Abstract
Antidepressant drugs produce therapeutic actions and many of their side effects via blockade of the plasma membrane transporters for serotonin (SERT/SLC6A2), norepinephrine (NET/SLC6A1) and dopamine (DAT/SLC6A3). Many antidepressants block several ofthese transporters; some are more selective. Mouse gene knockouts of these transporters provide interesting models for possible effects of chronic antidepressant treatments. To examine the role of monoamine transporters in models of depression DAT, NET and SERT KO mice and wildtype littermates were studied in the forced swim test (FST), the tail suspension test (TST) and for sucrose consumption. In order to dissociate general activity from the potential antidepressant effects three types of behavior were assessed in the FST: immobility, climbing and swimming. In confirmation of previous reports, both DAT KO and NET KO mice exhibited less immobility than wildtype littermates while SERT KO mice did not. Effects of DAT deletion were not simply due to hyperactivity as decreased immobility was observed in DAT +/- mice that were not hyperactive as well as in DAT -/- mice that displayed profound hyperactivity. Climbing was increased, while swimming was almost eliminated in DAT -/-mice, while a modest but similar effect was seen in NET KO mice, which showed a modest decrease in locomotor activity. Combined increases in climbing and decreases in immobility are characteristic of forced swim test results in antidepressant animal models, while selective effects on swimming are associated with the effects of stimulant drugs. Therefore, an effect on climbing is thought to more specifically reflect antidepressant effects, as has been observed in several other proposed animal models of reduced depressive phenotypes. A similar profile was observed in the TST, where DAT, NET and SERT knockouts were all found to reduce immobility, but much greater effects were observed in DAT KO mice. However, to further determine whether these effects of DAT KO in animal models of depression may be due to the confounding effects of hyperactivity, mice were also assessed in a sucrose consumption test. Sucrose consumption was increased in DAT KO mice consistent with reduced anhedonia, and inconsistent with competitive hyperactivity; no increases were observed in SERT KO or NET KO mice. In summary, the effects of DAT knockout in animal models of depression are larger than those produced by NET or SERT KO, and unlikely to be simply the result of the confounding effects of locomotor hyperactivity; thus, these data support reevaluation of the role that DAT expression could play in depression and the potential antidepressant effects of DAT blockade.
Keywords: depression, dopamine, norepinephrine, serotonin
INTRODUCTION
Inhibition of neurotransmitter reuptake by drugs acting at SERT, NET and/or DAT can produce antidepressant effects (Skolnick et al., 2003; Lucki and O'Leary, 2004), which, in part, has lead to hypotheses that dysfunction of serotonin, norepinephrine and/or dopamine systems might contribute to depression (Prange, 1964; Schildkraut, 1967; Akiskal and McKinney, 1973; van Praag, 1974; Willner, 1983a,b,c; Willner, 2000; Shelton, 2004; Dunlop and Nemeroff, 2007). Most drugs that are clinically effective antidepressants in humans produce reduced immobility in the rodent forced swim test (Porsolt, 1979). Modifications and extensions of the forced swim test paradigm (Armario et al., 1988; Cervo and Samanin, 1991; Detke et al., 1995; Detke and Lucki; 1996, Detke et al., 1997) have sought to improve its specificity and predictive validity for human antidepressant efficacy (for review see Cryan et al., 2005). These modified versions of the original test examine additional behavioral features and use slight modifications of the original apparatus that allow assessment of a broader behavioral repertoire. Such assessments may be especially important for studies of experimental manipulations that may alter both depressive behavior and general activity. In studies of Fawn Hooded rats that exhibit both hyperactivity (Hall et al., 1998) and reduced immobility in forced swim testing (Hall et al., 1998), for example, the examination of several behavioral endpoints allowed dissociation of hyperactivity from antidepressant-like effects. Such considerations might thus be especially useful in studies of rodents with genetic manipulations that could alter both general levels of motor activity and depressive-like symptoms, such as monoamine transporter knockout mice (Giros et al., 1996; Sora et al., 1998).
Monoamine transporter knockouts are potentially of interest in the study of antidepressant actions because they chronically elevate extracellular monoamine levels (Gainetdinov et al., 1998; Xu et al., 2000; Mathews et al., 2004; Shen et al., 2004) in a manner similar to antidepressant treatments (Abercrombie et al., 1988; Nomikos et al., 1990; Kreiss and Lucki, 1995). Effects of SERT, NET and DAT KO mice in antidepressant models may thus be of relevance in understanding the mechanisms that underlie antidepressant effects. Decreased immobility, a correlate of antidepressant activity, has been observed in NET KO mice (Xu et al., 2000) and DAT KO mice (Spielewoy et al., 2000). No difference or increased immobility has been observed SERT KO mice depending on the background strain examined (Holmes et al., 2002). However, all of these studies have only examined immobility in the FST. Examination of other behaviors in this test (e.g. climbing and swimming) should help to clarify the nature of these effects. In the case of DAT KO mice this is essential because DAT KO mice are hyperactive (Giros et al., 1996; Sora et al., 1998). In the previous study of DAT KO mice (Spielewoy et al., 2000) it was suggested that the increased activity, as opposed to immobility, of DAT KO mice in the FST was accounted for by increased “swimming”, but those authors did not in fact measure any other behavior in the test, nor differentiate between climbing and swimming. The nature of this behavior is rather important as it might reflect one of two possible effects, general activity or antidepressant activity.
Since hyperactivity may be a confounding issue in the FST it is important to validate findings in that model in other models of depression. Another model that has been extensively validated with a wide range of antidepressants is the tail suspension test (TST) (Cryan et al., 2005). SERT KO mice have reduced immobility in the TST, which was dependent on the background strain against which the knockout was expressed (Holmes et al., 2002), like effects in the FST. A similar profile has been observed in NET KO mice in the TST (Dziedzicka-Wasylewska et al., 2006), but has not yet been assessed in DAT KO mice. Thus, to clarify and extend these data, we now report examination of behavior in DAT, SERT and NET knockout mice using both the FST and the TST under identical experimental conditions so that comparisons may be made between the relative magnitude of the effects of each of these monoamine transporter gene knockouts.
However, the TST, as well as the FST, might be interpreted as being open to the confounding effects of hyperactivity. To further address this we examined another model, sucrose consumption, which should not be open to the same type of locomotor confound. The sucrose consumption model assesses a different aspect of depressive behavior, anhedonia, and was originally used to assess the depressive effects of chronic mild stress (Papp et al., 1991), but has since been used to assess the effects of several other models of depression (El Yacoubi et al., 2003; Wintink et al., 2003; Shumake et al., 2005).
EXPERIMENTAL PROCEDURES
Subjects
The knockout mice used in these experiments were from the DAT-SERT (Sora et al., 2001) and NET-SERT (Hall et al., 2002) double knockout lines described previously, which were produced from crossing the original DAT (Sora et al., 1998), SERT (Bengel et al., 1998) and NET (Wang et al., 1999) single knockout lines. The DAT mice used in these experiments from the DAT-SERT line were WT for SERT, the SERT mice from the DAT-SERT line were WT for DAT and NET mice from the NET-SERT line were WT for SERT. For simplicity the second genotype, always +/+, is not included in the descriptions. These knockout lines are therefore of a mixed C57BL/6J-129Sv background. Wild-type mice (+/+), heterozygote KO mice (+/-) and homozygote knockout mice (-/-) were genotyped by PCR, using two internal primers, one targeted at the knockout insertion sequence and one targeted at the WT gene, and one external primer, which generated two products identifying the WT and KO genes. The DAT and SERT transgenic knockout insertion sequences contained a neomycin gene (NEO), while the NET KO contained a green fluorescent protein gene insert (GFP). PCR using TaKaRa DNA polymerase (Takara Bio, Japan) was performed on DNA that was eluted from tail tip fragments after digestion overnight in Protease K. For DAT genotyping the external primer (5' AGT GTG TGC AGG GCA TGG TGT A 3') and the WT primer (5' TAG GCA CTG CTG ACG ATG ACT G 3') produced a 500 bp band, while the external primer and the NEO primer (5' CTC GTC GTG ACC CAT GGC GAT 3') produced a 600 bp band. For SERT genotyping the external primer (5' GCT CTC AGT CTT GTC TCC ATA AC 3') and the WT primer (5' TGC TGA CTG GAG TAC AGG CTA G 3') produced a 620 bp band, while the external primer and the NEO primer (5' CTC GTC GTG ACC CAT GGC GAT 3') produced an 800 bp band. For NET genotyping the external primer (5' GCT CTG TCC CTG TGC TTC ACG 3') and the WT primer (5' TGA GGC CTA AGC TGG AGC TCG 3') produced a 601 bp band, while the external primer and the GFP primer (5' CGG TGA ACA GCT CCT CGC CC 3') produced a 470 bp band. DAT KO, SERT KO and NET KO mice of all three genotypes (N=8-10 mice per genotype) were tested in a modified forced swim test as described below.
Behavioral Methods
Forced Swim Test
This experiment used the forced swim test (Porsolt et al., 1977) modified and validated in a manner similar to that previously described for rats (Hall et al., 1998). DAT, SERT and NET KO mice (N=8-10 / genotype) were placed in 3 L cylindrical beakers (diameter 19 cm) that were filled to a depth of 14 cm with 25 °C water on two successive days. On the pre-test day, mice were placed in the water for 15 minutes, towel-dried, placed under a warming lamp until completely dry and then returned to their home cages. On the test day, mice were placed into the water for five minutes and their behavior was recorded digitally. Their behavior was scored by observation ofthese recordings, blind to genotype. Durations of immobility, swimming, and climbing behaviors were measured using the TIMER behavioral scoring program (National Institutes of Health). Immobility was defined as being stationary with only enough motion of the tail or forepaws to keep the head above water. The forepaws usually remained at the animals' sides. Swimming was defined as active use of the forepaws with forward movement, in the center or along the sides of the cylinder, which did not involve lifting the paws above the surface of the water. The body was usually oriented parallel to the sides of the cylinder. Climbing was defined as active pawing of the side of the cylinder, lifting the paws above the surface of the water. The body was oriented with the head toward the wall and the body oriented perpendicularly to the side of the cylinder. Inter-rater reliability estimates for these three measures were calculated by comparing the scores of two observers for 32 subjects. Reliability estimates for immobility (r = 0.88, p<0.001), climbing (r = 0.86, p<0.001), and swimming (r = 0.81, p<0.001) were similar to the inter-rater reliability estimates obtained previously for rats (Hall et al., 1998).
Because strain has been shown to affect responses to SERT and NET blockers in the forced swim test (Lucki et al., 2001, David et al., 2003, Dulawa et al., 2004), an additional experiment was performed to determine the sensitivity of WT mice from the DAT-SERT line to selective transporter blockers. The effects of pretreatment with saline (1 mL/100g IP), fluoxetine (30 mg/kg IP), desipramine (20 mg/kg IP) or GBR12909 (20 mg/kg IP), 30 minutes prior to the 5 minute test on the second day were examined in WT mice (N=8-9 per condition). In all other respects the experiment was identical to that described above.
Tail Suspension Test
In the tail suspension test DAT, SERT and NET KO mice (N=8-13 / genotype) were suspended by the tail from a horizontal metal rod (8 mm diameter) for 5 minutes. The duration of immobility was measured by an observer with a stopwatch.
Sucrose Consumption Test
In the sucrose consumption test DAT KO (N = 7-11 per genotype), SERT KO (N = 7-13 per genotype), and NET KO (N = 6-10 per genotype), were placed in a sucrose consumption chamber (DM-8 lick counter, Columbus Instruments, Columbus, OH) each day with access to water and sucrose. The number of licks for sucrose and water were monitored for 30 minutes. In a preliminary experiment the volume consumed as well as the number of licks was monitored. The correlation between licks and volume was found to be r=0.85 (p<0.01); thus only the number of licks is presented. To account for differing weight of the subjects the number of licks is presented as licks / 100 g body weight. Subjects were tested over 9 days, 3 days at each concentration of sucrose (0.7%, 7% and 34% sucrose).
Locomotor Activity
Previous studies found that DAT -/- mice were profoundly hyperactive (Giros et al., 1996), while locomotor activity was reduced in NET KO mice (Xu et al., 2000). No basal differences in activity were found in SERT KO mice, nor in DAT +/- mice (Giros et al., 1996; Bengel et al., 1998; Xu et al., 2000). Nonetheless, because differences in locomotor activity between genotypes might be thought to affect the paradigms described above, basal locomotion was assessed in DAT, SERT and NET KO mice. For each gene knockout +/+, +/- and -/- mice (N=9-11 per genotype) were assessed for locomotor activity for 1 hour under novel conditions. Total distance traveled was measured in Optovarimax activity monitors (Columbus Instruments) under dark, sound-attenuated conditions using methods identical to our previous publications with DAT KO, SERT KO and DAT/SERT KO mice (Sora et al., 1998, Sora et al., 2001).
Data Analysis
Data were analyzed using analysis of variance with the between-subjects factor of GENOTYPE followed by Fisher's PLSD for post hoc comparisons were appropriate. For analysis of the sucrose consumption data the additional within subjects factors of SUCROSE (sucrose versus water) and CONCENTRATION (0.7 %, 7% and 34% sucrose). The data for the three days of testing at each concentration were averaged and the average values submitted to ANOVA. The effects of saline, fluoxetine, desipramine, and GBR12909 were compared by ANOVA followed by post hoc comparison with Fisher's PLSD.
RESULTS
Forced Swim Test
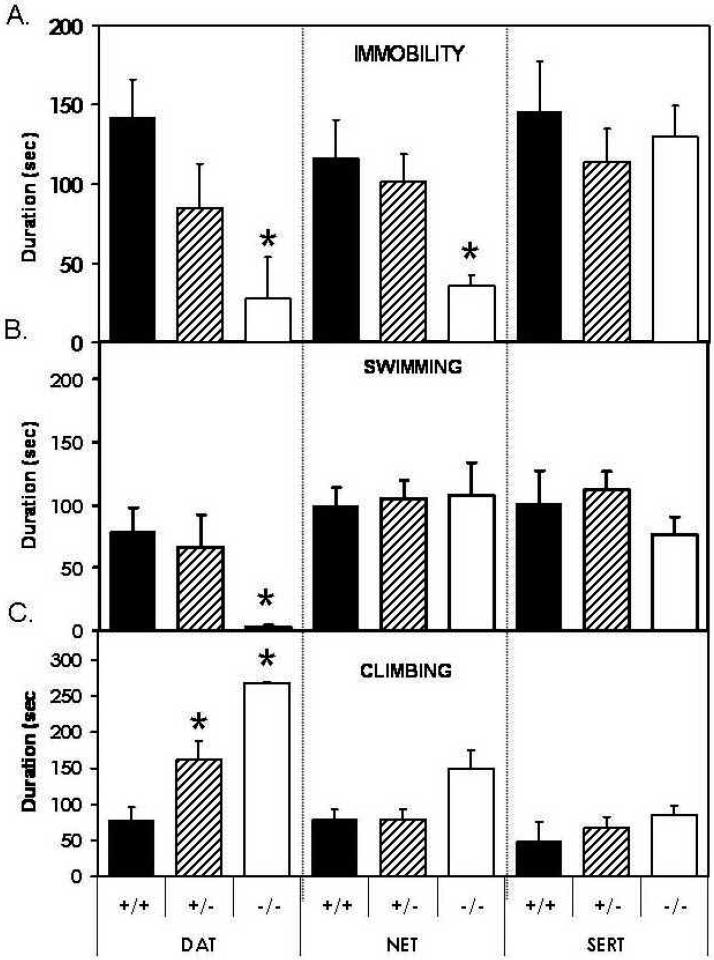
Deletion of the dopamine transporter gene profoundly affected forced swim test behavior. The duration of immobility (F[2,27]=4.9, p<0.02) was decreased by DAT KO (Fig. 1A) so that DAT -/- mice exhibited virtually no immobility. These results were significantly different from those obtained from wildtype littermate DAT +/+ mice (p<0.05). Immobility was also reduced in DAT +/- mice. Immobility in these mice was intermediate between values for DAT +/+ and DAT +/- mice, although post hoc comparisons did not reach statistical significance. The duration of swimming (F[2,27]=4.9, p<0.02) was also greatly reduced in DAT KO mice (Fig. 1B). DAT -/- mice exhibited virtually no swimming behavior. These differences achieved significance in comparison to DAT +/+ (p<0.05) and to DAT +/- mice (p<0.05). The duration of swimming was unaltered in DAT +/- mice compared to DAT +/+ mice. DAT -/- mice exhibited climbing behavior for almost the entire period of testing. Six of the ten DAT -/- subjects tested struggled to escape the cylinder for the entire session (Fig. 1C). The duration of climbing was greatly increased in DAT KO mice (F[2,27]=12.7, p<0.0001). In this case, as for immobility, DAT +/- mice exhibited behavior that was intermediate between DAT +/+ and DAT -/- mice. The duration of climbing was significantly greater in DAT -/- mice than in either DAT +/+ or DAT +/- mice (p<0.05). DAT +/- mice also exhibited significantly more climbing than wildtype DAT +/+ mice (p<0.05).
Figure 1. Behavior in the modified Forced Swim Test in DAT KO, SERT KO and NET KO mice.
The duration of immobility (A), swimming (B) and climbing (C) in DAT KO, SERT KO and NET KO mice (+/+, +/-, and -/- genotypes for each strain) expressed as mean ± the standard error of the mean. * Significant difference from +/+ mice, Fisher’s PLSD.
Deletion of the NET gene also reduced the duration of immobility in forced swim testing (Fig. 1A; F[2,27]=4.9, p<0.02). NET -/- mice exhibited substantially reduced immobility compared to NET +/+ mice (p<0.05). NET +/- mice did not differ significantly from wildtype mice. The duration of swimming was unaffected by NET KO (Fig. 1B; F[2,24]=0.1, ns). Although climbing showed a trend toward increase in NET -/- mice, this did not reach statistical significance (Fig. 1C; F[2,24]=2.1, ns).
Deletion of the SERT gene had no effect on any forced swim test behavior. Figs 1A-1C document that SERT KO failed to affect the duration of immobility (F[2,25]=0.3, ns), the duration of swimming (F[2,25]=0.8, ns), or the duration of climbing (F[2,25]=0.8, ns).
In wildtype mice there was a significant difference between treatment groups for immobility (Table 2; F[3,28]=10.2, p<0.0001), swimming (Table 2; F[3,28]=5.4, p<0.005), but not climbing (Table 2; F[3,28]=1.4, ns). Desipramine significantly reduced immobility (p<0.05 versus saline, Fisher's PLSD), and increased swimming (p<0.05 versus saline, Fisher's PLSD). Desipramine treatment also increased climbing but this difference was not statistically significant. By contrast, neither of the other two drugs, fluoxetine or GBR 12909, had any statistically significant effects, although there was a trend for GBR 12909 to decrease immobility and to increase climbing.
Table 2.
Effects of fluoxetine, desipramine and GBR 12909 in the modified Forced Swim Test in WT mice
| Drug | Behavioral Measure | ||
|---|---|---|---|
| Immobility | Swimming | Climbing | |
| The duration of immobility, swimming and climbing in WT mice treated with saline, fluoxetine, desipramine or GBR 12909 expressed as mean ± the standard error of the mean. | |||
| Saline | 181.5 ± 13.4 | 105.9 ± 12.6 | 11.1 ± 7.6 |
| Desipramine | 69.1 ± 15.7* | 193.5 ± 20.7* | 34.8 ± 10.0 |
| Fluoxetine | 183.6 ± 17.7 | 100.1 ± 19.4 | 14.1 ± 5.2 |
| GBR 12909 | 144.0 ± 19.6 | 121.0 ± 20.4 | 31.3 ± 14.7 |
Significant difference from saline treated mice mice, Fisher's PLSD.
Tail Suspension Test
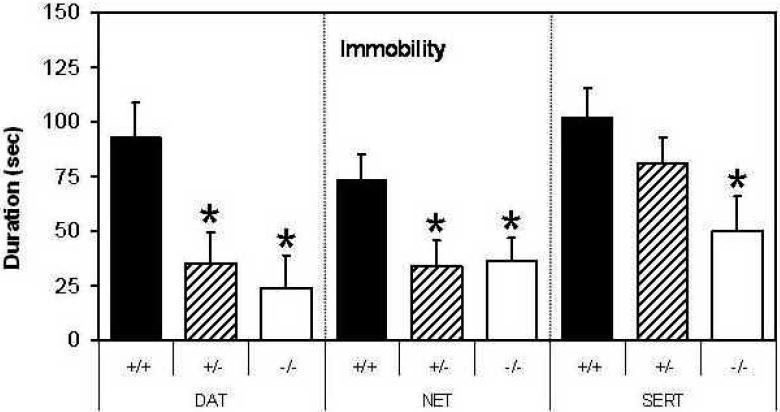
Deletion of all three monoamine transporter genes decreased immobility in the tail suspension test (Fig. 2): DAT (F[2,34]=6.5, p<0.01), SERT (F[2,31]=3.5, p<0.05) and NET (F[2,23]=3.9, p<0.05). However, in SERT KO mice only homozygous mice exhibited a significant reduction in immobility (p<0.05 Fisher's PLSD), while in DAT and NET KO mice both heterozygous and homozygous KO mice had reduced immobility compared to WT mice. The magnitude of the difference between +/+ and -/- mice differed between the knockout strains; the magnitude of the effect of a homozygous knockout was 74% in DAT KO mice, but only 51% in SERT KO and NET KO mice.
Figure 2. Immobility in the Tail Suspension Test in DAT KO, SERT KO and NET KO mice.
The duration of immobility in DAT KO, SERT KO and NET KO mice (+/+, +/-, and -/- genotypes for each strain) expressed as mean ± the standard error of the mean. * Significant difference from +/+ mice, Fisher's PLSD.
Sucrose Consumption Test
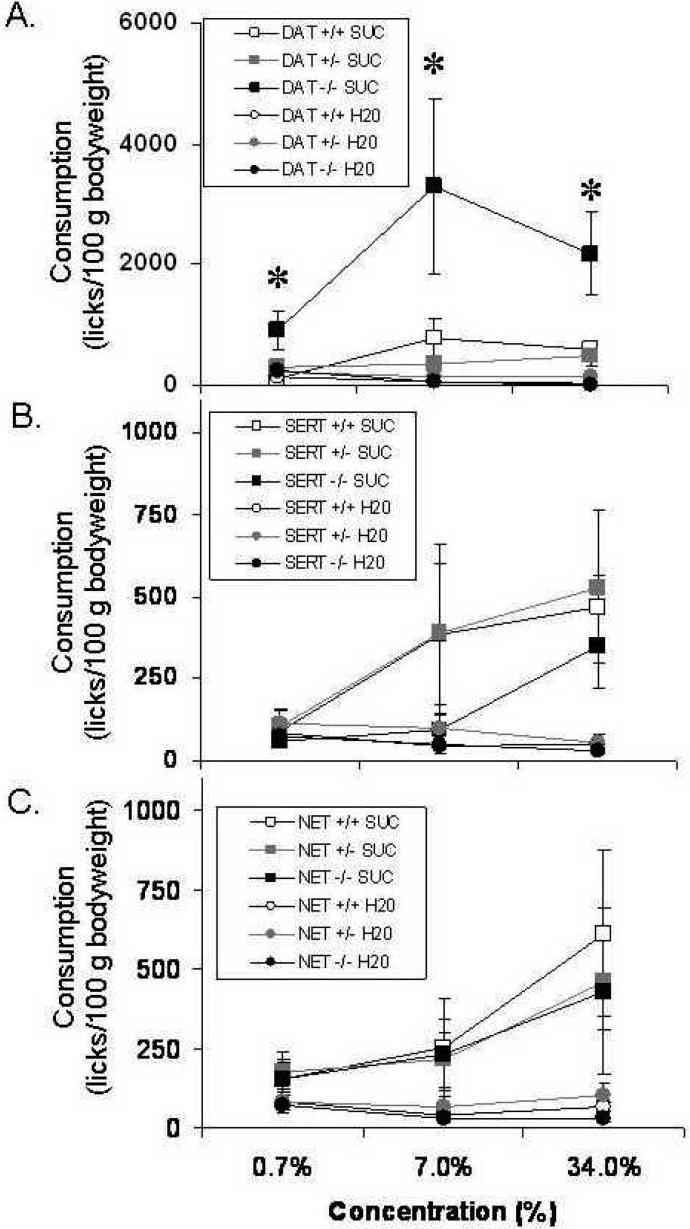
Deletion of the DAT gene increased sucrose consumption at all of the concentrations tested (Fig. 3A). There was an overall preference for sucrose over water for all subjects (SUCROSE: F[1,23]=16.9, p<0.001). DAT -/- had a greater fluid consumption overall (GENOTYPE: F[2,23]=6.2, p<0.01), which was the result of greater consumption of sucrose, but not water at all concentrations tested (GENOTYPE × SUCROSE: F[2,23]=6.4, p<0.01), especially at higher concentrations (GENOTYPE × SUCROSE × CONCENTRATION: F[4,46]=2.8, p<0.05). DAT +/- mice were not different from WT mice.
Figure 3. Sucrose consumption in DAT, SERT and NET KO mice.
Consumption of sucrose (0.7 %, 7.0 % and 34 %) and water in DAT (A), SERT (B) and NET (C) KO mice (+/+, +/- and -/- genotypes) expressed as average licks per 100 g body weight. The data is expressed as the mean ± the standard error of the mean. * Significant difference from +/+ mice, Fisher's PLSD.
Sucrose consumption was not affected by SERT KO (Fig. 3B) or NET KO (Fig. 3C). Although SERT KO mice showed a trend toward a reduction in sucrose consumption at the middle concentration, neither the main effect of GENOTYPE nor the interaction terms were significantly different in the ANOVA.
Locomotor Activity
DAT -/- mice were profoundly hyperactive (Table 1; GENOTYPE: F[2,27]=14.4, p<0.0001, p<0.05 Fisher's PLSD), but there was no difference in activity between DAT +/- and DAT +/+ mice. There were no significant differences in baseline activity in NET KO or SERT KO mice, although both had strong trends toward reduced locomotion (Table 1; NET: F[2,26]=3.2, p=0.06; SERT: F[2,27]=3.1, p=0.06).
Table 1.
Locomotor Activity in DAT, SERT and NET KO mice
| Knockout | Genotype | ||
|---|---|---|---|
| +/+ | +/- | -/- | |
| Locomotor activity in +/+, +/-, and -/- genotypes of DAT, SERT and NET KO mice. The data are expressed as the mean ± the standard error of the mean. | |||
| DAT KO | 12387 ± 1009 | 13140 ± 1365 | 32219 ± 4796* |
| NET KO | 8536 ± 1025 | 6622 ± 825 | 5108 ± 1015 |
| SERT KO | 8273 ± 768 | 10847± 2103 | 6191 ± 564 |
Significant difference from +/+ mice, Fisher's PLSD.
DISCUSSION
In the present study, deletion of the DAT gene produced changes in behavior in three behavioral tests sensitive to antidepressant-like phenotypes. These effects were quite profound. In the FST and the TST homozygous DAT KO mice spent nearly the entire duration of the test struggling to escape while sucrose consumption was greatly enhanced consistent with increased rewarding effects (e.g. reduced anhedonia). Contrary to what might be supposed based on naïve conceptions of the relative importance of each of the neurotransmitter systems in depression, and the mechanisms of the majority of commonly used antidepressant compounds, although similar effects were observed in NET KO mice in both the FST and TST and in SERT KO mice in the TST, these effects were less pronounced than those observed in DAT KO mice. Such a comparison must however be considered in light of potential interactions with genetic background, as discussed below, but suggests at the very least that under some circumstances DAT manipulations can have a greater effects on antidepressant-like phenotypes than previously appreciated.
DAT knockout almost completely eliminated immobility in the forced swim test, consistent with a previous finding (Spielewoy et al., 2000). That report suggested that increased immobility was associated with increased swimming, but those authors did not actually measure any other behavior; in particular, there was no attempt to dissociate general activity (swimming) from specific activity aimed at escaping from the cylinder (climbing). In the present experiment both immobility and swimming behavior were almost completely eliminated in DAT KO mice, replaced with persistent and almost continuous climbing (e.g. escape attempts). Because DAT KO mice are hyperactive (Giros et al., 1996; Sora et al., 1998), it might be supposed that differences in behavior in the FST between DAT +/+ and DAT -/- mice merely reflects this hyperactivity; indeed, this was the conclusion of the previously mentioned study (Spielewoy et al., 2000). Those authors interpreted the persistent escape attempts of DAT KO mice in the FST as “inappropriate” as opposed to the “adaptive” strategy of immobility, in accordance with one interpretation of the paradigm (Borsini et al., 1986). This interpretation is not generally accepted (see discussion of this subject in the review by (Cryan et al., 2005). To some extent the use of a single exposure forced swim paradigm in the Spielewoy et al. (2000) study, compared to a two exposure forced swim paradigm in the present study might lead to different interpretations of the results, the former relating more to acute stress, and the second to behavioral despair. Of course the type of paradigm used in the present study should be more related to antidepressant actions which was one of the conclusions of the review previously mentioned (Cryan et al., 2005).
Returning to the issue of hyperactivity several lines of evidence weigh against such a simple explanation of the effects of DAT KO in the FST. Reduced immobility and increased climbing were observed in both homozygous and heterozygous DAT KO mice, yet heterozygous DAT KO mice are not hyperactive (Table 1). Decreased immobility, albeit to a lesser extent, was also observed in NET KO mice, which are also not hyperactive (Table 1). In addition, decreased immobility in DAT KO mice was also observed in the TST, as was reduced immobility in both SERT KO and NET KO mice, consistent with previous reports (Holmes et al., 2002; Dziedzicka-Wasylewska et al., 2006), and hyperactivity was not observed in either of those knockout strains.
In any case, the description of DAT KO mice as hyperactive is probably not an accurate depiction of their behavior. This is based upon placement of DAT KO mice in a novel environment in which exploratory activity would be the dominant initial response tendency. More accurately, DAT KO, like amphetamine treatment (Evenden and Robbins, 1983), may increase the dominant response tendency. According to this view escape behavior in the TST and FST would be enhanced, and the present results would not be the result of hyperactivity per se. Behavior in a sucrose consumption test would not be confounded by locomotor hyperactivity in the same way as the other tests; indeed pronounced locomotor hyperactivity should reduce sucrose consumption by producing a competing behavior. By contrast, DAT KO mice had increased consumption of sucrose compared to WT mice. Increased sucrose consumption, particularly at low concentrations (e.g. 0.7% as in Fig. 3), has generally been taken to indicate differences the hedonic properties of sucrose (Papp et al., 1991; El Yacoubi et al., 2003; Wintink et al., 2003; Shumake et al., 2005). This additional evidence further indicates that the effects of DAT KO in the FST and TST are probably not the result of the confounding effects of hyperactivity. No differences were found in sucrose consumption in SERT or NET KO mice, consistent with the lack of effect of SERT KO in the FST, and somewhat reduced effects of NET KO in both tests.
The meaning of the relative lack of effect of NET KO and SERT KO in the present experiments must be interpreted with caution however, as substantial strain differences have been reported in the response to antidepressants in both the FST and the TST (Liu and Gershenfeld, 2001; David et al., 2003; Liu and Gershenfeld, 2003; Ripoll et al., 2003; Dulawa et al., 2004; Lucki and O'Leary, 2004; Crowley et al., 2005). In particular, both C57BL/6 and 129Sv mice do not exhibit antidepressant like effects in responses to fluoxetine in the FST (Lucki et al., 2001; Dulawa et al., 2004), although both strains appear to be responsive to a wide range of SERT, NET and DAT blockers in the TST (Ripoll et al., 2003; Crowley et al., 2005). In an interesting parallel to the present results one study found that C57BL/6 mice were unresponsive to NET or SERT blockers in the FST, but did respond to GBR 12909 (David et al., 2003). In the current experiments mixed C57BL/6-129Sv background WT mice did respond to desipramine in the FST, but not fluoxetine, consistent with findings in C57BL/6 mice (Lucki et al., 2001), so the effects of SERT KO in the present experiments should be treated with especial caution.
However, comparison of pharmacological antagonism of transporters and constituitive gene knockouts should be treated with caution in any case. Results of tests performed in adult knockout mice need to be potentially interpreted in light of the effects of absence of the gene during development as well as at the time of testing. In DAT KO mice in particular it might be supposed that differences in depressive phenotypes may largely reflect developmental consequences of the deletion. However, there is substantial evidence for a role of dopamine in depression and in depressive phenotypes in animal models of depression (Randrup et al., 1975; Willner, 1983a,b,c; Willner 2000; Dunlop and Nemeroff, 2007), much of it involving acute alterations in dopamine function. In humans the relatively selective DAT blocker bupropion, and its metabolites, produce clinically effective antidepressant actions (Ascher et al., 1995; Jefferson et al., 2005; Volkow et al., 2005). These findings are supported by numerous studies in animal models. Dopamine is also involved in anhedonia in the sucrose consumption model (Papp et al., 1991), and in the FST dopamine agonists and selective dopamine reuptake blockers have antidepressant-like effects (Cooper et al., 1980; Vaugeois et al., 1996; Hemby et al., 1997; Damaj et al., 2004; Siuciak and Fujiwara, 2004; Basso et al., 2005). In addition to reduced immobility, GBR 12909 selectively increased climbing behavior, but not swimming in the FST (Hemby et al., 1997). Dopamine agonists also enhance the antidepressant-like effects of serotonin selective reuptake inhibitors (Renard et al., 2001). The effects DAT blockers are not the result of generally enhanced activity as the effects of bupropion in the forced swim test occur at doses that do not affect spontaneous locomotion (Cooper et al., 1980; David et al., 2003).
The present findings are consistent with previous findings in DAT, SERT and NET knockout mice, while extending them in several respects. NET KO mice have been reported to have reduced immobility in the FST and the TST (Xu et al., 2000; Dziedzicka-Wasylewska et al., 2006), as was observed in the present study. The present data are also consistent with some previous results using SERT KO mice, which were reported to have decreased immobility in the TST, but increased immobility in the FST, when bred onto a 129S6 background, but did not display any differences in these tests when bred onto a C57BL/6J background (Holmes et al., 2002). It is interesting to note that in the TST the mixed background strain from the present study had reduced immobility in the TST, similar to SERT KO mice on a 129S6 background in the Holmes et al., (2002) study, while no effect was observed in the FST similar to C57BL/6J congenic mice in that study. This suggests a somewhat different underlying genetic substrates for the TST and FST consistent with some of the pharmacological findings in inbred strains discussed above.
In the forced swim test antidepressants that act primarily at the serotonin transporter have been reported to increase swimming, while those that act primarily at the norepinephrine transporter have been reported to increase climbing (Detke et al., 1995; Detke and Lucki, 1996; Detke et al., 1997; Page et al., 1999; Cryan et al., 2002; Cryan et al., 2003). This was not found in desipramine-treated WT mice in the present experiment. Nonetheless it appears that changes in either type of active behavior and concomitant reductions in immobility may be associated with antidepressant-like effects. However, several models of depression, including those produced by withdrawal from amphetamine, withdrawal from ovarian hormone treatments that simulate pregnancy and estrogen deficiencies caused by aromatase knockout increase forced swim test immobility and decrease climbing without affecting swimming (Galea et al., 2001; Cryan et al., 2003; Dalla et al., 2004). These depressive-like effects are mirrored by the opposite, antidepressant-like effects of amphetamine administration, pregnancy or estradiol: decreased immobility and increased climbing, in the absence of substantial changes in swimming (Galea et al., 2001; Molina-Hernandez and Tellez-Alcantara, 2001; Cryan et al., 2003). The pattern of antidepressant effects in these models is very similar to that observed in DAT KO mice, while the effects in models of depression are opposite to those observed in DAT KO mice.
The current results thus support a prominent role for DAT, and dopamine, in the mediation of antidepressant-like behavior as assessed by the models used here. The striking magnitude of the effects of DAT KO, particularly in comparison to the more modest consequences or lack of consequences of NET or SERT KO, each support re-evaluation of the clinically proven effects of DAT blockade on depression, and the role of the DAT gene or differences in DAT gene expression in individual differences in depression and responses to effective antidepressant treatments. The pronounced effects of background strain in both pharmacological and transgenic studies indicates that these very strong DAT effects may be limited to certain genetic backgrounds, but the data suggests that under these circumstances DAT may have more importance that SERT or NET. It remains to be seen whether such profound and prominent effects may be observed for NET or SERT gene knockout when it is expressed on other genetic backgrounds in which SERT and NET blockers have more pronounced effects. Taken together these data suggest that manipulations of DAT, SERT, and NET genes are highly dependent on genetic background and raise the possibility that this may influence both baseline depressive behavior and response to antidepressants acting selectively at each of these transporters.
Acknowledgement
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse (NIH/DHHS). The authors wish to thank the contract staff of Charles River Laboratories for their expert care of our mice and especially our breeder-geneticist Kriss Knestaut. We also wish to thank Joshua Schwarzbaum for his technical assistance in the later stages of these studies.
Abbreviations
- DAT
dopamine transporter
- NET
norepinephrine transporter
- SERT
serotonin transporter
- KO
knockout
- FST
forced swim test
- TST
tail suspension test
References
- Abercrombie ED, Keller RW, Jr., Zigmond MJ. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience. 1988;27:897–904. doi: 10.1016/0306-4522(88)90192-3. [DOI] [PubMed] [Google Scholar]
- Akiskal HS, McKinney WT., Jr. Depressive disorders: toward a unified hypothesis. Science. 1973;182:20–29. doi: 10.1126/science.182.4107.20. [DOI] [PubMed] [Google Scholar]
- Armario A, Gavalda A, Marti O. Forced swimming test in rats: effect of desipramine administration and the period of exposure to the test on struggling behavior, swimming, immobility and defecation rate. Eur J Pharmacol. 1988;158:207–212. doi: 10.1016/0014-2999(88)90068-4. [DOI] [PubMed] [Google Scholar]
- Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, Golden RN, Martin P, Potter WZ, Richelson E, et al. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry. 1995;56:395–401. [PubMed] [Google Scholar]
- Basso AM, Gallagher KB, Bratcher NA, Brioni JD, Moreland RB, Hsieh GC, Drescher K, Fox GB, Decker MW, Rueter LE. Antidepressant-like effect of D(2/3) receptor-, but not D(4) receptor-activation in the rat forced swim test. Neuropsychopharmacology. 2005;30:1257–1268. doi: 10.1038/sj.npp.1300677. [DOI] [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Borsini F, Volterra G, Meli A. Does the behavioral “despair” test measure “despair”? Physiol Behav. 1986;38:385–386. doi: 10.1016/0031-9384(86)90110-1. [DOI] [PubMed] [Google Scholar]
- Cervo L, Samanin R. Effect of chronic treatment with 8-OH-DPAT in the forced swimming test requires the integrity of presynaptic serotonergic mechanisms. Psychopharmacology (Berl) 1991;103:524–528. doi: 10.1007/BF02244253. [DOI] [PubMed] [Google Scholar]
- Cooper BR, Hester TJ, Maxwell RA. Behavioral and biochemical effects of the antidepressant bupropion (Wellbutrin): evidence for selective blockade of dopamine uptake in vivo. J Pharmacol Exp Ther. 1980;215:127–134. [PubMed] [Google Scholar]
- Crowley JJ, Blendy JA, Lucki I. Strain-dependent antidepressant-like effects of citalopram in the mouse tail suspension test. Psychopharmacology (Berl) 2005;183:257–264. doi: 10.1007/s00213-005-0166-5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Hoyer D, Markou A. Withdrawal from chronic amphetamine induces depressive-like behavioral effects in rodents. Biol Psychiatry. 2003;54:49–58. doi: 10.1016/s0006-3223(02)01730-4. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Page ME, Lucki I. Noradrenergic lesions differentially alter the antidepressant-like effects of reboxetine in a modified forced swim test. Eur J Pharmacol. 2002;436:197–205. doi: 10.1016/s0014-2999(01)01628-4. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Papadopoulou-Daifoti Z, Balthazart J, Bakker J. Oestrogen-deficient female aromatase knockout (ArKO) mice exhibit depressive-like symptomatology. Eur J Neurosci. 2004;20:217–228. doi: 10.1111/j.1460-9568.2004.03443.x. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Carroll FI, Eaton JB, Navarro HA, Blough BE, Mirza S, Lukas RJ, Martin BR. Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Mol Pharmacol. 2004;66:675–682. doi: 10.1124/mol.104.001313. [DOI] [PubMed] [Google Scholar]
- David DJ, Renard CE, Jolliet P, Hascoet M, Bourin M. Antidepressant-like effects in various mice strains in the forced swimming test. Psychopharmacology (Berl) 2003;166:373–382. doi: 10.1007/s00213-002-1335-4. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Johnson J, Lucki I. Acute and chronic antidepressant drug treatment in the rat forced swimming test model of depression. Exp Clin Psychopharmacol. 1997;5:107–112. doi: 10.1037//1064-1297.5.2.107. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Lucki I. Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: the effects of water depth. Behav Brain Res. 1996;73:43–46. doi: 10.1016/0166-4328(96)00067-8. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch. Gen. Psychiatr. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Dziedzicka-Wasylewska M, Faron-Gorecka A, Kusmider M, Drozdowska E, Rogoz Z, Siwanowicz J, Caron MG, Bonisch H. Effect of antidepressant drugs in mice lacking the norepinephrine transporter. Neuropsychopharmacology. 2006;31:2424–2432. doi: 10.1038/sj.npp.1301064. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, Bouali S, Popa D, Naudon L, Leroux-Nicollet I, Hamon M, Costentin J, Adrien J, Vaugeois JM. Behavioral, neurochemical, and electrophysiological characterization of a genetic mouse model of depression. Proc Natl Acad Sci U S A. 2003;100:6227–6232. doi: 10.1073/pnas.1034823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL, Robbins TW. Increased response switching, perseveration and perseverative switching following d-amphetamine in the rat. Psychopharmacology (Berl) 1983;80:67–73. doi: 10.1007/BF00427498. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Jones SR, Fumagalli F, Wightman RM, Caron MG. Reevaluation of the role of the dopamine transporter in dopamine system homeostasis. Brain Res Brain Res Rev. 1998;26:148–153. doi: 10.1016/s0165-0173(97)00063-5. [DOI] [PubMed] [Google Scholar]
- Galea LA, Wide JK, Barr AM. Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behav Brain Res. 2001;122:1–9. doi: 10.1016/s0166-4328(01)00170-x. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Hall FS, Huang S, Fong GF, Pert A. The effects of social isolation on the forced swim test in Fawn hooded and Wistar rats. J Neurosci Methods. 1998;79:47–51. doi: 10.1016/s0165-0270(97)00155-6. [DOI] [PubMed] [Google Scholar]
- Hall FS, Huang S, Fong GW, Pert A, Linnoila M. Effects of isolation-rearing on locomotion, anxiety and responses to ethanol in Fawn Hooded and Wistar rats. Psychopharmacology (Berl) 1998;139:203–209. doi: 10.1007/s002130050705. [DOI] [PubMed] [Google Scholar]
- Hall FS, Li XF, Sora I, Xu F, Caron M, Lesch KP, Murphy DL, Uhl GR. Cocaine mechanisms: enhanced cocaine, fluoxetine and nisoxetine place preferences following monoamine transporter deletions. Neuroscience. 2002;115:153–161. doi: 10.1016/s0306-4522(02)00379-2. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Lucki I, Gatto G, Singh A, Thornley C, Matasi J, Kong N, Smith JE, Davies HM, Dworkin SI. Potential antidepressant effects of novel tropane compounds, selective for serotonin or dopamine transporters. J Pharmacol Exp Ther. 1997;282:727–733. [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Murphy DL, Crawley JN. Evaluation of antidepressant-related behavioral responses in mice lacking the serotonin transporter. Neuropsychopharmacology. 2002;27:914–923. doi: 10.1016/S0893-133X(02)00374-3. [DOI] [PubMed] [Google Scholar]
- Jefferson JW, Pradko JF, Muir KT. Bupropion for major depressive disorder: Pharmacokinetic and formulation considerations. Clin Ther. 2005;27:1685–1695. doi: 10.1016/j.clinthera.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Kreiss DS, Lucki I. Effects of acute and repeated administration of antidepressant drugs on extracellular levels of 5-hydroxytryptamine measured in vivo. J Pharmacol Exp Ther. 1995;274:866–876. [PubMed] [Google Scholar]
- Liu X, Gershenfeld HK. Genetic differences in the tail-suspension test and its relationship to imipramine response among 11 inbred strains of mice. Biol Psychiatry. 2001;49:575–581. doi: 10.1016/s0006-3223(00)01028-3. [DOI] [PubMed] [Google Scholar]
- Liu X, Gershenfeld HK. An exploratory factor analysis of the Tail Suspension Test in 12 inbred strains of mice and an F2 intercross. Brain Res Bull. 2003;60:223–231. doi: 10.1016/s0361-9230(03)00033-9. [DOI] [PubMed] [Google Scholar]
- Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 2001;155:315–322. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- Lucki I, O'Leary OF. Distinguishing roles for norepinephrine and serotonin in the behavioral effects of antidepressant drugs. J Clin Psychiatry. 2004;65(Suppl 4):11–24. [PubMed] [Google Scholar]
- Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Methods. 2004;140:169–181. doi: 10.1016/j.jneumeth.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Molina-Hernandez M, Tellez-Alcantara NP. Antidepressant-like actions of pregnancy, and progesterone in Wistar rats forced to swim. Psychoneuroendocrinology. 2001;26:479–491. doi: 10.1016/s0306-4530(01)00007-5. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Damsma G, Wenkstern D, Fibiger HC. In vivo characterization of locally applied dopamine uptake inhibitors by striatal microdialysis. Synapse. 1990;6:106–112. doi: 10.1002/syn.890060113. [DOI] [PubMed] [Google Scholar]
- Page ME, Detke MJ, Dalvi A, Kirby LG, Lucki I. Serotonergic mediation of the effects of fluoxetine, but not desipramine, in the rat forced swimming test. Psychopharmacology (Berl) 1999;147:162–167. doi: 10.1007/s002130051156. [DOI] [PubMed] [Google Scholar]
- Papp M, Willner P, Muscat R. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl) 1991;104:255–259. doi: 10.1007/BF02244188. [DOI] [PubMed] [Google Scholar]
- Porsolt RD. Animal model of depression. Biomedicine. 1979;30:139–140. [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Prange AJ., Jr. The Pharmacology And Biochemistry Of Depression. Dis Nerv Syst. 1964;25:217–221. [PubMed] [Google Scholar]
- Randrup A, Munkvad I, Fog R, Gerlach J, Molander L, Kjellberg B, Scheel-Kruger J. Mania, Depression and brain dopamine. In: Essman WB, Vazelli L, editors. Current Developments in Pyschpharmacology. vol.2. Spectrum; New York: 1975. pp. 206–248. [Google Scholar]
- Renard CE, Fiocco AJ, Clenet F, Hascoet M, Bourin M. Is dopamine implicated in the antidepressant-like effects of selective serotonin reuptake inhibitors in the mouse forced swimming test? Psychopharmacology (Berl) 2001;159:42–50. doi: 10.1007/s002130100836. [DOI] [PubMed] [Google Scholar]
- Ripoll N, David DJ, Dailly E, Hascoet M, Bourin M. Antidepressant-like effects in various mice strains in the tail suspension test. Behav Brain Res. 2003;143:193–200. doi: 10.1016/s0166-4328(03)00034-2. [DOI] [PubMed] [Google Scholar]
- Schildkraut JJ. The catecholamine hypothesis of affective disorders. A review of supporting evidence. Int J Psychiatry. 1967;4:203–217. [PubMed] [Google Scholar]
- Shelton RC. The dual-action hypothesis: does pharmacology matter? J Clin Psychiatry. 2004;65(Suppl 17):5–10. [PubMed] [Google Scholar]
- Shen HW, Hagino Y, Kobayashi H, Shinohara-Tanaka K, Ikeda K, Yamamoto H, Yamamoto T, Lesch KP, Murphy DL, Hall FS, Uhl GR, Sora I. Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacology. 2004;29:1790–1799. doi: 10.1038/sj.npp.1300476. [DOI] [PubMed] [Google Scholar]
- Shumake J, Barrett D, Gonzalez-Lima F. Behavioral characteristics of rats predisposed to learned helplessness: reduced reward sensitivity, increased novelty seeking, and persistent fear memories. Behav Brain Res. 2005;164:222–230. doi: 10.1016/j.bbr.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, Fujiwara RA. The activity of pramipexole in the mouse forced swim test is mediated by D2 rather than D3 receptors. Psychopharmacology (Berl) 2004;175:163–169. doi: 10.1007/s00213-004-1809-7. [DOI] [PubMed] [Google Scholar]
- Skolnick P, Popik P, Janowsky A, Beer B, Lippa AS. “Broad spectrum” antidepressants: is more better for the treatment of depression? Life Sci. 2003;73:3175–3179. doi: 10.1016/j.lfs.2003.06.007. [DOI] [PubMed] [Google Scholar]
- Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, Wichems C, Lesch KP, Murphy DL, Uhl GR. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci U S A. 2001;98:5300–5305. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, Wichems C, Lesch KP, Murphy DL, Uhl GR. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci U S A. 2001;98:5300–5305. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, Lesch KP, Murphy DL, Uhl GR. Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc Natl Acad Sci U S A. 1998;95:7699–7704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielewoy C, Roubert C, Hamon M, Nosten-Bertrand M, Betancur C, Giros B. Behavioural disturbances associated with hyperdopaminergia in dopamine-transporter knockout mice. Behav Pharmacol. 2000;11:279–290. doi: 10.1097/00008877-200006000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag HM. Toward a biochemical classification of depression. Adv Biochem Psychopharmacol. 1974;11:357–368. [PubMed] [Google Scholar]
- Vaugeois JM, Pouhe D, Zuccaro F, Costentin J. Indirect dopamine agonists effects on despair test: dissociation from hyperactivity. Pharmacol Biochem Behav. 1996;54:235–239. doi: 10.1016/0091-3057(95)02131-0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Learned-Coughlin S, Yang J, Logan J, Schlyer D, Gatley JS, Wong C, Zhu W, Pappas N, Schueller M, Jayne M, Carter P, Warner D, Ding YS, Shea C, Xu Y. The slow and long-lasting blockade of dopamine transporters in human brain induced by the new antidepressant drug radafaxine predict poor reinforcing effects. Biol Psychiatry. 2005;57:640–646. doi: 10.1016/j.biopsych.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Wang YM, Xu F, Gainetdinov RR, Caron MG. Genetic approaches to studying norepinephrine function: knockout of the mouse norepinephrine transporter gene. Biol Psychiatry. 1999;46:1124–1130. doi: 10.1016/s0006-3223(99)00245-0. [DOI] [PubMed] [Google Scholar]
- Willner P. Dopamine and depression: a review of recent evidence. I. Empirical studies. Brain Res. 1983a;287:211–224. doi: 10.1016/0165-0173(83)90005-x. [DOI] [PubMed] [Google Scholar]
- Willner P. Dopamine and depression: a review of recent evidence. II. Theoretical approaches. Brain Res. 1983b;287:225–236. doi: 10.1016/0165-0173(83)90006-1. [DOI] [PubMed] [Google Scholar]
- Willner P. Dopamine and depression: a review of recent evidence. III. The effects of antidepressant treatments. Brain Res. 1983c;287:237–246. doi: 10.1016/0165-0173(83)90007-3. [DOI] [PubMed] [Google Scholar]
- Willner P. Dopaminergic mechanisms in depression and mania. In: Watson, editor. Psychopharmacology: The Fourth Generation of Progress. On-Line edition Lippincott Williams & Wilkins; New York: 2000. [Google Scholar]
- Wintink AJ, Young NA, Davis AC, Gregus A, Kalynchuk LE. Kindling-induced emotional behavior in male and female rats. Behav Neurosci. 2003;117:632–640. doi: 10.1037/0735-7044.117.3.632. [DOI] [PubMed] [Google Scholar]
- Xu F, Gainetdinov RR, Wetsel WC, Jones SR, Bohn LM, Miller GW, Wang YM, Caron MG. Mice lacking the norepinephrine transporter are supersensitive to psychostimulants. Nat Neurosci. 2000;3:465–471. doi: 10.1038/74839. [DOI] [PubMed] [Google Scholar]