Abstract
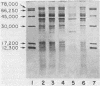
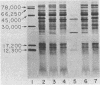
We evaluated the role of pili in the pathogenesis of disease due to Haemophilus influenzae type b (HiTb), using the infant rat model. Piliated and nonpiliated HiTb strains were isolated from the nasopharynx and cerebrospinal fluid, respectively, of three children. Infant rats inoculated intranasally with nonadherent HiTb developed bacteremia and meningitis more frequently (P = 0.005) than animals inoculated with companion adherent HiTb strains. When analyzed separately, only one HiTb pair (884/880) demonstrated significant differences in the incidence of bacteremia and meningitis between the adherent and nonadherent strains. Blood or cerebrospinal isolates recovered from infant rats inoculated with piliated adherent HiTb strains were not piliated and were not adherent in vitro. Adherent and nonadherent HiTb colonized the nasopharynx of infant rats equally. The piliated strains of HiTb were not adherent in vivo or in vitro to rat nasal or buccal epithelial cells, respectively. Piliated strains of HiTb have no apparent advantage over nonpiliated HiTb strains for colonization or invasion of infant rats. Furthermore, the loss of piliation is noted for cerebrospinal fluid, blood, and nasal isolates of HiTb cultured from infant rats inoculated with an adherent piliated HiTb strain. Thus, the loss or suppression of pili may be an important prerequisite for the invasion of the host by HiTb strains that are highly piliated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B., Eriksson B., Falsen E., Fogh A., Hanson L. A., Nylén O., Peterson H., Svanborg Edén C. Adhesion of Streptococcus pneumoniae to human pharyngeal epithelial cells in vitro: differences in adhesive capacity among strains isolated from subjects with otitis media, septicemia, or meningitis or from healthy carriers. Infect Immun. 1981 Apr;32(1):311–317. doi: 10.1128/iai.32.1.311-317.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson M., Medalia O., Schori L., Mirelman D., Sharon N., Ofek I. Prevention of colonization of the urinary tract of mice with Escherichia coli by blocking of bacterial adherence with methyl alpha-D-mannopyranoside. J Infect Dis. 1979 Mar;139(3):329–332. doi: 10.1093/infdis/139.3.329. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Craven D. E., Peppler M. S., Frasch C. E., Mocca L. F., McGrath P. P., Washington G. Adherence of isolates of Neisseria meningitidis from patients and carriers to human buccal epithelial cells. J Infect Dis. 1980 Oct;142(4):556–568. doi: 10.1093/infdis/142.4.556. [DOI] [PubMed] [Google Scholar]
- Guerina N. G., Langermann S., Clegg H. W., Kessler T. W., Goldman D. A., Gilsdorf J. R. Adherence of piliated Haemophilus influenzae type b to human oropharyngeal cells. J Infect Dis. 1982 Oct;146(4):564–564. doi: 10.1093/infdis/146.4.564. [DOI] [PubMed] [Google Scholar]
- Halsey N. A., Korock C., Johansen T. L., Glode M. P. Intralitter transmission of haemophilus influenzae type b in infant rats and rifampin eradication of nasopharyngeal colonization. J Infect Dis. 1980 Nov;142(5):739–743. doi: 10.1093/infdis/142.5.739. [DOI] [PubMed] [Google Scholar]
- Harber M. J., Chick S., Mackenzie R., Asscher A. W. Lack of adherence to epithelial cells by freshly isolated urinary pathogens. Lancet. 1982 Mar 13;1(8272):586–588. doi: 10.1016/s0140-6736(82)91749-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lampe R. M., Mason E. O., Jr, Kaplan S. L., Umstead C. L., Yow M. D., Feigin R. D. Adherence of Haemophilus influenzae to buccal epithelial cells. Infect Immun. 1982 Jan;35(1):166–172. doi: 10.1128/iai.35.1.166-172.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leunk R. D., Moon R. J. Association of type 1 pili with the ability of livers to clear Salmonella typhimurium. Infect Immun. 1982 Jun;36(3):1168–1174. doi: 10.1128/iai.36.3.1168-1174.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon E. R., Ostrow P. T. Haemophilus influenzae meningitis in infant rats: role of bacteremia in pathogenesis of age-dependent inflammatory responses in cerebrospinal fluid. J Infect Dis. 1977 Feb;135(2):303–307. doi: 10.1093/infdis/135.2.303. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Bacterial adherence. Adv Intern Med. 1980;25:503–532. [PubMed] [Google Scholar]
- Ofek I., Mosek A., Sharon N. Mannose-specific adherence of Escherichia coli freshly excreted in the urine of patients with urinary tract infections, and of isolates subcultured from the infected urine. Infect Immun. 1981 Dec;34(3):708–711. doi: 10.1128/iai.34.3.708-711.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichichero M. E., Loeb M., Anderson, Smith D. H. Do pili play a role in pathogenicity of Haemophilus influenzae type B? Lancet. 1982 Oct 30;2(8305):960–962. doi: 10.1016/s0140-6736(82)90161-1. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Hemagglutination by purified type I Escherichia coli pili. J Exp Med. 1977 Nov 1;146(5):1169–1181. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Morton G. Adherence of Neisseria meningitidis to human epithelial cells. Infect Immun. 1981 Jan;31(1):430–435. doi: 10.1128/iai.31.1.430-435.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinger D. S., Reed W. P. Pneumococcal adherence to human epithelial cells. Infect Immun. 1979 Feb;23(2):545–548. doi: 10.1128/iai.23.2.545-548.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Dreyer J. S., Schauer S. Effect of pili on susceptibility of Escherichia coli to phagocytosis. Infect Immun. 1979 Apr;24(1):218–223. doi: 10.1128/iai.24.1.218-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens D. S., Edwards K. M., Morris F., McGee Z. A. Pili and outer membrane appendages on Neisseria meningitidis in the cerebrospinal fluid of an infant. J Infect Dis. 1982 Oct;146(4):568–568. doi: 10.1093/infdis/146.4.568. [DOI] [PubMed] [Google Scholar]
- Stephens D. S., McGee Z. A. Attachment of Neisseria meningitidis to human mucosal surfaces: influence of pili and type of receptor cell. J Infect Dis. 1981 Apr;143(4):525–532. doi: 10.1093/infdis/143.4.525. [DOI] [PubMed] [Google Scholar]
- Tramont E. C., Sadoff J. C., Boslego J. W., Ciak J., McChesney D., Brinton C. C., Wood S., Takafuji E. Gonococcal pilus vaccine. Studies of antigenicity and inhibition of attachment. J Clin Invest. 1981 Oct;68(4):881–888. doi: 10.1172/JCI110343. [DOI] [PMC free article] [PubMed] [Google Scholar]