Abstract
Neurogenesis, tied to the proliferation, migration and differentiation of neural progenitor cells (NPC) is affected during neurodegenerative diseases, but how neurogenesis is affected during HIV-1 associated dementia (HAD) has not been fully addressed. Here we test the hypothesis that HIV-1-infected and/or immune-activated brain macrophages affect NPC proliferation and differentiation through the regulation of cytokines. We showed that human monocyte-derived macrophages (MDM) conditioned medium (MCM) induces a dose dependant increase in NPC proliferation. Conditioned media from lipopolysaccharide (LPS)-activated MDM (LPS-MCM) or HIV-infected MCM (HIV-MCM) induced a profound increase in NPC proliferation. HIV-infected and LPS-activated MCM (HIV+LPS-MCM) induced the most robust increase in NPC proliferation. Moreover, LPS-MCM and HIV+LPS-MCM decreased β-III-tubulin and increased GFAP expression, demonstrating an induction of gliogenesis and inhibition of neurogenesis. The increase of NPC proliferation and gliogenesis correlated with increases in production of TNF-α by infected/activated MDM. Although both IL-1β and TNF-α induced NPC proliferation and gliogenesis, these effects were only partially abrogated by soluble TNF-α receptors R1 and R2 (TNF-R1R2), but not by the IL-1 receptor antagonist (IL-1ra). This indicated that the HIV-1-infected/LPS-activated MCM-mediated effects were, in part, through TNF-α. These observations were confirmed in severe combined immunodeficient (SCID) mice with HIV-1 encephalitis (HIVE). In these HIVE mice, NPC injected with HIV-infected MDM showed more astrocyte differentiation and less neuronal differentiation compared to NPC injection alone. These observations demonstrated that HIV-1-infected and immune-activated MDM could affect neurogenesis through induction of NPC proliferation, inhibition of neurogenesis, and activation of gliogenesis.
Keywords: HIV-1, macrophage, neural progenitor cell, TNF-α, proliferation, differentiation
INTRODUCTION
Neurogenesis relies upon the proliferation, migration, and differentiation of neural progenitor cells (NPC), which are capable of self-renewal and are multipotent (Gage, 2002; Ming and Song, 2005). NPC give rise to neurons, astrocytes, and oligodendrocytes (Arvidsson et al., 2002; Gensert and Goldman, 1997; Parent, 2003). Active neurogenesis occurs throughout life in the subventricular zone (SVZ)of the lateral ventricle and in the subgranular zone (SGZ) of the dentate gyrus in the hippocampus. In response to brain insults, such as stroke, ischemia and seizure, NPCs in the neurogenic centers proliferate, and migrate to sites of pathology (Arvidsson et al., 2002; Nakatomi et al., 2002; Parent et al., 2002). In the case of neurodegenerative disorders, such as Alzheimer’s and Parkinson’s diseases (AD and PD), HIV-1-associated dementia (HAD) and multiple sclerosis, this process may be interrupted (Arvidsson et al., 2002; Burton et al., 2002; Haughey et al., 2002; Monje and Palmer, 2003; Monje et al., 2003; Poluektova et al., 2005). Brain inflammation, mainly resulting from immune activated brain mononuclear phagocytes (MP; perivascular macrophages and microglia), is a key component to the pathogenesis of both chronic neurodegenerative disorders like HAD, AD, PD, and acute brain insults such as stroke (Gendelman, 1997; Liu and Hong, 2003; Nelson et al., 2002; Stoll and Jander, 1999).
HAD is a neurodegenerative disease characterized by progressive cognitive, motor, and behavioral abnormalities (Maschke et al., 2000; McArthur et al., 2003). In HAD, infected MP migrates into the brain through a compromised blood brain barrier (BBB), infect and subsequently activate brain microglia. HIV-1-infected and/or immune-activated MP release a plethora of immune competent factors including cytokines (such as IL-1β and TNF-α), viral proteins and neurotoxins involved in neuronal injury and may affect neurogenesis (Conant et al., 1998; Gabuzda et al., 1998; Gendelman et al., 1997; Glass et al., 1995; He et al., 1997; Kaul et al., 2001; Kolson and Gonzalez-Scarano, 2000; Lipton and Gendelman, 1995; Masliah et al., 1997; Monje et al., 2003; Nath and Geiger, 1998; Strizki et al., 1996). How HIV-1-infected and/or immune-activated MP-derived factors affect the proliferation and differentiation of NPC remains unclear.
In our study, we used human cortical NPC to investigate the effects of soluble factors produced by HIV-1-infected and/or LPS-activated human monocyte-derived macrophages (MDM) on neurogenesis. Secretory factors produced by HIV-1-infected and/or LPS-activated MDM increased NPC proliferation, but it reduced β-III-tubulin and increased glial fibrillary acidic protein (GFAP) expression during differentiation. These effects are at least partially through the cytokine TNF-α, as the effects were partially blocked by TNF-α soluble receptors. In contrast, IL-1 receptor antagonist (IL-1ra) had no effect, indicating that IL-1β is not a major factor in this process. In severe combined immune deficient (SCID) mice with HIV-1 encephalitis (HIVE), HIV-1 infected MDM induced an increase in astrocyte differentiation. These observations provide evidence that HIV-1-infected and immune-activated macrophages affect neurogenesis through induction of NPC proliferation and gliogenesis and inhibition of neurogenesis.
MATERIALS AND METHODS
Monocyte Cell Culture and Conditioned Media Collection
Human monocytes were recovered from peripheral blood mononuclear cells (PBMCs) of HIV-1 and hepatitis B seronegative donors after leukopheresis and counter current centrifugal elutriation (Gendelman et al., 1988). Monocytes were cultured as adherent monolayers at a density of 1.1 × 106 cells/well in 24-well plates and cultivated in Dulbecco’s modified Eagles medium (DMEM, GIBCO Invitrogen, Carlsbad, CA) with 10% heat-inactivated pooled human serum (Cambrex Bio Science, Walkersville, MD), 50 μg/mL gentamicin and/or 10 μg/mL ciprofloxacin (Sigma-Aldrich, St. Louis, IL) and 1000 U/mL highly purified recombinant human macrophage colony stimulating factor (MCSF, a generous gift from Wyeth Institute, Cambridge, MA).
Seven days after plating, human MDM were infected with HIV-1 strain ADA at a multiplicity of infection (MOI) of 0.1 virus/target cell (Zheng et al., 2001). MDM were then treated with/without IL-1β (10 ng/mL, R&D Systems, Minneapolis, MN) or lipopolysaccharide (LPS) (Sigma, 0.1 μg/mL) for 3 h. Cells were then rinsed twice with fresh DMEM to remove residual IL-1β or LPS, and serum-free DMEM was placed onto the MDM for 24 h. The conditioned medium (MCM) was harvested, cleared of free-floating cells by centrifugation for 5 min at 1000 rpm, and stored at -80°C.
Neural Progenitor Cell Culture
Human cortical NPC were isolated from human brain tissue as previously described (Peng et al., 2004). Briefly, NPC were cultured in substrate-free tissue culture flasks and grown as spheres in neurosphere initiation medium (NPIM), which consisted of X-Vivo 15 (BioWhittaker, Walkersville, ME) with N2 supplement (Gibco Invitrogen), neural cell survival factor-1 (NSF-1, Bio Whittaker), basic fibroblast growth factor (bFGF, 20 ng/mL, Sigma-Aldrich), epidermal growth factor (EGF, 20 ng/mL, Sigma-Aldrich), leukemia inhibitory factor (LIF, 10 ng/mL, Chemicon, Temecula, CA), and 60 ng/mL N-acetylcysteine (Sigma-Aldrich). Cells were passaged at two-week intervals as previously described (Peng et al., 2004).
[3H] Thymidine Incorporation Assay (Proliferation Assay)
For proliferation studies, neurospheres (passage 4–10) were harvested and dissociated as previously described (Peng et al., 2004, 2005). Cells were seeded into 96-well plates at 10,000 cells/well in NPIM without growth factors. Replicates were treated with growth factors (EGF, bFGF), cytokines (IL-1β, TNF-α, R&D Systems) or MCM for 5 days before pulsing with [3H]thymidine (0.25 μCi/ well, PerkinElmer, Wellesley, MA) for 16 h. For the inhibition of TNF-α, MCM were preincubated with TNF-α soluble receptors R1 and R2 (TNF-R1R2, each 100 ng/mL, R&D Systems) for 1 h at 37°C. For the inhibition of IL-1β, NPC were pretreated with IL-1ra (100 ng/mL, R&D Systems) for 1 h before the addition of MCM. Cells were then lysed with 0.1% Triton X-100 (Sigma-Aldrich) and harvested using a FilterMate Cell Harvester (PerkinElmer). The incorporated radioactivity was measured using a 1450 Microbeta Plus liquid scintillation counter (PerkinElmer).
Cell Cycle Assay
NPC were treated with cytokines (TNF-α or IL-1β) or 25% MCM for 6 days. Neurospheres were harvested and trypsinized to make a single cell suspension. Cell cycle was examined by propidium iodide (PI, Sigma-Aldrich) staining. In brief, 5 × 105 cells were washed twice with Ca2+/Mg2+-free phosphate-buffered saline (PBS, Invitrogen), fixed overnight in 75% cold ethanol, digested with RNase A (Sigma-Aldrich) and stained with PI (100 μg/mL) (Liao et al., 2004). Data were obtained and analyzed by flow cytometry using the Cell Quest software on a FACScan (BD Biosciences, San Diego, CA).
Human Neural Progenitor Cell Differentiation
Following a protocol frequently used to induce neuronal differentiation of NPC (Peng et al., 2004), dissociated NPC were plated on poly-D-lysine-coated cell culture dishes or coverslips (Sigma-Aldrich). Cells were cultured in growth medium for 24 h and subsequently changed to serum-free Neurobasal medium (Gibco) supplemented with B27 (NB27) (Gibco) with or without MCM, or with TNF-α or IL-1β, for 6 days. MCM were pretreated with TNF-R1R2 (100 ng/mL), or NPC were pretreated with IL-1ra (100 ng/mL) for 1 h at 37°C to test for TNF-α and IL-1β involvement. Cells were collected for protein or fixed for immunocytochemical staining 1–6 days after treatment.
Immunocytochemistry
Cells were fixed in 1:1 methanol/acetone and washed in PBS as previously described by Peng et al. (2004). Subsequently, cells were incubated overnight with mouse or rabbit anti-nestin (1:200, Chemicon) anti-nestin or anti Sox-2 for the identification of NPC, mouse or rabbit anti-β-III-tubulin (Sigma, 1:400) for the identification of neurons or rabbit anti-GFAP (glial fibrillary acidic protein, Dako, Carpinteria, CA, 1:1000) or mouse anti-S100 (Sigma-Aldrich, 1: 1000) for the identification of astrocytes overnight, followed by Alexa Fluor secondary antibodies, goat antimouse IgG Alexa Fluor 488 and goat anti-rabbit IgG Alexa Fluor 594 (Molecular Probes, Eugene, OR, 1:200), for 1 h at room temperature. All antibodies were diluted in PBS with 0.1% Triton X-100, 2% BSA. Cells were counterstained with Hoechst 33342 (Sigma-Aldrich). Morphological changes were visualized and captured with a Nikon Eclipse E800 microscope equipped with a digital imaging system. Images were imported into Image-ProPlus, version 4.0 (Media Cybernetics, Sliver Spring, MD) for quantification. Fifteen random fields (total 300–500 cells/culture) of immunostained cells were manually counted using a 20× objective.
Western Blotting
Cells were rinsed twice with PBS and lysed by M-PER Protein Extraction Buffer (Pierce, Rockford, IL) containing 1× protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Protein concentration was determined using the BCA Protein Assay Kit (Pierce). Proteins (10–20 μg) were separated on a 10% SDS-polyacrylamide gel electrophoresis (PAGE) and then transferred to an Immuno-Blot polyvinylidene fluoride (PVDF) membrane (Bio-Rad, Hercules, CA). After blocking in TBS/Tween (0.1%) with 5% nonfat milk, the membrane was incubated with primary antibodies overnight at 4°C followed by horseradish peroxidase-conjugated secondary antibodies (1:10,000; Cell Signaling Technologies) and then developed using Enhanced Chemiluminescent (ECL) solution (Pierce). For data quantification the films were scanned with a CanonScan 9950F scanner and the acquired images were then analyzed on a Macintosh computer using the public domain NIH image program (developed at the U.S. National Institutes of Health and available on the internet at http://rsb.info.nih.gov/nih-image/).
NPC and MDM Injections into SCID Mice
Four-week-old male C.B.-17-SCID mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Animals were maintained in sterile microisolator cages under pathogen-free conditions in the Laboratory of Animal Medicine at UNMC in accordance with ethical guidelines for care of laboratory animals set forth by the National Institutes of Health. NPC were labeled with Qtracker following the manufacture’s protocol. NPC (5 × 105 cells in 5 μL), NPC with MDM (1:1, 5 × 105 cells in 5 μL), NPC with HIV-1ADA-infected MDM (1:1, 5 × 105 cells in 5 μL) were injected intracranially by stereotactic methods (Persidsky et al., 1996). Four animals were included in each group. Two weeks after injection, mice were euthanized with isoflurane and perfused transcardially with 25 mL of PBS and then 4% paraformaldehyde as described by Sato et al. (2006). The brains were rapidly removed and immersed in freshly depolymerized 4% paraformaldehyde for 48 h and then were cryoprotected by successive 24-h immersions in 10, 20, and 30% sucrose in Sorenson’s phosphate buffer immediately befor sectioning.
Immunohistochemistry and Image Analysis
Fixed, cryoprotected brains were frozen and sectioned in the horizontal plane at 30 μm using Cryostat (Leica Microsystems, Bannockburn, IL), with sections collected serially in PBS. Immunohistochemistry was performed using antibody to Vimentin intermediate filaments (clone Vim 3B4, Dako) was used for detection of human cells. Antibodies to CD68 and HIV-1 p24 (Dako Corp.) were used for detection of human MDM and HIV-1 infection. Antibodies to GFAP or β-III-tubulin were used for detection of astrocytes or neurons. Double-immunofluorescence staining was performed using Alexa Fluor 488 (green) and 594 (red) as secondary antibodies (Molecular Probes, Eugene, OR). All obtained images were imported into Image-ProPlus, version 4.0 (Media Cybernetics, Sliver Spring, MD) for quantifying levels of GFAP- and β-III-tubulin-positive staining and four sections from the injection site were analyzed.
Statistical Analyses
Data were expressed as means ± SD. The data were evaluated statistically by analysis of variance (ANOVA) followed by the Tukey-test for paired observations. Significance was considered to be P < 0.05. To account for any donor-specific differences, all experiments were performed with NPC from at least three donors. All assays were performed at least two times, with triplicate or quadruplicate samples in each.
RESULTS
Macrophage Conditioned Medium Induced NPC Proliferation
To assess the effects of brain MP on neurogenesis, HIV-1-infected and/or LPS-activated MDM were employed. Cultured human fetal cortical NPC (Peng et al., 2004, 2005) were used to test the effect of secreted factors from MDM on neurogenesis by measuring NPC proliferation and differentiation.
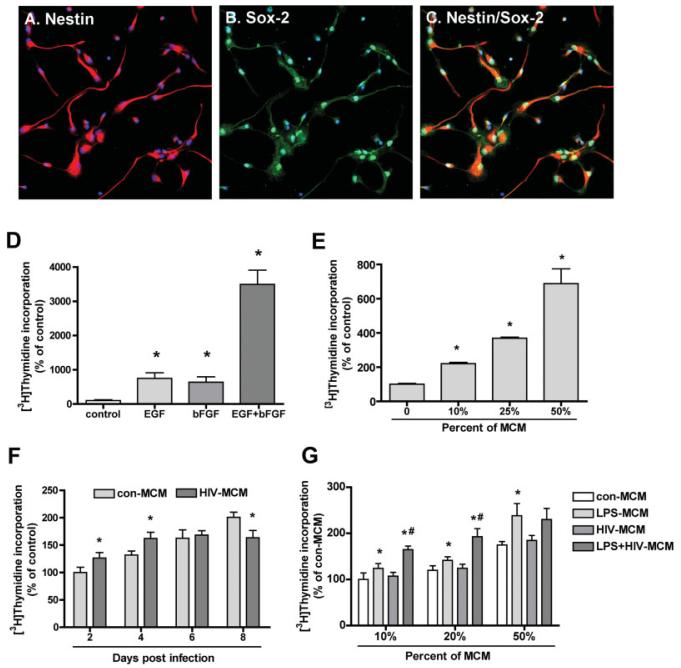
NPC culture purity was analyzed using specific stem-cell markers such as Nestin and Sox2 (Fig. 1A—C). Most of the NPCs were Nestin-immunoreactive (90%; Figs. 1A,C), a characteristic of neural stem cells, and they expressed the neural stem cell-specific transcription factor SOX2 (Figs. 1B,C). The expression of the differentiation markers β-III-tubulin (for neurons) and GFAP (for astrocytes) were less than 5% (data not shown).
Fig. 1.
Growth factors and conditioned media from HIV-1ADA infected and/or immune activated macrophages (MCM) induce NPC proliferation. (A—C) Characterization of human cortical NPCs. Human cortical NPCs were cultured in substrate-free tissue culture flasks and grown in suspension in NPIM as previously described (Peng et al., 2004). The dissociated cells were plated in poly-D-lysine-coated cover slips for 24 h. Cells were fixed and stained for Nestin (red, A) and Sox2 (green, B). Nuclei were stained using Hoechst 33342 (blue). Most of the cells analyzed were positive for Nestin (A) and Sox2 (B). C shows merge of A and B. Original magnification is ×20. Results are representative of two donors. (D, E) Human neural progenitor cells (NPC, passage 4—10) were dissociated and reseeded to 96-well plates. Cells were treated with growth factors (10 ng/mL EGF, 10 ng/mL bFGF) (D) or 10, 25, 50% of MCM (E) for 6 days before pulsing with [3H]thymidine (0.25 μCi/well) for 16 h. Cells were then lysed with 0.1% Triton X-100 and harvested using a FilterMate cell harvester. The incorporated radioactivity was measured using a β-liquid scintillation counter. Data is presented as a percentage of control, as mean ± SD. Results represent average of three donors. * P < 0.05 in comparison to control. (F, G) NPC were treated with 25% of MCM from different days post infection of HIV-1-infected MDM (F) or 10, 20 or 50% MCM from HIV-1 and/or LPS activated MDM (G) for 6 days. Cell proliferation was measured by [3H] thymidine incorporation assay. Data is presented as a percentage of control MCM, as mean ± SD. Results represent average of at least two donors. * P < 0.05 in comparison to con-MCM, # P < 0.05 in comparison to LPS-MCM.
Before testing the effect of conditioned medium derived from MDM (MCM) on NPC proliferation, the effects of standard growth factors were assessed. The neurospheres were dissociated and cultured in a 96-well plate in NPIM basal medium with or without 10 ng/mL EGF or bFGF for 6 days. Both EGF and bFGF showed a 500–600% increase in cell proliferation as assessed by [3H] thymidine incorporation. EGF and bFGF had a synergistic effect on NPC proliferation with an increase of 35-fold in cells treated with both factors (Fig. 1D). NPCs treated with different concentrations of MCM for 6 days exhibited a concentration-dependent increase in proliferation as compared to control cells without MCM (Fig. 1E). The effect of 50% MCM was comparable to that of EGF and bFGF alone. Thus, secreted factors from MDM increased NPC proliferation.
Immune Activated and/or HIV-1-Infected-MCM Induced NPC Proliferation
To further characterize the interaction of MDM and NPC, MCM from HIV-1-infected MDM were investigated for their ability to induce proliferation of NPC. Human NPC were treated with 25% of control MCM (con-MCM) and HIV-1-infected MCM (HIV-MCM). Cell proliferation was assayed by [3H] thymidine incorporation assay. HIV-MCM showed a dual effect on NPC proliferation as compared to con-MCM: at early stage of infection (2, 4 day-postinfection), HIV-MCM increased NPC proliferation; at late stage of infection (8 day-postinfection), HIVMCM inhibited NPC proliferation (Fig. 1F).
In HAD, MP are the principal cell types infected and HIV-1-infection of MDM also combined with the immune activation. Circulating LPS, which is an indicator of microbial translocation, was significantly increased in chronically HIV-infected individuals and in simian immunodeficiency virus (SIV)-infected rhesus macaques (Brenchley et al., 2006) and is a useful tool to mimic general MP activation (Peng et al., 2006). So we tested MCM from LPS-activated and/or HIV-1-infected MDM for their ability to induce proliferation of NPC. Human NPC were treated with 10, 20, and 50% of control MCM (con-MCM), HIV-1-infected MCM (HIV-MCM), LPS-activated MCM (LPSMCM) or HIV-1-infected and LPS-activated MCM (HIV+LPS-MCM) for 7 days. LPS-MCM induced a signifi-cant increase in NPC proliferation. Furthermore, HIV+LPS-MCM induced higher levels of NPC proliferation as compared to LPS-MCM at 10 and 20%, but not at 50% concentration (Fig. 1G). This may be due to saturation of the response with MCM at 50% concentration.
Cytokines Induced NPC Proliferation
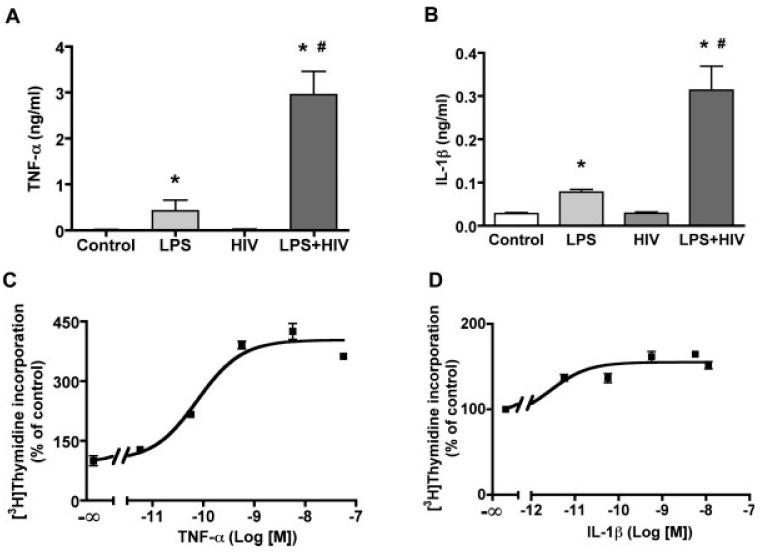
To assess the possible factors in MCM that contribute to NPC proliferation, two common proinflammatory cytokines IL-1β and TNF-α were quantified in MCM by ELISA. LPS induced IL-1β and TNF-α production by MDM, whereas HIV-1 infection alone did not induce a significant increase in neither IL-1β nor TNF-α production; however HIV-1 infection potentiated LPS-stimulated IL-1β and TNF-α production (Fig. 2A,B). Both IL-1β (Fig. 2D) and TNF-α (Fig. 2C) induced a dose-dependent stimulation of NPC proliferation (Fig. 2C,D), with TNF-α (EC50 5 1.35 ng/mL) being more potent than IL-1β (EC50 = 0.48 ng/mL).
Fig. 2.
Cytokine production and induced NPC proliferation. A-B. HIV-infected and/or LPS activated MCM were collected and measured for levels of TNF-α (A) and IL-1β (B) by ELISA. Data is presented as mean ± SD. Results represent the average of four donors. * P < 0.001 in comparison to con-MCM, # P < 0.001 in comparison to LPS-MCM. (C, D) NPC were treated with different concentrations of TNF-α (C) or IL-1β (D) for 6 days. Cell proliferation was measured by [3H]thymidine incorporation assay. Data is presented as a percentage of control, as mean ± SD. Results represent average of three donors.
TNF-α from Immune-Activated and/or HIV-1-Infected-MDM Induced NPC Proliferation
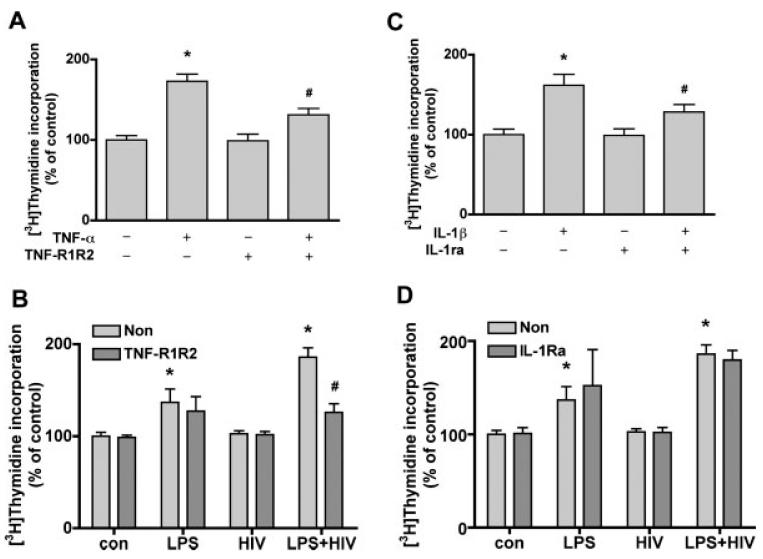
To test whether MCM-induced NPC proliferation is mediated by TNF-α or IL-1β, TNF-R1R2, and IL-1ra were used to block these effects. Treatment of cells with TNF-α plus TNF-R1R2 (100 ng/mL) reduced TNF-α induced proliferation by 71% (Fig. 3A); TNF-R1R2 did not affect IL-1β induced NPC proliferation (data not shown). Furthermore, TNF-R1R2 also reduced LPS+HIV MCM-induced NPC proliferation by 70% (Fig. 3B). Pretreatment with IL-1a (100 ng/mL) for 1 h reduced the effect of IL-1β induced proliferation by 55% (Fig. 3C); IL-1r did not affect TNF-α induced NPC proliferation (data not shown). However, IL-1ra did not affect MCM-induced NPC proliferation (Fig. 3D). Thus TNF-α, but not IL-1b present in LPS- and HIV+LPS-MCM can account for at least part of the increased NPC proliferation.
Fig. 3.
TNF-α soluble receptors block HIV-1 infected and/or immune activated MCM-induced NPC proliferation. (A) NPC were treated with TNF-α (10 ng/ mL) with or without TNF-R1R2 (100 ng/ mL) for 6 days. (B) NPC were treated with IL-1β (10 ng/mL) with or without IL-1ra (100 ng/mL) for 6 days. Cell proliferation was measured by [3H]thymidine incorporation assay. Data is presented as a percentage of control, as mean 6 SD. * P < 0.001 in comparison to control. # P < 0.001 in comparison to TNF-α (A) or IL-1β (B). (C,D) NPC were treated with HIV+LPS MCM with or w/o TNF-R1R2 (C) or IL-1ra (D) for 6 days. Cell proliferation was measured by [3H]thymidine incorporation assay. Data is presented as a percentage of con-MCM, as mean ± SD. * P < 0.001 in comparison to con-MCM. # P < 0.001 in comparison to without TNF-R1R2.
To further test this effect is through HIV-1 infection and brain inflammation, we treated NPC with MCM collected from brain proinflammatory factor, IL-1β, stimulated MDM (see Fig. 4). IL-1β alone did not induce a significant effect on TNF-α production, but HIV-1-infection significantly increased IL-1β-induced TNF-α production by MDM (Fig. 4A). HIV-1-infected and IL-1β-stimulated MCM induced an increase of NPC proliferation (Fig. 4B). Further, MCM induced NPC proliferation is correlated with TNF-α concentration in MCM (Fig. 4C).
Fig. 4.
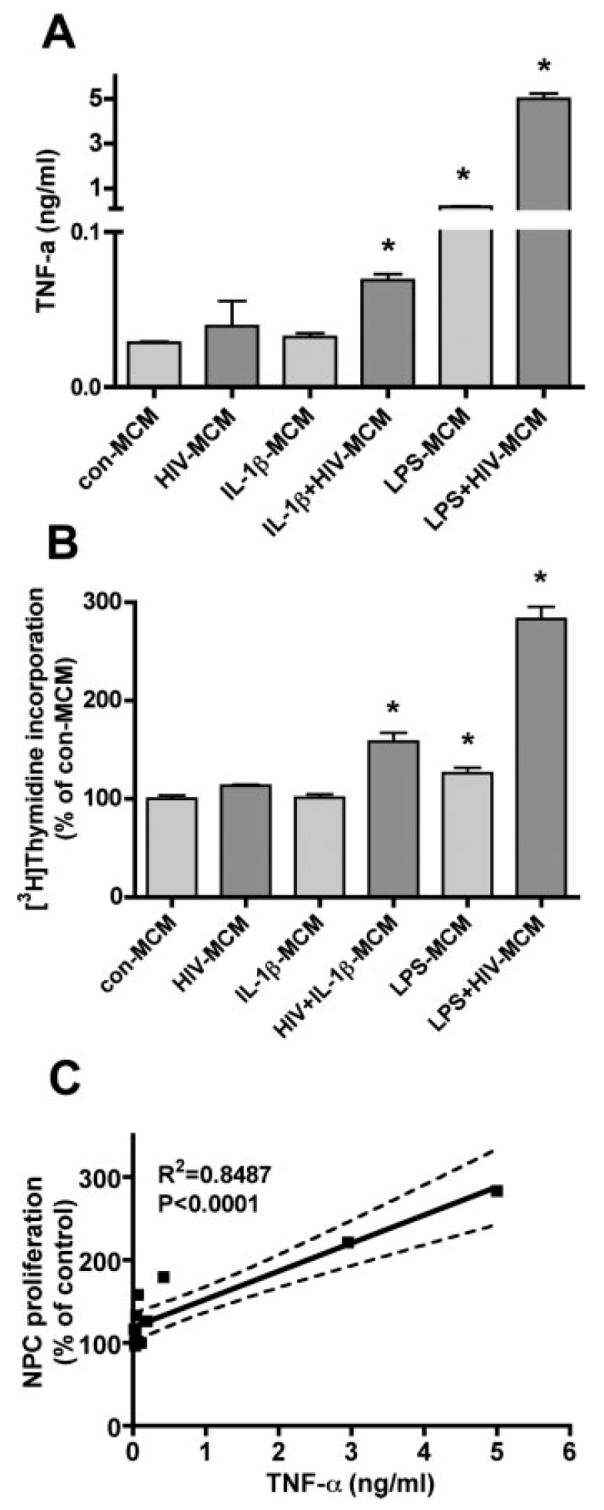
TNF-α production in HIV-1 and immune activated MCM and induced NPC proliferation. (A) HIV-infected and/or IL-1β, or LPS activated MCM were collected and measured for levels of TNF-α by ELISA. Data is presented as mean ± SD. Result is the represent of two donors. * P < 0.05 in comparison to con-MCM. (B) NPC were treated with 25% of MCM from HIV-1infected and/or IL-1β, or LPS activated MCM for 6 days. Cell proliferation was measured by [3H]thymidine incorporation assay. Data is presented as a percentage of con-MCM, as mean ± SD. * P < 0.05 in comparison to con-MCM. Result is the represent of two donors. (C) Result expresses the correlation of TNF-α production to NPC proliferation.
Next, we investigated the mechanism of TNF-α in NPC proliferation. Cell-cycle analysis showed TNF-α induced a dose-dependent increase of S-phase (Fig. 5A). Cyclin D1 is crucial for NPC cell cycle progression by promoting passage through the G1/S restriction point by formation of a complex between cyclin dependent kinase 4 (CDK4) and Cyclin D1 (Ferguson et al., 2000). Western blot showed TNF-α treatment leads to up-regulation of Cyclin D1 expression, resulting in increased proliferation (Fig. 5C). Cell cycle analysis of NPC treated with MCM also showed the same pattern as observed by [3H] thymidine incorporation assay: LPS-MCM induced a significant increase in NPC proliferation. HIV+LPS-MCM induced higher levels of NPC proliferation as compared to LPS-MCM (Fig. 5B).
Fig. 5.
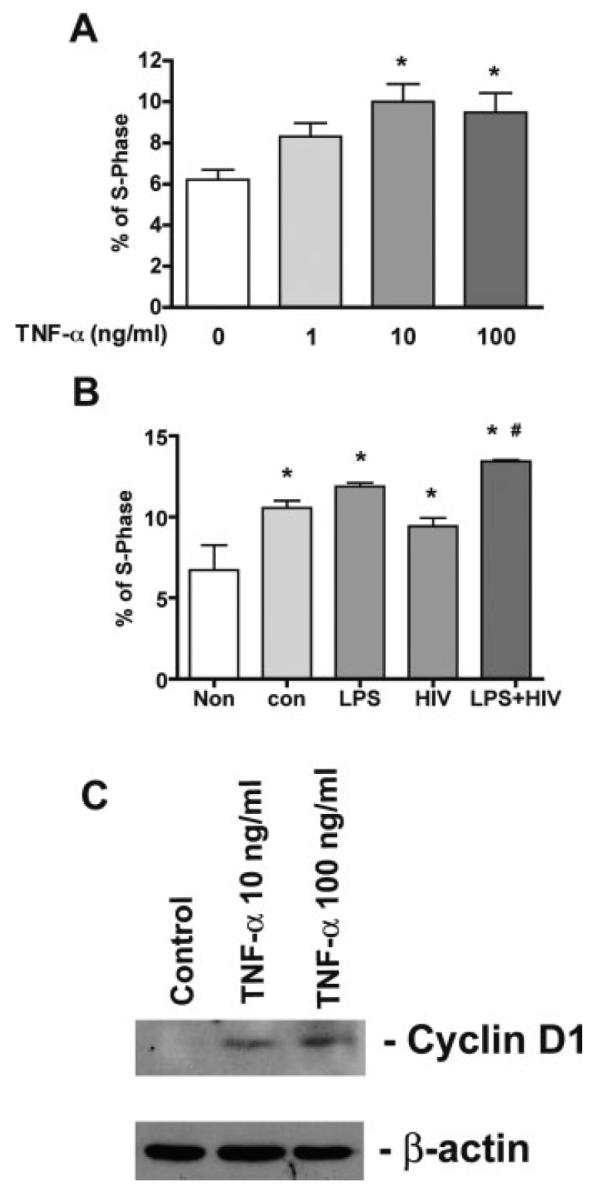
The effect of TNF-α and MCM on NPC by cell cycle analysis. A-B. NPC were treated with TNF-α (0, 1, 10, 100 ng/mL; A) or 25% HIV-1 and/or LPS activated MCM (B) for 6 days. Cell cycle was examined by propidium iodide staining. Data was obtained and analyzed by flow cytometry. * P < 0.05 in comparison to control, # P < 0.05 in comparison to con-MCM. (C) TNF-α treatment elevates Cyclin D1 expression in NPCs. NPCs were treated with TNF-α (10, 100 ng/mL) for 24 h. Expression of Cyclin D1 was detected by Western blotting of total cell lysates. β-actin was used as a loading control. Data is represent of three independent experiments.
Immune-Activated and/or HIV-1-Infected MCM Affect NPC Differentiation
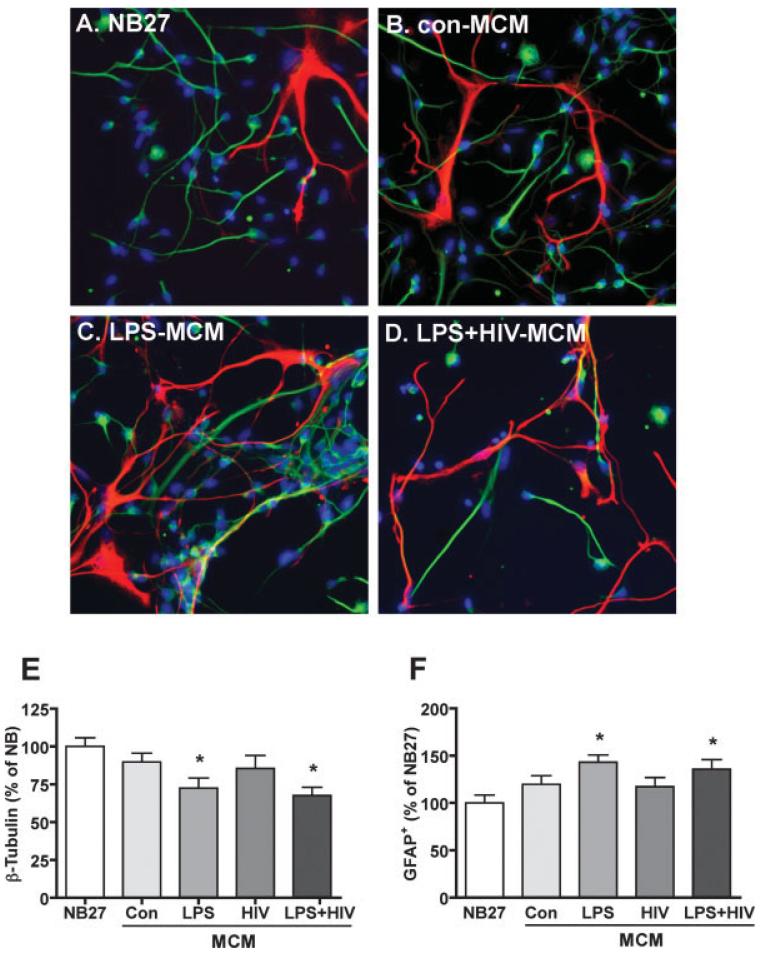
The ability of macrophage to influence the capacity of NPC to differentiate into neurons or astrocytes was tested. NPC were plated on poly-D-lysine-coated culture dishes and cells positive for b-III-tubulin (a marker for immature neurons) or GFAP were assessed by immunocytochemical staining (Fig. 6A–D). In cultures treated with LPS- and LPS+HIV-MCM, more astrocytes (GFAP+, red) were found as compared to control culture (NB27, Fig. 6F). Also, the proportions of neurons (β-III-tubulin1, green) were reduced in LPS and LPS+HIV MCM-treated cultures (Fig. 6E). Conditioned media from LPS or LPS+HIV MDM were both able to increase astrocyte differentiation and inhibit neuronal differentiation.
Fig. 6.
HIV-1infected and/or immune activated MCM increase astrocyte and decrease neuron proportions. NPC were differentiated in NB27 media with or without MCM for 6 days. Representative fluorescence overlay micrographs showing the morphology of neurons (green) and astrocytes (red) in NB27 (A), con-MCM (B), LPS-MCM (C) or HIV+LPS-MCM (D). Nuclei were stained with Hoechst (blue). β-III-tubulin (E) or GFAP (F) positive cells was quantified, data is presented as a percent of NB27 expression. * P < 0.05 in comparison to NB27.
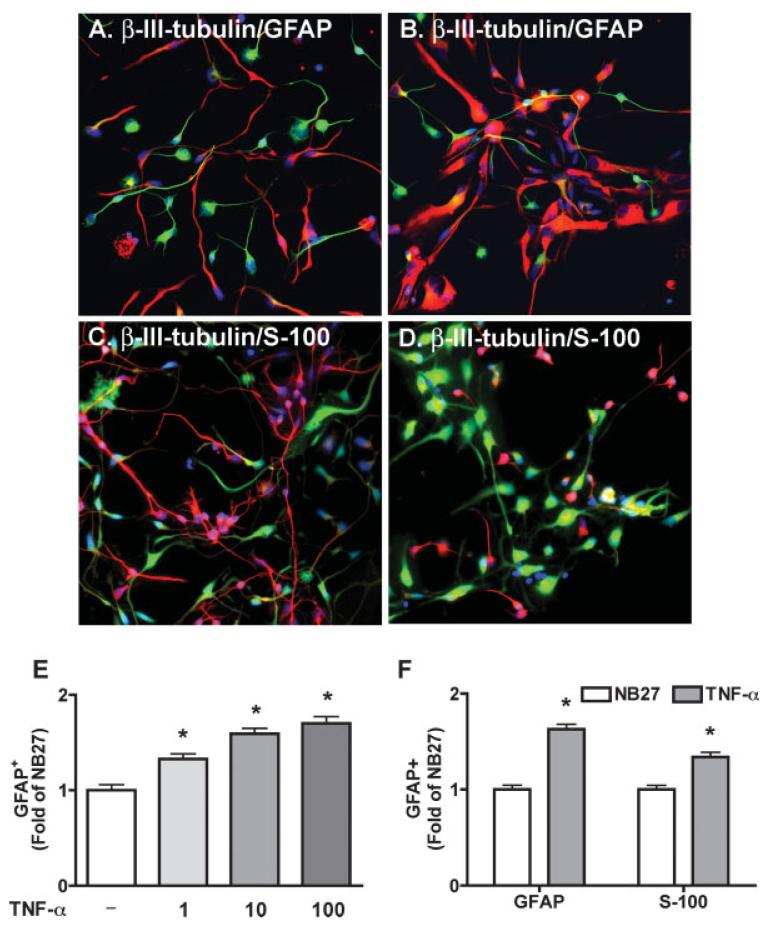
To test the effects of TNF-α on NPC differentiation, NPC were exposed to recombinant TNF-α for 6 days and the expression of β-III-tubulin (for neurons) and S-100 (for astrocyte precursors, Fig 7C,D) or GFAP (for astrocytes, Fig. 7A,B) were assessed by immunocytochemical staining (Fig. 7A–D). TNF-α showed a dose-dependent increase of GFAP-positive cell expression (Fig. 7E) and the same pattern of both S-100 and GFAP expression (Fig. 7F), indicating TNF-α stimulates astrocyte differentiation.
Fig. 7.
TNF-α increases astrocyte and decreases neuron proportions. NPC were differentiated in NB27 media with or without TNF-α for 6 days. Representative fluorescence overlay micrographs showing the morphology of neurons (β-III-tubulin, A,B in green, C,D in red) and astrocytes (GFAP, A-B in red; S-100, C-D in green) in NB27 (A and C) and TNF-α (B and D). Nuclei were stained with Hoechst (blue). GFAP (E) or GFAP and S-100 (F) positive cells was quantified, data is presented as fold of NB27 expression. * P < 0.05 in comparison to NB27.
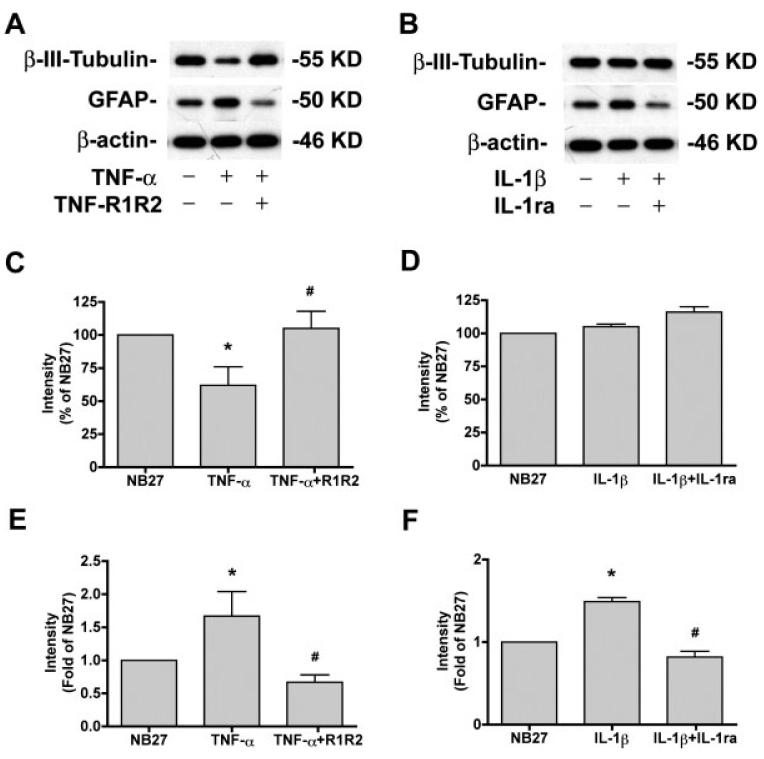
The effects of TNF-α and IL-1β on NPC differentiation were further examined by Western blotting. As shown in Fig. 8, TNF-α (A, C, E) and IL-1β (B, D, F) reduced β-III-tubulin expression (Fig. 8C,D) and increased GFAP expression (Fig. 8E,F), indicating both agents inhibit neuronal differentiation and stimulate astrocyte differentiation. These effects were blocked by pretreatment with TNF-R1R2 or IL-1ra, respectively.
Fig. 8.
TNF-α and IL-1β decrease β-III-tubulin and increase GFAP expression. (A,B) NPC were differentiated in NB27 with TNF-α (10 ng/mL) with or without TNF-R1R2 (A), or IL-1β (10 ng/ mL) with or without IL-1ra (B) for 6 days. Expression of β-III-tubulin or GFAP was detected by Western blotting of total cell lysates. β-actin was used as a loading control. (C–F) The films were scanned and the acquired images were analyzed using the public domain NIH image program for data quantification. Expression of β-III-tubulin (C, D) or GFAP (E, F) was normalized to β-actin as a loading control. Data is presented as a percent of NB27 expression (C, D) or Folds of NB27 expression (E, F). Results are representative of two independent experiments.
Treatment of NPC with MCM for 6 days also resulted in a reduction of β-III-tubulin (Fig. 9A,B) and increased GFAP (Fig. 9A,C), especially by LPS- or HIV+ LPS-MCM. Treatment with TNF-R1R2 partially restored the neuronal differentiation (71% for LPS-MCM, 40% for HIV+LPS-MCM) and reduced the glial differentiation (from 10- to 6-fold for LPS-MCM, form 15- to 9-fold for HIV+LPS-MCM) induced by HIV-1 and/or LPS MCM (see Fig. 9). Treatment with IL-1ra did not significantly restore the neuronal differentiation and reduce the glial differentiation induced by HIV-1 and/or LPS MCM (see Fig. 9).
Fig. 9.
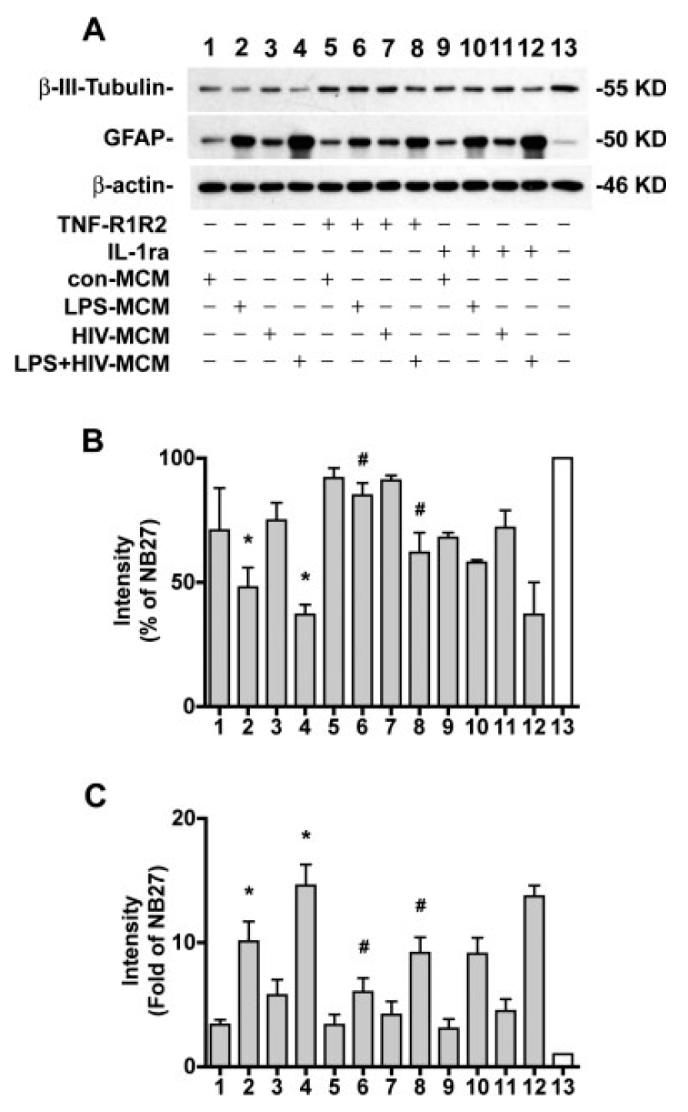
HIV-1 infected and/or LPS activated MCM decrease β-III-tubulin and increase GFAP expression through TNF-α.(A) NPC were differentiated in NB27 with 20% MCM with or without TNF-R1R2 (100 ng/mL), or IL-1ra (100 ng/mL) for 6 days. Expression of β-III-tubulin or GFAP was detected by Western blotting of total cell lysates. β-actin was used as a loading control. (B,C) The films were scanned and the acquired images were analyzed using the public domain NIH image program for data quantification. Expression of β-III-tubulin (B) or GFAP (C) was normalized to β-actin as a loading control. Data is presented as a percent of NB27 expression (B) or Folds of NB27 expression (C). Results are representative of two independent experiments.
TNF-α could induce the cell death of NPC or differentiating cells, which might affect the differentiation pattern of NPC. We next examined the cell death of NPC and differentiating cells treated with TNF-α or conditioned media. Notably, in our studies HIV-infected and/or LPS-activated MCM did not show a significant effect on NPC apoptosis (less than 1% of sub G1 for each different condition). TNF-α, the major player for HIV-infected and/or LPS-activated MCM-induced gliogenesis, did not induce a significant effect on NPC apoptosis by TUNEL assay (Supplement Fig. 1). This excluded the effect of apoptosis on decrease of neurogenesis.
HIV-1-Infected MDM Affect NPC Differentiation in HIVE Mice
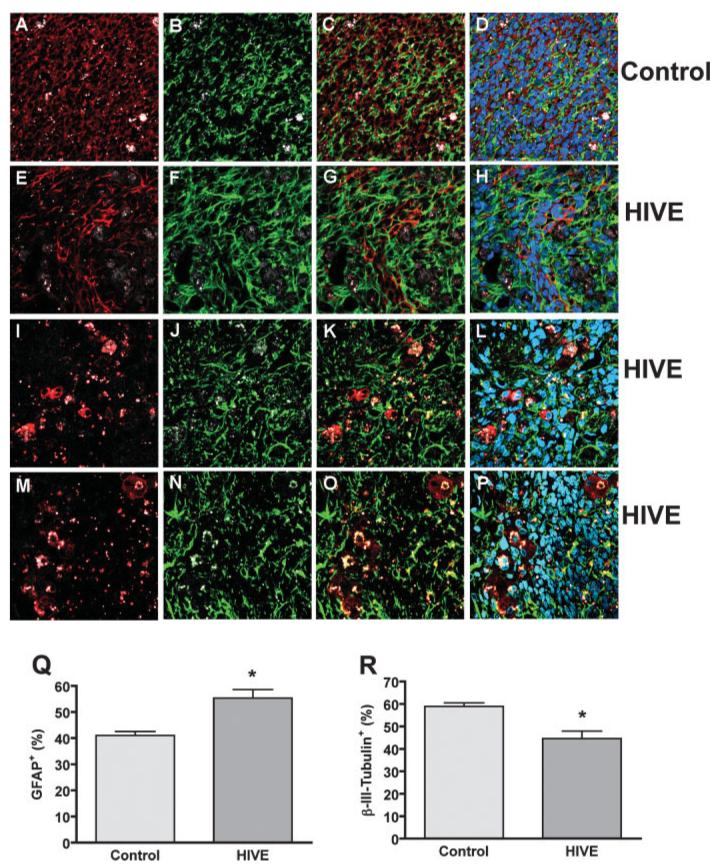
To investigate to role of HIV-1-infected macrophage in mediating NPC differentiation, we used a HIVE SCID mouse model to human disease. Qtracker-labeled human NPCs were intracranially injected into the basal ganglia of SCID mice with/without HIV-1ADA-infected MDM. Fourteen days after injection, NPC differentiation was identified through the injected hemisphere by immuno-staining in serial 30 μm brain sections (see Fig. 10). Confocal images showed human NPC survived and differentiated into neurons (β-III-tubulin-positive, Figs. 10A,C,D) and astrocytes (GFAP-positive, Fig. 10B,C,D). In HIV-1-infected MDM and NPC injection sections, HIV-1-infeced MDM were found in the injection area as shown by CD68 (Fig. 10I,K,L) or P24 (Fig. 10M,O,P) staining. HIV-1-infected MDM induced more astrocyte differentiation (Fig. 10F,G,H,Q) and less neuronal differentiation (Fig. 10E,G,H,R) of NPC. Neuronal or astrocyte differentiation was quantified by determining the β-III-tubulin-positive or GFAP-positive area as a percentage of total β-III-tubulin-positive and GFAP-positive area. HIV-1-infected MDM induced NPC astrocyte differentiation, as indicated by GFAP-positive (Fig. 10Q) and less neuronal differentiation, as indicated by β-III-tubulin-positive (Fig. 10R). This data further confirm HIV-1-infected MDM affect neurogenesis through inducing NPC differentiation.
Fig. 10.
HIV-1-infected MCM affect NPC differentiation in HIVE mice. Qtracker labeled human NPCs were intracranially injected into the basal ganglia of SCID mice with/without HIV-1ADA-infected MDM. Fourteen days after injection, NPC differentiation was identified using immunostaining in serial 30 μm brain sections. (A–H) Serial 30 μm paraformaldehyde-fixed floating brain sections were immunolabeled with antibodies to GFAP (green; B-D, F-H) β-III-tubulin (red; A, C, D, E, G, and H) in both NPC injection group (control, A–D) and NPC injected with HIV-1-infected MDM group (HIVE, E–H). (I–L) HIVE brain sections were stained with antibodies to CD68 (red; I, K and L) and GFAP (green, J-L) or antibodies to P24 (red; M, O and P) and GFAP (green; N-P) to identify HIV-1-infected MDM. Original magnification is ±60. (Q,R) GFAP and (β-III-tubulin expression were quantified by determining the GFAP-positive or β-III-tubulin-positive area as a percentage of the sum area and calculated for a window of the injection site as indicated by Qtracker. Data were shown as the percentage of GFAP-positive area (Q), and the percentage of β-III-tubulin area (R). Data represent the mean SEM. * P < 0.05 in comparison to control.
DISCUSSION
Our studies show that LPS-activated and/or HIV-1-infected MCM substantially increase NPC proliferation (Figs. 1, 4, 5). This appears to be partially through TNF-α, since this effect is partially abrogated by soluble TNF-R1R2 (see Fig. 3). Treatment of NPC with TNF-α, IL-1β, or MCM decreased β-III-tubulin and increased GFAP expression, indicating an inhibition in neurogenesis and increase in gliogenesis (Figs. 6–8). Compared to control MCM, HIV+LPS MCM significantly decreased β-III-tubulin expression and increased GFAP expression and this effect can be blocked by TNF-R1R2 but not by IL-1ra (see Fig. 9). In a murine HIVE model, injection of HIV-1-infected MDM induced a significant increase of NPC astrocyte differentiation and inhibition of neuronal differentiation (see Fig. 10). These observations provide the first evidence that HIV-1-infected and immune-activated macrophages may affect neurogenesis through induction of human NPC proliferation while inhibiting neurogenesis and activating gliogenesis. These studies also identify TNF-α, produced by HIV-1 infection and activation of macrophages, as a contributor to this process.
Recent studies have shown soluble factors released from microglial cells direct the migration and differentiation neural precursor cells (Aarum et al., 2003). Whether and how HIV-infected and immune activated-MP affect NPC proliferation and differentiation in HAD were the focus of this work. Here we have shown that HIV-1-infected MCM has a dual effect on NPC proliferation: at early stage of infection (2 and 4 day-post-infection), HIV-MCM increased NPC proliferation; whereas, at the late stage of infection (8 day-postinfection), HIV-MCM decreased NPC proliferation. At the early stage of infection, HIV-1 may activate MDM to produce inflammatory factors and growth factors that induce NPC proliferation. But at the late stage, viral proteins including gp120 induce quiescence and inhibit NPC proliferation (Krathwohl and Kaiser, 2004; Okamoto et al., 2007). Also cell death and the multitude of released factors (including cytokine TNF-α) could potentially have led to changes in NPC proliferation.
We further investigated the effect of immune activation of HIV-infected MDM on NPC proliferation. Here LPS-activated MCM induced a substantial increase in NPC proliferation and gliogenesis. HIV-1-infected MDM activated with LPS or proinflammatory cytokine IL-1β derived MCM further increase NPC proliferation and gliogenesis when compared to immune activation alone (Figs. 1, 4–6). These effects are correlated to the proinflammatory cytokines TNF-α production: LPS-activated-MDM-increased TNF-α production; HIV-1-infected MDM activated with LPS or IL-1β induce the most TNF-α production (Figs. 2A,B, 4A). Although either TNF-α or IL-1β induced an increase in NPC proliferation and gliogenesis, only soluble TNF-R1R2 but not IL-1ra blocked MCM-induced NPC proliferation and gliogenesis (Figs. 3, 9). Furthermore, NPC proliferation is significantly correlated with TNF-α production (Fig. 4C). These findings suggest TNF-α, but not IL-1β, is a major factor in HIV-1-infected and/or immune activated MCM-induced NPC proliferation and astrocyte differentiation.
To date, conclusions about the action of TNF-α on neural stem or progenitor cells (NSC, NPC) have been conflicting more likely relied to different models, methods and use of cell derived from different brain regions (Ben-Hur et al., 2003; Iosif et al., 2006; Wu et al., 2000). Several studies have indicated negative control of neuronal cell survival/differentiation by TNF-α in NPC culture. TNF-α was found to suppress cell proliferation in neurospheres from neonatal rat striatum in vitro (Ben-Hur et al., 2003). Our study demonstrated that TNF-α induced a dose-dependent increase in human NPC proliferation assessed by [3H]thymidine incorporation assay (Fig. 2C). Cell cycle analysis also showed a dose-dependent increase of S-phase with TNF-α treatment (Fig. 5A). We further demonstrated an up-regulation of Cyclin D1 expression after TNF-α treatment (Fig. 5C). This echoes recent experimental evidence by Widera et al., which suggests TNF-α modulates the proliferation of adult neural stem cells via IKK/NF-κB signaling (Widera et al., 2006; Wu et al., 2000). It is well established that Cyclin dependent kinase 4 and 6 (CDK4/6) signaling is crucial for NPC cycle regulation (Ferguson et al., 2000). The formation of a complex between CDKs 4 and 6 and Cyclin D1 is necessary for cell cycle progression. Further, Widera et al. showed TNF-mediated activation of IKK-β resulted in activation of nuclear factor-kappa B (NF-κB) and was followed by upregulation of the target gene Cyclin D1 and NPC proliferation (Widera et al., 2006). Clearly, the signaling pathways involved in TNF-α-induced human NPC proliferation need further investigation.
Differentiation of NPC was observed by both immunocytochemical staining for cell portion and Western blotting for protein expression. In both experimental systems HIV+LPS-MCM induced a higher proportion of astrocytes (indicated by GFAP expression) than would be expected from spontaneous differentiation alone (NB27). This effect is partially through TNF-α, as indicated by the Western blotting showing HIV-infected and/or LPS-activated MCM-induced GFAP expression was blocked by soluble TNF-R1R2. There are at least three different mechanisms that could be the basis for the increase of gliogenesis in HIV-infected and/or LPS-activated MCM-treated cultures.
First, MDM produce factors that are toxic to newly generated neurons. It has been shown that most new hippocampal progenitor cells (HPCs) in the dentate gyrus die within the first week after epileptic insults through a caspase-mediated apoptotic mechanism (Ekdahl et al., 2001). Further data indicate that increased production of TNF-α by microglial cells, which accompanies hippocampal inflammation (Vezzani et al., 2002), could be a major reason for the death of newly generated HPC. However, in our studies HIV-infected and/or LPS-activated MCM did not show a significant effect on NPC apoptosis (less than 1% of sub G1 for each different condition). TNF-α, the major player for HIV-infected and/or LPS-activated MCM-induced gliogenesis, did not induce a significant effect on NPC apoptosis by TUNEL assay (Supplement Fig. 1). This excluded the effect of apoptosis on decrease of neurogenesis.
Secondly, MCM may selectively promote the survival or proliferation of astocytes. It has been shown that IL-1β could induce astrocyte proliferation. However, blocking IL-1β in the MCM by IL-1ra could not abrogate HIV-infected and/or LPS-activated MCM induced glio-genesis. This suggests that inducing astrocyte proliferation is not important in HIV-infected and/or LPS-activated MCM-induced gliogenesis.
Finally, MCM may directly affect NPC cell fate by inducing the NPC to be committed for a glia fate. From our preliminary observation, TNF-α induced an increase of the inhibitory bHLH transcription factor Hes1 and a decrease expression of the bHLH determination factor Mash1, suggesting that TNF-α in MCM may directly regulate NPC cell fate through bHLH regulation (data not shown). Still, we would like to emphasize that the mechanisms responsible for the increased proportion of astrocytes in HIV-infected and/or LPS-activated MCM-treated NPC cultures remains to be further investigated.
The clinical presentation and progression of neuroAIDS in the developing brain of children is distinct from that seen in adult patients. Neuroimaging and autopsy findings in pediatric patients show impaired brain growth, reactive gliosis, myelin pallor, calcification of the basal ganglia, cortical and cerebral atrophy with neuronal loss, and ventricular enlargement, and abnormalities of cerebral vasculature (Schwartz and Major, 2006). One difference between the adult and child brain is that there are more NPC in the child brain. NPC are a population of CNS cells critical to brain development and response to injury and inflammation (Schwartz and Major, 2006). Recent evidence has shown that HAD patients manifest fewer adult NPC in the dentate gyrus of the hippocampus, an important center for memory and learning, than noninfected subjects or HIV-infected patients without dementia (Krathwohl and Kaiser, 2004).
It may suggest neuroregeneration generated by NPC may contribute to the neuropathogenesis of HAD. A previous study found that injection of human MDM into the basal ganglia of SCID mice inhibited hippocampal neurogenesis (Poluektova et al., 2005). The attenuation of neurogenesis was associated with MP inflammatory responses, especially the high sensitivity of proliferating PSA-NCAM+ neuronal precursors to inflammatory products (Poluektova et al., 2005). However, the limited numbers of endogenous NPC in SCID mice, prevented a thorough study of neurogenesis in vivo. We have adapted the SCID mouse model by injecting HIV-1-infected-MDM with NPC into the SCID mouse brain. HIV-1-infected MDM induced more astrocyte differentiation and less neuronal differentiation as compared to NPC injection alone (see Fig. 10). Taken together, the current studies further demonstrated that HIV-1-infected and immune-activated macrophages affect neurogenesis through induction of human NPC proliferation, while inhibiting neurogenesis and activating gliogenesis partially through cytokine TNF-α. To our knowledge, this study shows for the first time the direct effect of HIV-1-infected and immune activated macrophages on human NPC function, which could contribute to the neuropathogenesis of HAD.
Neural stem/progenitor cells, which have been isolated from both the developing brain and the adult CNS, may differ in their regenerative potential dependent upon the developmental stage at which they were isolated and the site from where they were obtained (Gage, 2000; Kennea and Mehmet, 2002; Rao, 1999). Our work has demonstrated that MDM affect human fetal NPC proliferation and differentiation whether or not MDM may also affect adult NPC function during HAD awaits further investigation.
This study provides evidence that in HAD, activated microglia/macrophage may play a complex regulatory role in the proliferation and differentiation of NPC. HIV-1-infected and immune-activated macrophages affect neurogenesis through induction of NPC proliferation, inhibition of neurogenesis and activation of gliogenesis. These findings may have widespread relevance to other neurodegenerative disorders, such as PD and AD, in which inflammation is induced as a consequence of microglia activation. Moreover, our data on the ability of the proinflammatory cytokines TNF-α and IL-1β to inhibit neuronal differentiation and stimulate glial differentiation suggest that these cytokines could become drug targets for preservation of the endogenous regenerative capability of the CNS that is normally repressed in the inflamed brain.
Supplementary Material
ACKNOWLEDGMENTS
The authors kindly acknowledge Dr. Anuja Ghorpade, Ms. Jane Kennedy and Li Wu who provided technical support for this work. Drs. Myron Toews, Howard E. Gendelman and Tsuneya Ikezu and Mr. Nathan Erd-mann provided valuable comments and suggestions about the manuscript. Dr. Charles Kuszynski and Ms. Linda Wilkie performed the flow cytometry support. Ms. Julie Ditter, Johna Belling, Robin Taylor, Myhanh Che, Na Ly and Emilie Scoggins provided outstanding administrative support.
Grant sponsor: National Institutes of Health; Grant number: R01 NS 41858, P20 RR15635, P01 NS043985.
REFERENCES
- Aarum J, Sandberg K, Haeberlein SL, Persson MA. Migration and differentiation of neural precursor cells can be directed by micro-glia. Proc Natl Acad Sci USA. 2003;100:15983–15988. doi: 10.1073/pnas.2237050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Ben-Hur T, Ben-Menachem O, Furer V, Einstein O, Mizrachi-Kol R, Grigoriadis N. Effects of proinflammatory cytokines on the growth, fate, and motility of multipotential neural precursor cells. Mol Cell Neurosci. 2003;24:623–631. doi: 10.1016/s1044-7431(03)00218-5. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar B, Rodriguez B, Teixeira-Johnson L, Landay A, Martin J, Hecht F, Picker L, Lederman D, Deeks S, Douek D. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Burton TR, Dibrov A, Kashour T, Amara FM. Anti-apoptotic wild-type Alzheimer amyloid precursor protein signaling involves the p38 mitogen-activated protein kinase/MEF2 pathway. Brain Res Mol Brain Res. 2002;108:102–120. doi: 10.1016/s0169-328x(02)00519-3. [DOI] [PubMed] [Google Scholar]
- Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Mohapel P, Elmer E, Lindvall O. Caspase inhibitors increase short-term survival of progenitor-cell progeny in the adult rat dentate gyrus following status epilepticus. Eur J Neurosci. 2001;14:937–945. doi: 10.1046/j.0953-816x.2001.01713.x. [DOI] [PubMed] [Google Scholar]
- Ferguson KL, Callaghan SM, O’Hare MJ, Park DS, Slack RS. The Rb-CDK4/6 signaling pathway is critical in neural precursor cell cycle regulation. J Biol Chem. 2000;275:33593–33600. doi: 10.1074/jbc.M004879200. [DOI] [PubMed] [Google Scholar]
- Gabuzda D, He J, Ohagen A, Vallat A. Chemokine receptors in HIV-1 infection of the central nervous system. Immunology. 1998;10:203–213. doi: 10.1006/smim.1998.0133. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gage FH. Neurogenesis in the adult brain. J Neurosci. 2002;22:612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman HE. The neuropathogenesis of HIV-1-dementia. In: Gendelman HE, Lipton SA, Epstein LG, Swindells S, editors. The neurology of AIDS. Chapman and Hall; New York: 1997. pp. 1–10. [Google Scholar]
- Gendelman HE, Orenstein JM, Martin MA, Ferrua C, Mitra R, Phipps T, Wahl LA, Lane HC, Fauci AS, Burke DS. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167:1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman HE, Persidsky Y, Ghorpade A, Limoges J, Stins M, Fiala M, Morrisett R. The neuropathogenesis of the AIDS dementia complex. Aids. 1997;11(Suppl A):S35–S45. [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocyto-chemical quantitation of human immunodeficiency virus in the brain: Correlations with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Chan SL, Borchard AC, Rao MS, Mattson MP. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer’s disease. J Neurochem. 2002;83:1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay C, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of micro-glia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, Jacobsen SE, Lindvall O. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neuro-genesis. J Neurosci. 2006;26:9703–9712. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kennea NL, Mehmet H. Neural stem cells. J Pathol. 2002;197:536–550. doi: 10.1002/path.1189. [DOI] [PubMed] [Google Scholar]
- Kolson DL, Gonzalez-Scarano F. HIV and HIV dementia. J Clin Invest. 2000;106:11–13. doi: 10.1172/JCI10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krathwohl MD, Kaiser JL. HIV-1 promotes quiescence in human neural progenitor cells. J Infect Dis. 2004;190:216–226. doi: 10.1086/422008. [DOI] [PubMed] [Google Scholar]
- Liao XD, Wang XH, Jin HJ, Chen LY, Chen Q. Mechanical stretch induces mitochondria-dependent apoptosis in neonatal rat cardiomyocytes and G2/M accumulation in cardiac fibroblasts. Cell Res. 2004;14:16–26. doi: 10.1038/sj.cr.7290198. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Gendelman HE. Dementia associated with the acquired immunodeficiency syndrome. New Engl J Med. 1995;16:934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: Mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003;304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- Maschke M, Kastrup O, Esser S, Ross B, Hengge U, Hufnagel A. Incidence and prevalence of neurological disorders associated with HIV since the introduction of highly active antiretroviral therapy (HAART) J Neurol Neurosurg Psychiatry. 2000;69:376–380. doi: 10.1136/jnnp.69.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan A, Nelson JA, Atkinson JH, Grant I. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. Ann Neurol. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N. Human immunodeficiency virus-associated dementia: An evolving disease. J Neurovirol. 2003;9:205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Monje ML, Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol. 2003;16:129–134. doi: 10.1097/01.wco.0000063772.81810.b7. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- Nath A, Geiger J. Neurobiological aspects of human immunodeficiency virus infection: Neurotoxic mechanisms. Prog Neurobiol. 1998;54:19–33. doi: 10.1016/s0301-0082(97)00053-1. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Soma LA, Lavi E. Microglia in diseases of the central nervous system. Ann Med. 2002;34:491–500. doi: 10.1080/078538902321117698. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Kang Y, Christopher B, Siviglia E, Russo R, Clemente A, Harrop A, McKercher S, Kaul M, Lipton SA. HIV/gp120 decreases adult neural progenitor cell proliferation via checkpoint kinase-mediated cell-cycle withdrawal and G1 arrest. Cell Stem Cell. 2007;1:1–7. doi: 10.1016/j.stem.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Parent JM. Injury-induced neurogenesis in the adult mammalian brain. Neuroscientist. 2003;9:261–272. doi: 10.1177/1073858403252680. [DOI] [PubMed] [Google Scholar]
- Parent JM, Valentin VV, Lowenstein DH. Prolonged seizures increase proliferating neuroblasts in the adult rat subventricular zone-olfactory bulb pathway. J Neurosci. 2002;22:3174–3188. doi: 10.1523/JNEUROSCI.22-08-03174.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Erdmann N, Whitney N, Dou H, Gorantla S, Gendelman HE, Ghorpade A, Zheng J. HIV-1-infected and/or immune activated macrophages regulate astrocyte SDF-1 production through IL-1beta. Glia. 2006;54:619–629. doi: 10.1002/glia.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Huang Y, Duan Z, Erdmann N, Xu D, Herek S, Zheng J. Cellular IAP1 regulates TRAIL-induced apoptosis in human fetal cortical neural progenitor cells. J Neurosci Res. 2005;82:295–305. doi: 10.1002/jnr.20629. [DOI] [PubMed] [Google Scholar]
- Peng H, Huang Y, Rose J, Erichsen D, Herek S, Fujii N, Tamamura H, Zheng J. Stromal cell-derived factor 1 mediated CXCR4 signaling in rat and human cortical neural progenitor cells. J Neurosci Res. 2004;76:35–50. doi: 10.1002/jnr.20045. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Limoges J, McComb R, Bock P, Baldwin T, Tyor W, Patil A, Nottet HS, Epstein L, Gelbard H, et al. Human immunodeficiency virus encephalitis in SCID mice. Am J Pathol. 1996;149:1027–1053. [PMC free article] [PubMed] [Google Scholar]
- Poluektova L, Meyer V, Walters L, Paez X, Gendelman HE. Macrophage-induced inflammation affects hippocampal plasticity and neuronal development in a murine model of HIV-1 encephalitis. Glia. 2005;52:344–353. doi: 10.1002/glia.20253. [DOI] [PubMed] [Google Scholar]
- Rao MS. Multipotent and restricted precursors in the central nervous system. Anat Rec. 1999;257:137–148. doi: 10.1002/(SICI)1097-0185(19990815)257:4<137::AID-AR7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Sato S, Cerny RL, Buescher JL, Ikezu T. Tau-tubulin kinase 1 (TTBK1), a neuron-specific tau kinase candidate, is involved in tau phosphorylation and aggregation. J Neurochem. 2006;98:1573–1584. doi: 10.1111/j.1471-4159.2006.04059.x. [DOI] [PubMed] [Google Scholar]
- Schwartz L, Major EO. Neural progenitors and HIV-1-associated central nervous system disease in adults and children. Curr HIV Res. 2006;4:319–327. doi: 10.2174/157016206777709438. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol. 1999;58:233–247. doi: 10.1016/s0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- Strizki JM, Albright AV, Sheng H, O’Connor M, Perrin L, Gonzalez-Scarano F. Infection of primary human microglia and monocyte-derived macrophages with human immunodeficiency virus type 1 isolates: Evidence of differential tropism. J Virol. 1996;70:7654–7662. doi: 10.1128/jvi.70.11.7654-7662.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Moneta D, Richichi C, Aliprandi M, Burrows SJ, Ravizza T, Perego C, De Simoni MG. Functional role of inflammatory cytokines and antiinflammatory molecules in seizures and epileptogenesis. Epilepsia. 2002;43(Suppl 5):30–35. doi: 10.1046/j.1528-1157.43.s.5.14.x. [DOI] [PubMed] [Google Scholar]
- Widera D, Mikenberg I, Elvers M, Kaltschmidt C, Kaltschmidt B. Tumor necrosis factor alpha triggers proliferation of adult neural stem cells via IKK/NF-kappaB signaling. BMC Neurosci. 2006;7:64. doi: 10.1186/1471-2202-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JP, Kuo JS, Liu YL, Tzeng SF. Tumor necrosis factor-alpha modulates the proliferation of neural progenitors in the subventricular/ventricular zone of adult rat brain. Neurosci Lett. 2000;292:203–206. doi: 10.1016/s0304-3940(00)01472-5. [DOI] [PubMed] [Google Scholar]
- Zheng J, Thylin MR, Persidsky Y, Williams CE, Cotter RL, Zink W, Ryan L, Ghorpade A, Lewis K, Gendelman HE. HIV-1 infected immune competent mononuclear phagocytes influence the pathways to neuronal demise. Neurotoxi Res. 2001;3:461–484. doi: 10.1007/BF03033204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.