Abstract
Whereas tuberculous granulomas, comprised of infected macrophages and other immune cells, were considered impermeable structures, recent studies showed that superinfecting Mycobacterium marinum traffic rapidly to established fish and frog granulomas by host-mediated and Mycobacterium-directed mechanisms. The present study shows that superinfecting Mycobacterium tuberculosis and Mycobacterium bovis BCG similarly home to established granulomas in mice. Furthermore, two prominent mycobacterial virulence determinants, Erp and ESX-1, do not impact this cellular trafficking. These findings suggest that homing of infected macrophages to sites of infection is a general feature of tuberculosis pathogenesis, with important consequences for therapeutic strategies.
Keywords: Mycobacterium, granulomas, caseum, ESX-1, Erp, reinfection
Introduction
Long-term persistence of mycobacteria in the face of an apparently robust immune response thwarts tuberculosis control, and may explain the inefficacy of the BCG vaccine. Tuberculosis begins with the phagocytosis of mycobacteria by macrophages and dendritic cells at the site of infection, and the migration of infected cells into deeper tissues where they aggregate with additional macrophages and other immune cells to form characteristic structures called granulomas[1]. The tuberculous granuloma has long been considered an impenetrable barrier structure that contains and restricts mycobacterial infection, and this presumed impenetrability is invoked to explain re-infection tuberculosis, which is increasingly appreciated to occur in immunocompetent individuals capable of forming granulomas[2]. It has been hypothesized that superinfecting or re-infecting bacteria are able to survive by evading established granulomas where pre-existing immune elements are concentrated[3].
This view of the granuloma was called into question by a study that used a Mycobacterium marinum infection model to show that superinfection of chronically infected frogs resulted in the rapid trafficking of new bacteria to established granulomas[4]. Newly infecting organisms were transported by host cells to established granulomas and persisted long-term therein. Moreover, super-infecting M. marinum similarly penetrated necrotic caseous centers of pre-existing zebrafish granulomas, demonstrating that infected phagocytes readily access this compartment. These findings highlighted the dynamic nature of tuberculous granulomas, and suggested the existence of communication between mycobacteria, infected host cells, and granulomas. This same study showed that trafficking of infected cells is likely induced by a Mycobacterium-specific signal; mycobacterial traffic into granulomas is greater than that of latex beads or of Salmonella enterica serovar arizonae, another macrophage pathogen. Therefore, the unique pattern of host-cell trafficking elicited by mycobacterial infection likely involves specific bacterial determinants.
While intriguing, these results left open the possibility that the trafficking of superinfecting mycobacteria is unique to M. marinum pathogenesis of the fish and amphibian models used. Genome comparisons of M. marinum and M. tuberculosis reveal their close genetic relationship[5], explaining the conservation of their essential framework of infection, granuloma formation and persistence[1]. The present study was undertaken to establish whether superinfecting M. tuberculosis and M. bovis BCG also home to established granulomas of mice, and to probe the mechanisms by which mycobacteria influence phagocyte trafficking.
Methods
M. tuberculosis strain Erdman (gift of W. Bishai) and M. bovis BCG substrain Russia[6] were rendered green and red fluorescent by transformation with plasmids pMSP12::gfp and pMSP12::dsRed2[4], respectively. M. marinum strain M (ATCC# BAA-535) was rendered red-fluorescent by transformation with plasmid pMSP12::dsRed2, while M. marinum strains RD1-6 (esx1::ΔRD1[7]) and KK33 (erp::aph[8]) were rendered green fluorescent by transformation with plasmid pGFPHYG2 (hygromycin-resistant derivative of pMSP12::gfp). Bacteria were grown in static culture in Middlebrook 7H9 medium supplemented with 0.2% glycerol, 0.005% oleic acid, 0.2% dextrose, 0.5% BSA, 0.085% NaCl, and 0.05% Tween-80, in the presence of 20 μg/ml kanamycin sulfate or 50 μg/ml hygromycin B as appropriate. Colony forming units (CFU) were enumerated on Middlebrook 7H10 agar supplemented with 0.5% glycerol, 0.005% oleic acid, 0.2% dextrose, 0.5% BSA and 0.085% NaCl. M. marinum was grown at 33°C, and M. tuberculosis and M. bovis BCG at 37°C.
6–8 week-old female C57B/6 mice (Charles River) were infected by intraperitoneal (IP) injection of red fluorescent M. bovis BCG or M. tuberculosis Erdman for approximately five weeks to allow granuloma formation, and then superinfected by IP injection with the corresponding green fluorescent strain. Three to seven days later, their organs were removed and fixed overnight in 4% paraformaldehyde in 1X PBS. Tissues were rehydrated and frozen for cryosectioning as described[9]. Wild-caught small male leopard frogs (Rana pipiens, Nasco) were infected with red fluorescent M. marinum by IP injection for six weeks and superinfected with green fluorescent M. marinum or Fluoresbrite Carboxylate YG 1.0 μm microspheres (Polysciences, Inc) for three days at which time their organs were removed and processed as described[9].
CFU from liver homogenates were enumerated on 7H10 agar. Beads were enumerated by spotting aliquots of tissue homogenate on a slide and visual counting using fluorescence microscopy. 10-μm thick frozen sections were stained with Slow Fade Gold Anti-Fade reagent with DAPI (Molecular Probes) and imaged using a Cool-Snap HQ camera (Photometrics). Established granulomas were defined by both the presence of red-fluorescent bacteria, and a dense clustering of DAPI-stained host cell nuclei, which served as a conservative measure of their boundaries[4]. Localization of superinfecting particles was scored by visual inspection of sections; all green fluorescent bacteria or beads were counted and scored as having migrated into granulomas as defined here, or not.
Results
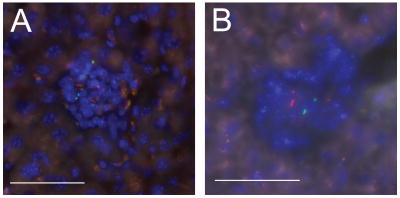
To examine the trafficking of newly infected cells with respect to established granulomas in the mouse model of tuberculosis, C57B/6 mice were first infected with M. bovis BCG that constitutively express the red fluorescent protein, dsRed via IP injection. This method of inoculation has been used for superinfection studies in frogs[4]. Five weeks after the initial infection, a timepoint at which bacterial counts have stabilized due to the onset of adaptive immunity[10], livers were found to contain small compact paucibacillary granulomas typical of this organ[11, 12]. The mice were then superinfected with green fluorescent M. bovis BCG. At both three and seven days post-infection, superinfecting bacteria were present in established granulomas (Fig. 1A and data not shown). Similar rapid trafficking of superinfecting green-fluorescent M. tuberculosis Erdman bacteria was observed into granulomas established using red-fluorescent M. tuberculosis Erdman (Fig. 1B). These data demonstrate that mammalian granulomas induced by M. tuberculosis complex organisms are permeable to cellular traffic and superinfection. Thus, the propensity of superinfecting mycobacteria to home to granulomas is a general feature of tuberculosis pathogenesis, and not specific to M. marinum, fish or frogs.
Figure 1. M. tuberculosis.
and M. bovis BCG traffic to established mouse granulomas. A. C57B/6 mice were infected with 4 × 106 red-fluorescent M. bovis BCG bacteria and were then superinfected with 2 × 107 green-fluorescent M. bovis BCG five weeks later. Livers were harvested three and seven days post-secondary infection and were processed as described in Methods. Seven day post-infection granuloma shown is representative of mixed lesions found in one mouse at three days post-infection and in three mice at seven days post-infection. B. A C57B/6 mouse was infected with 107 red-fluorescent M. tuberculosis Erdman, and was then superinfected with green fluorescent M. tuberculosis Erdman 4.5 weeks later. Liver was harvested four days post-secondary infection and processed as described in Methods. Scale bar, 50 μm.
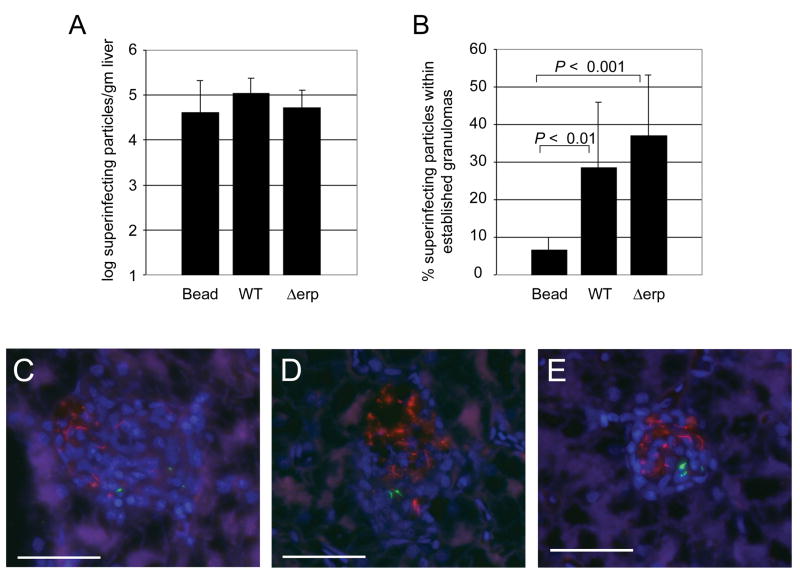
To probe the mechanism of Mycobacterium-directed phagocyte trafficking, two mycobacterial virulence determinants, ESX-1 and Erp, shared by M. tuberculosis and M. marinum, were analyzed in the frog model. These determinants mediate virulence by distinct mechanisms. Erp acts earlier in infection and is required for intracellular growth and resistance to host defenses[8, 13, 14]. The ESX-1 locus is not required for bacterial growth within individual macrophages but mediates granuloma formation, macrophage death and intercellular spread of bacteria[7, 15]. Frogs infected with red fluorescent wild-type M. marinum for six weeks were superinfected with green fluorescent beads, wild type or Δerp M. marinum. As expected[4], at three days post-infection bacteria were four-fold more likely than beads to be associated with established granulomas (Fig. 2B,C), despite comparable numbers of beads and bacteria infiltrating the surrounding tissue (Fig. 2A). Similar proportions of Δerp and wild-type bacteria were found within granulomas (Fig. 2A, B, and D), indicating that this bacterial determinant is not required for induction of host cell trafficking to granulomas. Furthermore, this result suggests that intracellular bacterial replication is dispensable for homing of infected cells to pre-existing granulomas. In a separate experiment, the ESX-1-deficient ΔRD1 mutant also trafficked to granulomas within three days (Fig. 2E and data not shown) but the small sample size precluded quantitative comparison to wild-type bacteria. Nevertheless, this experiment shows that the ESX-1 locus is not required for rapid delivery of mycobacteria to established granulomas.
Figure 2.
Wild type, erp::aph and ΔRD1 M. marinum traffic to established granulomas. A–D) Frogs were infected with 3 × 106 CFU red fluorescent wild-type M. marinum. After six weeks frogs were superinfected with 4.5 × 106 green fluorescent beads (11 frogs), 1.1 × 106 green fluorescent wild type bacteria (6 frogs), or 1.6 × 106 green fluorescent Δerp::aph bacteria (8 frogs). Livers were harvested three days post-secondary infection and were processed for CFU and microscopy as described in Methods. (A) Green fluorescent particles (latex beads or bacteria) per gram liver tissue. Differences were not statistically significant (ANOVA with Tukey’s post test). (B) Proportion of green fluorescent particles found within established granulomas. Error bars represent standard deviations. Means compared using ANOVA with Tukey’s Post test. Wild-type vs. erp::aph, not significant. C) Wild-type and D) erp::aph M. marinum found inside of established granulomas. E) Frogs were infected with 1.9 × 105 CFU red fluorescent wild-type M. marinum. After 10.5 weeks frogs were superinfected with 2.0 × 106 green fluorescent ΔRD1 M. marinum. Livers were harvested 3 days post-secondary infection and were processed for CFU and microscopy as described in the Methods. Scale bar, 50 μm.
Discussion
The earlier finding that superinfecting mycobacteria home to established granulomas in M. marinum-infected frogs and fish challenged long-held assumptions about the nature of the tuberculous granuloma[4]. This study demonstrated that granulomas, including their caseous centers, are permeable entities and that the concentration of immune elements at these foci fail to protect against naïve superinfecting bacteria, which persist long term therein. These findings revealed the limits of anti-tuberculous immunity, drawing into question whether vaccine strategies that use a more persistent or immunogenic strain would be successful. However, while M. marinum and M. tuberculosis pathogenesis in their respective ectotherm and mammalian hosts appear to operate via a common functional framework[1], it was unclear whether M. tuberculosis complex bacilli would behave similarly. First, M. marinum-specific determinants absent from M. tuberculosis might direct newly infected macrophages to granulomas. Alternatively, differences in the granulomas of fish/amphibians versus mammals might be responsible for the homing behavior of infected cells. Although M. marinum infection is moderated by adaptive immunity, fish and frog granulomas contain many fewer lymphocytes than do mammalian granulomas[16]. A recent study of M. bovis BCG granulomas in mouse liver (the tissue used in this study) shows that T lymphocytes are abundant and highly motile therein[11]. Thus, it was possible that the paucity of lymphocytes in fish and amphibian granulomas, while not affecting the ability of the granuloma to restrict pre-existing bacteria, might make them more permeable to newly infected phagocytes. Finally, it was possible that M. marinum-infected fish and amphibian phagocytes might have intrinsically higher motility than, or other trafficking differences from, mammalian ones, as has been suggested[11]. Therefore, it was important to determine whether this phenomenon occurs in mammalian tuberculosis. The present study establishes that the propensity of superinfecting mycobacteria to home to pre-existing granulomas is a general feature of tuberculosis pathogenesis and opens the possibility that it may occur in humans as well. One aspect of mammalian disease that is not addressed by this study is whether lung granulomas are similarly permeable to superinfecting traffic. Attempts to demonstrate homing of superinfecting organisms using aerosol infection of mice were unsuccessful (data not shown), likely due to technical hurdles. Specifically, the number of superinfecting organisms that was achieved with the aerosolization apparatus used was likely too low to allow detection. Alternately, this failure may reflect a biological difference between liver and lung granulomas, or between peritoneal and alveolar macrophages. However, this explanation seems less likely given the permeability of frog lung granulomas to superinfecting bacteria delivered intraperitoneally[4].
Mycobacteria have been shown to be both susceptible to host killing by macrophages in vivo[13] but to also utilize these cells to promote their spread and dissemination[7, 17]. Elucidating the mechanisms by which Mycobacterium infection alters phagocytes traffic may help to define more clearly the seemingly disparate roles of the mature granuloma, as both a host and pathogen-beneficial structure. Thus, a mechanistic understanding of this surprising phenomenon, and its consequences for infection, will depend upon the elucidation of the mycobacterial determinants required to induce the host-cell trafficking now confirmed for both M. marinum and M. tuberculosis. In this study, two bacterial virulence determinants shared by both pathogens were examined for their ability to influence host-cell trafficking behavior. Erp, a cell surface protein of unknown function, acts early in the pathogenesis program in the M. marinum-zebrafish embryo model, being required for bacterial growth and/or survival beginning early in infection. Data presented here show that erp-deficient bacteria traffic to granulomas as well as do wild type bacteria. This observation demonstrates that Erp is not involved in modulating host cell trafficking and further suggests that the ability to grow and/or survive intracellularly is similarly not required. The second determinant tested, the ESX-1 secretion system, is a virulence determinant in both M. tuberculosis and M. marinum[7, 15, 18]. Indeed, the primary attenuating deletion in M. bovis BCG, referred to as region-of-difference 1 (RD1), removes a substantial portion of the ESX-1 locus[6, 19]. In the present study, both BCG and a M. marinum ΔRD1 mutant were shown to traffic into mature granulomas. While it remains possible that a quantitative difference exits, this result demonstrates that an intact ESX-1 locus is not absolutely required for the induction of phagocyte trafficking to granulomas.
As both attenuated strains (Erp and ESX-1 mutants) exhibit some level of persistence in vivo[6, 8, 14] and also home to existing granulomas, this suggests an intriguing new therapeutic route. If properly modified–for example, for phage[20] or drug delivery—these strains could rapidly deliver anti-bacterial cargo directly to pre-existing granulomas, including caseum, where the bacilli are thought to be hardest to eradicate[21].
Acknowledgments
The authors thank Rosa Kim and Jeremy Freeman for help with infections and tissue preparation, Graham Hatfull, Mark Troll, and Lynn Connolly for valuable discussions on the implications of these findings and Lynn Connolly for critical reading of the manuscript. This work was supported by the NIH grant AI36396 and a Burroughs Wellcome Award to LR and the NIH grant HL64550 to DRS.
Footnotes
The authors do not have a commercial or other association that might pose a conflict of interest.
Some of these data were previously presented at the 2005 Keystone Meeting “Tuberculosis: Integrating Host and Pathogen Biology, April 2-7, 2005. Whistler Resort, Whistler, BC, Canada. Abstract number 1055.
References
- 1.Tobin DM, Ramakrishnan L. Comparative pathogenesis of Mycobacterium marinum and Mycobacterium tuberculosis. Cell Microbiol. 2008 doi: 10.1111/j.1462-5822.2008.01133.x. [DOI] [PubMed] [Google Scholar]
- 2.van Rie A, Warren R, Richardson M, et al. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N Engl J Med. 1999;341:1174–9. doi: 10.1056/NEJM199910143411602. [DOI] [PubMed] [Google Scholar]
- 3.Balasubramanian V, Wiegeshaus EH, Taylor BT, Smith DW. Pathogenesis of tuberculosis: pathway to apical localization. Tuber Lung Dis. 1994;75:168–78. doi: 10.1016/0962-8479(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 4.Cosma CL, Humbert O, Ramakrishnan L. Superinfecting mycobacteria home to established tuberculous granulomas. Nat Immunol. 2004;5:828–35. doi: 10.1038/ni1091. [DOI] [PubMed] [Google Scholar]
- 5.Stinear TP, Seemann T, Harrison PF, et al. Insights from the complete genome sequence of Mycobacterium marinum on the evolution of Mycobacterium tuberculosis. Genome Res. 2008 doi: 10.1101/gr.075069.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis KN, Liao R, Guinn KM, et al. Deletion of RD1 from M. tuberculosis mimics BCG attenuation . J Inf Dis. 2003;187:117–23. doi: 10.1086/345862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volkman HE, Clay H, Beery D, Chang JC, Sherman DR, Ramakrishnan L. Tuberculous granuloma formation is enhanced by a Mycobacterium virulence determinant. PLoS Biol. 2004;2:e367. doi: 10.1371/journal.pbio.0020367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cosma CL, Klein K, Kim R, Beery D, Ramakrishnan L. Mycobacterium marinum Erp is a virulence determinant required for cell wall integrity and intracellular survival. Infect Immun. 2006;74:3125–33. doi: 10.1128/IAI.02061-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosma CL, Davis JM, Swaim LE, Volkman H, Ramakrishnan L. Zebrafish and Frog Models of Mycobacterium marinum Infection. Current Protocols in Microbiology: John Wiley adn Sons, Inc. 2006:10B.2.1–10B.2.33. doi: 10.1002/0471729256.mc10b02s3. [DOI] [PubMed] [Google Scholar]
- 10.Flynn JL. Lessons from experimental Mycobacterium tuberculosis infections. Microbes and Infection. 2006;8:1179–1188. doi: 10.1016/j.micinf.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 11.Egen JG, Rothfuchs AG, Feng CG, Winter N, Sher A, Germain RN. Macrophage and T Cell Dynamics during the Development and Disintegration of Mycobacterial Granulomas. Immunity. 2008;28:271–284. doi: 10.1016/j.immuni.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogan LH, Macvilay K, Barger B, et al. Mycobacterium bovis Strain Bacillus Calmette-Guerin-Induced Liver Granulomas Contain a Diverse TCR Repertoire, but a Monoclonal T Cell Population Is Sufficient for Protective Granuloma Formation. J Immunol. 2001;166:6367–6375. doi: 10.4049/jimmunol.166.10.6367. [DOI] [PubMed] [Google Scholar]
- 13.Clay H, Davis JM, Beery D, Huttenlocher A, Lyons SE, Ramakrishnan L. Dichotomous role of the macrophage in early Mycobacterium marinum infection of the zebrafish. Cell Host Microbe. 2007;2:29–39. doi: 10.1016/j.chom.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berthet FX, Lagranderie M, Gounon P, et al. Attenuation of virulence by disruption of the Mycobacterium tuberculosis erp gene. Science. 1998;282:759–62. doi: 10.1126/science.282.5389.759. [DOI] [PubMed] [Google Scholar]
- 15.Guinn KM, Hickey MJ, Mathur SK, et al. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol Microbiol. 2004;51:359–70. doi: 10.1046/j.1365-2958.2003.03844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swaim LE, Connolly LE, Volkman HE, Humbert O, Born DE, Ramakrishnan L. Mycobacterium marinum infection of adult zebrafish causes caseating granulomatous tuberculosis and is moderated by adaptive immunity. Infect Immun. 2006;74:6108–17. doi: 10.1128/IAI.00887-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis JM, Clay H, Lewis JL, Ghori N, Herbomel P, Ramakrishnan L. Real-time visualization of Mycobacterium-macrophage interactions leading to initiation of granuloma formation in zebrafish embryos. Immunity. 2002;17:693–702. doi: 10.1016/s1074-7613(02)00475-2. [DOI] [PubMed] [Google Scholar]
- 18.Hsu T, Hingley-Wilson SM, Chen B, et al. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. PNAS. 2003;100:12420–25. doi: 10.1073/pnas.1635213100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pym AS, Brodin P, Brosch R, Huerre M, Cole ST. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol Microbiol. 2002;46:709–17. doi: 10.1046/j.1365-2958.2002.03237.x. [DOI] [PubMed] [Google Scholar]
- 20.Danelishvili L, Young LS, Bermudez LE. In vivo efficacy of phage therapy for Mycobacterium avium infection as delivered by a nonvirulent Mycobacterium. Microb Drug Resist. 2006;12:1–6. doi: 10.1089/mdr.2006.12.1. [DOI] [PubMed] [Google Scholar]
- 21.Connolly LE, Edelstein PH, Ramakrishnan L. Why is long-term therapy required to cure tuberculosis? PLoS Med. 2007;4:e120. doi: 10.1371/journal.pmed.0040120. [DOI] [PMC free article] [PubMed] [Google Scholar]