Abstract
Fast-flow electron spin resonance (ESR) spectroscopy has been used to detect a free radical formed from the reaction of l-tryptophan with Ce4+ in an acidic aqueous environment. Computer simulations of the ESR spectra from l-tryptophan and several isotopically modified forms strongly support the conclusion that the l-tryptophan radical cation has been detected by ESR for the first time. The hyperfine coupling constants (HFCs) determined from the well-resolved isotropic ESR spectra support experimental and computational efforts to understand l-tryptophan's role in protein catalysis of oxidation-reduction processes. l-tryptophan HFCs facilitated the simulation of fast-flow ESR spectra of free radicals from two related compounds, tryptamine and 3-methylindole. Analysis of these three compounds' β-methylene hydrogen HFC data along with equivalent l-tyrosine data has led to a new computational method that can distinguish between these two amino acid free radicals in proteins without dependence on isotope labeling, electron nuclear double resonance or high-field ESR. This approach also produces geometric parameters (dihedral angles for the β-methylene hydrogens) which should facilitate protein site assignment of observed l-tryptophan radicals as has been done for l-tyrosine radicals.
Keywords: free radical, β-methylene hydrogens, hyperfine coupling constants, tyrosine
INTRODUCTION
The role of l-tryptophan (Fig. 1) in protein electron transport continues to be studied 1-3. The assignment of observed spectral data from electron spin resonance (ESR) and electron nuclear double resonance (ENDOR) is complicated by l-tryptophan's possible free radical formation either as the neutral (deprotonated) free radical or as the protonated cation free radical 4. While most studies conclude that the species observed is the neutral free radical, two publications 4, 5 demonstrate the formation of the cation free radical. Adding further uncertainty is the possibility that some observed ESR spectra actually originate from l-tyrosine radicals 6. Recent work by Pogni et al. 7, 8 involving versatile peroxidase, which contains no tyrosines, established that the observed ESR spectra originated from an l-tryptophan neutral radical formed after the enzyme was treated with hydrogen peroxide. They also speculated that the precursor of that species was the l-tryptophan radical cation, which then deprotonated. Computational efforts, either accompanying some of the experimental work 4 or standing alone 9, generally support the assignment of the radical identity to either the neutral or radical cation, but have areas of disagreement with regard to unpaired electron spin density predictions.
Fig. 1. l-Tryptophan radical cation ESR spectrum.
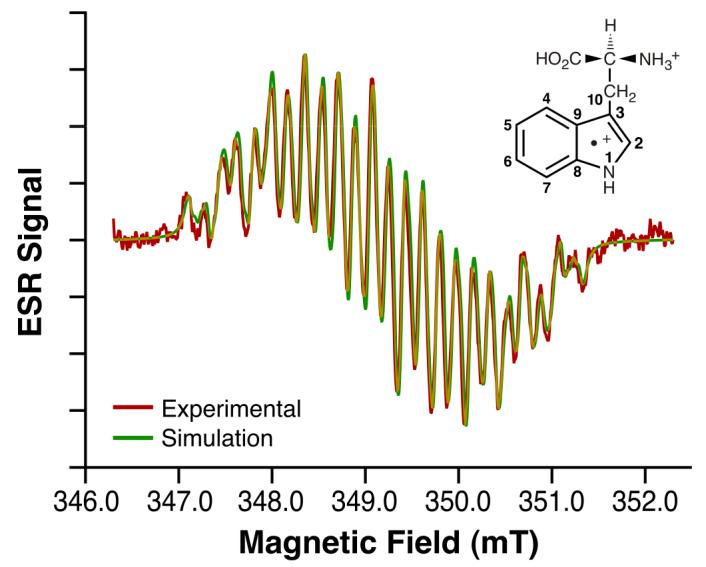
ESR fast-flow spectrum of l-tryptophan radical cation produced in a system of l- tryptophan, Ce4+, and H2SO4 having concentrations of 0.5 mM, 0.25 mM, and 0.225 M, respectively. Equal volumes of aqueous solutions of l-tryptophan/H2SO4 and Ce4+/ H2SO4 were mixed milliseconds before entering the ESR flat cell at a total flow rate of 60 mL/min. ESR spectra were recorded at 20 mW microwave power, 0.1mT field modulation, 6.00 mT field sweep width, 163 ms time constant, 81 ms conversion time and 86 scans of 1024 data points (Red). Also shown is the l-tryptophan radical cation simulation spectrum with coupling constants given in Table 1 (Green).
Information about the ESR spectral properties of the free radical forms of the naturally occurring amino acid would greatly facilitate the interpretive efforts of protein free radical studies. Even at room temperature, the l-tryptophan radical in proteins is at least partially immobilized on the ESR time scale, resulting in broad, poorly resolved spectra. The free radical of the amino acid in aqueous solution gives a motionally narrowed, high resolution spectrum that inherently provides more definitive spectroscopic information. In this work, we have used a fast-flow ESR system with acidic Ce4+ as the oxidizing agent to obtain a highly resolved ESR spectrum that we attribute to the l-tryptophan radical cation. (The species observed is actually a dication because of the protonation of the amino group of l-tryptophan and the presence of a positive charge on the indole ring due to the one-electron oxidation with Ce4+, but to maintain consistency with protein ESR study terminology, it will be referred to as a cation.)
Comparison of ESR results from structurally similar indole compounds not only facilitated the characterization of the observed species as the l-tryptophan radical cation but also enabled development of a new method of discerning which amino acid, tryptophan or tyrosine, was being observed in ESR of free radicals in proteins. This new approach involves comparison of parameters derived from using appropriate hyperfine coupling constants (HFCs) to calculate the dihedral angles of the β-methylene hydrogens in immobilized tryptophan and tyrosine protein radicals. This new approach involves comparison of parameters determined from calculations of the dihedral angles of the β-methylene hydrogens in immobilized tryptophan and tyrosine protein radicals using the appropriate hyperfine coupling constants (HFCs).
EXPERIMENTAL PROCEDURES
Chemicals and Biochemicals
l-tryptophan, tryptamine, 3-methyl indole, sulfuric acid, deuterosulfuric acid, cerium sulfate and activated carbon (Norit) were obtained from Sigma-Aldrich (St. Louis, MO). l-tryptophan 2H-5 and 2H2O were obtained from Isotec, Inc. (St. Louis, MO). l-tryptophan 2 15N was obtained from Cambridge Isotope Laboratories, Inc. (Andover, MA). 2H2O was purified by two cycles of mixing with Norit for 24 hours, followed by filtration and fractional distillation.
ESR Experiments
Free radical instability necessitated the use of fast-flow mixing of the reagent solutions in the ESR cavity to generate adequate ESR spectra. Details of fast-flow methods are described elsewhere 10, 11 . In these experiments, reagents (solution A: 4.0 L 0.5 mM Ce4+, 0.225 M H2SO4; solution B: 4.0 L 1.0 mM l-tryptophan or its equivalent, 0.225 M H2SO4) were placed in solution reservoirs elevated 1.8 m relative to the ESR cavity, deoxygenated by bubbling nitrogen through them, equilibrated to ambient temperature, and allowed to flow through plastic hoses to a quartz, fast-flow mixing chamber flat cell (Type WG-804, 10 mm width, Wilmad Glass Co., Buena, N.J.) at rates controlled by Gilmont compact flow meters. Experiments at temperatures other than ambient involved flowing the solutions through two 3 m glass heat exchange coils immersed in a Precision RDL 40 temperature bath (Chicago, IL) immediately prior to the mixing chamber. The temperature of the system was monitored with a Fisher Digi-Thermo temperature probe inserted into the exit tube of the mixing cell.
EPR measurements were made using either a Bruker EMX or Elexsys EPR spectrometer operating at a frequency near 9.7 GHz and a magnetic field of approximately 348.0 mT (Bruker Biospin, Billerica, MA). Signal detection utilized a 100 kHz field modulation and an EPR 4122 super hi-Q microwave cavity (Bruker Biospin). The instrumental software was used to collect the spectra, which were then analyzed using locally produced software 12 Sigmadraw and Canvas X software were used to prepare ESR figures.
RESULTS
The oxidation of l-tryptophan by Ce4+ is energetically highly favorable based on the reduction potentials of 1.15 V for l-tryptophan 13 and 1.44V for Ce4+ 14 when the systems are maintained at low pH.
l-Tryptophan radical cation
The ESR spectrum presented in Fig. 1 was obtained when l-tryptophan and Ce4+ reacted in acid media. The spectrum was simulated (Fig. 1) using couplings for the relevant hydrogens that were chosen based on reported values from protein studies and theoretical calculations 15 (Table 1). Notable HFCs include a comparatively low value of 0.195 mT for the indole nitrogen and large and unequal couplings, 0.71 and 1.07 mT, attributed to the β-methylene hydrogens. In another experiment seeking information concerning the origins of the differences of the β-methylene hydrogens, the temperature was varied (10 °C to 40 °C); the resulting spectra were identical to that shown in Fig. 1 (data not shown). This apparent lack of temperature dependence could be due to the limited temperature range dictated by free radical stability and the masking of small changes because of the significant overlap. Nevertheless, we can conclude that the observed spectral similarities mean that the spectrum is not in an intermediate exchange rate, which can give complex spectra that are difficult to interpret.
Table 1.
ESR HFCs of l-tryptophan and related cation free radicalsa
| Cation radical | aN-1 | aHN-1 | aHβ(1) | aHβ(2) | aHC-4b | aHC-2b | aHC-6b | aHC-5b | aHC-7b |
|---|---|---|---|---|---|---|---|---|---|
| l-tryptophan | 0.20 | 0.35 | 0.71 | 1.07 | 0.54 | 0.51 | 0.42 | 0.13 | 0.06 |
| l-[15N2]tryptophan | 0.28c | 0.35 | 0.71 | 1.08 | 0.54 | 0.51 | 0.42 | 0.12 | 0.06 |
|
l-[indole N-2H]- tryptophan |
0.19 | 0.05d | 0.70 | 1.08 | 0.56 | 0.52 | 0.42 | 0.14 | 0.05 |
|
l-[2,4,5,6,7-2H5]- tryptophan |
0.19 | 0.37 | 0.69 | 1.05 | 0.08d | 0.08 d | 0.07 d | 0.02 d | 0.01 d |
| tryptamine | 0.21 | 0.37 | 1.04 | 1.04 | 0.54 | 0.50 | 0.42 | 0.11 | 0.07 |
| 3-methylindole | 0.23 | 0.39 | 1.42e | --- | 0.46 | 0.46 | 0.45 | 0.10 | 0.05 |
Isotopic substitution studies
Use of several types of isotopically substituted l-tryptophan or deuterium oxide as a solvent enabled us to make specific assignments for several of the hyperfine coupling values.
l-[15N2]tryptophan
Ce4+ oxidation of l-tryptophan containing two 15N atoms produced an ESR spectrum (Fig. 6 in supporting information (SI)), which could be simulated with hydrogen coupling constants from normal l-tryptophan (Table 1) provided that the coupling constant for the indole nitrogen was changed from 0.195 mT to 0.275 mT, and the N nuclear spin was changed from 1 to ½. The change in aN was as expected because the different magnetogyric ratios for 15N and 14N have a ratio of 15N /14N = 1.40. Only one nitrogen coupling is listed in Table 1. Presumably, the nitrogen of the amino acid portion of the molecule had an unresolvably small hyperfine coupling.
2H2O
Oxidation of l-tryptophan in 2H2O/2H2SO4 was expected to yield a cation free radical in which the exchangable H on the indole nitrogen was replaced by deuterium 4. The decrease in the N-2H hyperfine coupling by a factor of 6.5 from the corresponding 1H value and the increase in nuclear spin state from ½ to 1 resulted in a dramatically different ESR spectrum (Fig. 7 in SI). The only significant change in the simulation coupling values resulted from the deuteron/proton exchange. This experiment clearly established that the observed free radical is cationic with a protonated indole nitrogen and, furthermore, established the assignment of the hydrogen coupling as 0.35 mT in the original l-tryptophan spectrum (Table 1).
l-[2,4,5,6,7-2H5]tryptophan
Confirmation of the assignment of the HFCs for the l-tryptophan's methylene hydrogens was achieved through oxidation of l-[2,4,5,6,7-2H5]tryptophan and simulation of the observed ESR spectrum (Fig. 8 in SI). Complete deuteration of the indole ring resulted in decreased ring hyperfine couplings; the associated ESR lines then overlapped to produce composite ESR lines with large linewidths. After adjustment for the effects of deuteration, the simulation was accomplished using coupling values very similar to those in the original l-tryptophan calculation for the four remaining nuclei. Prior assignment of the hyperfine couplings of the nitrogen and its hydrogen support the conclusion that the two remaining couplings of 1.07 and 0.71 mT must be assigned to the β-methylene hydrogens (Table 1).
ESR analyses of related compounds
To facilitate the interpretation of the l-tryptophan radical β-methylene hydrogen couplings, we obtained ESR spectra of the radical cations of two l-tryptophan-related indole compounds, tryptamine and 3-methylindole (Fig. 2 and 3). The ESR spectrum of tryptamine radical cation, a decarboxylated analog of l-tryptophan, was readily simulated using the l-tryptophan hyperfine couplings with the exception that the β-methylene hydrogen couplings (Fig. 2) were now changed to equivalent values (1.04 mT). Similarly, oxidation of 3-methylindole gave an ESR spectrum (Fig. 3) which was simulated based on the l-tryptophan coupling constants with the exception that three equivalent hydrogens (aH=1.42 mT) had to be substituted for the β-methylene hydrogens (Table 1).
Fig. 2. Tryptamine radical cation ESR spectrum.
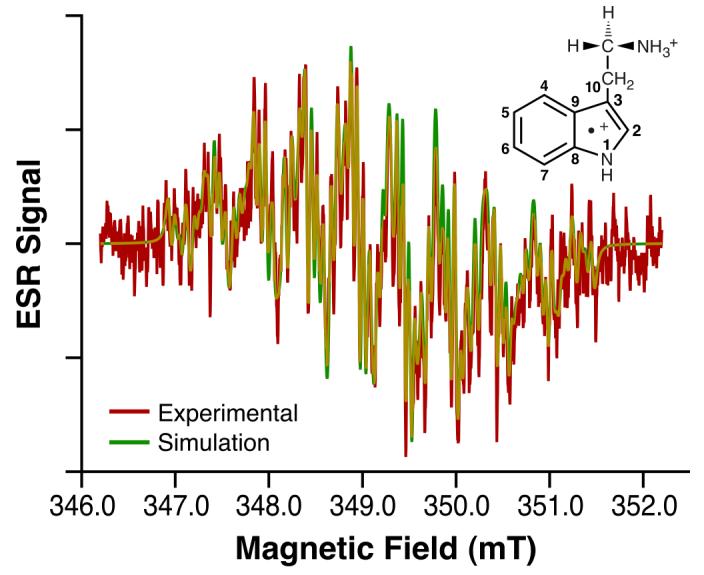
ESR fast-flow spectrum of tryptamine radical cation produced in a system of tryptamine, Ce4+, and H2SO4 having concentrations of 0.5 mM, 0.25 mM, and 0.225 M, respectively. Equal volumes of aqueous solutions of tryptamine/H2SO4 and Ce4+/H2SO4 were mixed milliseconds before entering the ESR flat cell at a total flow rate of 30 mL/min. ESR spectra were recorded at 8 mW microwave power, 0.025 mT field modulation, 6.00 mT field sweep width, 163 ms time constant, 81 ms conversion time, and 230 scans of 2048 data points (Red). Also shown is the tryptamine radical cation simulation spectrum with coupling constants given in Table 1 (Green).
Fig. 3. 3-methylindole radical cation ESR spectrum.
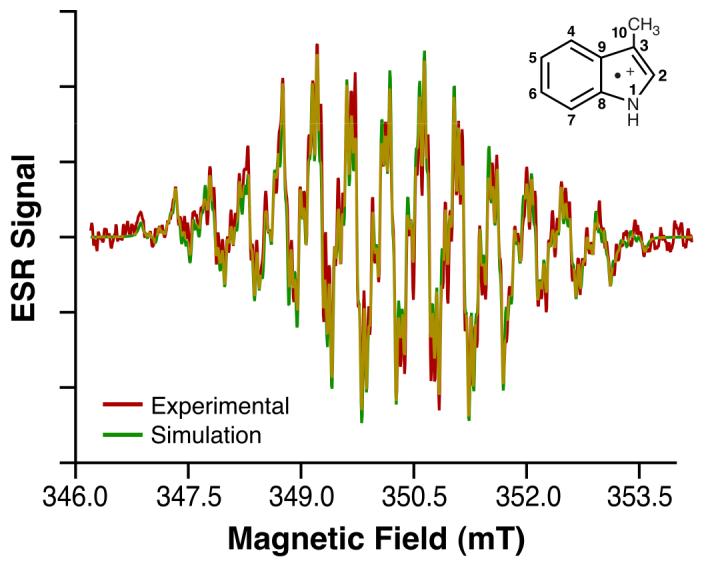
ESR fast-flow spectrum of 3-methyl indole radical cation produced in a system of 3-methyl indole, Ce4+, acetic acid, and H2SO4 having concentrations of 0.5 mM, 0.25 mM, 1.75 M, and 0.113 M, respectively. Equal volumes of aqueous solutions of 3-methyl indole/acetic acid and Ce4+/H2SO4 were mixed milliseconds before entering the ESR flat cell at a total flow rate of 50 mL/min. ESR spectra were recorded at 20 mW microwave power, 0.10 mT field modulation, 8.00 mT field sweep width, 163 ms time constant, 81 ms conversion time, and 79 scans of 1024 data points (Red). Also shown is the 3-methyl- indole radical cation simulation spectrum with coupling constants given in Table 1 (Green).
The internally consistent HFCs (Table 1) strongly support the conclusion that the observed ESR spectrum is produced by the l-tryptophan radical cation. Isotopically substituted l-tryptophan ESR spectra support the presence of key molecular components in the observed free radical. HFCs (Table 1) from ESR spectra of the l-tryptophan radical containing 15N in the indole ring (Fig. 6 in SI) and using D2O as the solvent (Fig. 7 in SI) substantiate the presence of the protonated indole nitrogen in the free radical. Similarly, the ESR spectrum of the l-tryptophan radical cation containing a perdeuterated indole ring exhibits β-methylene hydrogen couplings identical to those of l-tryptophan (Fig. 8 in SI). The notable inequivalence of these β-methylene hydrogen couplings demonstrates the presence of the chiral center in the free radical as is the case in l-tyrosine 16. Loss of either the carboxyl or amino group would remove the asymmetry and make the β-methylene hydrogens equivalent as observed in the ESR spectrum of tryptamine (Fig. 2).
DISCUSSION
A recent chemically induced dynamic nuclear polarization study17 that determined relative hydrogen HFC's for the tryptophan radical cation in aqueous solution, but not their magnitude, lends support to the conclusion that the ESR data is produced by the l-tryptophan radical cation. In that work, values were reported for indole ring hydrogens bonded to carbon and the β-methylene hydrogens. Comparison of our results in the same format (italics) to those reported (in parentheses) agree very well: H(C-2) 0.48 (0.525); H(C-4) 0.50 (0.495); H(C-5) 0.12 (0.145); H(C-6) 0.39 (0.49); H(C-7) 0.06 (0.07); Hβ1 1.00 (1.00); Hβ2 0.66 (0.69).
The similarity of the indole ring hyperfine couplings for l-tryptophan, tryptamine, and 3-methylindole radical spectra (Table 1) supports previous assertions 9, 15 that results from theoretical studies involving simpler compounds such as 3-methylindole and tryptamine can serve as models for l-tryptophan studies. Several reports on spin density computations 4, 9, 15 can be compared with spin densities determined in these experiments. Determination of such spin densities necessitates using McConnell's equation 18:
| (1) |
Using our HFCs (aH) for the indole ring Hs and a Q value of 2.8 mT 19 suited to radical cations, we calculated the spin density values (ρCπ) tabulated in the first three columns of Table 2. Since these spin densities compare favorably with calculations by Jensen et al. for 3-methylindole 9 and by Walden et al. for indole 15 (columns 4 and 5, Table 2), we feel confident assigning them to indole carbons as indicated in Tables 1 and 2. Included at the bottom of Table 2 are spin density values for the indole nitrogen calculated from the N-H hydrogen's HFC. In this case, a Q value of 2.8 mT 20 was used in the equivalent of Eqn.1 to calculate those spin densities. Once again, agreement is reasonably good, especially between the 3-methylindole values. A separate discussion concerning the spin density on carbon C-3 and its effect on the C-10 β-methylene hydrogen hyperfine couplings is presented below.
Table 2.
Spin density values of l-tryptophan and related cation free radicals
β-Methylene Hydrogen Hyperfine coupling constants
Numerous protein ESR studies have used the HFCs of the β methylene hydrogens of l-tyrosine and l-tryptophan radicals to support arguments concerning these amino acids' participation in electron transport processes through free radical formation 21-24. In those experiments, the l-tyrosine or l-tryptophan is immobilized in the protein and the β methylene hydrogens have a fixed position relative to the plane of the aromatic ring. The HFCs (aβH) can be evaluated using the Heller-McConnell equation (Eqn. 2) 25.
| (2) |
In this equation, ρCπ is the unpaired electron spin density on the ring carbon adjacent to the methylene group, B′ is a small constant which is usually set equal to 0, B″ is a proportionality constant which varies with the type of free radical (neutral, anion, or cation 26) and θ is the dihedral angle between the α-carbon pz axis and the projected CβHβ bond (Fig. 4) 27. Setting B′ = 0, we find that the simplified equation (Eqn. 3) gives hyperfine couplings that are maximal when θ = 0° and approach zero as the β-methylene hydrogen becomes co-planar to the indole ring plane. Use of this equation either to justify the observed inequality of the methylene Hs through θ determinations or to predict aβH for those hydrogens based on variations in geometry has been central to numerous studies 21, 23, 24.
| (3) |
Care must be taken to differentiate between the protein studies involving fixed amino acids as mentioned above and interpretations of the β-methylene HFCs associated with free radicals in solution as presented in this work. Such solution free radicals exhibit intramolecular movement, which causes the β-methylene hydrogen position relative to the indole ring to be a distribution of values rather than a single angle as in proteins at low temperature.
Fig. 4.
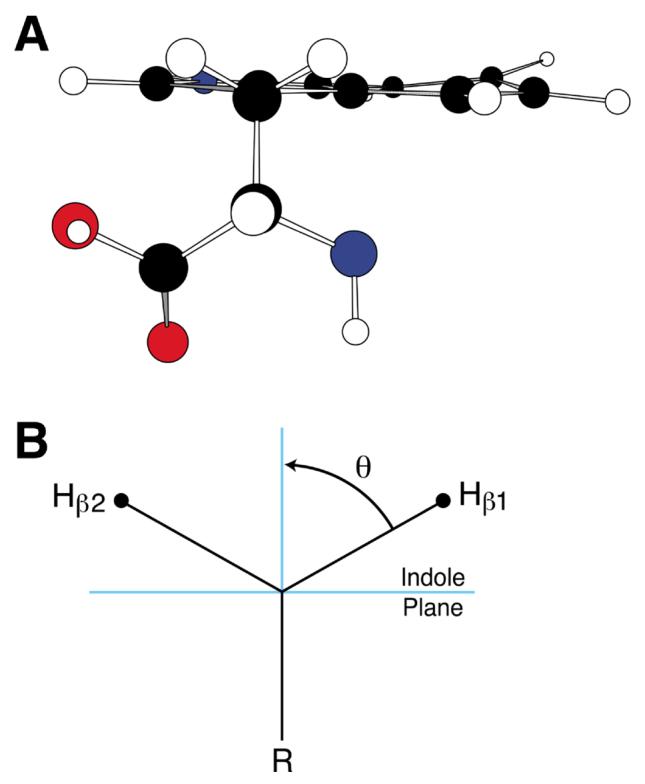
l- Tryptophan molecular model (A) and corresponding β-methylene hydrogen representation (B).
To evaluate the effect of intramolecular motion on the aβHs, we compared values for 3-methylindole, tryptamine and l-tryptophan radicals and assumed that the spin density on C-3 is the same for all three species. In the simplest molecule, 3-methylindole, the methyl group will have unhindered rotation relative to the indole ring so that the average angle relative to the ring normal is 45°. A value of 2.84 mT for the product ρCπB″ is obtained by substituting cos2 θ = 0.5 into Eqn. 3. In contrast to 3-methylindole, interpretation concerning tryptamine must take into consideration the rotational hindering effect of the large R group attached to C-10 (Fig. 2). As substantiated by experimental and theoretical data, such a large group will orient perpendicular to the indole ring, forcing the β-methylene hydrogens to occupy equilibrium positions at θ = 60° to the ring normal 16. If the β-methylene hydrogens were fixed at θ = 60°, the expected aβHs calculated from Eqn. 3 using cos2θ = 0.25 and B″= 2.84 mT would be 0.71 mT.
The difference between the expected value of 0.71 mT and the observed value of 1.04 mT for tryptamine can be explained using the work of Stone and Maki 28, who studied the effect of intramolecular motion on β-methylene aβHs in alkylaryl radicals. They approximated the intramolecular motion in such rotationally hindered molecules as simple harmonic oscillation about the rotating group's equilibrium position (β-methylene Hs at 60 degrees for tryptamine). In such a moving system, the cos2θ is replaced by a time averaged form, <cos2θ>. Their calculations showed that as the extent of oscillation increased toward free rotation, the value of <cos2θ> increased. Thus the tryptamine radical aβH value is greater than the fixed orientation value because of significant oscillatory motion around the 60° equilibrium position. Using their data, the β-methylene hydrogens can be shown to oscillate around their θ = 60° equilibrium position by approximately 45° in either direction. Thus, the relatively large aβH value for tryptamine can be understood as resulting from the oscillation causing increased interaction of the hyperconjugation-derived molecular orbital of the β-methylene hydrogens with the unpaired electron spin density in the 2pz orbital on C-3 of the indole ring.
To interpret the β-methylene hydrogen aβHs of the tryptophan radical cation, the effect of the adjacent asymmetric carbon center must be considered in addition to the oscillatory effect discussed above. The unequal aβH values of 0.71 mT and 1.07 mT imply that the equilibrium orientation of the bulky constituent on C-10 (Fig. 1) is shifted slightly away from the ring normal, thereby causing unequal hyperconjugative-2pz interactions between the two methylene hydrogens and the indole ring. This shift occurs because of the differences in steric interactions in two key conformers shown in Fig. 5. The two conformers (A and B) represent the sterically favored orientations of the amino and carboxyl groups for two possible orientations with respect to the indole ring. As these groups oscillate between the orientations A and B in Fig. 5, the differences in steric interactions experienced by the amino and carboxyl groups with respect to the indole ring components will result in different tilts of the whole bulky substituent on C-10 away from the ring normal as represented in Fig 5 by δθ′ and δθ″. Thus, the unequal β-methylene hydrogen aβHs result from the influence of these unequal steric interactions.
Fig. 5.
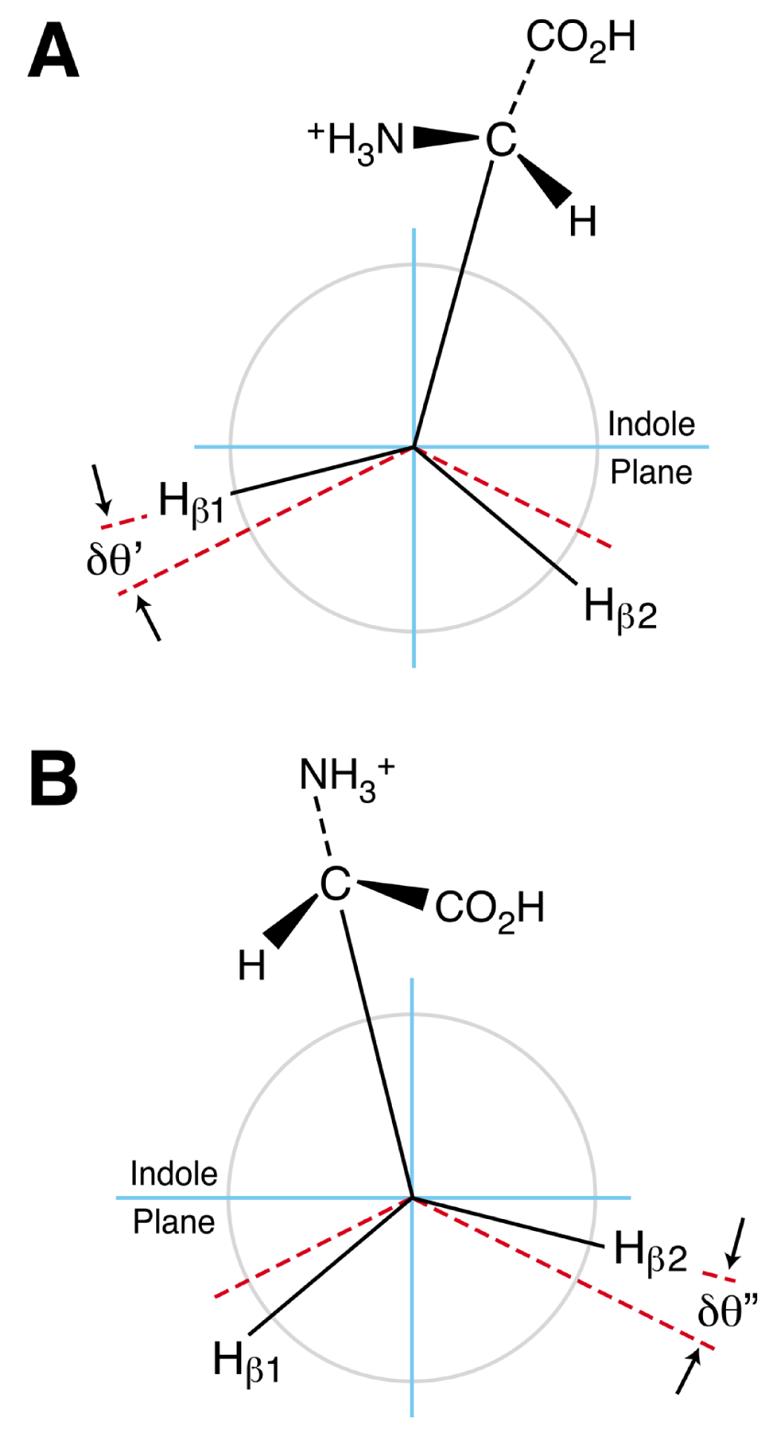
l-Tryptophan conformers that contribute to β-methylene aβH inequivalency.
Characterization of the average position of the bulky constituent should also include the effect of the asymmetry of the indole ring. Such a consideration would include contributions to the final positional average from two more key conformers like A and B in Fig. 5 with the indole ring rotated 180 deg about the C3-C10 axis. Thus, the β-methylene aβHs also include the effects of steric interactions of these conformers. Furthermore, consideration of such averaging can also be used to show that an inversion of chirality does not affect the average orientation of the bulky substituent to the ring normal. We confirmed this expectation by obtaining an ESR spectrum identical to that shown in Fig. 1 by oxidizing d-tryptophan (data not shown).
Estimation of the equilibrium orientations of the β-methylene hydrogens necessitates separation of the effect of the simple harmonic oscillation discussed above for tryptamine cation radical from the tilting of the bulky substituent away from the indole ring normal. Since the tilting of the bulky substituent causes the inequality in aβHs, a suitable approximation of the tilt's effect can be approximated by Δ aβH = (aβH (1) - aβH (2))/2. Assuming that the asymmetric carbon's effect on aβHs is independent of the effect of simple harmonic oscillation, “immobilized” aβH values of 0.71 ± Δ aβH (0.89 and 0.53 mT) can be used to calculate θs for the β-methylene hydrogens. Calculation of these values can be accomplished using Eqn. 4, which is derived from the expression
| (4) |
Use of this equation with “immmobilized” aβH values gives θ values of 55.7° and 64.3° for the tilt caused by the unequal steric interactions. An approximate value of 30° for the bulky constituent's oscillation around its equilibrium position can be obtained from the data of Stone and Maki by using the average of the experimental aβH s, 0.89 mT. This decrease in intramolecular motion in comparison to tryptamine's value of 45° appropriately represents decreased oscillation of the amino acid appendage due to its greater size and coulombic repulsion of the two positive charges.
l-tryptophan and l-tyrosine free radicals in proteins
A frequent issue in the assignment of free radicals in proteins concerns differentiating l-tyrosine from l-tryptophan radicals. Currently, the most effective methods for making these assignments involve ENDOR and high-field ESR either separately or in combination. High-field ESR spectroscopy exploits the significant difference in g-tensor values between the two radicals.
Another method for such differentiation can be developed from a quantitative comparison of our data for the l-tryptophan radical cation and equivalent data for l-tyrosine. We first note the difference in aβHs for the 3-methyl indole radical cation (1.42 mT) and the 4-methylphenoxyl radical (1.25 mT) 29. The unhindered rotation of the methyl group in these free radicals results in a <cos2 θ > value of 0.5 which leads to calculated values of ρCπ B″ of 2.84 and 2.5 mT, respectively. These values for ρCπ B″ represent the effect of the two aromatic ring systems' structural differences on the unpaired electron spin density on the ring carbon that is β to the β-methylene hydrogens. Since the ρCπ B″ values calculated from protein situations tend to be lower than the ρCπ B″ values for the methyl substituted rings listed above, the structural conversion to the amino acid must change the values of ρCπ B″. Computations by Walden and Wheeler 30 indicate that about 10% of the electron spin density exists on the amino acid portion of the radical system around the chiral carbon. As a consequence of this difference between the methyl and amino acid constituents, the spin density on the aromatic ring could be reduced by approximately 10%, thereby reducing ρCπ B″ to values similar to those we determined.
Using Eqn. 4 and experimental data, we find that the differences in ρCπ B″ between l-tyrosine and l-tryptophan radicals provide the means to determine the identity of the species providing the ESR signal. To exploit these differences, experimental β-methylene hyperfine couplings from protein ESR spectral simulations were used in Eqn. 4 to calculate two pairs of θ's for each data set. (Since Eqn. 4 is the solution of a quadratic equation, using it produces two sets of angles.) The θ values and the hyperfine couplings were then used in Eqn. 3 to calculate values of ρCπ B″. Of the two possible pairs of results, those ρCπ B″ values in the range of 1.9 to 3.0 mT were considered appropriate values based on comparison of our calculated values with those reported in the literature. Most values of ρCπ B″ for l-tyrosine range from 1.95 to 2.35 mT whereas the corresponding values for l-tryptophan range from 2.4 to 2.95 mT. Results concerning tryptophan are presented in Table 3 below and the much more extensive results for tyrosine are contained in Table 4 in supporting information.
Table 3.
Values of dihedral angles (θ1 and θ1-120°) and ρCπ B″(mT) calculated from reported β-methylene hydrogen HFCs (aβH (1) and aβH (2) in mT) for l-tryptophan radicals in proteins.
| Protein- Radical |
l-tryptophan | free | a(1) | a(2) | Θ 1 | Θ 1- 120 |
ρCπ B″ | Reference |
|---|---|---|---|---|---|---|---|---|
| B. adusta VPd | 2.25 | 1.88 | −25.4 | −145.4 | 2.77 | 7 | ||
| E coli CCPb | 0.75 | 0.46 | 56.0 | −64.0 | 2.41 | 4 | ||
| E. coli RNR R2 Y122Fc | 2.76 | 1.38 | −13.3 | −133.3 | 2.92 | 31 | ||
| E. coli RNR R2 Y122F Wac | 2.83 | 1.36 | −12.4 | −132.4 | 2.98 | 27 | ||
| E. coli RNR Y122Wc | 2.80 | 1.30 | −11.7 | −131.7 | 2.93 | 32 | ||
| E. coli RNRd | 2.75 | 1.38 | −13.4 | −133.4 | 2.92 | 31 | ||
| Human SODd | 0.13 | 1.43 | 77.3 | −42.7 | 2.63 | 33 | ||
| M. tuberculosis KatG | 1.60 | 0.10 | 40.8 | −79.2 | 2.81 | 34 | ||
| Mouse RNR R2 Y177Wd | 2.25 | 0.15 | 15.6 | −104.4 | 2.43 | 31 | ||
| Mouse RNR R2 Y177Wd | 2.25 | 0.15 | 15.6 | −104.4 | 2.43 | 35 | ||
| Mouse RNR Y177Wd | 2.25 | 1.50 | −19.9 | −139.9 | 2.55 | 35 | ||
| P. erygii VPc | 2.60 | 1.15 | −10.6 | −130.6 | 2.70 | 8 | ||
| E. coli RNR R2 Y122F Wbc,e | 0.20 | 1.60 | 75.4 | −44.6 | 3.15 | 27 | ||
| Yeast CCPb,e | 0.44 | 0.72 | 63.9 | −56.1 | 2.30 | 5 | ||
| Bovine CCOa,b | 0.41 | 0.67 | 64.0 | −56.0 | 2.14 | 5 | ||
| Bovine CCO pH= 8.5a,b | 0.43 | 0.62 | 63.0 | −57.0 | 2.09 | 5 | ||
Assignments questioned by Svistunenko6 and this work (irregular ρCπ B″ values)
Cation radical
Neutral radical with H-bonded proton
Neutral radical
Irregular ρCπ B″ values for unknown reasons
Abbreviations: VP, versatile peroxidase; CCP, cytochrome C peroxidase; RNR, ribonucleotide reductase; SOD, superoxide dismutase; KatG, catalase-peroxidase; CcO, cytochrome c oxidase
While determining ρCπ B″ values from HFCs reported in the literature, we found that some data gave confusing values but, upon reflection, could be either disqualified or re-evaluated. One common situation in early work occurred where the C-4 ρCπ for l-tyrosine was believed to be about 0.49 36. This value along with a B″ of 5.8 mT was used to calculate a linewidth-obscured small β-methylene coupling. The reverse calculation involved in our approach would then give back a larger value of ρCπ B″. Later work that used a reduced value of the spin density showed good agreement with our expected values. Only those later data are included in the ranges cited above (Table 3 above and Table 4 in SI). For cases where methylene aβHs are determined from spectral simulations rather than spin density-dependent computations, this new spin density-independent approach provides ρCπ B″ values that have less uncertainty than values obtained from spin density-dependent methods 37. Alternatively, data on small couplings from ENDOR studies could be included in those tables.
The range of ρCπ B″ values for l-tyrosine radicals in proteins of 1.95 to 2.35 mT (Table 4 in SI) can be attributed to a combination of HFC value uncertainty and variations in polarity of the surrounding protein environment. While the quality of simulations varies from case to case, the problem of HFC uncertainty with regard to the parameters most critical to this method, the β-methylene hydrogen HFCs, is relatively small in most cases because, in general, β-methylene hydrogen HFCs are large enough to have readily observable effects on the ESR spectra37 , although, when one of the β-methylene HFCs is comparable to the linewidth, relatively large errors in its determination can result. In this case, since the calculations of the dihedral angle depends on the ratio of the β-methylene hydrogen HFCs, large errors can result. Calculation of the effect of a hyperfine uncertainty of 0.01 mT in the value of the HFC's causes variation in the ρCπ B″ value ranging from 0.06 mT for the case where the magnitude of one of the β-methylene hydrogen couplings is near that of the line width (1.9 and 0.1 mT) to 0.006 mT for the case where the couplings are similar ( 1.57 and 1.50 mT). The polarity effect can be estimated by comparison of the β methyl HFCs for the 4-methyl phenoxyl radical in nonpolar benzene (1.175 mT) 29 with the aqueous solution value (1.25 mT). The corresponding ρCπ B″ range of 0.15 mT could contribute about one third of the observed variability in ρCπ B″ values (Table 3). Although recent reports 38, 39 focus on the influence of the protein environment on free radical formation and stability, proton transfer, and protein catalytic capabilities, the fact that extreme solvent polarity differences such as between benzene and water have a relatively slight effect on the observed β-methyl HFCs means that the method of analysis presented here cannot distinguish between protonated and unprotonated forms of radicals of such species. While the solvent polarity differences for l-tryptophan are not available, analogous relationships would be expected to apply in that case also.
A third issue concerning this new evaluation method involves the characterization of l-tryptophan protein radicals as cation free radicals or neutral, N-deprotonated free radicals. To date, only two studies 4, 5 have reported indole nitrogen proton HFCs indicative of an l-tryptophan radical cation. Several of the studies reporting the neutral radical also include evidence of a proton hydrogen-bonded to the indole N 8, 27, 35. As is evident in the ρCπ B″ values listed in Table 4, there is not a consistent difference in ρCπ B″ values for the two types of radicals. The applicability of this evaluation method to neutral l-tryptophan protein radicals as well as the corresponding cation radicals can be justified to some degree by consideration of the separate parameters, B″ and ρCπ . The values of B″ have been found to be higher for cation radicals compared to the value for the equivalent neutral radical 26. In contrast, the calculated values of ρCπ for the neutral tryptophan radical range from 0.41 to 0.61 and are always greater than the corresponding values of 0.27 to 0.41 for the tryptophan cation radical 4, 7, 26. Thus, these effects tend to cancel in the ρCπ B″ product for the two charge states of l-tryptophan radicals.
Thus, our computation method, which involves only experimental β-methylene hyperfine couplings, can facilitate determination of whether an l-tryptophan or l-tyrosine radical is being detected by ESR. As demonstrated in Table 3 above and Table 4 in SI, most current work correctly identifies the l-tyrosine or l-tryptophan radical.
Recently, Svistunenko et al. showed how a set of l-tyrosine parameters could be used to compute an ESR spectrum 6 which matched an ESR spectrum attributed to an l-tryptophan radical in cytochrome c oxidase 5. Our calculated results for the ESR data of that work gave a ρCπ B″ of 2.14 mT (Table 3), which is more appropriate for an l-tyrosine radical than for an l-tryptophan radical. Thus, our method also questions this assignment to l-tryptophan.
In addition to enabling l-tryptophan and l-tyrosine free radical assignments in proteins, the dihedral angles calculated using Eqn. 4 may make possible the protein site assignments to a specific tryptophan as has been done for tyrosine 21, 37.
CONCLUSIONS
In this paper, we report the first observation of a free radical of l-tryptophan, thereby lending support to studies of the participation of l-tryptophan in protein electron transport. This work provides a new method for the use of spectroscopic data for β-methylene hydrogen HFCs to determine whether the free radical site in a protein involves l-tryptophan or l-tyrosine without relying on unpaired electron spin density calculations. Furthermore, the dihedral angles associated with the β-methylene hydrogens of the l-tryptophan radical determined from this data manipulation may be used to assign the protein sites of such free radicals in cases where the protein geometry is known.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
The authors wish to thank Jean Corbett, Jill Flachskam, Mary Mason, Sue Edelstein and Dr. Ann Motten for assistance with editing and manuscript preparation and Lusine Tonoyan for help with computations.
Footnotes
Supporting Information Available:
Table 4. Values of dihedral angles (θ1 and θ1 - 120°) and ρCπ B″ (mT) calculated from reported β-methylene hydrogen HFCs (aβH (1) and aβH (2) in mT) for l-tyrosine radicals in proteins.
Fig. 6 l-[15N2]tryptophan radical cation ESR spectrum.
Fig. 7 l-Tryptophan radical cation in 2H2O ESR spectrum.
Fig. 8 l-[2,4,5,6,7-2H5]tryptophan radical cation ESR spectrum.
REFERENCES
- 1.Di Bilio AJ, Crane BR, Wehbi WA, Kiser CN, Abu-Omar MM, Carlos RM, Richards JH, Winkler JR, Gray HB. J. Am. Chem. Soc. 2001;123(13):3181–3182. doi: 10.1021/ja0043183. [DOI] [PubMed] [Google Scholar]
- 2.Miller JE, Gradinaru, Crane BR, Bilio, Wehbi WA, Un S, Winkler C, Gray HB. J. Am. Chem. Soc. 2003;125(47):14220–140221. doi: 10.1021/ja037203i. [DOI] [PubMed] [Google Scholar]
- 3.Immoos CE, Di Bilio AJ, Cohen MS, Van der Veer W, Gray HB, Farmer PJ. Inorg. Chem. 2004;43(12):3593–3596. doi: 10.1021/ic049741h. [DOI] [PubMed] [Google Scholar]
- 4.Huyett JE, Doan PE, Gurbiel R, Houseman ALP, Sivaraja M, Goodin DB, Hoffman BM. J. Am. Chem. Soc. 1995;117(35):9033–9041. [Google Scholar]
- 5.Rigby SEJ, Junemann S, Rich PR, Heathcote P. Biochemistry. 2000;39(20):5921–5928. doi: 10.1021/bi992614q. [DOI] [PubMed] [Google Scholar]
- 6.Svistunenko DA, Wilson MT, Cooper CE. Biochim. Biophys. Acta, Bioenerg. 2004;1655(13):372–380. doi: 10.1016/j.bbabio.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Pogni R, Baratto MC, Giansanti S, Teutloff C, Verdin J, Valderrama B, Lendzian F, Lubitz W, Vazquez-Duhalt R, Basosi R. Biochemistry. 2005;44(11):4267–4274. doi: 10.1021/bi047474l. [DOI] [PubMed] [Google Scholar]
- 8.Pogni R, Baratto MC, Teutloff C, Giansanti S, Ruiz-Duenas FJ, Choinowski T, Piontek K, Martinez AT, Lendzian F, Basosi R. J. Biol. Chem. 2006;281(14):9517–9526. doi: 10.1074/jbc.M510424200. [DOI] [PubMed] [Google Scholar]
- 9.Jensen GM, Goodin DB, Bunte SW. J. Phys. Chem. 1996;100(3):954–959. [Google Scholar]
- 10.West PR, Harman LS, Josephy PD, Mason RP. Biochem. Pharmacol. 1984;33(18):2933–2936. doi: 10.1016/0006-2952(84)90222-3. [DOI] [PubMed] [Google Scholar]
- 11.Fischer V, Harman LS, West PR, Mason RP. Chem.-Biol. Interactions. 1986;60:115–127. doi: 10.1016/0009-2797(86)90021-9. [DOI] [PubMed] [Google Scholar]
- 12.Duling DR. J. Mag. Res. Series B. 1994;104(2):105–110. doi: 10.1006/jmrb.1994.1062. [DOI] [PubMed] [Google Scholar]
- 13.Harriman A. J. Phys. Chem. 1987;102:8221–8228. [Google Scholar]
- 14.Milazzo G, Caroli S, Sharma VK. Tables of Standard Electrode Potentials. Wiley; London: 1978. [Google Scholar]
- 15.Walden SE, Wheeler RA. J. Phys. Chem. 1996;100(5):1530–1535. [Google Scholar]
- 16.Sealy RC, Harman L, West PR, Mason RP. J. Am. Chem. Soc. 1985;107(12):3401–3406. [Google Scholar]
- 17.Kiryutin AS, Morozova, Olga B, Kuhn, Lars T, Yurkovskaya, Alexandra V, Hore PJ. J. Phys. Chem. B. 2007;111(38):11221–11227. doi: 10.1021/jp073385h. [DOI] [PubMed] [Google Scholar]
- 18.McConnell HM, Chesnut DB. J. Chem. Phys. 1958;28(1):107–117. [Google Scholar]
- 19.Gerson F, Huber W. Electron Spin Resonance Spectroscopy of Organic Radicals. Wiley-VCH Verlag GmbH&Co.; Weinheim: 2003. p. 58. [Google Scholar]
- 20.Gerson F, Huber W. Electron Spin Resonance Spectroscopy of Organic Radicals. Wiley-VCH Verlag GmbH&Co; Weinheim: 2003. p. 69. [Google Scholar]
- 21.Gunther MR, Sturgeon BE, Mason RP. Free Radical Biol. Med. 2000;28(5):709–719. doi: 10.1016/s0891-5849(00)00164-7. [DOI] [PubMed] [Google Scholar]
- 22.Lassmann G, Odenwaller R, Curtis JF, Degray JA, Mason RP, Marnett LJ, Eling TE. J. Biol. Chem. 1991;266(30):20045–20055. [PubMed] [Google Scholar]
- 23.Degray JA, Lassmann G, Curtis JF, Kennedy TA, Marnett LJ, Eling TE, Mason RP. J. Biol. Chem. 1992;267(33):23583–23588. [PubMed] [Google Scholar]
- 24.Lendzian F. Biochim. Biophys. Acta, Bioenerg. 2005;1707(1):67–90. doi: 10.1016/j.bbabio.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Heller C, McConnell HM. J. Chem. Phys. 1960;32(5):1535–1539. [Google Scholar]
- 26.Gerson F, Huber W. Electron Spin Resonance Spectroscopy of Organic Radicals. Wiley-VCH Verlag GmbH& Co.; Weinheim: 2003. p. 62. [Google Scholar]
- 27.Lendzian F, Sahlin M, MacMillan F, Bittl R, Fiege R, Potsch S, Sjoberg BM, Graslund A, Lubitz W, Lassmann G. J. Am. Chem. Soc. 1996;118(34):8111–8120. [Google Scholar]
- 28.Stone EW, Maki AH. J. Chem. Phys. 1962;37(6):1326–1333. [Google Scholar]
- 29.Dixon WT, Moghimi M, Murphy D. J. Chem. Soc.-Faraday Trans. 1974;70(10):1713–1720. [Google Scholar]
- 30.Walden SE, Wheeler RA. J. Am. Chem. Soc. 1997;119:3175–3176. [Google Scholar]
- 31.Bleifuss G, Kolberg M, Potsch S, Hofbauer W, Bittl R, Lubitz W, Graslund A, Lassmann G, Lendzian F. Biochemistry. 2001;40(50):15362–15368. doi: 10.1021/bi010707d. [DOI] [PubMed] [Google Scholar]
- 32.Katterle B, Sahlin M, Schmidt PP, Potsch S, Logan DT, Graslund A, Sjoberg BM. J. Biol. Chem. 1997;272(16):10414–10421. doi: 10.1074/jbc.272.16.10414. [DOI] [PubMed] [Google Scholar]
- 33.Karunakaran C, Zhang H, Crow JP, Antholine WE, Kalyanaraman B. J. Biol. Chem. 2004;279(31):32534–32540. doi: 10.1074/jbc.M314272200. [DOI] [PubMed] [Google Scholar]
- 34.Zhao XB, Girotto S, Yu SW, Magliozzo RS. J. Biol. Chem. 2004;279(9):7606–7612. doi: 10.1074/jbc.M311884200. [DOI] [PubMed] [Google Scholar]
- 35.Potsch S, Lendzian F, Ingemarson R, Hornberg A, Thelander L, Lubitz W, Lassmann G, Graslund A. J. Biol. Chem. 1999;274(25):17696–17704. doi: 10.1074/jbc.274.25.17696. [DOI] [PubMed] [Google Scholar]
- 36.Barry BA, El-deeb MK, Sandusky PO, Babcock GT. J. Biol. Chem. 1990;265(33):20139–20143. [PubMed] [Google Scholar]
- 37.Svistunenko DA, Cooper CE. Biophys. J. 2004;87(1):582–595. doi: 10.1529/biophysj.104.041046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sibert R, Josowicz M, Porcelli F, Veglia G, Range K, Barry BA. J. Am. Chem. Soc. 2007;129:4393–4400. doi: 10.1021/ja068805f. [DOI] [PubMed] [Google Scholar]
- 39.Rogers MS, Tyler EM, Akyumani N, Kurtis CR, Spooner RK, Deacon SE, Tamber S, Firbank SJ, Mahmoud K, Knowles PF. Biochemistry. 2007;46:4606–4618. doi: 10.1021/bi062139d. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


