Abstract
Background. In a previous study we demonstrated that mild metabolic alkalosis resulting from standard bicarbonate haemodialysis induces hypotension. In this study, we have further investigated the changes in systemic haemodynamics induced by bicarbonate and calcium, using non-invasive procedures.
Methods. In a randomized controlled trial with a single-blind, crossover design, we sequentially changed the dialysate bicarbonate and calcium concentrations (between 26 and 35 mmol/l for bicarbonate and either 1.25 or 1.50 mmol/l for calcium). Twenty-one patients were enrolled for a total of 756 dialysis sessions. Systemic haemodynamics was evaluated using pulse wave analysers. Bioimpedance and BNP were used to compare the fluid status pattern.
Results. The haemodynamic parameters and the pre-dialysis BNP using either a high calcium or bicarbonate concentration were as follows: systolic blood pressure (+5.6 and −4.7 mmHg; P < 0.05 for both), stroke volume (+12.3 and +5.2 ml; P < 0.05 and ns), peripheral resistances (−190 and −171 dyne s cm−5; P < 0.05 for both), central augmentation index (+1.1% and −2.9%; ns and P < 0.05) and BNP (−5 and −170 ng/l; ns and P < 0.05). The need of staff intervention was similar in all modalities.
Conclusions. Both high bicarbonate and calcium concentrations in the dialysate improve the haemodynamic pattern during dialysis. Bicarbonate reduces arterial stiffness and ameliorates the heart tolerance for volume overload in the interdialytic phase, whereas calcium directly increases stroke volume. The slight hypotensive effect of alkalaemia should motivate a probative reduction of bicarbonate concentration in dialysis fluid for haemodynamic reasons, only in the event of failure of classical tools to prevent intradialytic hypotension.
Keywords: alkalosis, bicarbonate, calcium, haemodialysis, haemodynamics
Introduction
Despite the fact that the severity of chronic metabolic acidosis secondary to end-stage renal failure in haemodialysis patients can vary widely depending on dialysis frequency and quality and on protein intake, the bicarbonate concentration in the dialysis fluid is often set arbitrarily somewhere between 32 and 35 mmol/l [1,2]. This strategy is the result of a compromise between the advantages of correcting pre-dialysis acidosis and the side effects of the transient peri- and post-dialysis alkalosis resulting from bicarbonate transfer. The undesirable consequences, on the one hand, of metabolic acidosis on bone metabolism [3–5] and nitrogen balance [6,7] and, on the other, of metabolic alkalosis on neuromuscular excitability and on cerebral blood flow [8–10] are well known. Furthermore, the positive inotropic effect induced by alkalaemia in experimental models [11,12], if effective in humans, could be at least in part counterbalanced by the secondary decrease of serum ionized calcium concentration [13]. In fact, the direct relationship, mediated by its inotropic effect (a concomitant increase in peripheral resistance is controversial), between an acute rise or fall in ionized calcium and blood pressure has been exhaustively demonstrated in haemodialysis [14–20]. With reference to the haemodynamic consequences of bicarbonate transfer, a symptomatic intradialytic hypotensive effect of alkalaemia has been observed in two previous studies, the first one (n = 4) performed with 40 mmol/l and the second one (n = 26) with 32 mmol/l of bicarbonate in the dialysis fluid [21,22]. As a consequence, even though the correction of chronic metabolic acidosis is an important goal of dialysis, it was suggested, in patients showing a significant peri- and post-dialysis alkalaemia and in the event of failure of the classical tools to prevent intradialytic hypotension, to consider a reduction of the bicarbonate concentration in the dialysis fluid [22].
However, in a further study designed to ascertain whether the calcium shift secondary to alkalaemia explained the observed haemodynamic consequences, the decrease in blood pressure was not significant, probably due to an insufficient statistical power (243 versus 468 dialysis sessions) [19]. Moreover, the haemodynamic pattern of the postulated hypotensive effect was, in the above-cited studies, not investigated in detail. For these reasons, we designed a new study in which we (i) analysed non-invasively the haemodynamic consequences of sequentially changing the dialysis fluid bicarbonate and calcium concentrations and (ii) monitored the occurrence of intradialytic hypotensive episodes as well as their impact on acid–base, electrolyte and fluid status.
Methods and patients
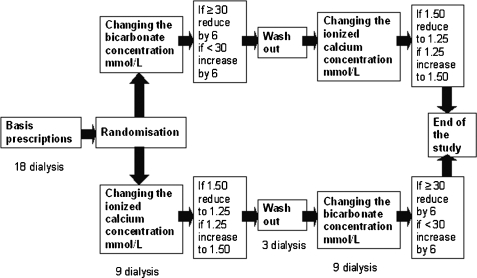
Twenty-one chronic haemodialysis patients (10 males and 11 females) were enrolled in the study. Each patient was dialyzed for 4 hs three times a week, was clinically stable and had no intercurrent illnesses. Using a single-blind, cross-over design, after a baseline observation phase of 6 weeks, the patients were randomized in the two arms of the study, beginning by changing either the bicarbonate or the calcium concentration in the dialysis fluid. This first 3-week intervention phase was followed by a week of wash out going back to the baseline dialysis prescriptions and then, switching round the bicarbonate and calcium prescriptions compared to the initial randomization, by a second 3-week intervention phase (for a schematic representation see Figure 1). The adaptation of the bicarbonate and calcium concentrations in the dialysis fluid in the intervention phases was done as follows: in one arm, patients with a baseline bicarbonate ≥30 mmol/l were dialyzed reducing the concentration by 6 mmol/l while patients initially treated with a bicarbonate <30 mmol/l were dialyzed increasing the concentration by 6 mmol/l; in the other arm, the ionized calcium concentration was changed from 1.25 to 1.50 mmol/l or vice versa. The haemodialyses were performed using a 4008 H machine, calibrated to deliver a bicarbonate concentration of between 24 and 40 mmol/l from a sodium bicarbonate bag Bibag©, and a high flux polysulfone membrane, all from Fresenius Medical Care (Bad Homburg, Germany). The prescribed, dialyzer effective surface area, dialysis fluid conductibility, temperature and composition and effective blood flow were recorded at the moment of enrolment in the study. These potential variables, with the exception of the dialysate bicarbonate and calcium concentrations, were then left unchanged for the following 13 weeks. The medications of the patients (including phosphate binders) were also kept constant.
Fig. 1.
Study design. Schematic representation of the study design.
Serum BUN and creatinine were measured at the beginning and at the end of the first dialysis session of the third week of each study phase. Whole blood pH, ionized calcium, sodium and potassium were measured at the beginning of each dialysis session and at the end of the 3rd dialysis sessions of each week using a point of care ionometer by direct ionometry. The brain natriuretic peptide (BNP) concentration was determined at the beginning of each dialysis session. Blood samples were taken from the arterial limb of the shunt.
The ionized sodium concentration of the dialysis fluid produced by each dialysis machine was determined once at the beginning of each treatment modality by indirect ionometry.
Systolic and diastolic blood pressures and heart rate were measured before starting the session, every 30 min throughout the dialysis and 5 min after the end of the procedure with an automated Blood Pressure Monitor 4008 (Fresenius Medical Care, Bad Homburg, Germany) integrated in the dialysis machine. Stroke volumes (integrated mean of the flow waveform between the current upstroke and the dicrotic notch) and peripheral resistances (ratio of mean arterial pressure to stroke volume multiplied by heart rate) were evaluated before starting the session and then every hour using a finger beat-to-beat monitor Finometer© (Finapres Medical Systems BV, Arnhem, The Netherlands). As a quality control, a pulse wave analysis with calculation of the central aortic indices (augmentation index, central augmented pressure, central systolic and diastolic BP, end systolic BP, ejection and diastolic duration) was performed three times in each study phase in the middle 2 h of the dialysis session using a Sphigmocor© (Atcor Medical, West Ryde, Australia). A body impedance analyser BIA 101© and the software Bodygram 1.3 (both from Akern Bioresearch, Pontassieve, Italy) were used before starting each dialysis session to estimate the total body, intracellular and extracellular water (TBW, ICW and ECW respectively). The use of isotonic saline (100–200 ml) infusions to treat symptomatic hypotension or symptoms related to intravascular hypovolaemia was registered.
Kt/V was calculated using a second-generation single-pool Daugirdas formula (Kt/V = −ln(R − 0.03) + [(4 − 3.5 × R) × (UF/W)], where R = post-dialysis BUN/ pre-dialysis BUN, UF = net ultrafiltration and W = weight).
The free water deficit at the end of the dialysis session was calculated on the basis of pre- and post-dialysis serum sodium and the total body water (TBW) measured by bioimpedance, whereas the sodium removal was estimated using the following formula: (TBW × pre-dialysis sodium) – (TBW – weight loss during dialysis) × post-dialysis sodium.
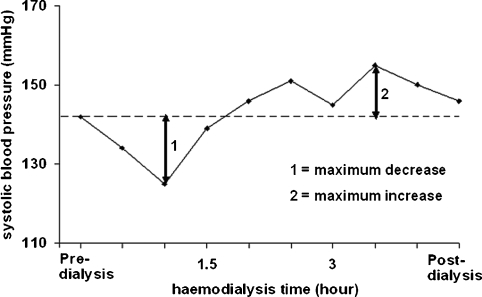
Statistical analyses were performed using a statistical software package (SPSS 12.0; SPSS Inc., Chicago, IL, USA). Results were expressed as mean ± SD. An evaluation of the maximum increase and decrease in each parameter was added to the data analysis, with the intention of ameliorating the understanding of the haemodynamic changes (see Figure 2 for an explanation of the calculation method). Comparisons between the results of the laboratory tests (BNP, ionized calcium, bicarbonate, potassium, sodium and pH) and between haemodynamic and bioimpedance parameters were carried out with a paired t-test. Blood pressure profiles as a function of the dialysis time were compared using a trapezoidal estimation of the area under the curves followed by a Wilcoxon signed-rank test. In all cases, a P ≤ 0.05 was considered statistically significant; P was expressed as ns (not significant), 0.05, <0.05, <0.01 and <0.001.
Fig. 2.
Analysis of the haemodynamic parameter fluctuations. Schematic representation of the method used to calculate the maximum increase and decrease of each measured parameter during the dialysis session.
The protocol of the study was approved by the local ethical committee. All the patients gave informed consent prior to enrolment in the study.
Results
Characteristics of the studied population
The (n = 21) characteristics of the studied population at the moment of enrolment were as follows (mean ± SD): age 69.1 ± 9.9 years, weight 69.8 ± 15.5 kg and male/female ratio 0.91. The basis of haemodialyses prescriptions were dialyzer effective surface area 1.88 ± 0.20 m2; Kt/V 1.59 ± 0.26; dialysis fluid conductibility 13.8 ms/cm; dialysis fluid temperature 36.5°C; effective blood flow 334 ± 69 ml/min; dialysis fluid flow rate 600 ml/min and dialysis fluid concentration for potassium 2.64 ± 0.65 mmol/l, magnesium 0.5 mml/l, acetate 3 mmol/l and glucose 1 g/l. See Table 1 for details including underlying nephropathies, comorbidities and antihypertensive drugs in use.
Table 1.
Characteristics of the studied population
| Patient no. | Sex (M/F) | Age (years) | Underlying nephropathy | Comorbidities | Medication | Dry weight (kg) | Dialyzer surface | Kt/V | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| area (m2) | ||||||||||||
| Ischaemic | Diabetes | Beta | Calcium | Alpha | ACE-inhibitors | |||||||
| cardiomyopathy | mellitus | blockers | antagonists | blockers | or ARB | |||||||
| 1 | F | 74 | Diabetic nephropathy | Y | Y | Y | N | N | Y | 70.0 | 1.8 | 1.59 |
| 2 | F | 87 | Nephroangiosclerosis | N | N | Y | Y | N | Y | 63.0 | 1.8 | 1.38 |
| 3 | M | 71 | Diabetic nephropathy | Y | Y | N | N | N | Y | 85.0 | 1.8 | 1.28 |
| 4 | F | 84 | Rapidly progressive glomerulonephritis | Y | N | Y | Y | Y | Y | 57.5 | 1.8 | 1.88 |
| 5 | M | 79 | Diabetic nephropathy | Y | Y | N | N | N | N | 62.0 | 1.8 | 1.28 |
| 6 | F | 56 | Focal segmental glomerulosclerosis | N | N | Y | Y | N | N | 104.0 | 1.8 | 1.81 |
| 7 | M | 58 | Focal segmental glomerulosclerosis | N | Y | Y | Y | N | N | 80.0 | 2.2 | 1.46 |
| 8 | F | 79 | Rapidly progressive glomerulonephritis | Y | N | Y | N | N | N | 44.0 | 1.8 | 1.85 |
| 9 | M | 64 | Focal segmental glomerulosclerosis | N | N | Y | N | N | N | 81.0 | 1.8 | 1.49 |
| 10 | F | 73 | Diabetic nephropathy | N | Y | N | Y | N | N | 83.0 | 1.8 | 1.32 |
| 11 | M | 63 | Nephroangiosclerosis | Y | N | Y | N | N | Y | 95.0 | 2.2 | 1.28 |
| 12 | M | 73 | Nephroangiosclerosis | N | N | N | N | N | N | 72.5 | 1.8 | 1.64 |
| 13 | F | 63 | Focal segmental glomerulosclerosis | N | N | N | N | N | N | 42.5 | 1.4 | 1.69 |
| 14 | M | 71 | Diabetic nephropathy | Y | Y | N | N | N | Y | 79.0 | 1.8 | 1.29 |
| 15 | M | 82 | Relapsing nephrolithiasis | N | Y | N | N | N | N | 63.0 | 1.8 | 1.61 |
| 16 | F | 47 | IgA nephropathy | N | Y | N | N | N | N | 71.5 | 2.2 | 2.0 |
| 17 | M | 72 | Nephroangiosclerosis and bilateral a.renalis stenosis | Y | N | Y | Y | N | Y | 68.25 | 2.2 | 1.32 |
| 18 | F | 63 | Relapsing pyelonephritis | N | N | Y | Y | N | N | 57.0 | 1.8 | 1.84 |
| 19 | M | 67 | Nephroangiosclerosis | N | N | N | N | N | Y | 72.0 | 2.2 | 1.6 |
| 20 | F | 62 | Nephroangiosclerosis | N | N | Y | N | N | Y | 64.0 | 1.8 | 1.65 |
| 21 | F | 64 | Diabetic nephropathy | Y | Y | Y | Y | Y | Y | 51.0 | 1.8 | 2.18 |
Characteristics of the cohort at the beginning of the study.
M: male; F: female; Y: yes; N: no; ACE: angiotensin-converting enzyme; ARB: angiotensin receptor antagonists.
Effect of bicarbonate and calcium concentrations on systemic haemodynamics
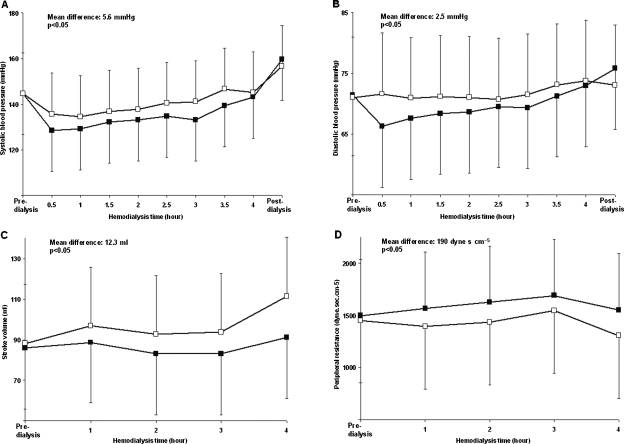
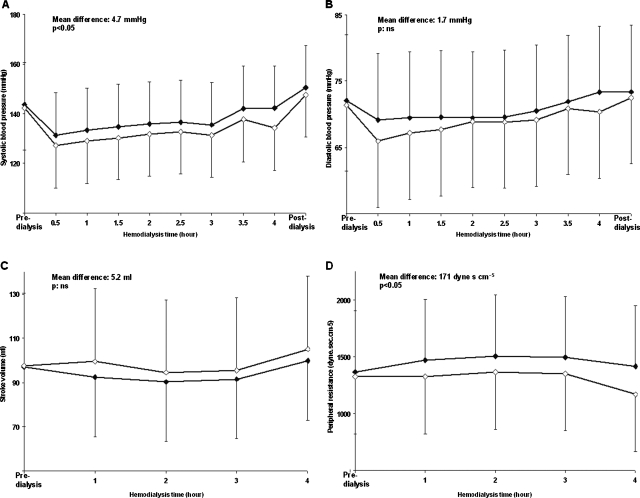
Blood pressure, heart rate, stroke volume and peripheral resistance fluctuations during dialysis using a low or a high dialysate calcium or bicarbonate concentration are shown in Tables 2 and 3. Systolic and diastolic pressures, stroke volume and peripheral resistance as a function of the haemodialysis time using a dialysate ionized calcium concentration of 1.25 and 1.50 mmol/l and a low or a high bicarbonate concentration are depicted graphically in Figures 3 and 4, respectively.
Table 2.
Systemic haemodynamics
| Dialysate ionized calcium concentration (mmol/l) | t-test | |||
|---|---|---|---|---|
| 1.25 | 1.5 | |||
| Blood volume (%) | Max. decrease | 6.2 ± 4.0 | 6.9 ± 3.9 | ns |
| Systolic BP (mmHg) | Max. decrease | 29.8 ± 11.9 | 23.4 ± 14.1 | <0.05 |
| Max. increase | 12.0 ± 9.5 | 16.4 ± 12.1 | <0.05 | |
| Diastolic BP (mmHg) | Max. decrease | 13.1 ± 4.9 | 11.7 ± 5.3 | ns |
| Max. increase | 8.3 ± 4.6 | 9.0 ± 4.7 | ns | |
| Pulse pressure (mmHg) | Max. decrease | 20.7 ± 11.9 | 19.1 ± 9.8 | ns |
| Max. increase | 12.2 ± 8.9 | 14.5 ± 8.3 | ns | |
| Heart rate (beat/min) | Max. decrease | 7.0 ± 3.3 | 8.0 ± 3.7 | ns |
| Max. increase | 10.3 ± 15.6 | 7.0 ± 10.8 | ns | |
| Stroke volume (ml) | Max. decrease | 19.7 ± 9.3 | 17.2 ± 12.9 | ns |
| Max. increase | 28.3 ± 15.7 | 32.1 ± 18.1 | ns | |
| Peripheral resistance (dyne s cm-5) | Max. decrease | 311 ± 187 | 341 ± 266 | ns |
| Max. increase | 950 ± 639 | 541 ± 331 | 0.05 | |
| Dialysate bicarbonate concentration (mmol/l) | t-test | |||
| Low (range 26–29) | High (range 32–35) | |||
| Blood volume (%) | Max. decrease | 7.3 ± 4.3 | 6.5 ± 3.3 | ns |
| Systolic BP (mmHg) | Max. decrease | 25.9 ± 12.9 | 27.3 ± 16.1 | ns |
| Max. increase | 12.9 ± 7.1 | 13.9 ± 10.3 | ns | |
| Diastolic BP (mmHg) | Max. decrease | 12.9 ± 6.4 | 13.8 ± 7.2 | ns |
| Max. increase | 8.1 ± 4.2 | 8.7 ± 5.5 | ns | |
| Pulse pressure (mmHg) | Max. decrease | 20.6 ± 8.4 | 22.0 ± 10.0 | ns |
| Max. increase | 11.8 ± 5.5 | 11.3 ± 7.8 | ns | |
| Heart rate (beat/min) | Max. decrease | 8.2 ± 3.9 | 7.1 ± 3.5 | 0.05 |
| Max. increase | 7.3 ± 10.6 | 9.0 ± 14.2 | ns | |
| Stroke volume (ml) | Max. decrease | 18.6 ± 11.8 | 20.6 ± 13.6 | ns |
| Max. increase | 24.6 ± 8.8 | 27.3 ± 12.1 | ns | |
| Peripheral resistance (dyne s cm−5) | Max. decrease | 345 ± 288 | 286 ± 198 | ns |
| Max. increase | 520 ± 200 | 690 ± 455 | ns | |
Blood volume, blood pressure (BP), heart rate, stroke volume and peripheral resistance fluctuations during dialysis using low or high dialysate calcium and bicarbonate concentrations.
Significant differences are highlighted in bold; ns: non significant.
Table 3.
Acid–base status, fluid balance and serum electrolytes
| Dialysate ionized calcium concentration (mmol/l) | t-test | |||
|---|---|---|---|---|
| 1.25 | 1.5 | |||
| BNP (ng/l) | Pre-dialysis | 863 ± 719 | 858 ± 810 | Ns |
| ECW (l) | Pre-dialysis | 21.4 ± 4.5 | 20.8 ± 4.1 | Ns |
| ICW (l) | Pre-dialysis | 16.9 ± 4.8 | 17.0 ± 4.8 | Ns |
| Weight loss during dialysis (kg) | 1.23 ± 0.64 | 1.28 ± 0.70 | Ns | |
| Ultrafiltered volume (l) | 1.34 ± 0.65 | 1.43 ± 0.68 | Ns | |
| Free water deficit (ml) | Post-dialysis | −174 ± 447 | 0 ± 450 | <0.05 |
| Dialyses with need of staff intervention (%) | 12 ± 17 | 10 ± 13 | Ns | |
| Sodium, whole blood (mmol/l) | Pre-dialysis | 134.1 ± 2.1 | 134.6 ± 2.5 | Ns |
| Post-dialysis | 133.3 ± 1.4 | 134.6 ± 1.5 | <0.001 | |
| Potassium, whole blood (mmol/l) | Pre-dialysis | 5.49 ± 1.58 | 4.92 ± 0.55 | Ns |
| Post-dialysis | 3.86 ± 0.30 | 3.88 ± 0.26 | ns | |
| pH, whole blood | Pre-dialysis | 7.39 ± 0.03 | 7.38 ± 0.03 | ns |
| Post-dialysis | 7.48 ± 0.04 | 7.45 ± 0.04 | <0.01 | |
| Calcium, ionized, whole blood (mmol/l) | Pre-dialysis | 1.13 ± 0.12 | 1.10 ± 0.08 | Ns |
| Post-dialysis | 1.11 ± 0.08 | 1.19 ± 0.05 | <0.001 | |
| Dialysate bicarbonate concentration (mmol/l) | ||||
| Low (range 26–29) | High (range 32–35) | |||
| BNP, pre-dialysis (ng/l) | Pre-dialysis | 924 ± 905 | 754 ± 703 | 0.05 |
| ECW (l) | Pre-dialysis | 20.9 ± 4.0 | 21.5 ± 4.3 | 0.05 |
| ICW (l) | Pre-dialysis | 17.0 ± 4.8 | 16.8 ± 4.7 | ns |
| Weight loss during dialysis (kg) | 1.40 ± 0.83 | 1.36 ± 0.79 | ns | |
| Ultrafiltered volume (l) | 1.67 ± 1.07 | 1.65 ± 1.12 | ns | |
| Free water deficit (ml) | Post-dialysis | −13 ± 557 | −42 ± 524 | ns |
| Sodium removal (mmol) | 184.6 ± 106.8 | 197.7 ± 109.5 | ns | |
| Dialyses with need of staff intervention (%) | 9 ± 12 | 10 ± 20 | ns | |
| Sodium, whole blood (mmol/l) | Pre-dialysis | 134.5 ± 2.4 | 134.5 ± 2.7 | ns |
| Post-dialysis | 134.6 ± 1.6 | 134.1 ± 1.6 | <0.05 | |
| Potassium, whole blood (mmol/l) | Pre-dialysis | 5.00 ± 0.54 | 5.22 ± 1.63 | ns |
| Post-dialysis | 3.91 ± 0.23 | 3.74 ± 0.24 | <0.001 | |
| pH, whole blood | Pre-dialysis | 7.36 ± 0.05 | 7.41 ± 0.09 | 0.05 |
| Post-dialysis | 7.44 ± 0.06 | 7.51 ± 0.04 | <0.001 | |
| Calcium, ionized, whole blood (mmol/l) | Pre-dialysis | 1.14 ± 0.09 | 1.12 ± 0.11 | ns |
| Post-dialysis | 1.19 ± 0.08 | 1.14 ± 0.06 | <0.01 | |
Pre-dialysis BNP, extra- and intra-cellular body water (ECW and ICW, respectively), free water deficit at the end of dialysis,% of dialyses with need of staff intervention, pH in whole blood and serum ionized calcium, potassium and sodium at the beginning and at the end of the dialysis sessions using low or high dialysate calcium and bicarbonate concentrations.
Significant differences are highlighted in bold; ns: non significant.
Fig. 3.
Panels A–D: Haemodynamic consequences of changing the calcium concentration in dialysis fluids. Systolic (Panel A) and diastolic (Panel B) pressure, stroke volume (Panel C) and peripheral resistance (Panel D) as a function of the haemodialysis time using a dialysate ionized calcium concentration of 1.25 (black squares) and 1.50 (empty squares) mmol/l, respectively. The mean differences between the curves and the statistical significances are superimposed in the figures.
Fig. 4.
Panels A–D: haemodynamic consequences of changing the bicarbonate concentration in dialysis fluids. Systolic (Panel A) and diastolic (Panel B) pressure, stroke volume (Panel C) and peripheral resistance (Panel D) as a function of the haemodialysis time using a high (empty diamonds) and a low (black diamonds) bicarbonate concentration in the dialysis fluid, respectively. The mean differences between the curves and the statistical significances are superimposed in the figures.
The mean difference in heart rate between the two calcium and bicarbonate schedules was not significant (1.1 and 1.2 beat/min, respectively).
The results of the pulse wave analysis with calculation of the central aortic indices using the four dialysis schedules are shown in Table 4.
Table 4.
Pulse wave analysis
| Dialysate ionized calcium concentration (mmol/l) | t-test | ||
|---|---|---|---|
| 1.25 | 1.50 | ||
| Operator quality index | 82.7 ± 19.3 | 89.0 ± 8.4 | ns |
| Systolic BP, peripheral (mmHg) | 134.2 ± 18.9 | 144.9 ± 21.6 | <0.05 |
| Diastolic BP, peripheral (mmHg) | 68.2 ± 10.8 | 73.9 ± 10.8 | <0.05 |
| Heart rate (beat/min) | 65.1 ± 8.7 | 61.4 ± 9.5 | <0.01 |
| Sistolic BP, central (mmHg) | 123.4 ± 19.2 | 133.9 ± 20.4 | <0.05 |
| Diastolic BP, central (mmHg) | 68.8 ± 10.9 | 74.5 ± 11.2 | <0.05 |
| Ejection duration, adjusted (ms) | 308.7 ± 26.1 | 315.3 ± 27.0 | <0.05 |
| Diastolic duration, central (ms) | 631.1 ± 113.4 | 685.2 ± 148.7 | <0.05 |
| Augmentation index, central (%) | 151.6 ± 22.9 | 152.7 ± 18.6 | ns |
| Augmented pressure, central (mmHg) | 18.5 ± 9.4 | 20.8 ± 10.0 | ns |
| Dialysate bicarbonate concentration (mmol/l) | |||
| Low (range 26–29) | High (range 32–35) | ||
| Operator quality index | 87.5 ± 11.8 | 82.3 ± 20.8 | ns |
| Systolic BP, peripheral (mmHg) | 142.4 ± 16.8 | 134.0 ± 16.4 | <0.05 |
| Diastolic BP, peripheral (mmHg) | 72.5 ± 10.9 | 67.4 ± 8.3 | <0.01 |
| Heart rate (beat/min) | 60.9 ± 9.4 | 63.2 ± 8.8 | ns |
| Sistolic BP, central (mmHg) | 131.1 ± 16.4 | 123.1 ± 16.3 | <0.05 |
| Diastolic BP, central (mmHg) | 73.0 ± 11.2 | 68.3 ± 8.5 | <0.05 |
| Ejection duration, adjusted (ms) | 315.9 ± 25.0 | 313.1 ± 25.3 | ns |
| Diastolic duration, central (ms) | 693.1 ± 143.6 | 652.9 ± 125.9 | ns |
| Augmentation index, central (%) | 152.5 ± 22.9 | 149.6 ± 21.4 | <0.05 |
| Augmented pressure, central (mmHg) | 20.0 ± 9.4 | 18.2 ± 8.9 | ns |
Results of the pulse wave analysis with calculation of the central aortic indices using a low and high dialysate calcium and bicarbonate concentrations, respectively.
Significant differences are highlighted in bold; ns: non significant.
Effect on acid–base status, fluid balance and serum electrolytes
No differences were noticed in the dialysate ionized sodium concentration among the different treatment modalities (137.8 ± 3.7, 137.2 ± 3.8, 137.4 ± 4.1 and 137.7 ± 3.8 with low and high bicarbonate and calcium concentrations, respectively).
Using a low or a high dialysate calcium (Table 2) or bicarbonate (Table 3) concentration, the following values were recorded blood volume fluctuations during dialysis, pre-dialysis BNP, extra-cellular, intra-cellular and total body water, free water deficit at the end of dialysis, sodium removal, percent of dialyses with a need of staff intervention, pH and ionized calcium, potassium and sodium in whole blood at the beginning and at the end of the dialysis sessions.
Discussion
Summary observations
Using three independent methods giving concordant results (automated blood pressure monitor, finger beat- to-beat monitor and pulse wave analyser), we demonstrated that (i) increasing the bicarbonate concentration in the dialysis fluid, despite a favourable effect on heart function expressed by an increased tolerance for interdialytic volume overload, produces a slight decrease in blood pressure via a reduction in peripheral resistances and (ii) increasing the calcium concentration ameliorates both left ventricular systolic and diastolic functions and, despite a concomitant reduction in peripheral resistances, results in an increased blood pressure and stroke volume. Contrarily to the effect of calcium that did not persist until the following dialysis session, some elements suggest that increasing the bicarbonataemia generates a sustained positive effect on cardiac function. Furthermore, a positive bicarbonate balance could reduce arterial stiffness.
Essential results of the previous studies
In a previous study conducted with a similar design, we demonstrated that mild metabolic alkalosis resulting from standard bicarbonate haemodialysis (32 compared to 26 mmol/l) may induce symptomatic hypotension [22]. The haemodynamic consequences were documented monitoring the lowest (−3.5 mmHg) and the mean systolic (−6.1 mmHg) blood pressures along with registering the incidence of hypotensive episodes (+3.8%) and the need for extra isotonic saline or hypertonic glucose infusions (+7.2%) [20]. As expected, the pH at the end of the dialysis sessions (7.42 versus 7.48) was associated with a contrary behaviour of the serum ionized calcium concentration (1.37 versus 1.32) [22,23]. Given that alkalaemia and calcaemia are partially dependent and are expected to be associated with opposite cardiovascular effects [14–22] and that the observed haemodynamic instability could have been partly a consequence of the secondary decrease in serum ionized calcium, we performed a second study with the same design aimed at verifying whether the calcium shift secondary to alkalaemia explained the consequences of bicarbonate transfer on blood pressure [19]. Unfortunately, as a consequence of an insufficient statistical power, no significant differences in blood pressures and incidence of intra-dialytic hypotensive episodes were observed in this second study. However, on the basis of the monitoring of the systolic blood pressure and of the serum ionized calcium, it was possible to estimate the mean increase in the dialysate calcium concentration that would have been necessary to counterbalance the haemodynamic effect of the mild alkalaemia induced by standard bicarbonate haemodialysis. Knowing that a mean increase in serum ionized calcium concentration of 0.18 mmol/l was obtained with a 0.25 mmol/l calcium supplementation in the dialysis fluid, a correction of the systolic blood pressure fall induced by standard bicarbonate haemodialysis would have required an increase in dialysate calcium concentration of ∼0.22 mmol/l. Given that, in both studies, the mean difference in serum ionized calcium concentration between the dialyses performed with a high or low bicarbonate concentration was of only 0.05 mmol/l (justifying a mean increase in systolic blood pressure of about 1 mmHg), the haemodynamic consequences could not have been explained by the calcium shift alone.
Effect of bicarbonate and calcium concentration on systemic haemodynamics in the present study
We confirmed that the concentrations of both bicarbonate and calcium in the dialysis fluid have significant repercussions during haemodialysis. However, contrarily to the first study performed with a similar design, in a group of stable patients with a low requirement of staff intervention during dialysis, unfavourable consequences of a positive bicarbonate balance on the haemodialysis tolerance were not observed. Confirming this observation, analysing the maximum fluctuations during dialysis of the studied haemodynamic parameters, no differences were evident between the dialysis performed with a low or high concentration of bicarbonate. In contrast, analysing the data of the two calcium schedules, a significant difference in the maximum excursions of the systolic blood pressure, with a higher increase (16.4 versus 12.0 mmHg) and a lower decrease (23.4 versus 29.8 mmHg) using the supraphysiologic calcium concentration, was observed (see Table 2).
As expected, an increase in systolic and diastolic blood pressures was observed using either a high calcium (+5.6 and +2.5 mmHg, respectively) or a low bicarbonate (+4.7 and +1.7 mmHg, respectively) concentration. The increased calcium concentration was associated primarily with changes in stroke volume (+12.3 ml) and the low bicarbonate with changes in peripheral resistances (+171 dyne s cm−5) (see Figures 2–4). Despite the resultant slight reduction in blood pressure, the increased tolerance for the interdialytic volume overload documented by the pre-dialysis BNP (the significance of this finding is underlined by an analogous weight gain and a discordant extra-cellular water expansion between groups; see Tables 2 and 3) suggests a favourable effect of the positive bicarbonate balance on cardiac function.
Returning to the haemodynamic effect of a high dialysate calcium concentration, as expected, a positive effect on left ventricular diastolic function was also documented via an increase in the ejection duration (+6.6 ms) using pulse wave analysis.
With the same method, in patients treated with a high bicarbonate concentration in the dialysis fluid, a significant reduction in the central augmentation index in favour of a same behaviour of the arterial stiffness was noticed (see Table 4). Whether or not the decrease in arterial stiffness was fully dependent on the blood pressure reduction cannot be stated in the present study.
Effect on acid–base status, fluid balance and serum electrolytes
The effects of the changes in the dialysate composition on acid–base status, ionized calcium and serum potassium confirmed the results of the two previous studies [19–22] (see Tables 2 and 3). As expected, the difference in the pH at the end of the dialysis sessions (7.44 versus 7.51) was associated with a contrary behaviour of the whole blood ionized calcium concentration (1.19 versus 1.14 mmol/l). The lower pre-dialysis pH using a dialysate bicarbonate in the low range (7.36 versus 7.41) suggests a less efficient acidosis correction. Concerning the significant and measurable sequestration of potassium into the intracellular space (serum potassium at the end of the dialysis session with the low and high bicarbonate concentration 3.91 versus 3.74 mmol/l, respectively), the independent impact on systemic haemodynamic of the potassium kinetic during dialysis, demonstrated in a previous study [24], was not investigated.
Surprisingly, an unexplained, less pronounced deficit in free water at the end of dialysis was observed; this suggests a transient water shift towards the intracellular space using the higher calcium concentration (see Tables 2 and 3).
Finally, the significant lower post-dialysis whole blood sodium concentration (134.1 versus 134.6 mmol/l) and the trend towards both a lower dialysate ionized sodium concentration (137.2 versus 137.8 mmol/l) and a more pronounced sodium removal (197.7 versus 184.6 mmol) noticed using a dialysate bicarbonate in the high range (32 to 35 mmol/l) may be explained by the effect of bicarbonate in the dialysis fluids on its total-to-ionized sodium ratio. In fact, adding 6 mmol/l of bicarbonate in a dialysate with a total sodium concentration of 140 mmol/l results for physical/chemical reasons in a reduction of ∼0.5 mmol/l in its ionized sodium concentration [25]. In the present study, it cannot be stated to what extent the cited consequences on sodium removal during dialysis could have contributed to the haemodynamic changes of using a dialysate bicarbonate in the high range.
The discrepancy between, on the one hand, the pre- and post-dialysis plasma water ionized sodium concentration (134.4 ± 2.4 and 134.2 ± 1.5 mmol/l at the beginning and at the end of the dialysis sessions, respectively; see Table 3) and, on the other hand, the dialysate ionized sodium concentration (137.5 ± 4.1 mmol/l) has been referred to the different laboratory methods used: indirect ionometry for the dialysate and point of care direct ionometry on whole blood for the plasma.
In conclusion, both high bicarbonate and calcium concentrations in the dialysate improve the haemodynamic pattern during dialysis. Bicarbonate reduces arterial stiffness and ameliorates the heart tolerance for volume overload in the interdialytic phase, whereas calcium directly increases stroke volume. The slight hypotensive effect of alkalaemia should motivate a probative reduction of bicarbonate concentration in dialysis fluid for haemodynamic reasons, only in the event of failure of classical tools to prevent intradialytic hypotension.
The implications on systemic haemodynamics are also to be considered when performing haemodialyses and continuous renal replacement therapies with citrate anticoagulation, a method that exposes patients to both metabolic alkalosis (citrate is converted in bicarbonate) and hypocalcaemia [26].
Acknowledgments
This work was funded by a grant from the Fondazione Ettore Balli, Locarno, Switzerland. The study sponsors had no role in study design, data collection, data analysis, data interpretation or in the writing of the report.
Conflict of interest statement. None declared.
References
- 1.Oettinger CW, Oliver JC. Normalization of uremic acidosis in hemodialysis patients with a high bicarbonate dialysate. J Am Soc Nephrol. 1993;3:1804–1807. doi: 10.1681/ASN.V3111804. [DOI] [PubMed] [Google Scholar]
- 2.Rault R. Optimal dialysate bicarbonate during hemodialysis. ASAIO Trans. 1991;37:M372–M373. [PubMed] [Google Scholar]
- 3.Kraut JA, Mishler DR, Singer FR, et al. The effects of metabolic acidosis on bone formation and bone resorption in the rat. Kidney Int. 1986;30:694–700. doi: 10.1038/ki.1986.242. [DOI] [PubMed] [Google Scholar]
- 4.Cochran M, Wilkinson R. Effect of correction of metabolic acidosis on bone mineralization rate in patients with renal osteomalacia. Nephron. 1975;15:98–110. doi: 10.1159/000180501. [DOI] [PubMed] [Google Scholar]
- 5.Movilli E, Zani R, Carli O, et al. Direct effect of the correction of acidosis on plasma parathyroid hormone concentrations, calcium and phosphate in hemodialysis patients: a prospective study. Nephron. 2001;87:257–262. doi: 10.1159/000045923. [DOI] [PubMed] [Google Scholar]
- 6.May RC, Kelly RA, Mitch WE. Mechanisms for defects in muscle protein metabolism in rats with chronic uremia. Influence of metabolic acidosis. J Clin Invest. 1987;79:1099–1103. doi: 10.1172/JCI112924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papadoyannakis NJ, Stefanidis CJ, McGeown M. The effect of correction of metabolic acidosis on nitrogen and potassium balance of patients with chronic renal failure. Am J Clin Nutr. 1984;40:623–627. doi: 10.1093/ajcn/40.3.623. [DOI] [PubMed] [Google Scholar]
- 8.Sethi D, Curtis JR, Topham DL, et al. Acute metabolic alkalosis during haemodialysis. Nephron. 1989;51:119–120. doi: 10.1159/000185265. [DOI] [PubMed] [Google Scholar]
- 9.Kaye M, Somerville PJ, Lowe G, et al. Hypocalcemic tetany and metabolic alkalosis in a dialysis patient : an unusual event. Am J Kidney Dis. 1997;30:440–444. doi: 10.1016/s0272-6386(97)90292-4. [DOI] [PubMed] [Google Scholar]
- 10.Arieff AI, Defronzo RA. Fluid, Electrolyte and Acid–Base Disorders. New York: Churchill Livingstone; 1995. Metabolic alkalosis; pp. 159–198. [Google Scholar]
- 11.Streisand RL, Gourin A, Stuckey JH. Respiratory and metabolic alkalosis and myocardial contractility. J Thorac Cardiovasc Surg. 1971;62:431. [PubMed] [Google Scholar]
- 12.Clancy RL, Cingolani HE, Taylor RR, et al. Influence of sodium bicarbonate on myocardial performance. Am J Physiol. 1967;212:917. doi: 10.1152/ajplegacy.1967.212.4.917. [DOI] [PubMed] [Google Scholar]
- 13.Hernich WL, Hunt JM, Nixon JV. Increased ionized calcium and left ventricular contractility during hemodialysis. N Engl J Med. 1984;310:19–23. doi: 10.1056/NEJM198401053100105. [DOI] [PubMed] [Google Scholar]
- 14.Kaye M, Vasilevsky M, Ketis M. The effect on blood pressure of an acute fall in ionized calcium during hemodialysis. A randomized study in two patients. Clin Nephrol. 1998;50:361–366. [PubMed] [Google Scholar]
- 15.Van Der Sande FM, Cheriex EC, Van Kuijk WHM, et al. Effect of dialysate calcium concentrations on intradialytic blood pressure course in cardiac-compromised patients. Am J Kidney Dis. 1998;32:125–131. doi: 10.1053/ajkd.1998.v32.pm9669433. [DOI] [PubMed] [Google Scholar]
- 16.Leunissen KML, Van Den Berg BW, van Hooff JP. Ionized calcium plays a pivotal role in controlling blood pressure during haemodialysis. Blood Purif. 1989;7:233–239. doi: 10.1159/000169600. [DOI] [PubMed] [Google Scholar]
- 17.Sherman RA, Bialy GB, Gazinski B, et al. The effect of dialysate calcium levels on blood pressure during hemodialysis. Am J Kidney Dis. 1986;8:244–247. doi: 10.1016/s0272-6386(86)80033-6. [DOI] [PubMed] [Google Scholar]
- 18.Maynard JC, Cruz C, Kleerekoper M, et al. Blood pressure response to changes in serum ionized calcium during hemodialysis. Ann Intern Med. 1986;104:358–361. doi: 10.7326/0003-4819-104-3-358. [DOI] [PubMed] [Google Scholar]
- 19.Gabutti L, Ross V, Duchini F, et al. Does Bicarbonate transfer have relevant hemodynamic consequences in standard hemodialysis? Blood Purif. 2005;23:365–372. doi: 10.1159/000087193. [DOI] [PubMed] [Google Scholar]
- 20.Locatelli F, Covic A, Chazot C, et al. Optimal composition of the dialysate, with emphasis on its influence on blood pressure. Nephrol Dial Transplant. 2004;19:785–796. doi: 10.1093/ndt/gfh102. [DOI] [PubMed] [Google Scholar]
- 21.Graziani G, Casati S, Passerini P, et al. Pathophysiology and clinical consequences of metabolic alkalosis in hemodialyzed patients. Arch Ital Urol Nefrol Androl. 1987;59:105–111. [PubMed] [Google Scholar]
- 22.Gabutti L, Ferrari N, Giudici G, et al. Unexpected haemodynamic instability associated with standard bicarbonate haemodialysis. Nephrol Dial Transplant. 2003;18:2369–2376. doi: 10.1093/ndt/gfg383. [DOI] [PubMed] [Google Scholar]
- 23.Pederson KO. The effect of bicarbonate, PCO2 and pH on serum calcium fractions. Scand J Clin Lab Invest. 1971;27:145. doi: 10.3109/00365517109080201. [DOI] [PubMed] [Google Scholar]
- 24.Dolson GM, Ellis KJ, Bernardo MV, et al. Acute decreases in serum potassium augment blood pressure. Am J Kidney Dis. 1995;26:321–326. doi: 10.1016/0272-6386(95)90652-5. [DOI] [PubMed] [Google Scholar]
- 25.Sargent JA, Gotch FA. Principles and Biophysics of Dialysis. In: Jacobs C, Kjellstrand CM, Koch KM, Winchester JF, editors. Replacement of Renal Function by Dialysis. Vol. 2. Dordrecht, The Netherlands: Kluwer; 1996. pp. 74–76. [Google Scholar]
- 26.Gabutti L, Marone C, Colucci G, et al. Citrate anticoagulation in continuous venovenous hemofiltration: a metabolic challenge. Intensive Care Med. 2002;28:1419–1425. doi: 10.1007/s00134-002-1443-y. [DOI] [PubMed] [Google Scholar]