Abstract
Background. Thrombotic thrombocytopenic purpura (TTP) and haemolytic uraemic syndrome (HUS) are thrombotic microangiopathies (TMAs). They are generally diagnosed and treated by plasmapheresis in the presence of non-immune haemolytic anaemia and thrombocytopenia. Yet, many individuals admitted in our hospital for athrombocytopenic renal failure of unknown cause were reported to have TMA as main lesion on kidney biopsies.
Methods. Speculating that this presentation is not uncommon and that the underlying lesion might not be suspected because of current diagnostic criteria, we determined its prevalence and other accompanying features through a retrospective single-centre cohort of 50 cases where TMA had been identified histologically.
Results. At presentation, normal serum platelets were common (44%) but still accompanied by abnormal serum LDH in most subjects. End-stage renal disease and mortality at 5 years were also high especially in the athrombocytopenic group, but unrelated to the underlying aetiology of TMA. Importantly, several subjects in both groups received and apparently responded to plasmapheresis.
Conclusion. In the absence of thrombocytopenia, TMA should still be contemplated when renal failure is associated with high serum LDH and its possible treatment with plasmapheresis assessed through prospective trials.
Keywords: haemolytic uraemic syndrome, plasmapheresis, renal failure, serum LDH, thrombotic microangiopathies
Introduction
Thrombotic microangiopathy (TMA) was identified initially as the lesion causing thrombotic thrombocytopenic purpura (TTP), which presents with non-immune haemolytic anaemia, thrombocytopenia and tissue dysfunction [1], and was identified later on also as the lesion causing haemolytic uraemic syndrome (HUS), which resembles TTP except for affecting the kidney predominantly [2]. Although the pathogenic mechanisms of TTP appear to differ from those of HUS, it is still common usage to consider both disorders as the same entity, and ‘non-immune haemolytic anaemia’ and ‘thrombocytopenia’ as main diagnostic criteria [3,4].
On several occasions, colleagues in our Division have reported identifying TMA lesions in athrombocytopenic patients only after a kidney biopsy had been obtained for renal failure of unknown aetiology. They have also reported that even if the TMA lesions occurred in the absence of underlying condition and were apparently plasmapheresis responsive, haematologists frequently argued that this treatment was of unproven benefit when one of the diagnostic criteria for TTP/HUS was missing.
Based on our colleagues’ experience, and considering that plasmapheresis is a well-tolerated and effective treatment for TTP/HUS [5,6], we became concerned that the criteria used to diagnose these disorders might lead, in being too exclusive, to undertreat many individuals with actual TMA lesions. This worry was justified further by studies in which HUS was found to be associated with a worse renal outcome than TTP [7–10].
Subjects and methods
To confirm that TMA lesions had often presented atypically in our centre, we analysed a 30-year retrospective cohort who underwent kidney biopsies before 2007 and were found to have intimal proliferation and/or endothelial swelling with luminal fibrin deposition in arterial or capillary beds but no signs of acute rejection, vasculitis or extensive microthromboses. Subjects with progressive systemic sclerosis, diarrhoeal HUS or bone marrow transplantation were excluded since disseminated intravascular coagulation and malignant hypertension appeared unlikely in all cases.
Clinical data were extracted from charts at both presentations and at up to 5 years following diagnosis and regrouped for analyses according to variables such as platelet count (PLT) at presentation. Data between the ‘low PLT’ and ‘normal PLT’ groups were analysed further by Fisher's exact tests and Kaplan–Meier survival analyses.
Results
Fifty subjects constituted the cohort. In all cases, TMA affected arteriolar beds predominantly and were accompanied by secondary mesangiolysis, wrinkling of capillary basement membranes or double contour images. As seen in Table 1 (columns 1–3), the subjects were relatively young (46 years) with a majority of females (70%) and had frequently no underlying condition (46%). As expected, thrombocytopenia was found in only 56% but serum LDH was still elevated in most (mean 619 U/L). When the cohort was divided according to PLT, the most important difference observed was the higher percentage of athrombocytopenic subjects who required dialysis although it did not reach statistical significance. Importantly, TMA lesions in the absence of underlying condition were associated with similar findings (columns 4–6) and were of equal occurrence between the ‘low PLT’ and ‘normal PLT’ groups (columns 1–3).
Table 1.
Presenting characteristics of subjects with biopsy-proven TMA
| All subjects | No underlying causes | |||||
|---|---|---|---|---|---|---|
| Columns | (1) | (2) | (3) | (4) | (5) | (6) |
| Subgroups | Total | ↓ PLT | N PLT | Total | ↓ PLT | N PLT |
| n subjects | 50 | 28 | 22 | 23 | 13 | 10 |
| Age (y) | 46 ± 17 | 47 ± 18 | 44 ± 16 | 47 ± 19 | 46 ± 21 | 47 ± 16 |
| Males (%) | 30 | 32 | 27 | 39 | 38 | 40 |
| No underlying causes (%) | 46 | 46 | 45 | 100 | 100 | 100 |
| Underlying cause (%) | 54 | 54 | 55 | 0 | 0 | 0 |
| Low Hb (%) | 94 | 100 | 86 | 96 | 100 | 90 |
| Mechanic haemolysis (%) | 94 | 100 | 86 | 96 | 100 | 90 |
| Low platelets (%) | 56 | 100 | 0§ | 56 | 100 | 0§ |
| Low Hb and PLT (%) | 56 | 100 | 0§ | 56 | 100 | 0§ |
| High LDH (%) | 86 | 89 | 82 | 87 | 92 | 80 |
| High creatinine (%) | 90 | 93 | 86 | 100 | 100 | 100 |
| Dialysis (%) | 50 | 43 | 59 | 65 | 62 | 70 |
| Plasmapheresis (%) | 58 | 61 | 55 | 78 | 87 | 70 |
Potential underlying causes included stage IV lupus nephritis (12%), mixed connective tissue disease with or without polymyositis (14%), drugs (14%), cyclosporine (0%), neoplasias (6%), renal transplantation (4%) and pregnancy (4%). For this study serum creatinine >1.2 mg/dL, Hb <120 g/L (female) or <140 g/L (male), PLT <150 × 109/L and LDH >220 U/L were considered abnormal (low or elevated). Mechanic haemolysis was defined as follows: anaemia + low haptoglobin or high indirect bilirubin + negative Coomb's test. In all cases, INR was < 1.26 and in no cases, abnormal serum D-dimers or fibrinogen was found. Based on Fisher's exact tests, values indicated by the § sign were significantly different statistically (P < 0.03) between the ‘low PLT’ and ‘normal PLT’ groups.
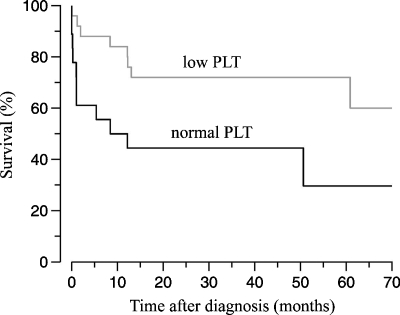
The number of subjects for whom data were available 5 years post-diagnosis decreased to 34 because TMA lesions had been identified beyond 2002 or because of loss to follow-up. Table 2 shows that the most striking long-term outcome was a lower renal and patient survival in the ‘normal PLT’ group (columns 1–3) and that this outcome was similar in the absence of underlying condition (columns 4–6) although no statistical difference was seen due to population size. Plasmapheresis initiated at the time of diagnosis appeared to offer no long-term benefit (compare % between the ‘all subjects’ and ‘plasmapheresis-treated subjects’ groups) but to correlate with lower serum creatinine at 1 year (not shown), suggesting that subjects lost to follow-up subjects had better outcomes. The predictive value of PLT at presentation is suggested further by lower 60-month cumulative survival rates in the ‘normal PLT’ group (Figure 1).
Table 2.
Characteristics of subjects with biopsy-proven TMA 5 years after diagnosis
| All subjects | No underlying causes | |||||
|---|---|---|---|---|---|---|
| Columns | (1) | (2) | (3) | (4) | (5) | (6) |
| Subgroups | Total | ↓ PLT | N PLT | Total | ↓ PLT | N PLT |
| All subjects (n) | 34 | 18 | 16 | 17 | 9 | 8 |
| ↓ CR > 1.5 mg/dL (%) | 12 | 17 | 6 | 24 | 22 | 13 |
| ESRD (%) | 50 | 44 | 56 | 65 | 56 | 75 |
| Death (%) | 18 | 6 | 31 | 6 | 0 | 13 |
| ESRD or death (%) | 68 | 50 | 88§ | 71 | 56 | 88 |
| Plasmapheresis (n)§ | 23 | 14 | 9 | 13 | 8 | 5 |
| No underlying causes (%) | 56 | 57 | 56 | 100 | 100 | 100 |
| ↓ CR > 1.5 mg/dL (%) | 17 | 21 | 11 | 31 | 38 | 20 |
| ESRD (%) | 48 | 43 | 56 | 54 | 50 | 60 |
| Death (%) | 17 | 7 | 33 | 8 | 0 | 20 |
| ESRD or death (%) | 65 | 50 | 89 | 62 | 50 | 80 |
§ = at the time of diagnosis, CR = serum creatinine and ESRD = end-stage renal disease. Cohort characteristics were similar to those reported in Table 1 legend. Based on Fisher's exact tests, values indicated by the § sign were significantly different statistically between the ‘low PLT’ and ‘normal PLT’ groups (P < 0.03).
Fig. 1.
Patient survival following histological diagnosis of TMA. Based on a Kaplan–Meier analysis, curves were significantly different statistically between the ‘low PLT’ and ‘normal PLT’ groups (P = 0.023).
Discussion
This study highlights the usefulness of retrospective cohorts to confirm clinical impressions acquired through personal experience and the importance of serum LDH or tissue biopsies to identify atypical cases of TMA. Perhaps in this regard, newer biological markers of TTP/HUS such as serum levels of high molecular weight Von Willebrand factors and ADAMTS-13 will also prove useful in the diagnosis of TMA when PLT is normal [10,11].
In contrast to statements that were made in the recent articles [3,4], our study has shown that TMA lesions could present with normal PLT and lead to substantial renal damage quite commonly. These observations raise the concern that athrombocytopenic TMA may not be considered for timely therapeutic interventions based on current diagnostic criteria or because of delayed identification. They should thus be seen as an incentive to conduct prospective trials aimed at determining the usefulness of plasmapheresis in atypical forms of TMA.
Through consensus reports, a simplified clinical definition of TTP/HUS has been proposed in the 1990s [12]. In our opinion, it did not rest on solid evidence and implied that TMA was always easy to diagnose. This impression is now supported by the current analysis and is in keeping with newer concepts that have emerged from the molecular and clinical investigation of TTP/HUS. For instance, it is now known that several pathogenic mechanisms are involved and that they tend to differ even when manifestations are alike [13–15]. Whether idiopathic athrombocytopenic TMA is a unique entity in this regard needs to be confirmed.
Conflict of interest statement. None declared.
References
- 1.Amorosi EL, Ultmann JE. Thrombotic thrombocytopaenic purpura: report of 16 cases and review of the literature. Medicine. 1966;45:139–159. [Google Scholar]
- 2.Gasser C, Gautier E, Steck A, et al. Hämolytisch-urämische syndrome: bilaterale Nierenrindennekrosen bei akuten erworbenen hämolytischen anämien. Schweiz Med Wochenschr. 1955;85:905–909. [PubMed] [Google Scholar]
- 3.Franchini M. Thrombotic microangiopathies: an update. Hematology. 2006;11:139–146. doi: 10.1080/10245330600667583. [DOI] [PubMed] [Google Scholar]
- 4.George JN. Thrombotic thrombocytopenic purpura. N Engl J Med. 2006;354:1927–1935. doi: 10.1056/NEJMcp053024. [DOI] [PubMed] [Google Scholar]
- 5.Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. N Engl J Med. 1991;325:393–407. doi: 10.1056/NEJM199108083250604. [DOI] [PubMed] [Google Scholar]
- 6.Bell WR, Braine HG, Ness PM, et al. Improved survival in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. N Engl J Med. 1991;325:398–403. doi: 10.1056/NEJM199108083250605. [DOI] [PubMed] [Google Scholar]
- 7.Ruggenenti P, Noris M, Remuzzi G. Thrombotic microangiopathies. In: Brady HR, Wilcox CS, editors. Therapy in Nephrology and Hypertension. Philadelphia, PA: Saunders; 2003. pp. 283–295. [Google Scholar]
- 8.No authors listed Adult haemolytic uremic syndrome with renal microangiopathy. Outcome according to therapeutic protocol in 53 cases. French Cooperative Study Group for Adult HUS. Ann Med Interne (Paris) 1992;143:27–32. [PubMed] [Google Scholar]
- 9.Schieppati A, Ruggenenti P, Comejo RP, et al. Renal function at hospital admission as a prognostic factor in adult hemoytic uremic syndrome. J Am Soc Nephrol. 1992;2:1640–1644. doi: 10.1681/ASN.V2111640. [DOI] [PubMed] [Google Scholar]
- 10.Vesely SK, George JN, Lämmle B, et al. ADAMTS13 activity in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: relation to presenting features and clinical outcomes in prospective cohort of 142 patients. Blood. 2003;102:60–68. doi: 10.1182/blood-2003-01-0193. [DOI] [PubMed] [Google Scholar]
- 11.Veyradier A, Obert B, Houllier A, et al. Specific von Willebrand factor-cleaving protease in thrombotic microangiopathies: a study of 111 cases. Blood. 2001;98:1765–1772. doi: 10.1182/blood.v98.6.1765. [DOI] [PubMed] [Google Scholar]
- 12.George JN, Gilcher RO, Smith JW, et al. Thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: diagnosis and management. J Clin Apheresis. 1998;13:120–125. doi: 10.1002/(sici)1098-1101(1998)13:3<120::aid-jca5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.Tsai HM. Advances in the pathogenesis, diagnosis, and treatment of thrombotic thrombocytopaenic purpura. J Am Soc Nephrol. 2003;14:1072–1081. doi: 10.1097/01.asn.0000060805.04118.4c. [DOI] [PubMed] [Google Scholar]
- 14.Nangaku M, Nishi H, Fujita T. Pathogenesis and prognosis of thrombotic microangiopathy. Clin Exp Nephrol. 2007;11:107–114. doi: 10.1007/s10157-007-0466-7. [DOI] [PubMed] [Google Scholar]
- 15.Jokiranta TS, Zipfel PF, Fremeaux-Bacchi V, et al. Where next with atypical hemolytic uremic syndrome? Mol Immunol. 2007;44:3889–3900. doi: 10.1016/j.molimm.2007.06.003. [DOI] [PubMed] [Google Scholar]