Abstract
Limited knowledge about human oligodendrogenesis prompted us to explore the lineage relationship between cortical radial glia (RG) cells and oligodendrocytes in the human fetal forebrain. RG cells were isolated from cortical ventricular/subventricular zone and their progeny was followed in vitro. One portion of RG cells differentiated into cells of oligodendrocyte lineage identified by cell-type specific antibodies, including PDGFRα, NG2, O4, MBP and MOG. Moreover, using Cre Lox fate mapping (BLBP-Cre/Floxed-YFP) we established a direct link between RG cells and oligodendrocyte progenitors. In vitro generation of RG-derived O4+ oligodendrocytes progenitors was enhanced by addition of Sonic hedgehog (SHH) and reduced by SHH inhibitor-cyclopamine, suggesting the role of SHH-signaling in this process. In summary, our in vitro experiments revealed that a portion of cortical RG cells isolated from human forebrain at the second trimester of gestation generate oligodendrocyte progenitors and suggest a role of SHH in this process.
Keywords: oligodendrocyte progenitors, myelination, primate brain development, LeX immunocytochemistry, transfection, Cre-LoxP fate mapping
INTRODUCTION
Oligodendrocytes (OLs) are necessary for myelination of axons in the central nervous system, and thus essential for proper brain function. In rodents, most OLs are generated during late embryogenesis and early postnatal life (Kessaris et al., 2006; Pringle and Richardson, 1993; Rowitch, 2004). Several well established markers are used to demonstrate gradual differentiation of OL lineage, from early oligodendrocyte progenitor cells (OPCs), immunolabeled by chondroitin sulfate proteoglycan (NG2 or Cspg4) and platelet-derived growth factor receptor-alpha (PDGFRα), to late OPCs labeled with O4 antibody, and finally to premyelinating and myelinating OLs expressing myelin proteins, MBP (myelin basic protein) and MOG (myelin oligodendrocyte glycoprotein) (Hardy and Reynolds, 1991; Pfeiffer et al., 1993).
Several cell types are described as the initial oligodendrocyte progenitors: common oligodendrocyte and astrocyte progenitors (2A–O cells) (Raff et al., 1983), glial-restricted precursors (Gregori et al., 2002; Rao et al., 1998), a common neuronoligodendrocyte progenitor in the ventral spinal cord (Richardson et al., 2000; Rowitch et al., 2002), and adult subventricular zone (Menn et al., 2007). Ventral origin of OLs in the spinal cord depends critically on the morphogene SHH (Pringle et al., 1996; Miller et al., 1999; Spassky et al., 2001). However, SHH-independent oligodendrogenesis from dorsal progenitors has been reported both in vivo (Cai et al., 2005; Kessaris et al., 2006; Vallstedt et al., 2005) and in vitro (Chandran et al., 2004; Kessaris et al., 2004; Nery et al., 2001).
Lately, radial glia (RG) cells were demonstrated to be progenitor cells for cortical pyramidal neurons in rodents (Malatesta et al., 2000; Miyata et al., 2001; Noctor et al., 2001) as well as in humans (Mo et al., 2007a). In rodents, RG cells were also suggested to differentiate into OLs (Casper and McCarthy, 2006; Fogarty et al., 2005; Malatesta et al., 2003). It has been demonstrated that the progeny of dorsal RG include oligodendrocytes by anatomical fate-mapping strategy in postnatal mice (Ventura and Goldman, 2007). This lineage relationship, however, was not reported in humans.
In the human forebrain, oligodendrocyte progenitors originate both in the ventral telencephalon (ganglionic eminence) and in the cortical subventricular zone (Rivkin et al., 1995; Back et al., 2001; Ulfig et al., 2002; Rakic and Zecevic, 2003; Jakovcevski and Zecevic, 2005a,b). We previously labeled human RG on fetal brain cryosections with GFAP (glial fibrillary acidic protein), BLBP (brain lipid binding protein) and vimentin (Howard et al., 2006; Zecevic et al., 1999; Zecevic, 2004), in agreement with studies in primates (Cameron and Rakic, 1991; Dahl et al., 1981; Kadhim et al., 1988; Levitt et al., 1981). Occasionally, however, human RG cells can be co-labeled with markers of early OL progenitors (Jakovcevski and Zecevic, 2005b; Mo and Zecevic, 2007a). These results suggested a lineage relationship of RG and OL cell populations, but direct proof of this relationship has not been established. We now demonstrate that in vitro human cortical RG may generate a subpopulation of OL lineage cells. Together with our previous report (Mo et al., 2007a,b) this study suggests that human RG cells could generate all three neural cell types: astrocytes, cortical neurons, and oligodendrocytes. Partial results of this study were presented in the abstract form (Mo and Zecevic, 2007b).
MATERIALS AND METHODS
Human Fetal Brain Tissue
Human fetuses (n=7), ranging in age from 15 to 21 gestational weeks (gw, 15gw, n=1; 16gw, n=1; 17gw, n=2; 19gw, n=1; 20gw, n= 1; 21gw, n=1)., were obtained from the Tissue Repository of The Albert Einstein College of Medicine (Bronx, NY) with proper parental consent and the approval of the Ethics Committees. Brain tissue was collected in oxygenized Hank’s Balanced Salt Solution (HBSS, Invitrogen, Carlsbad, CA) and transported on ice. Dissociated cell cultures were prepared from the ventricular and the subventricular zones (VZ/SVZ) of the fetal forebrain as described before (Zecevic et al., 2005).
Immunopanning and cell cultures
We used immunopanning with a surface marker LeX, to enrich human RG cells according to a procedure described earlier (Mo et al., 2007a). In short, 100mm tissue culture dishes were pre-coated with secondary antibody [(goat anti-mouse IgM, 10 µg/ml in 5 ml Tris (50 mM, pH 9.5), SouthernBiotech, Birmingham, AL)] overnight at 4°C . The next day dishes were rinsed with phosphate-buffered-saline (PBS), incubated with 5 ml of anti- LeX antibody (1:100, Lab Vision, Fremont, CA) in PBS with 0.2% BSA at room temperature for 2 h, followed by another PBS wash. The dissociated cells (107) suspended in 10 ml DMEM/F12/N27 medium (Invitrogen) supplemented with 10 ng/ml of basic fibroblast growth factor (FGF2, Peprotech, Rocky Hill, NJ), were incubated on the anti-LeX coated dishes for 20 minutes at room temperature with gentle agitation. Thereafter, the non-adherent cells were removed by rinsing, whereas adhered LeX+ cells were detached from the dish with trypsin-EDTA (Invitrogen), counted, plated on 12mm pre-coated poly-L-lysine cover slips (Carolina Biological Supply, Burlington, NC). The purity of immunopanned cells was initially 95%. The cells were cultured in the expansion medium (DMEM/F12/N2, 10 ng/ml FGF2) up to 7 days. Subsequently cells were transferred to a differentiation medium (DMEM/F12/N2, without FGF2) and kept up to 21 days in culture. Cultures were then treated with either SHH (N-terminal SHH, 200ng/ml, Abcam, Cambridge, MA,) or its receptor inhibitor, cyclopamine (Biomol, Plymouth meeting, PA; 1 µM), and incubated for 7 days (7 div).
Plasmids and LeX+ Cell Transfection
To selectively label RG cells and trace their progeny, we transfected RG cells either with enhanced green fluorescent protein (EGFP) under RG specific promoter brain lipid binding protein (BLBP; Schmid et al., 2006), or with BLBP-Cre and Floxed yellow fluorescent protein (YFP) plasmids (gifts from K. Ikenaka and J. Li). LeX+ cells were cultured onto 12 mm coverslips in the expansion medium for 3 div, and then transfected or co-transfected with plasmids using lipofectamine 2000 (Invitrogen) according to manufacturer’s protocol
Immunostaining
Cultures of human fetal brain cells were fixed in 4% paraformaldehyde, and stained with following antibodies diluted in blocking solution [1% bovine serum albumin, 5% normal goat serum, and 0.5% Tween-20 in PBS]: anti-BLBP (1: 2,000, gift, N. Heintz, The Rockefeller University), anti-vimentin (1:1,000, Sigma, St. Louis, MO, USA), anti-GFAP (1:25, Dako, Carpinteria, CA), anti-PDGFRα (Pharmingen, San Diego, CA, 1:25), anti-NG2 (Chemicon, Tamecula, CA, 1:100), anti-Olig1 or anti-Olig2 (gift Ch. Stiles, 1:5,000), anti-SHH (Neuromics, Bloomington, MN, 1: 10), anti-Patched1 (Ptc1, Abnova, Taiwan, 1:100), and O4 antibody (gift S. Pfeiffer, 1:25). In the case of double staining for a surface marker O4, cells were first immunostained with O4 antibody and then fixed briefly before another primary antibody was applied. Premyelinating OLs were identified by antibodies to MBP (mouse, 1:100, Covance, Emeryville, CA) and MOG (mouse, gift S. Pfeiffer, 1:25). A positive immunoreaction in all cases was accompanied by typical OL morphology (Pfeiffer et al., 1993). Primary antibodies were applied overnight at 4°C, followed by corresponding secondary antibodies (Jackson ImmunoResearch Lab., West Grove, PA) for 2 hours, and a short incubation in a nuclear stain bisbenzamide (Sigma). Coverslips were viewed with the Carl Zeiss Axioplan microscope.
Terminal uridine deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
Apoptosis in cell cultures was determined by “In Situ Cell Death Detection kit”, according to manufacturer’s instructions (Roche, Germany). Identification of apoptotic nuclei was done using terminal deoxynucleotidyltransferase (TdT) enzymatic reaction for the incorporation of rhodamine-labeled nucleotides into DNA strand breaks in situ.
Cell counting and statistical analysis
Cells co-stained with the nuclear stain bisbenzamide (Sigma) and various cellular markers were visualized with Zeiss fluorescence microscope using Axiovision software and photographed with a digital camera. Ten predesignated adjacent optical fields of view were examined at magnification 10x (0.5mm2 surface area), counts of immunolabled cells were pooled together, expressed as means ± SEMs and analyzed using t-test or Chi-squared ( χ2 ) test. The criterion for significance was set at 5%.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated using Trizol reagent (Invitrogen) according to manufacturer’s instructions. Identical amounts of RNA were reverse transcribed into cDNA, which was subsequently amplified by the polymerase chain reaction (PCR) with different primers, SHH - forward: 5'-ccaattacaaccccgacatc-3', reverse: 5'-ccgagttctctgctttcacc-3' (339bp); Ptc1 – forward, 5'-gcttcccgtgcttttgtctt-3', reverse, 5'-tcctgcagctcaatgacttc-3' (792bp); Smoothened (Smo)- forward, 5′-caccctggccacattcgt-3′, reverse-5′-aagtgtgccaggcataggt-3’ (284bp); GAPDH - forward, 5’-ggtgaaggtcggagtcaacgga-3’, reverse: 5’tcttccaggagcgagatcc ctc3’(240bp). Thirty five cycles of amplification were performed in a Peltier Thermal Cycler (PTC-100, MJ Research).
RESULTS
1.The expression of OPC markers by RG cells
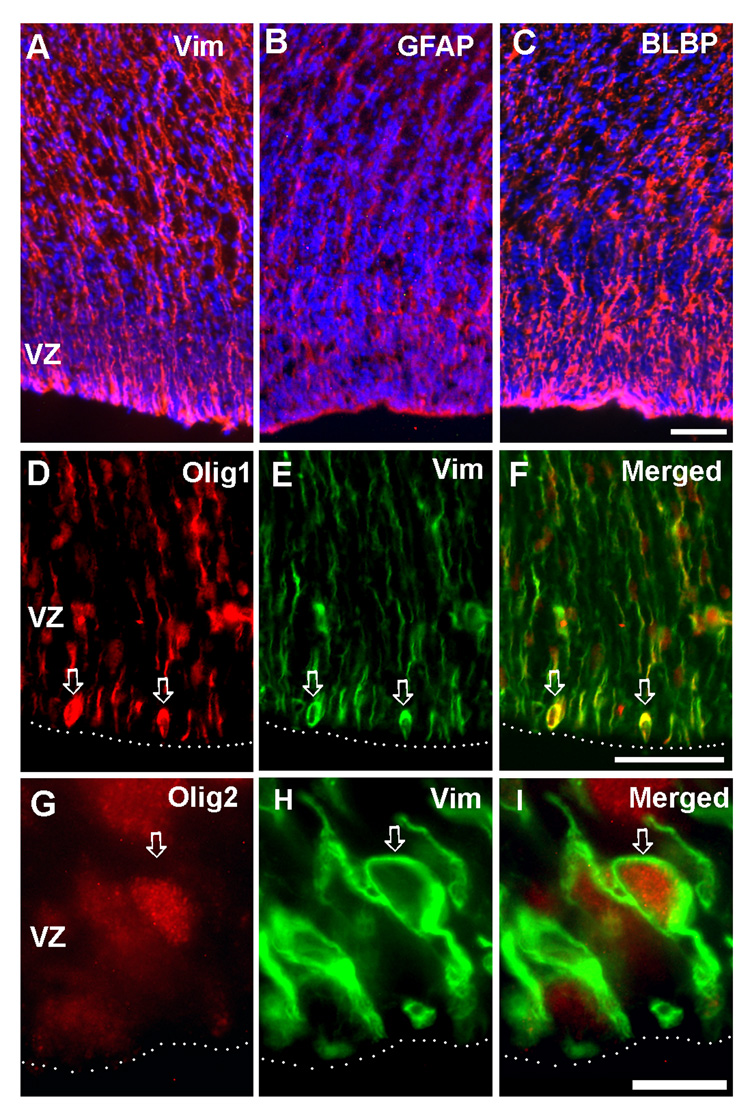
At 16 gw telencephalon, long, radial fibers labeled with typical immunomarkers of RG, vimentin (Fig.1A), GFAP (Fig. 1B) and BLBP (Fig.1C), extended from the ventricular surface towards pia, as previously reported for the embryonic (Zecevic, 2004) and fetal forebrain (Howard et al., 2006, Howard et al., 2008). Double-labeling experiments on cryosections of fetal forebrain demonstrated that vimentin and transcription factors Olig 1 (Fig. 1D–F) and Olig 2 (Fig. 1G–I) could be co-expressed in around 1% of cells of the cortical ventricular zone (Fig. 1D–F). The presence of these double-labeled cells might suggest transition from RG cells to OL lineage. In the same gestational age studied here, oligodendrocyte progenitors in various stages of differentiation were reported in the VZ/SVZ and emerging white matter (Jakovcevski and Zecevic, 2005a,b).
Fig. 1. Co-expression of RG and oligodendrocyte progenitors markers in vivo.
On cryosections of 15 g.w. fetal brain, in the cortical ventricular/ subventricular zones (VZ/SVZ), A) Vimentin+ B) GFAP+, and C) BLBP+ cells have typical mophorlogy of RG cells; Vimentin+ (Vim) RG cells on the ventricular surface can be co-labeled with D–F) Olig1 (arrows), and G–I) Olig2 (arrow). Dashed line marks ventricular surface. Scale bars: A–C, 50µm; D–F, 50µm; G–I) 10µm.
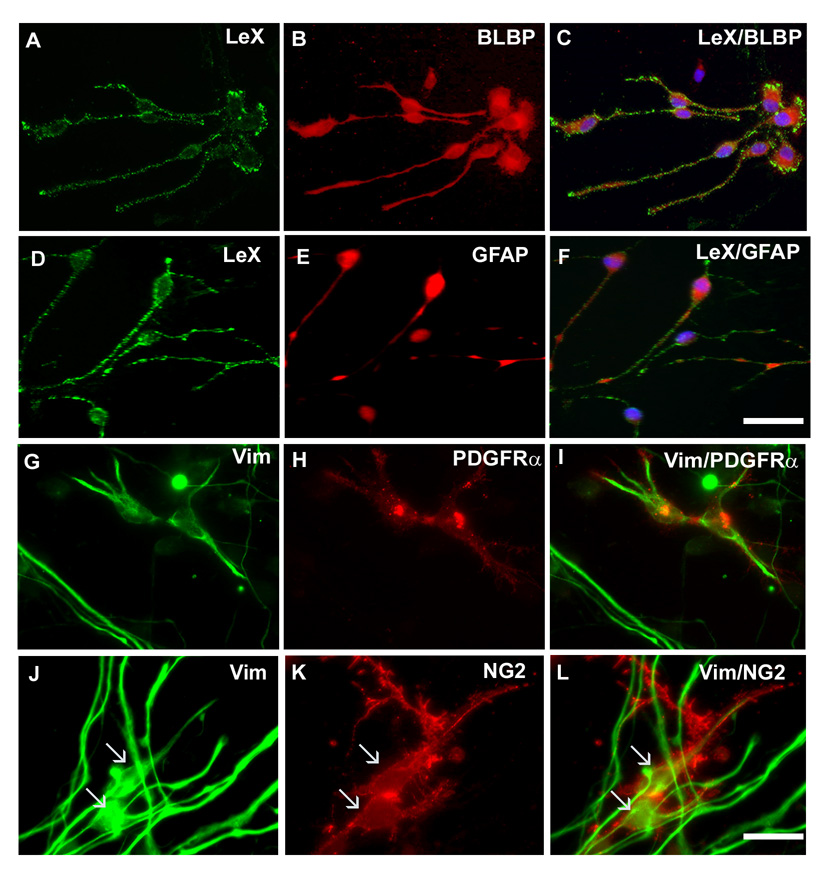
To study whether indeed RG cells generate cells of OL lineage, we followed cell fate of RG cells enriched from the cortical VZ/SVZ of 16–19 g.w. fetal brains (n=4). We used a previously described method of immunopanning with LeX antibody to enrich RG cells in vitro (Mo et al., 2007a). Majority of the LeX+ cells (95%) grown in expansion medium can be co-labeled with RG markers, BLBP (Fig. 2A–C), vimentin (not shown) and GFAP (Fig. 2D–F). High doses of FGF2 in the medium have been shown to have proliferative effect on LeX+ cells but did not change their identity up to 7 days in culture (Mo et al., 2007a).
Fig. 2. Co-expression of RG and oligodendrocyte progenitors markers in vitro.
In the expansion medium, the majority of LeX+ cells are co-labeled with RG markers: A–C) BLBP and D–F) GFAP. Some cells co-express vimentin and G–I) PDGFRα or J–L) NG2 (arrows). Scale bars, A–I: 25 µm; G–L: 10µm.
After 3 div in the expansion medium, among isolated cells labeled with vimentin, 19±1.8% co-expressed early OL progenitor markers, PDGFRα (Fig. 2G–I), 16.2±1.9% Olig1 (not shown), and 14.1±1.5% NG2 (Fig. 2J–L). PDGFRα and NG2 cell populations are partially overlapping in the SVZ at this fetal age (Jakovcevski and Zecevic, 2005a). Notably, less than 0.1% of cells were labeled only with NG2 or PDGFRα and not co-labeled with vimentin. Moreover, O4 or MBP antibodies, markers of more differentiated forms of oligodendrocyte progenitors, did not appear in cell cultures at 3 div. Thus, initially LeX+ RG cells were the main progenitor cell type in our cultures, whereas the amount of OL restricted progenitors was negligible. Cells that expressed only vimentin may represent astroglial or neuronal progenitors (Mo et al., 2007a).
2. Radial Glia cells generate oligodendrocytes in vitro
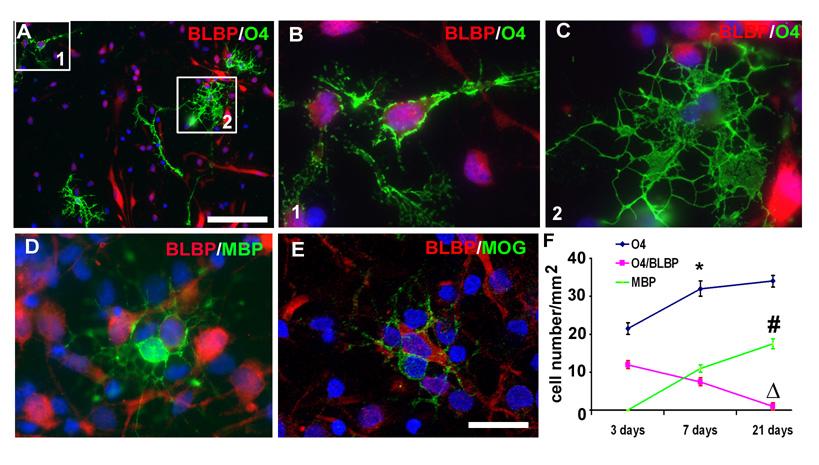
When culture medium was changed from expansion (with FGF-2) to differentiation medium (without the FGF2), the situation changed dramatically. After 3 days in the differentiation medium double-immunolabeling with astroglial marker BLBP and O4 antibody, revealed a large number of BLBP+/O4− cells, which is consistent with their astrocyte linage, but also a subpopulation of BLBP+/O4+ cells (23.1±2.5 cells/mm2). Closer analysis of the O4+ population of cells revealed that they consisted of either immature looking cells often co-labeled with BLBP (Fig. 3A, B) or more mature looking cells with multiple branches, which were not co-labeled with BLBP antibody (Fig. 3A, C). Double labeled BLBP+/O4+ cells might represent RG cells differentiating into oligodendrocytes. With longer period of differentiation, the number of O4+ cells increased by 34%, from 23.1±2.5 cells/mm2 at 3 div to 31.2±2.7 cells/mm2 at 7 div, whereas the number of double labeled BLBP+/O4+ cells during the same time period decreased by 42%, from 12.4±1.1 cells/mm2 to 7.1±0.5 cells/mm2 (p<0.05) (Fig. 3F). Cells labeled with either MBP or MOG antibodies were observed for the first time after 7 div differentiation, and these cells did not co-express BLBP, probably due to its down-regulation with the progression along OLs differentiation pathway. The number of MBP+ and MOG+ cells further increased after 21 div of differentiation (Fig. 3D–F). During two weeks period, from 7 to 21 div, the number of MBP+ cells increased by almost 60%, from 10.5±1.2 cells/mm2 to 17.2±1.6 cells/mm2 (p < 0.05, Fig. 3F).
Fig. 3. Progression of cultured RG cells through oligodendrocytes differentiation steps.
A–C) LeX+ cells maintained in differentiation medium for 7 div generate O4+ (green) cells. Boxed areas (1, 2) are presented in B and C. B) Immature looking O4+ cells co-labeled with BLBP. C) O4+ cells with a more mature, branched morphology are not co-labeled with BLBP. D, E) After 21 div in differentiation medium, a number of D) MBP+ (green) and E) MOG+ (green) cells are present, but are never co-labeled with BLBP (red). Blue-bisbenzamide nuclear stain. F) Number of the immuno-positive cells per surface area (mm2) at 3, 7 and 21 div in a differentiation medium; * and △, significant difference compared with the values at 3 div; # compared to 7 div (n-4). Scale bars: D: 50 µm; B–E: 10 µm.
On the other hand, number of O4+ cells did not significantly change in the same time period, from 31.2±2.7 cells/mm2 to 34.6±2.4 cells/mm2 (p > 0.05) (Fig. 3F), in contrast to a sharp increase seen in the previous period, from 3 to 7 div. This suggests that the capacity of RG cells to generate oligodendrocyte progenitors decreases over time in culture. Simultaneously, the BLBP+ cells seem to terminally differentiate into astrocytes, as seen by the change of their morphology into large and flat cells (Fig. 3D). The relative small increase of O4+ cells in these cultures might also be due to their progressive differentiation into mature OLs (MBP+ or MOG+ cells).
3. Selective labeling of RG cells: Plasmid transfection
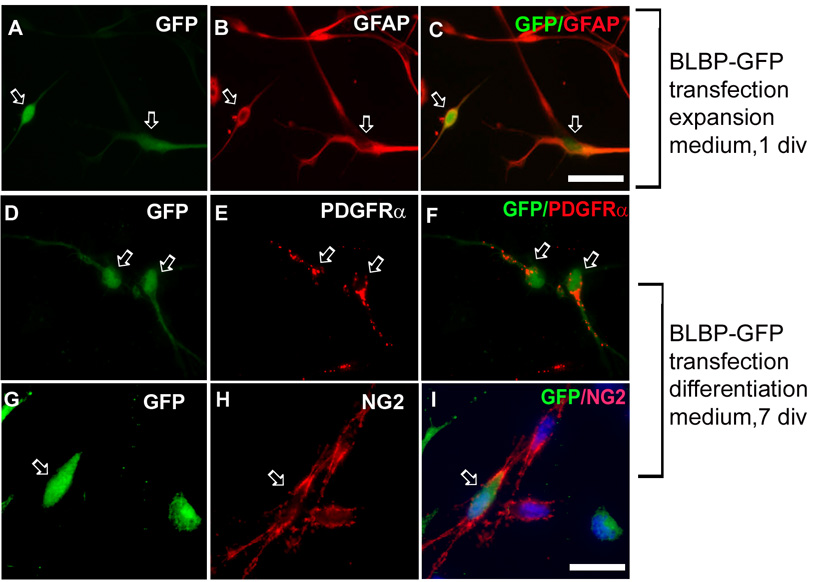
To confirm that we followed the progeny of RG cells and not that of a small number (0.1%) of oligodendrocyte restricted progenitors initially present in LeX+ cultures, we selectively labeled RG cells by transfecting them with a BLBP-EGFP plasmid specific for RG cells (Schmid et al., 2006; Mo et al., 2007a). 24 hours after transfection, almost all transfected (green) cells were co-labeled with RG markers, GFAP (Fig. 4A–C) and BLBP, but not with OPCs markers (not shown), suggesting specific transfection of RG cells. After 7 days of differentiation, however, a small percentage of green cells, was co-labeled with early OPCs markers, PDGFRα (Fig. 4D–F) or NG2 (Fig. 4G–I), but not with markers of more advanced OL differentiation, such as O4 or MBP (not shown). We speculate that the lack of green signal in more differentiated OL lineage cells (O4+, MBP+) was due to down-regulation of BLBP promoter activity with the progression of OL differentiation. The earlier observed BLBP+/O4+ cells (Fig.3) express BLBP protein for some time even after the promoter down regulation, whereas in this experiment the green signal critically depends on promoter activity and thus we could not see any EGFP+/O4+ cells. Note that some NG2+ cells were not green (Fig. 4 G–I) due to 10% transfection efficiency resulting in a large subpopulation of non-transfected RG cells.
Fig. 4. Specific transfection of RG cells.
Enriched LeX+ cells transfected with BLBP-EGFP plasmid. A–C) After 1 div in the expansion medium, transfected (green) cells are co-labeled with GFAP (arrows). After 7 div differentiation, a number of green cells are co-labeled with D–F) PDGFRα (red, arrows), and G–I) NG2 antibody (red, arrow). Blue - bisbenzamide nuclear stain. Scale bars: A–C: 20 µm; D–I: 10 µm
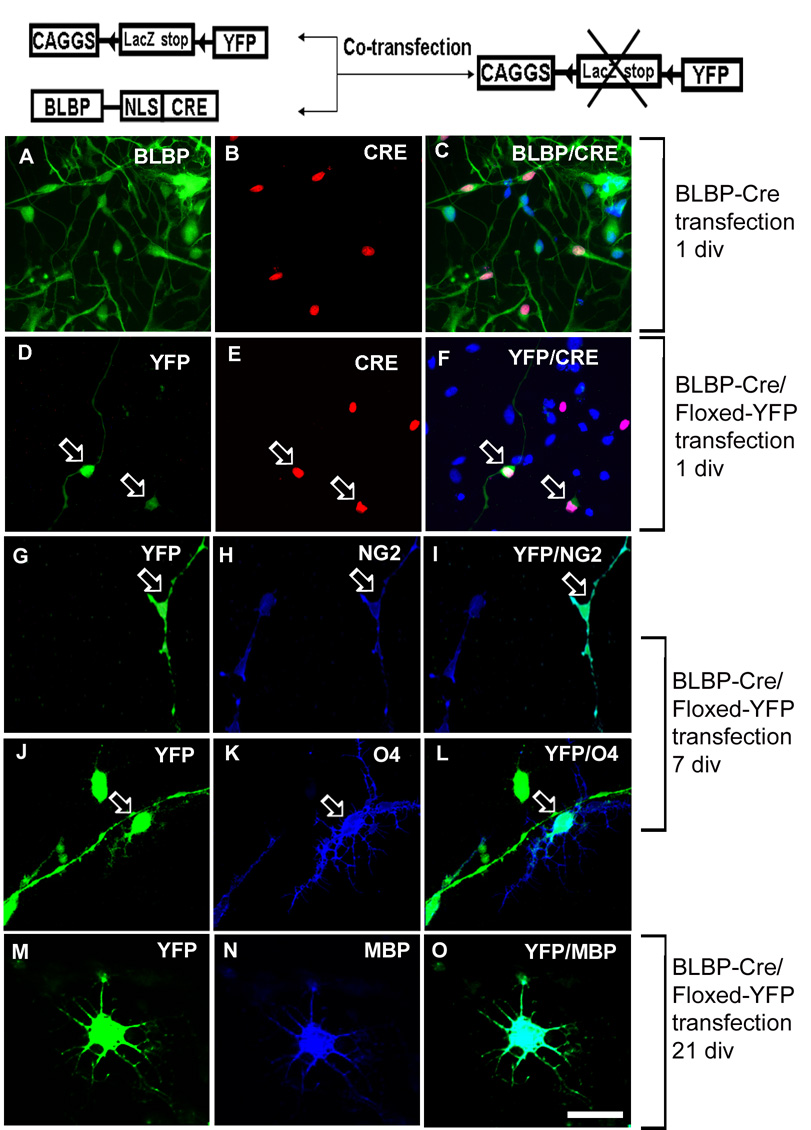
To establish a direct lineage relationship between RG and more mature OPCs, we used co-transfection of isolated LeX+ cells with the BLBP-Cre and Floxed-YFP under β-actin based promoter (Mo et al., 2007a). Only in cells that contain Cre the stop signal preceding YFP can be removed which results in YFP expression and green fluorescence (Fig. 5).
Fig. 5. Transfection of RG cells with BLBP-Cre/Floxed-YFP.
Schematic diagram of the pCAGGS-LoxP-LacZ-LoxP-YFP and BLBP-Cre plasmids. CAGGS is a β-actin based promoter; LoxP sites are indicated by triangles; NLS-nuclear localization sequence. A–C) One day after transfection, the CRE activity is only shown in BLBP+ cells, and D–F) YFP signal is detected exclusively in CRE+ cells (arrows). G–I) After 7div differentiation, transfected RG cell (green YFP+) give rise to NG2+ (blue, arrow) or J–L) O4+ cell (arrow). M–O) After 21 div, the YFP+ cell is co-labeled with MBP antibody (blue) and has a typical multi-branched morphology of a pre-myelinating oligodendrocyte. Scale bar: 20µm.
To confirm the specificity of BLBP-Cre, we performed a series of double labeling experiments with Cre/BLBP, Cre/PDGFRα or Cre/NG2 antibodies one day after transfection. The Cre activity was directed to RG cells by BLBP promoter and has been shown only in BLBP+ cells (Fig. 5A–C), and not in PDGFRα+ or NG2+ cells. Furthermore, YFP was expressed only in Cre+ cells (Fig. 5D–F) demonstrating specificity of BLBP promoter for RG cells. After 7 days of differentiation, 15.5±1.8% of the YFP transfected cells were co-labeled with NG2 (Fig. 5G–I) and 2.0±0.9% were co-labeled with O4 antibody (Fig. 5J–L). Longer culturing (21 div) resulted in differentiation of green cells into small number of MBP+ cells (Fig. 5M–O). Hence, the genetic fate-mapping experiments in vitro strongly suggest that a subset of human cortical RG cells may generate cells of OL lineage, including MBP+ premyelinating oligodendrocytes. The already mentioned low transfection efficiency limits us from conclusively stating the absolute number of RG cells that generate cells of OL lineage.
4. Is differentiation of RG cells into OL lineage sonic hedgehog dependent?
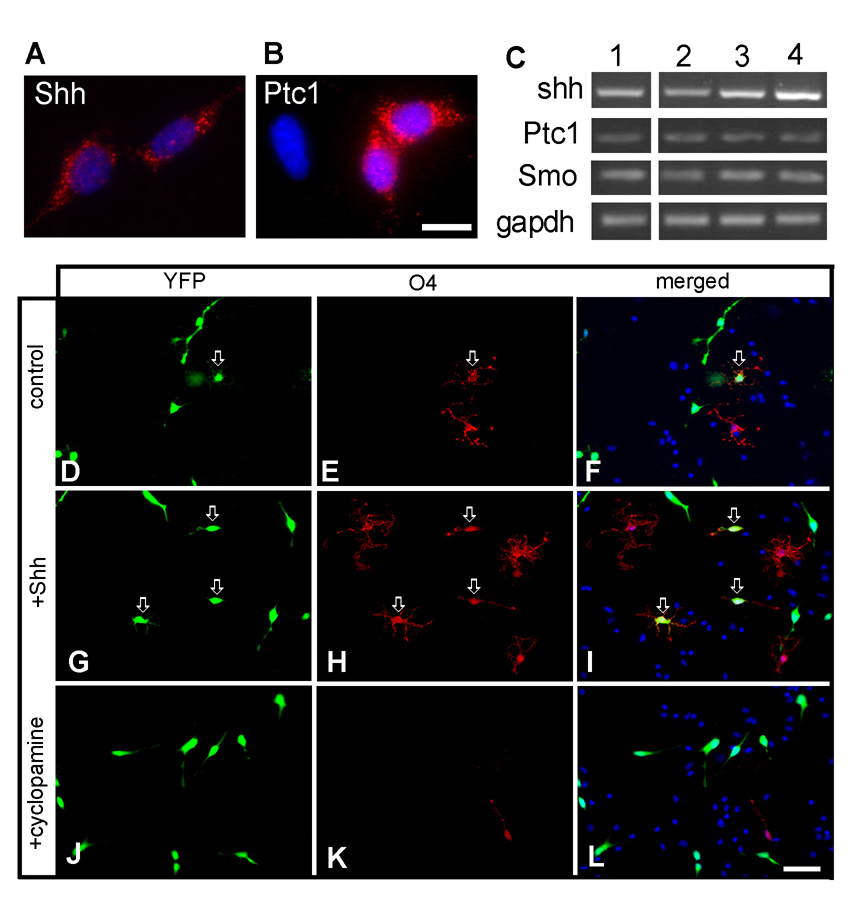
We further investigated the signaling requirements for the generation of OLs from a subset of cortical RG cells in vitro. Immunolabeling of cultured LeX+ cells revealed that SHH, a known regulator of OL lineage specification, was expressed by almost all cultured cells (Fig. 6A), whereas SHH receptor Patched1 (Ptc1) was expressed by 30% of cultured cells (122 Ptc+ cells/ of 406 counted) (Fig. 6B). In addition, the RT-PCR analyses of mRNAs isolated from cultured cortical LeX+ cells showed the expression of mRNAs for SHH, Ptc1, and smoothened (Smo), another SHH receptor (Fig. 6C). SHH mRNA expression was 2-folds lower in the cortical VZ/SVZ than in medial and lateral ganglionic eminence (MGE and LGE, Fig. 6C) suggesting regional specificity of SHH expression within the human fetal forebrain. When LeX+ cultures from 16 gw VZ/SVZ were maintained in differentiation medium (without FGF2) and treated with 200 ng/ml SHH for 7 div, the number of O4+ cells increased almost four times in comparison to non-treated cultures (Supplemental Fig.1). To study whether SHH treatment affects RG differentiation, we quantified the number of O4+ cells that originated from BLBP-Cre/Floxed-YFP co-transfected cells (O4/YFP). In this experiment cells were cultured for 7 div with either SHH (200ng/ml) or the SHH inhibitor, cyclopamine (1µM). In the control cultures (without any treatment), O4/YFP progenitors appeared within 7 div (Fig.6D–F), and their percentage increased 3.8 fold (from 2.1% to 7.9%) with the addition of SHH (Fig. 6G–I, M, p<0.01). Furthermore, when SHH signaling was blocked by cyclopamine (1µM) percentage of O4/YFP cells was reduced by more than 85% (Fig. 6J–L, M, p < 0.01). Cell death visualized by TUNEL method was not increased in these culture conditions (not shown). These results are consistent with both autocrine and paracrine function of SHH in generation of O4+ cells from a subpopulation of RG cells.
Fig. 6. The generation of oligodendrocyte progenitors from a subtype of RG cells is sonic hedgehog dependant.
LeX+ cells isolated from cortical VZ/SVZ of a 21 gw fetus are immunolabeled with antibodies to A) SHH and its receptor B) Patched1 (Ptc1). C) The RT-PCR analysis (n=3) shows that - Lane 1): cortical LeX+ cells express mRNAs for SHH, Ptc1 and Smo; SHH, Ptc1 and Smo mRNA expression in cortical VZ/SVZ (Lane 2), medial (Lane 3) and lateral (Lane 4) ganglionic eminence. D–L) Co-transfected BLBP-Cre/Floxed-YFP cells are labeled with anti-O4 antibody 7 days after being cultured in differentiation medium D–F) without SHH (control), G–I) with SHH (200ng/ml ,7 div) or J–L) with the SHH inhibitor cyclopamine (1µM, 7 div). Note the increased number of YFP/O4+ as well as progenitor cells labeled only with O4 antibody in SHH treated cultures (I) and the lack of YFP/O4+ or O4+ cells after cyclopamine treatment (L). Arrows point to transfected YFP+ cells co-labeled with O4 antibody. M) Quantification of O4+ cells generated from a subtype of RG cells. Scale bars: A–B: 10 µm; D–L: 50 µm.
DISCUSSION
Application of a Cre-loxP system for genetic labeling of RG cells allowed us to demonstrate, for the first time in the human fetal brain cell cultures, a direct lineage relationship between subpopulation of RG progenitors and pre-myelinating oligodendrocytes. A subpopulation of RG cells obtained from the cortical VZ/SVZ of the human fetal forebrain at the second trimester of gestation can in vitro generate cells that express markers of OL lineage. Local secretion of SHH influenced this type of oligodendrogenesis, as seen by a decreased number of generated OPCs after treatment with its inhibitor, cyclopamine. In addition, one third of cultured RG cells expressed functional receptors for SHH, Ptc1 and Smo, and might represent the subpopulation of cortical RG that have potential to give rise to OLs. These cells probably reacted to SHH treatment by increase generation of O4+ oligodendrocyte progenitors. As the number of generated OPCs is smaller than the number of cells immunopositive for Ptc1, other factors may be involved in suppressing this type of oligodendrogenesis.
Oligodendrogenesis in the human forebrain
Oligodendrogenesis starts in the ventral spinal cord, and spreads to the ventral forebrain (MGE), which was identified as a major source of OLs in rodents (He et al., 2001; Marshall and Goldman, 2002; Nery et al., 2001, Kessaris et al., 2006). It has been shown, however, that the site of oligodendrogenesis depends on the stage of development. In rodents, early in development OPCs are generated in the ventral telencephalon, but around birth there is a switch to the cortical VZ/SVZ site (Gorski et al., 2002, Ivanova et al., 2003; Kessaris et al., 2006; Richardson et al., 2006), which continues to generate OLs postnatally (Levison and Goldman, 1993; Ventura and Goldman, 2007). Furthermore, later cortical myelination depends largely on dorsal progenitors as shown by spatially restricted ablation of Olig2 function (Yue et al., 2006).
Studies concerning OLs origin in human forebrain are lagging behind animal studies, due to technical difficulties of applying cutting edge methods of molecular biology and genetics in humans. On cryosections of the human fetal forebrain of the same gestational age as studied here (16–19 gw), we previously described cells labeled with markers specific for oligodendrocyte lineage (PDGFRα, NG2, MOG, MBP) distributed in a region- and time-specific manner (Jakovcevski and Zecevic, 2005a; Rakic and Zecevic, 2003; Zecevic et al., 2005; Jakovcevski et al., 2007). Similar results were reported by other laboratories and suggested OL origin in both ganglionic eminence and the cortical SVZ (Back et al., 2001; Ulfig et al., 2002).
In addition, early expression of transcription factors Olig1 and Olig2 most likely plays a role in the initial specification of the OL lineage, both in human (Jakovcevski and Zecevic, 2005b), and in animal models (e.g., Zhou et al., 2000; Lu et al., 2000).
In vitro studies have previously revealed generation of human OLs from neural precursors (Hajihosseini et al., 1996; Murray and Dubois-Dalcq, 1997; Svendsen et al., 1999; Zhang et al., 2000), glia-restricted progenitors (Dietrich et al., 2002), and human embryonic stem cells (Izrael et al., 2007). In addition, dividing OPCs were found by us and others in dissociated cell and slice cultures from the human fetal forebrain (Filipovic and Zecevic, 2008; Satoh and Kim, 1994; Wilson et al., 2003).
RG cells generate a subpopulation of human oligodendrocytes in vitro
Co-expression of RG and OL lineage markers on cryosections of the fetal forebrain at midgestation, signaled a lineage relationship of these two cell classes (Jakovcevski and Zecevic, 2005b). It was, however, impossible to firmly establish this relationship since we could not follow subsequent differentiation of RG cells on fixed fetal brain tissue, so we established an in vitro system. To be certain that we are following RG progeny, we used Cre/lox P fate mapping system, which allowed us to demonstrated that RG cells isolated from human fetal VZ/SVZ at midgestation can generate MAP+ or MOG+ premyelinating oligodendrocytes. In vitro sequence of gradual differentiation along OL linage corresponds well with in vivo human fetal studies (Back et al., 2001; Jakovcevski and Zecevic, 2005a,b; Rakic and Zecevic, 2003; Ulfig et al., 2002 ). Moreover, timing of RG cells differentiation into O4+ cells after 7 div, and MBP+ and MOG+ premyelinating oligodendrocytes at 21 div, is similar to that reported for rodents (Murray et al., 2002) and human cell cultures (Zhang et al., 2000). The small percentage of generated MBP+ cells in our experiments probably underestimates their real number, due to limited time window of development that we studied and a relatively small transfection efficacy of around 10%. Although it is hard to determine the exact percentage or the subtype of RG cells that can generate oligodendrocytes, our results suggest that this number could be between 15–20% (Fig. 2). Capacity of cortical RG cells to generate oligodendrocyte progenitors declined with time in culture, consistent with a gradual decrease of the multipotential capacity of progenitor cells in vitro (Murray and Dubois-Dalcq, 1997).
Does SHH induce oligodendrocyte fate in human fetal RG cells?
Sonic hedgehog is a potent morphogene secreted early during development by a notochord and floor plate, and later on, by progenitor cells in ventral telencephalic regions, such as the medial ganglionic eminence and zona interthalamica (Marti and Bovolenta, 2002; Shimamura and Rubenstein, 1997). SHH acts through its receptor complex, Ptc1 and Smo (e.g., McMahon 2000). SHH not only acts locally to regulate establishment of the ventro-dorsal axis of the embryo, but also on distance to regulate brain size through the promotion of progenitor cells proliferation (Gritli-Linde et al., 2001; Marti and Bovolenta, 2002; Xu et al., 2005). Through Gli activators SHH influences the expression of transcription factors, Nkx 2.1, and Olig1 and Olig2, which regulate ventral oligodendrogenesis (Lu et al., 2000; Nery et al., 2001; Rowitch 2004; Takebayashi et al., 2000; Tekki-Kessaris et al., 2001). Some of later dorsal sources of OLs, however, are SHH independent (Cai et al., 2005, Vallstedt et al., 2005). Others have reported that even in rostral telencephalon appearance of oligodendrocytes is under SHH control (Spassky et al., 2001). Moreover, both Ptc1 and Smo are expressed also dorsally in mice, in the cortical progenitors, and treatment with SHH influences dorsally located cortical progenitors to adopt OLs fate (Murray et al., 2002; Nery et al., 2001; Xu et al., 2005).
Dependence of oligodendrogenesis on SHH signaling is largely unknown in the human fetal brain. Distribution of SHH has been reported only in human embryonic ventral spinal cord and mesencephalon (Hajihosseini et al., 1996; Orentas et al., 1999), or in relation to brain defects such as holoprosencephaly or cyclopia (Schell-Apacik et al., 2003). We here demonstrate that a subpopulation of human RG cells isolated from dorsal forebrain in the second trimester of gestation, express both SHH and its receptors, Ptc1 and Smo. Moreover, treatment with SHH increased the number of generated O4 progenitor cells, indicating functional Ptc1 receptor in our cultured RG cells. In contrast, treatment with cyclopamine reduced the number of generated oligodendrocyte progenitors, suggesting local production of SHH by RG cells. These experiments suggest that SHH either influence proliferation and/or differentiation of earlier progenitors into O4+ late progenitors. The role for SHH in human dorsal oligodendrogenesis should be further investigated. If confirmed, this result would be in agreement with reports in rodents (Incardona et al. 2000; Murray et al., 2001; Spassky et al., 2001; Tekki-Kessaris et al., 2001). In future studies, it would be particularly important to determine whether the same subtype of human RG cells can produce all three neural cell types, and also whether RG cells are the only progenitors or other classes of progenitors exist for forebrain oligodendrocytes in a time- and region-specific manner.
Supplementary Material
Late oligodendrocyte progenitors labeled with O4 antibody (red) in LeX+ cell cultures enriched from cortical VZ/SVZ of a 16 gw fetal brain. After 7 div in differentiation medium A) control culture- without SHH and B) culture with 200 ng/ml of SHH, where the number of O4+ cells increased almost four folds, from 0.7% to 2.6% of total cells seen with bisbenzamide nuclear labeling in blue. Scale bar: 50µm.
Acknowledgments
We are grateful to E.Anton (University of North Carolina, Chapel Hill, NC), J. Li (University of Connecticut Health Ctr., Farmington, CT) and K. Ikenaka (National Institute for Physiological Sciences 5-1-Higashiyama, Myodaiji, Okazaki, Japan) for generous gifts of plasmids, E.Barbarese for critically reviewing an earlier draft of the manuscript, and Nazia Sindhi for editing the text. Midgestational human tissue was obtained from B. Poulos at the Albert Einstein College of Medicine, Tissue Repository, Bronx, NY, USA; Supported by NIH NS 41489/07 and the National Soc. for Multiple Sclerosis RG 3083C4/1.
References
- Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21:1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Qi Y, Hu X, Tan M, Liu Z, Zhang J, Li Q, Sander M, Qiu M. Generation of oligodendrocyte precursor cells from mouse dorsal spinal cord independent of Nkx6 regulation and Shh signaling. Neuron. 2005;45:41–53. doi: 10.1016/j.neuron.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Cameron RS, Rakic P. Glial cell lineage in the cerebral cortex - a review and synthesis. Glia. 1991;4:124–137. doi: 10.1002/glia.440040204. [DOI] [PubMed] [Google Scholar]
- Casper KB, McCarthy KD. GFAP-positive progenitor cells produce neurons and oligodendrocytes throughout the CNS. Mol Cell Neurosci. 2006;31:676–684. doi: 10.1016/j.mcn.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Chandran S, Kato H, Gerreli D, Compston A, Svendsen CN, Allen ND. FGF-dependent generation of oligodendrocytes by a hedgehog-independent pathway. Development. 2004;130:6599–6609. doi: 10.1242/dev.00871. [DOI] [PubMed] [Google Scholar]
- Dahl D, Rueger DC, Bignami A, Weber K, Osborn M. Vimentin, the 57.000 molecular weight protein of fibroblast filaments, is the major cytoskeleton component in immature glia. Eur J Cell Biol. 1981;24:191–196. [PubMed] [Google Scholar]
- Dietrich J, Noble M, Mayer-Proschel M. Characterization of A2B5+ glial precursor cells from cryopreserved human fetal brain progenitor cells. Glia. 2002;40:65–77. doi: 10.1002/glia.10116. [DOI] [PubMed] [Google Scholar]
- Filipovic R, Zecevic N. Lipopolysaccharide affects golli expression and promotes proliferation of oligodendrocyte progenitors. Glia. 2005;49:457–466. doi: 10.1002/glia.20125. [DOI] [PubMed] [Google Scholar]
- Fogarty M, Richardson WD, Kessaris N. A subset of oligodendrocytes generated from radial glia in the dorsal spinal cord. Development. 2005;132:1951–1959. doi: 10.1242/dev.01777. [DOI] [PubMed] [Google Scholar]
- Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, Jones KR. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J. Neurosci. 2002;22:6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregori N, Proschel C, Noble M, Mayer-Proschel M. The tripotential glial-restricted precursor (GRP) cell and glial development in the spinal cord: generation of bipotential oligodendrocyte-type-2 astrocyte progenitor cells and dorsal-ventral differences in GRP cell function. J Neurosci. 2002;22:248–256. doi: 10.1523/JNEUROSCI.22-01-00248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritli-Linde A, Lewis P, McMahon AP, Linde A. The whereabouts of a morphogen: direct evidence for short- and graded long-range activity of hedgehog signaling peptides. Dev Biol. 2001;236:364–386. doi: 10.1006/dbio.2001.0336. [DOI] [PubMed] [Google Scholar]
- Hajihosseini M, Tham TN, Dubois-Dalcq M. Origin of oligodendrocytes within the human spinal cord. J. Neurosci. 1996;16:7981–7994. doi: 10.1523/JNEUROSCI.16-24-07981.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R, Reynolds R. Proliferation and differentiation potential of rat forebrain oligodendroglial progenitors both in vitro and in vivo. Development. 1991;111:1061–1080. doi: 10.1242/dev.111.4.1061. [DOI] [PubMed] [Google Scholar]
- He W, Ingraham C, Rising L, Goderie S, Temple S. Multipotent stem cells from the mouse basal forebrain contribute GABAergic neurons and oligodendrocytes to the cerebral cortex during embryogenesis. J. Neurosci. 2001;21:8854–8862. doi: 10.1523/JNEUROSCI.21-22-08854.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard B, Chen Y, Zecevic N. Cortical progenitor cells in the developing human telencephalon. Glia. 2006;53:57–66. doi: 10.1002/glia.20259. [DOI] [PubMed] [Google Scholar]
- Howard B, Mo Zh, Filipovic R, Moore AR, Antic SD, Zecevic N. Radial glia cells in the developing human brain. The Neuroscientist. 2008 doi: 10.1177/1073858407313512. May 2008 http://nro.sagepub.com/cgi/rapidpdf/1073858407313512v1. [DOI] [PMC free article] [PubMed]
- Incardona JP, Lee JH, Robertson CP, Enga K, Kapur RP, Roelink H. Receptor-mediated endocytosis of soluble and membrane-tethered Sonic hedgehog by Patched-1. Proc. Natl . Acad. Sci U S A. 2000;97:12044–12049. doi: 10.1073/pnas.220251997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova A, Nakahira E, Kagawa T, Oba A, Wada T, Takebayashi H, Spassky N, Levine J, Zalc B, Ikenaka K. Evidence for a second wave of oligodendrogenesis in the postnatal cerebral cortex of the mouse. J. Neurosci. Res. 2003;73:581–592. doi: 10.1002/jnr.10717. [DOI] [PubMed] [Google Scholar]
- Izrael M, Zhang P, Kaufman R, Shinder V, Ella R, Amit M, Itskovitz-Eldor J, Chebath J, Revel M. Human oligodendrocytes derived from embryonic stem cells: Effect of noggin on phenotypic differentiation in vitro and on myelination in vivo. Mol Cell Neurosci. 2007;34:310–323. doi: 10.1016/j.mcn.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Jakovcevski I, Mo Z, Zecevic N. Down-regulation of the axonal PSA-NCAM expression coincides with the onset of myelination in the human fetal forebrain. Neuroscience. 2007;149:328–337. doi: 10.1016/j.neuroscience.2007.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovcevski I, Zecevic N. Sequence of oligodendrocyte development in the human fetal telencephalon. Glia. 2005a;49:480–491. doi: 10.1002/glia.20134. [DOI] [PubMed] [Google Scholar]
- Jakovcevski I, Zecevic N. Olig transcription factors are expressed in oligodendrocyte and neuronal cells in human fetal CNS. J Neurosci. 2005b;25:10064–10073. doi: 10.1523/JNEUROSCI.2324-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadhim HJ, Gadisseux J-F, Edvrard P. Topographical and cytological evolution of the glial phase during prenatal development of the human brain: histochemical and electron microscopic study. J Neuropath Exp Neurol. 1988;47:166–188. doi: 10.1097/00005072-198803000-00009. [DOI] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessaris N, Jamen F, Rubin L, Richardson WD. Cooperation between sonic hedgehog and fibroblast growth factor/MAPK signalling pathways in neocortical precursors. Development. 2004;131:1289–1298. doi: 10.1242/dev.01027. [DOI] [PubMed] [Google Scholar]
- Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10:201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- Levitt P, Cooper ML, Rakic P. Coexistence of neural and glial precursor cells in the cerebral ventricular zone of the fetal monkey: an ultrastuctural immunoperoxidase analysis. J Neurosci. 1981;1:27–39. doi: 10.1523/JNEUROSCI.01-01-00027.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu QR, Yuk D-I, Alberta JA, Zhu Z, Pawlitzky I, Chan J, McMahon AP, Stiles CD, Rowitch DH. Sonic hedgehog-regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25:317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hartfuss E, Götz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127:5253–5263. doi: 10.1242/dev.127.24.5253. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hack MA, Hartfuss E, Kettenmann H, Klinkert W, Kirchhoff F, Götz M. Neuronal or glial progeny: regional differences in radial glia fate. Neuron. 2003;37:751–764. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- Marshall CA, Goldman JE. Subpallial Dlx2-expressing cells give rise to astrocytes and oligodendrocytes in the cerebral cortex and white matter. J. Neurosci. 2002;22:9821–9830. doi: 10.1523/JNEUROSCI.22-22-09821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martí E, Bovolenta P. Sonic hedgehog in CNS development: one signal, multiple outputs. Trends Neurosci. 2002;25:89–96. doi: 10.1016/s0166-2236(02)02062-3. [DOI] [PubMed] [Google Scholar]
- McMahon AP. More surprises in the Hedgehog signaling pathway. Cell. 2000;100:185–188. doi: 10.1016/s0092-8674(00)81555-x. [DOI] [PubMed] [Google Scholar]
- Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RH, Hayes JE, Dyer KL, Sussman CR. Mechanisms of oligodendrocyte commitment in the vertebrate CNS. Int. J. Dev. Neurosci. 1999;17:753–763. doi: 10.1016/s0736-5748(99)00068-4. [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–741. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- Mo Z, Moore AR, Filipovic R, Ogawa Y, Kazuhiro I, Antic SD, Zecevic N. Human cortical neurons originate from radial glia and neuron-restricted progenitors. J Neurosci. 2007a;27:4132–4145. doi: 10.1523/JNEUROSCI.0111-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Z, Zecevic N. Human fetal oligodendrocytes are generated from a subpopulation of Radial glia cells. J. Neurochem. 2007b;102(Suppl.1):126. [Google Scholar]
- Mo Z, Zecevic N. Is Pax6 Critical for Neurogenesis in the Human Fetal Brain? Cereb Cortex. 2008;18:1455–1465. doi: 10.1093/cercor/bhm181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K, Calaora V, Rottkamp C, Guicherit O, Dubois-Dalcq M. Sonic hedgehog is a potent inducer of rat oligodendrocyte development from cortical precursors in vitro. Mol. Cell. Neurosci. 2002;19:320–332. doi: 10.1006/mcne.2001.1079. [DOI] [PubMed] [Google Scholar]
- Murray K, Dubois-Dalcq M. Emergence of oligodendrocytes from human neural spheres. J Neurosci Res. 1997;50:146–156. doi: 10.1002/(SICI)1097-4547(19971015)50:2<146::AID-JNR4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Nery S, Wichterle H, Fishell G. Sonic hedgehog contributes to oligodendrocyte specification in the mammalian forebrain. Development. 2001;128:527–540. doi: 10.1242/dev.128.4.527. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Orentas DM, Hayes JE, Dyer KL, Miller RH. Sonic hedgehog signaling is required during the appearance of spinal cord oligodendrocyte precursors. Development. 1999;126:2419–2429. doi: 10.1242/dev.126.11.2419. [DOI] [PubMed] [Google Scholar]
- Pfeiffer SE, Warrington AE, Bansal R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993;3:191–197. doi: 10.1016/0962-8924(93)90213-k. [DOI] [PubMed] [Google Scholar]
- Pringle NP, Richardson WD. A singularity of PDGF alpha-receptor expression in the dorsoventral axis of the neural tube may define the origin of the oligodendrocyte lineage. Development. 1993;117:525–533. doi: 10.1242/dev.117.2.525. [DOI] [PubMed] [Google Scholar]
- Pringle NP, Yu WP, Guthrie S, Roelink H, Lumsden A, Peterson AC, Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on the culture medium. Nature. 1983;303:389–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Rakic S, Zecevic N. Early oligodendrocyte progenitor cells in the human fetal telencephalon. Glia. 2003;41:117–127. doi: 10.1002/glia.10140. [DOI] [PubMed] [Google Scholar]
- Rao MS, Noble M, Mayer-Proschel M. A tripotential glial precursor cell is present in the developing spinal cord. Proc Natl Acad Sci USA. 1998;95:3996–4001. doi: 10.1073/pnas.95.7.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson WD, Kessaris N, Pringle N. Oligodendrocyte wars. Nat Rev Neurosci. 2006;7:11–18. doi: 10.1038/nrn1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson WD, Smith HK, Sun T, Pringle NP, Hall A, Woodruff R. Oligodendrocyte lineage and the motor neuron connection. Glia. 2000;29:136–142. doi: 10.1002/(sici)1098-1136(20000115)29:2<136::aid-glia6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Rivkin MJ, Flax J, Mozell R, Osathanondh R, Volpe JJ, Villa-Komaroff L. Oligodendroglial development in human fetal cerebrum. Ann Neurol. 1995;38:92–101. doi: 10.1002/ana.410380116. [DOI] [PubMed] [Google Scholar]
- Rowitch DH. Glia specification in the vertebrate neural tube. Nature Neurosci Reviews. 2004;5:409–419. doi: 10.1038/nrn1389. [DOI] [PubMed] [Google Scholar]
- Rowitch DH, Lu QR, Kessaris N, Richardson WD. An ‘oligarchy’ rules neural development. Trends Neurosci. 2002;25:417–422. doi: 10.1016/s0166-2236(02)02201-4. [DOI] [PubMed] [Google Scholar]
- Satoh J, Kim SU. Proliferation and differentiation of fetal human oligodendrocytes in culture. J Neurosci Res. 1994;39:260–272. doi: 10.1002/jnr.490390304. [DOI] [PubMed] [Google Scholar]
- Schell-Apacik C, Rivero M, Knepper JL, Roessler E, Muenke M, Ming JE. Sonic Hedgehog mutations causing human holoprosencephaly impair neural patterning activity. Hum. Genet. 2003;113:170–177. doi: 10.1007/s00439-003-0950-4. [DOI] [PubMed] [Google Scholar]
- Schmid RS, Yokota Y, Anton ES. Generation and characterization of brain lipid-binding protein promoter-based transgenic mouse models for the study of radial glia. Glia. 2006;53:345–351. doi: 10.1002/glia.20274. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Rubenstein JL. Inductive interactions direct early regionalization of the mouse forebrain. Development. 1997;124:2709–2718. doi: 10.1242/dev.124.14.2709. [DOI] [PubMed] [Google Scholar]
- Spassky N, Heydon K, Mangatal A, Jankovski A, Olivier C, Queraud-Lesaux F, Goujet-Zalc C, Thomas JL, Zalc B. Sonic hedgehog-dependent emergence of oligodendrocytes in the telencephalon: evidence for a source of oligodendrocytes in the olfactory bulb that is independent of PDGFR alpha signaling. Development. 2001;128:4993–5004. doi: 10.1242/dev.128.24.4993. [DOI] [PubMed] [Google Scholar]
- Svendsen CN, Caldwell MA, Ostenfeld T. Human neural stem cells: isolation, expansion and transplantation. Brain. Pathol. 1999;9:499–513. doi: 10.1111/j.1750-3639.1999.tb00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi H, Yoshida S, Sugimori M, Kosako H, Kominami R, Nakafuku M, Nabeshima Y. Dynamic expression of basic helix-loop-helix Olig family members: implication of Olig2 in neuron and oligodendrocyte differentiation and identification of a new member, Olig3. Mech. Dev. 2000;99:143–148. doi: 10.1016/s0925-4773(00)00466-4. [DOI] [PubMed] [Google Scholar]
- Tekki-Kessaris N, Woodruff R, Hall AC, Gaffield W, Kimura S, Stiles CD, Rowitch DH, Richardson WD. Hedgehog-dependent oligodendrocyte lineage specification in the telencephalon. Development. 2001;128:2545–2554. doi: 10.1242/dev.128.13.2545. [DOI] [PubMed] [Google Scholar]
- Ulfig N, Briese M, Bohl J. Expression of oligodendrocyte-specific protein (OSP/claudin-11) in the human fetal forebrain. Neuroembryology. 2002;1:48–53. [Google Scholar]
- Vallstedt A, Klos JM, Ericson J. Multiple dorsoventral origins of oligodendrocyte generation in the spinal cord and hindbrain. Neuron. 2005;45:55–67. doi: 10.1016/j.neuron.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Ventura RE, Goldman JE. Dorsal Radial glia generate olfactory bulb interneurons in the postnatal murine brain. J. Neurosci. 2007;27:4297–4302. doi: 10.1523/JNEUROSCI.0399-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HC, Onischke C, Raine CS. Human oligodendrocyte precursor cells in vitro: phenotypic analysis and differential response to growth factors. Glia. 2003;44:153–165. doi: 10.1002/glia.10280. [DOI] [PubMed] [Google Scholar]
- Xu Q, Wonders CP, Anderson SA. Sonic hedgehog maintains the identity of cortical interneuron progenitors in the ventral telencephalon. Development. 2005;132:4987–4998. doi: 10.1242/dev.02090. [DOI] [PubMed] [Google Scholar]
- Yue T, Xian K, Hurlock E, Xin M, Kernie S, Parada L, Lu QR. A Critical Role for Dorsal Progenitors in Cortical Myelination. J. Neurosci. 2006;26:1275–1280. doi: 10.1523/JNEUROSCI.4717-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N. Specific characteristics of radial glia in the human fetal telencephalon. Glia. 2004;48:27–35. doi: 10.1002/glia.20044. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Chen Y, Filipovic R. Contributions of cortical subventricular zone to the development of the human cerebral cortex. J.Comp.Neurol. 2005;491:109–122. doi: 10.1002/cne.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Wang S, Anderson DJ. Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron. 2000;25:331–343. doi: 10.1016/s0896-6273(00)80898-3. [DOI] [PubMed] [Google Scholar]
- Zhang SC, Ge B, Duncan ID. Tracing human oligodendroglial development in vitro. J Neurosci Res. 2000;59:421–429. doi: 10.1002/(SICI)1097-4547(20000201)59:3<421::AID-JNR17>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Late oligodendrocyte progenitors labeled with O4 antibody (red) in LeX+ cell cultures enriched from cortical VZ/SVZ of a 16 gw fetal brain. After 7 div in differentiation medium A) control culture- without SHH and B) culture with 200 ng/ml of SHH, where the number of O4+ cells increased almost four folds, from 0.7% to 2.6% of total cells seen with bisbenzamide nuclear labeling in blue. Scale bar: 50µm.