.3).
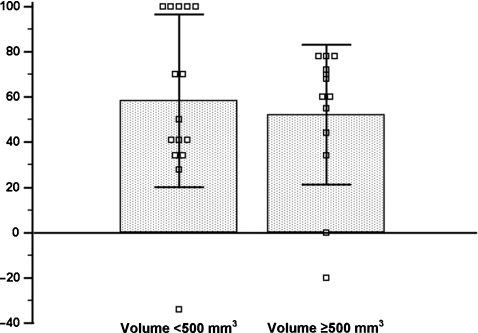
Fig. 4.
Percentage of volume reduction in glands with a baseline volume <500 and ≥500 mm3, means and SDs.
Thirteen glands had a baseline volume ≥500 mm3. The mean volume ± SD of these glands did not change statistically significantly during the course of the study (from 1036 ± 1062 mm3 at baseline to 837 ± 1290 mm3; P = 0.3) (Figure 3B and Table 4), with a mean reduction percentage of volume of 54% (Figure 4). By the end of the follow-up period, 11 glands had reduced in volume, 1 gland had no change in volume and 1 gland was enlarged, due to the appearance of extended anechoic areas of cystic degeneration. Figure 5 shows 1 of the 11 glands that had reduced in volume: at baseline, 12 months following treatment with cinacalcet and at the end of the follow-up period. Of the 13 glands analysed, 8 acquired a hyperechoic pattern and 8 glands showed extended anechoic areas of cystic degeneration. During the course of the study, six glands became hypovascularized and seven became avascularized. A summary of the vascular pattern changes is outlined in Figure 6.
Fig. 5.
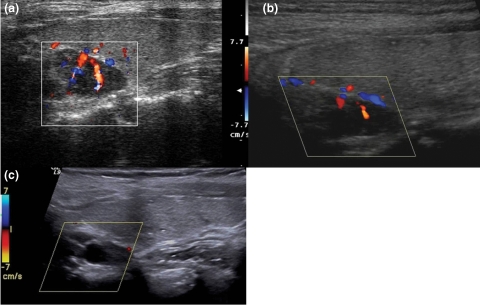
Longitudinal scans of the right thyroid lobe before and after cinacalcet treatment. A parathyroid gland with a nodular, hypoechoic pattern is shown behind the thyroid lobe. (a) Baseline image acquired with high-resolution colour Doppler (CD) showing a vascular hilum with ray-like centrifugal vessels. Baseline iPTH was 1623 pg/ml and gland volume was 1337 mm3. (b) The same gland after 12 months, showing a reduced volume and a hypovascular pattern. (c) CD sampling at low pulse repetition frequency (800 kHz) at the end of follow-up, showing a significant volume reduction (1337 mm3 versus 608 mm3), the complete absence of endonodular perfusion and the appearance of an anechoic area of cystic degeneration. Patient intact parathyroid hormone was 148 pg/ml.
Fig. 6.
Vascular pattern of glands at baseline and at the end of follow-up.
Effect of interruption of cinacalcet treatment
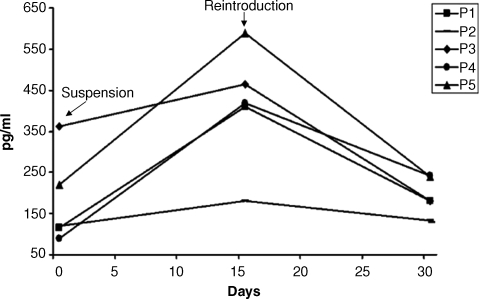
After a 2-week suspension, a rebound of serum iPTH levels occurred in all patients, even in those who had shown major involutive phenomena and lower values of iPTH at the end of the follow-up. The reintroduction of cinacalcet led to another reduction in serum iPTH levels in all patients. The results of this experiment are given in Figure 7.
Fig. 7.
Variations in serum intact parathyroid hormone (iPTH) levels following the suspension and the reintroduction of cinacalcet.
Discussion
Phase II and III studies have shown that cinacalcet successfully lowers serum iPTH in MHD patients with sHPT, simultaneously controlling parameters associated with the Ca–P balance [21,22]. Long-term studies have given similar results [30]. An experimental study showed that stabilization of mineral metabolism parameters was accompanied by a decrease in parathyroid hyperplasia in uraemic rat models treated with cinacalcet, with no evidence of increased apoptosis [31]. To date, there has been a lack of experimental models or studies focused on the effect of cinacalcet on nodular hyperplasia. Furthermore, no data have been published regarding the effect of cinacalcet on potential morphological variations in hyperplastic parathyroid glands in CKD patients with severe sHPT. Clinical evaluation of sHPT is usually carried out by the assessment of biochemical parameters rather than via morphological measurements. The aim of this observational study was to investigate the effects of long-term therapy with cinacalcet, in association with conventional therapy, on the morphology of hyperplastic parathyroid glands.
We show here that cinacalcet had a more rapid effect on the biochemical profile of sHPT than on the morphology of parathyroid glands. Changes in volume, vascularization and sonographic pattern were observed after 12–18 months of therapy. After this time, we observed a progressive change in the structural pattern of hyperplastic glands. Glands with a baseline volume ≥500 mm3 had a tendency to show involutive phenomena, such as anechoic areas of cystic degeneration. This was associated with a decrease in glandular volume in all but one case, where gland size increased. In glands with a baseline volume <500 mm3, there were more significant changes in volume. There was a tendency for the structural pattern of these glands to change from hypoechoic (suggesting that they were hyperplastic with a rich cellularity) to hyperechoic (suggesting that they were quiescent with an increased number of adipose cells). In the most significant cases, glands became very small and, like normal parathyroid glands, were indistinguishable from the thyroid parenchyma.
We have noted a relationship between major involutive phenomena and a major reduction in iPTH and Ca serum levels, suggesting that a lower dose of cinacalcet may have been used. Interestingly, independent of baseline volume, glands that became hypovascularized following cinacalcet treatment inversely reproduced the progression of the parathyroid gland to hyperplasia (i.e. from a hypovascularized to a hypervascularized pattern).
Critical analysis of these data suggests that stabilization of the biochemical profile of sHPT, as well as the involutive phenomena in the glands, was observed only after the introduction of cinacalcet in the therapeutical schema. These observations suggest that cinacalcet is able to modulate the biological response of parathyroid cells in synergy with vitamin D and phosphate binders. So, the degenerative effects that are observed during long-term treatment have to be considered as a result of a combined pharmacological effect.
Cystic degeneration and hypovascularization were evident in glands with diffuse and nodular hyperplasia. Physiopathological mechanisms responsible for glandular degeneration are unknown and should be the objective of further studies. At the moment, findings do not support or exclude the possibility that glandular degeneration is due to enhanced apoptosis [32] as well as to an indirect effect of cinacalcet on the vessel tree [33]. Nevertheless, it should be noted that a recent study has reported a pro-apoptotic effect of cinacalcet in glands with nodular hyperplasia excised in patients not previously treated with phosphate binders or vitamin D [34].
To determine whether cinacalcet could be considered the trigger of the functional response of hyperplastic glands, cinacalcet therapy was interrupted temporarily in a small number of patients. After 2 weeks of suspension, serum iPTH levels increased in all patients, even in those who had the major involutive phenomena. Interestingly, the increase in serum iPTH values was smaller as compared with initial PTH levels (mean values close to 1200 pg/ml). One explanation might be that PTH secretion was lower after long-term cinacalcet therapy due to a reduction in the number of functional parathyroid cells (mass reduction). These data, although not definitive, are interesting: they demonstrate the efficacy of the drug on biochemical and functional profiles, but they are contradictory because they do not demonstrate a clear association between involutive phenomena and a reduction in function of the glands. Possible explanations for this could be linked to the persistence of glandular cellular masses that are no longer inhibited after the suspension of the drug or to the possibility that the apparent cellular necrosis is not sufficiently complete to withstand interruption of the drug. Furthermore, the response seen in iPTH after discontinuation and reintroduction of cinacalcet suggests that the remnant parathyroid tissue responded to stimulation of the CaR. These findings confirm the evidence from pre-clinical studies that show that calcimimetics inhibit parathyroid hyperplasia [35] in subtotally nephrectomized rats [31]. However, it should be noted that cellular cultures in vitro [36] and the post-5/6 nephrectomy rat model of secondary HPT are not a true reflection of the progression of nodular hyperplasia in humans [31].
The main limitations of this study were the small number of patients and that data for the control patients were collected retrospectively. In addition, some analyses were based on the number of glands, but the changes in gland volume within a patient are unlikely to be independent, and this has not been accounted for in the statistical analyses.
In summary, the practical consequences of these preliminary data are the need for a combination of cinacalcet with conventional therapy and also the monitoring of the glandular response with morphological parameters. Finally, the reductions in glandular volume and vascularization, particularly in smaller glands, provide a strong argument for the early use of cinacalcet (and at lower doses) in combination with standard therapy. Early ‘pharmacological PTX’, using cinacalcet in combination with conventional therapy, could reduce the number of patients who eventually require a surgical PTX.
Acknowledgments
We thank Amgen Europe for providing funding for editing support.
Conflict of interest statement. The authors have no involvement, financial or otherwise, that may potentially bias this work. The study was conducted without any external financial support, but editing support was funded by Amgen (Europe).
(See related article by H. Komaba and M. Fukagawa. Regression of parathyroid hyperplasia by calcimimetics—fact or illusion? Nephrol Dial Transplant 2009; 24: 707–709.)
References
- 1.Drueke TB. The pathogenesis of parathyroid gland hyperplasia in chronic renal failure, Nephrology Forum. Kidney Int. 1995;48:259–272. doi: 10.1038/ki.1995.292. [DOI] [PubMed] [Google Scholar]
- 2.Parfitt AM. The hyperparathyroidism of chronic renal failure: a disorder of growth. Kidney Int. 1997;52:3–9. doi: 10.1038/ki.1997.297. [DOI] [PubMed] [Google Scholar]
- 3.Drueke TB. Cell biology of parathyroid gland hyperplasia in chronic renal failure. J Am Soc Nephrol. 2000;11:1141–1152. doi: 10.1681/ASN.V1161141. [DOI] [PubMed] [Google Scholar]
- 4.Arnold A, Brown MF, Urena P, et al. Monoclonality of parathyroid tumors in chronic renal failure and in primary parathyroid hyperplasia. J Clin Invest. 1995;95:2047–2053. doi: 10.1172/JCI117890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salem M. Hyperparathyroidism in the hemodialysis population: a survey of 612 patients. Am J Kidney Dis. 1997;29:862–865. doi: 10.1016/s0272-6386(97)90459-5. [DOI] [PubMed] [Google Scholar]
- 6.Stanbury SW, Lumb GA. Parathyroid function in chronic renal failure. Q J Med. 1966;33:1–23. [PubMed] [Google Scholar]
- 7.Fukagawa M. Cell biology of parathyroid hyperplasia in uremia. Am J Med Sci. 1999;317:377–382. doi: 10.1097/00000441-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Indridason OS, Heath H, III, Khosla S, et al. Non-suppressible parathyroid hormone secretion is related to gland size in uremic secondary hyperparathyroidism. Kidney Int. 1996;50:1663–1667. doi: 10.1038/ki.1996.483. [DOI] [PubMed] [Google Scholar]
- 9.Bannister LH, Berry MM, Collins P. Gray's Anatomy. The Parathyroid Glands. New York: Churchill Livingstone; 1995. [Google Scholar]
- 10.O’Doherty MJ, Kenle AG. Symposium on parathyroid localization. Parathyroid imaging: preoperative localization. Nucl Med Commun. 2003;24:125–131. doi: 10.1097/00006231-200302000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Fukagawa M, Kitaoka M, Yi H, et al. Serial evaluation of parathyroid size by sonography is another useful marker for the long-term prognosis of calcitriol pulse therapy in chronic dialysis patients. Nephron. 1994;68:221–228. doi: 10.1159/000188261. [DOI] [PubMed] [Google Scholar]
- 12.Katoh N, Nakayama M, Shigematsu T, et al. Presence of sonographically detectable parathyroid gland can predict resistance to oral pulsed-dose calcitriol treatment of secondary hyperparathyroidism. Am J Kidney Dis. 2000;35:466–468. doi: 10.1016/s0272-6386(00)70199-5. [DOI] [PubMed] [Google Scholar]
- 13.NKF-K/DOQI Clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;85:S111–S114. [PubMed] [Google Scholar]
- 14.de Francisco ALM. Secondary hyperparathyroidism: review of the disease and its treatment. Clin Ther. 2004;26:1976–1993. doi: 10.1016/j.clinthera.2004.12.011. de Francisco ALM. New strategies for the treatment of hyperparathyroidism incorporating calcimimetics. Expert Opin Pharmacother 2008; 9: 795–811. [DOI] [PubMed] [Google Scholar]
- 15.Rostand SG, Drueke TB. Parathyroid hormone vitamin D, and cardiovascular disease in chronic renal failure. Kidney Int. 1999;56:383–392. doi: 10.1046/j.1523-1755.1999.00575.x. Tentori F, Blayney MJ, Albert JM et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2008; 52: 519–530. [DOI] [PubMed] [Google Scholar]
- 16.Pavlovic D. Prevention and treatment of secondary hyperparathyroidism: still a challenge for the nephrologist? Nephrol Dial Transplant. 2003;18:S45–S46. doi: 10.1093/ndt/gfg1045. [DOI] [PubMed] [Google Scholar]
- 17.Schomig M, Ritz E. Indications for parathyroidectomy. Nephrol Dial Transplant. 2000;15:S25–S29. doi: 10.1093/ndt/15.suppl_5.25. [DOI] [PubMed] [Google Scholar]
- 18.Tominaga Y, Tanaka Y, Sato K, et al. Histopathology, pathophysiology and indications for surgical treatment of renal hyperparathyroidism. Semin Surg Oncol. 1997;13:78–86. doi: 10.1002/(sici)1098-2388(199703/04)13:2<78::aid-ssu3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 19.Grahame JE. Parathyroidectomy in the calcimimetic era. Nephrology. 2005;10:511–515. doi: 10.1111/j.1440-1797.2005.00476.x. [DOI] [PubMed] [Google Scholar]
- 20.Nemeth EF, Heaton WH, Miller M, et al. Pharmacodynamics of the type II calcimimetic compound cinacalcet HCl. J Pharmacol Exp Ther. 2004;308:627–635. doi: 10.1124/jpet.103.057273. [DOI] [PubMed] [Google Scholar]
- 21.Block GA, Martin KJ, de Francisco AL, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350:1516–1525. doi: 10.1056/NEJMoa031633. [DOI] [PubMed] [Google Scholar]
- 22.Lindberg JS, Culleton B, Wong G, et al. Cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: a randomized, double-blind, multicenter study. J Am Soc Nephrol. 2005;16:800–807. doi: 10.1681/ASN.2004060512. [DOI] [PubMed] [Google Scholar]
- 23.Messa P, Macário F, Yaqoob M, et al. The OPTIMA study: assessing a new cinacalcet (Sensipar/Mimpara) treatment algorithm for secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2008;3:36–45. doi: 10.2215/CJN.03591006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang CA. The anatomic basis of parathyroid surgery. Ann Surg. 1976;183:271–275. doi: 10.1097/00000658-197603000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilmour JR. The normal histology of the parathyroid glands. Pathol Bacteriol. 1937;45:507–22. and 48: 187–222. [Google Scholar]
- 26.Akerstrom G, Malmaeus J, Bergstrom R. Surgical anatomy of human parathyroid glands. Surgery. 1984;95:14–21. [PubMed] [Google Scholar]
- 27.Solbiati L, Osti V, Cova L, et al. Ultrasound of thyroid, parathyroid glands and neck lymph nodes. Eur Radiol. 2001;11:2411–2424. doi: 10.1007/s00330-001-1163-7. [DOI] [PubMed] [Google Scholar]
- 28.Meola M, Barsotti M, Lenti C, et al. Color-Doppler in the imaging work-up of primary hyperparathyroidism. J Nephrol. 1999;12:270–274. [PubMed] [Google Scholar]
- 29.Lane MJ, Desser TS, Weigel RJ, et al. Use of colour and power Doppler sonography to identify feeding arteries associated with parathyroid adenomas. Am J Roentgenol. 1998;171:819–823. doi: 10.2214/ajr.171.3.9725323. [DOI] [PubMed] [Google Scholar]
- 30.Moe SM, Cunningham J, Bommer J, et al. Long term treatment of secondary hyperparathyroidism with the calcimimetic cinacalcet HCl. Nephrol Dial Transplant. 2005;20:2186–2193. doi: 10.1093/ndt/gfh966. [DOI] [PubMed] [Google Scholar]
- 31.Colloton M. Cinacalcet HCl attenuates parathyroid hyperplasia in a rat model of secondary hyperparathyroidism. Kidney Int. 2005;67:467–476. doi: 10.1111/j.1523-1755.2005.67103.x. [DOI] [PubMed] [Google Scholar]
- 32.Mizobuchi M, Ogata H, Hatamura I, et al. Activation of calcium-sensing receptor accelerates apoptosis in hyperplastic parathyroid cells. Biochem Biophys Res Commun. 2007;362:11–16. doi: 10.1016/j.bbrc.2007.07.177. [DOI] [PubMed] [Google Scholar]
- 33.Fryer RM, Segreti JA, Widomski DL, et al. Systemic activation of the calcium sensing receptor produces acute effects on vascular tone and circulatory function in uremic and normal rats: focus on central versus peripheral control of vascular tone and blood pressure by cinacalcet. J Pharmacol Exp Ther. 2007;323:217–226. doi: 10.1124/jpet.107.123901. [DOI] [PubMed] [Google Scholar]
- 34.Lomonte C, Vernaglione L, Chimienti D, et al. Does vitamin D receptor and calcium receptor activation therapy play a role in the histopathologic alterations of parathyroid glands in refractory uremic hyperparathyroidism? Clin J Am Soc Nephrol. 2008;3:794–799. doi: 10.2215/CJN.04150907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drueke T, Martin D, Rodriguez M. Can calcimimetics inhibit parathyroid hyperplasia? Evidence from preclinical studies. Nephrol Dial Transplant. 2007;22:1828–1839. doi: 10.1093/ndt/gfm177. [DOI] [PubMed] [Google Scholar]
- 36.Kawata T, Imanishi Y, Kobayashi K, et al. Direct in vitro evidence of the suppressive effect of cinacalcet HCl on parathyroid hormone secretion in human parathyroid cells with pathologically reduced calcium-sensing receptor levels. J Bone Miner Metab. 2006;24:300–306. doi: 10.1007/s00774-006-0687-y. [DOI] [PubMed] [Google Scholar]