Abstract
Activation of p38 MAP kinase (MAPK) in the spinal cord has been implicated in the development and maintenance of pain states. In this study, we tested whether p38 MAPK is involved in the response to first-degree burn of the hindpaw. This injury induces central sensitization leading to tactile allodynia, mediated by activation of Ca2+ permeable AMPA/kainate receptors through PKC and PKA (Jones and Sorkin 2005). We demonstrate that p38 MAPK is rapidly and robustly activated in the superficial spinal dorsal horn after mild thermal injury to the hindpaw. Activated p38 MAPK was localized primarily to microglia and to a lesser extent in oligodendrocytes and lamina II neurons. Astrocytes were not involved in the p38 MAPK response. Intrathecal pretreatment of pharmacological inhibitors of p38 MAPK (SB203580, SD-282) dose-dependently blocked development of tactile allodynia, a characteristic of the first-degree burn model. The effects of the inhibitors on tactile allodynia were lost when they were administered post-injury. These studies identify p38 MAPK as a major mediator of tactile allodynia, most likely activated downstream of AMPA/kainate receptors.
Keywords: burn injury, cell signaling, pain related behaviors
Introduction
Activation of mitogen activated protein kinases (MAPK) within the spinal cord has been implicated in a variety of enhanced pain states (Ji et al. 1999; Ma and Quirion 2002; Milligan et al. 2001; Svensson et al. 2003b; Tsuda et al. 2004; Zhuang et al. 2005). p38 MAPK is activated in spinal glia in models of inflammatory pain (intraplantar carrageenan) and peripheral nerve injury (Svensson et al. 2003b; Scholz et al. 2008). The mechanism by which p38 MAPK is activated remains incompletely understood; however, p38 MAPK is activated by stressful conditions and by inflammatory mediators, such as IL-1β and TNF-α. Once activated, P38 MAPK targets an assortment of pivotal downstream targets including ATF-2 and ELK1. p38 MAPK also can phosphorylate other protein kinases such as MAPKAK2/3 and thereby amplify intracellular signaling (Cohen 1997). Pharmacological antagonism of p38 MAPK prior to spinal nerve ligation inhibits development of neuropathic pain (Schafers et al. 2003; Jin et al. 2003). Thus, p38 MAPK may play a role in development and/or maintenance of chronic neuropathic pain. However, the role of spinal p38 MAPK in allodynia arising from a mild thermal injury (first degree burn) has not been tested.
Following a first degree burn of the heel, spinally mediated tactile allodynia, but not thermal hyperalgesia, develops at the base of the toes outside of the burned area (Nozaki-Taguchi and Yaksh 1998). This spinal component appears to be mediated by activation of spinal Ca2+ permeable AMPA/kainate receptors and not by NMDA receptors (Nozaki-Taguchi and Yaksh 2002; Sorkin et al. 1999; Sorkin et al. 2001). It is not known at this time whether AMPA receptor activation leads to phosphorylation of p38 MAPK.
Unlike NMDA dependent models of pain, first-degree burn-induced tactile allodynia is not dependent on CamKinase IIα (Jones and Sorkin 2005) or activation of either cyclooxygenase or nitric oxide synthase (Sorkin et al. 2008). Thus, other second messengers and signal transduction cascades may be activated following activation of AMPA receptors. The goal of the present study was to identify downstream signaling cascades associated with this model. Our data indicate that first-degree burn of the hindpaw results in acute and robust activation of p38 MAPK, primarily in spinal microglia. Allodynia in this model was sensitive to pharmacological antagonism of p38 MAPK. These studies are consistent with a model in which microglial p38 MAPK activation plays a significant role as a mediator of pain behavior initiated by Ca2+ permeable AMPA/kainate receptor activation.
Materials and Methods
Reagents
A proprietary, highly specific ATP-competitive indole-5-carboxamide, ATP competitive inhibitor of p38α/β inhibitor, SD-282 (Koppelman et al. 2008) (Scios Corporation, Sunnyvale, CA) was dissolved in 5% dimethylsulfoxide (DMSO) and 5% Cremephor EL (Sigma, St. Louis, MO) in sterile saline. SD-282 is a small molecule with low activity against p38 δ and p38 λ. This agent does not inhibit members of the JNK or ERK MAP kinase families (Koppelman et al. 2008) nor does it inhibit the activity of cyclooxygenase 1 or 2 (Svensson et al. 2003a; Svensson et al. 2003b). In addition, a second p38α/β inhibitor, SB203580 (CalBiochem, La Jolla, CA) (Jin et al. 2003; Svensson et al. 2003b), which also binds within the ATP pocket, was dissolved in a saline vehicle.
Animals and first degree burn
Male Holtzman rats (250-300 g, Harlan Industries, Indianapolis, IN) were maintained on a 12:12 h light:dark cycle. Food and water were made available, ad libitum, except during recovery from surgery and during behavioral testing. Efforts were made to minimize animal discomfort and reduce numbers of animals used. All studies were carried out in accordance with protocols approved by the Animal Care and Use Committee of the University of California, San Diego. Rats were lightly anesthetized with isoflurane and the heel of the left hindpaw was held on a 52.5°C metal surface for 45 sec. A 10 g sand bag was placed on the dorsal surface of the paw to maintain constant pressure. This application produced transient redness (without blister formation). Animals were then returned to their home cage (western blot or immunohistochemistry) or original test compartments (behavior) where recovery from anesthesia took approximately 2-3 min.
SDS-PAGE and Immunoblotting
Animals intended for immunoblotting of P-p38 received a first-degree burn and were allowed to waken prior to sacrifice. Control animals were anesthetized for the same duration during a sham burning procedure using a room temperature metal surface, as it was determined in preliminary experiments that true naïve controls had a tendency towards slightly higher levels of P-MAPKs than those exposed to the volatile anesthetic.
Preliminary experiments examined post-injury survival times of 5-30 min (data not shown); 10 min survival produced the highest level of P-p38 MAPK and was used for the reported experiments. Rats were anesthetized with isoflurane and decapitated. The spinal cord was quickly removed by hydroextrusion with chilled saline and a 1 cm length of the lumbar enlargement centered around L4/5 was divided into ipsilateral and contralateral halves and placed in 200 μl cold lysis buffer containing protease and phosphatase inhibitors (Campana et al. 1996). Samples were sonicated on ice until dissolved and centrifuged at 15,000 × g. The spinal cord was directly homogenized in boiling sample buffer (100 nM Tris, pH 6.8, 2% SDS, 20% glycerol) as described previously (Ji and Rupp 1997). The protein content of each lysate was determined by BCA (Pierce, Rockville, IL). Subsequently, β-mercaptoethanol (10%) and bromphenol blue (0.1%) was added to each sample and 50 μg protein was loaded into each lane of a 10% SDS polyacrylamide gel. Proteins were transferred onto nitrocellulose membranes (Millipore, Bedford, MA) as previously described (Campana et al. 1998). Membranes were blocked in 5% milk for one hour at room temperature and incubated overnight at 4°C with polyclonal antibodies for P-p38 (1:1000, New England BioLabs). The blots were rinsed and then incubated for 1 h at room temperature with HRP-conjugated secondary antibody (1:2000, New England Biolabs) and visualized in ECL solution (NEN, Boston, MA) for 1 min and exposed onto hyperfilms (Amersham Biosciences) for 1-30 min. Blots were stripped and re-probed with polyclonal anti-total p38 antibody (1:1000; New England Biolabs) as a loading control for P-p38. Data are expressed as phosphorylated/total protein levels for p38. Autoradiographs were scanned by Canoscan (Lake Success, NY) and the optical density of each band was analyzed by NIH Image 1.62.
Immunohistochemistry
Animals were deeply anesthetized with isoflurane and transcardially perfused 10 min post-injury with 200 ml heparinized saline followed by 300 ml cold, fresh 4% paraformaldehyde in 0.1 M phosphate buffer saline (PBS). The L4-L5 segments were removed and post-fixed in perfusate at 4°C overnight before transfer to 30% sucrose for cryoprotection. Transverse 30-μm-thick sections were cut on a cryostat and stored as free-floating sections in 0.1 M PBS. Immunostaining was performed as previously described (Hefferan et al. 2007). Sections were blocked in 5% normal goat serum (NGS) for 1 h at room temperature and then transferred to primary antibody solution containing rabbit anti-P-p38 (1:200 in 1% bovine serum albumin, 0.1 M PBS-0.3% Triton X-100) for 72 h at 4°C on a shaker. For cell identification, NeuN (neuronal marker 1:1000, Chemicon, Temecula, CA), OX-42 (microglia, 1:500, Serotec, Raleigh, NC), GFAP (astrocyte, 1:400 Sigma, St. Louis, MO) or APC (oligodendrocytes, 1:500, EMD Chemicals, Gibbstown NJ) was added to the primary antibody solution. After incubation, sections were washed with 0.1 M PBS and reacted with goat anti-mouse 488 and goat anti-rabbit 546 secondary antibodies for 2 h at room temperature on a shaker; DAPI was added as a general cellular nuclear stain. After washing, sections were mounted on glass slides, air-dried and covered.
For brightfield imaging, some sections were treated with 0.3% H2O2 in methanol for 15 min, washed with 0.1 M PBS, and incubated with rabbit anti-P-p38 (1:500) for 72 h at 4°C on a shaker. Bound antibody was detected using biotinylated donkey anti-rabbit (1:500; Amersham, Piscataway, NJ), the ABC Elite kit (Vector Laboratories, Burlingame, CA) and diaminobenzidine (Vector Laboratories, Burlingame, CA) as the chromogen. Sections were mounted on glass slides, air dried, dehydrated and covered. Controls include omission of the primary antibody or incubation with isotype-matched non-specific antibody, none of which resulted in specific immunoreactivity. Images were captured using a Leica DMLB microscope with a Zeiss Axiocam MRm monochrome camera or a Leica SP2 confocal microscope. Any post-processing was done with Adobe CS3 (Adobe Systems, Inc., San Jose, CA) with equal adjustments to any images being compared. At least three random sections, subjected to identical antibody treatment, were taken from each animal. Reported results were observed consistently in a minimum of three animals under each condition (burned and sham).
Behavioral Studies
Surgery
Animals intended for behavioral studies were deeply anesthetized with 4% isoflurane and anesthesia was maintained with 2% isoflurane. Polyethylene catheters (PE-5, Baxter Healthcare Corporation, Deerfield, IL) were inserted through the atlanto-occipital membrane and guided through the subarachnoid space, ending at the rostral lumbar enlargement (Yaksh and Rudy 1976). Rats received 5 mL intraperitoneal Lactated Ringer’s solution (Baxter HealthCare Corporation, Deerfield, IL) immediately after surgery and again on the following day. Animals were housed individually after surgery. Behavioral experiments were carried out 5-8 days after catheter implantation. Rats were monitored daily for sensory and motor disturbances and weight loss. Presentation of behavioral or motor deficits or loss of cannula patency was justification for removal of the animal from the study (less than 5% were excluded).
Behavioral Paradigm
Animals were acclimated to their testing chambers for 30 min periods on two days prior to the experiment. On the day of the experiment, after baseline behavior measurements were obtained, inhibitor or appropriate vehicle was injected into the intrathecal space in 10 μl volumes followed by 10 μl of isotonic sterile saline. Ten min post-injection, animals were anesthetized and burned. Nociceptive testing resumed 15 or 30 min post-injury and animals were then retested at 15 or 30 min intervals for the next 90-120 min. All of the testing was performed blinded.
Nociceptive Testing
Secondary mechanical allodynia: Rats were placed in individual compartments (26 × 11× 20 cm) with wire mesh bottoms for two days prior to the experiment. Mechanical withdrawal thresholds were tested at a site approximately 2 cm distal to the intended thermal injury. Following a first degree burn to the heel, this area is sensitized to mechanical, but not to thermal stimuli (Nozaki-Taguchi and Yaksh 1998). This classical pattern of secondary hyperalgesia (LaMotte et al. 1982) has been ascribed to changes in central excitability (Simone et al. 1991). Baseline withdrawal thresholds were measured with calibrated von Frey filaments (Stoelting, Wood Dale, IL) having buckling forces between 0.41 and 15.2 g. Each filament was applied to the mid plantar paw, just proximal to the base of the toes on skin totally distinct from the injured area. Testing began with the 2.0 g filament and proceeded according to the up-down method described by Chaplan (Chaplan et al. 1994). Stimuli were separated by several seconds or until the animal was calm with all four paws placed on the mesh. The 50% probability withdrawal threshold was determined.
Primary hyperalgesia (thermal): Rats were placed in a modified Hargreaves apparatus (Hargreaves et al. 1988) with a heated glass plate maintained at 30°C. After a 30 min acclimation period, basal withdrawal latency from a light beam focused on the midheel of the left hindpaw was measured. Two basal measures, 5 min apart, were made and the results averaged. Ten min following intrathecal injection of p38 antagonist or vehicle, the left heel was placed on the heated metal surface as described. Animals were returned to the testing apparatus and thermal withdrawal latencies within the area of primary hyperalgesia, were measured. Different groups of animals were used for testing mechanical thresholds and thermal latencies.
Statistical Analysis
All data is presented as mean ± SEM. One and two-way ANOVA followed by Bonferroni post-test was used to determine statistical significance in behavioral measures. Differences in P-p38 MAPK following the first degree burn or exposure to the neutral temperature plate (sham) were determined with unpaired t-tests. In all cases, p < 0.05 was considered to be significant. Statistical analysis was performed using Prism or InStat (San Diego, CA) statistical software packages.
Results
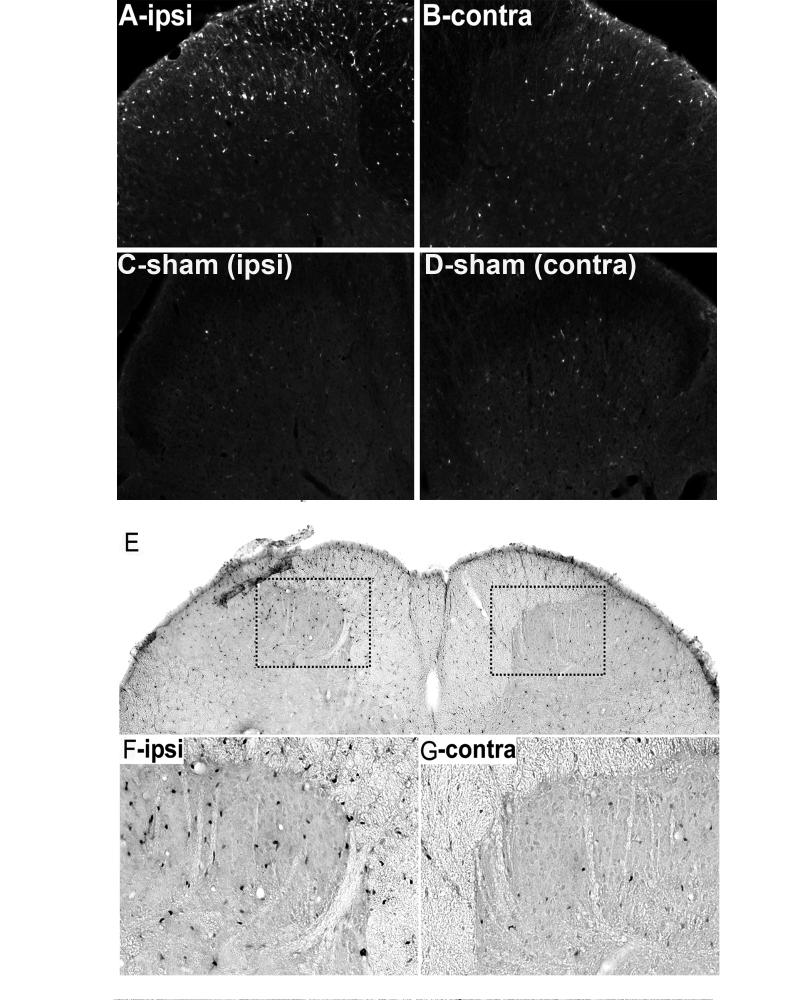
To determine whether p38 MAPK is activated by first-degree burn to the hindpaw, we performed immunohistochemistry, analyzing frozen sections of spinal cord dorsal horns to detect phosphorylated p38 MAPK (P-p38 MAPK). In sham-injured animals, immunoreactivity for P-p38 MAPK was infrequently detected in the superficial dorsal horn (Fig. 1). After first-degree burn, ipsilateral P-p38 MAPK immunoreactivity became pronounced. The highest concentration of P-p38 MAPK immunoreactive profiles was in the ipsilateral medial superficial dorsal horn in the somatotopic area which represents C-fiber input from the heel (Woolf and Fitzgerald 1986). There were also increased numbers of immunopositive cells in the ipsilateral lateral superficial dorsal horn, dorsal columns, ventral lateral funiculus, and when found, in attached dorsal rootlets (results not shown). Interestingly, there was consistent evidence of increased P-p38 MAPK immunostaining in the contralateral dorsal horn compared to those of sham-injured animals. Figure 1F and G show higher power images of P-p38 MAPK immunoreactivity in the superficial dorsal horns of a burn injured rat.
Figure 1.
p38 MAPK phosphorylation in the spinal dorsal horn after a first-degree burn. Immunofluorescence of P-p38 MAPK 10 min after burn in ipsilateral (A) and contralateral (B) spinal dorsal horns. Immunofluorescence of P-p38 MAPK after sham injury in ipsilateral (C) and contralateral (D) spinal dorsal horn. A similar pattern was noted when the immunogen was detected by brightfield methodology (E-G). Scale bar (G) is 100 μm for A-D, 200 μm for E and 75 μm for F, G.
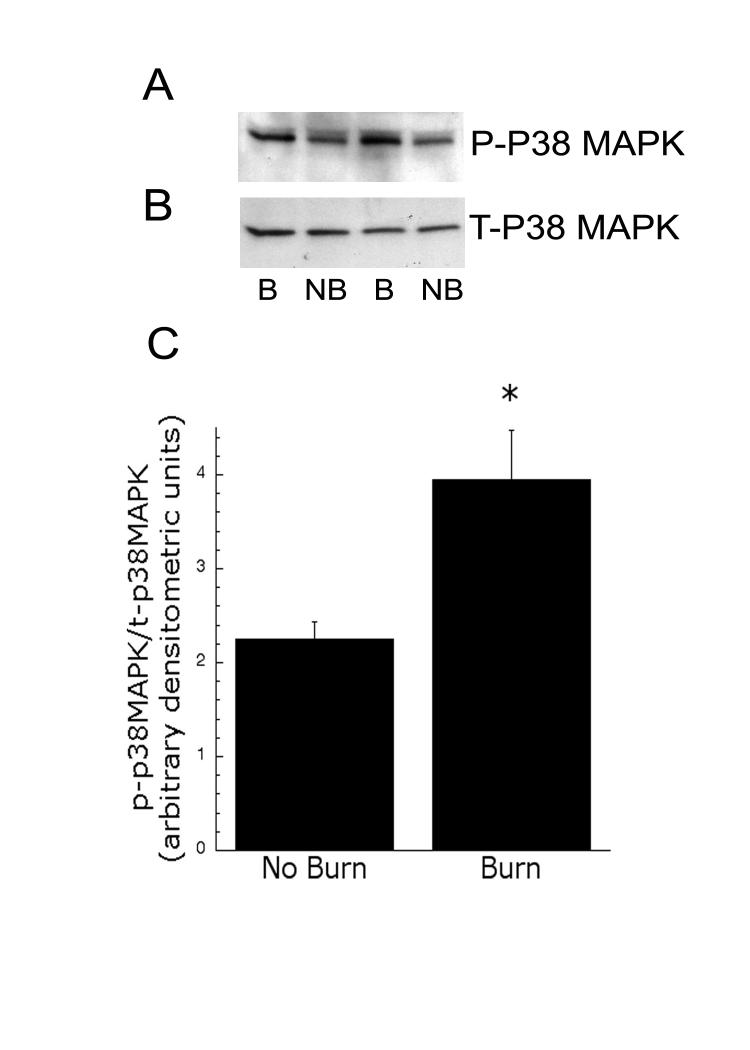
To confirm our immunohistochemistry results, we performed immunoblot analysis of dorsal spinal cord homogenates, obtained from injured and control rats, using P-p38 MAPK-specific antibody. Preliminary time course studies, assessing samples obtained 5-30 min post burn-injury, indicated that maximal P-p38 MAPK occurred 10 min after injury. At this time, P-p38 MAPK was consistently increased in the ipsilateral dorsal spinal cord of injured rats (B, burn), compared with tissue from rats that received an equivalent amount of anesthesia, but no burn (NB). Representative blots of P-p38 MAPK and total p38 MAPK from the ipsilateral lumbar enlargement are shown in Fig. 2. Densitometric analysis demonstrated a significant (p<0.05) increase in P-p38/total p38 ratio after first-degree burn.
Figure 2.
Immunoblots of P-p38 MAPK (A) and T-p38 MAPK (B) in ipsilateral dorsal horn extracts. 50 μg of total protein of spinal homogenates were loaded in each lane. NB = no burn, B = burn from 2 animals. (C) Densitometric analysis of immunoblots for the ratio of P-p38 MAPK and T-p38 MAPK (n=4) after burn. * indicates significance p<0.05.
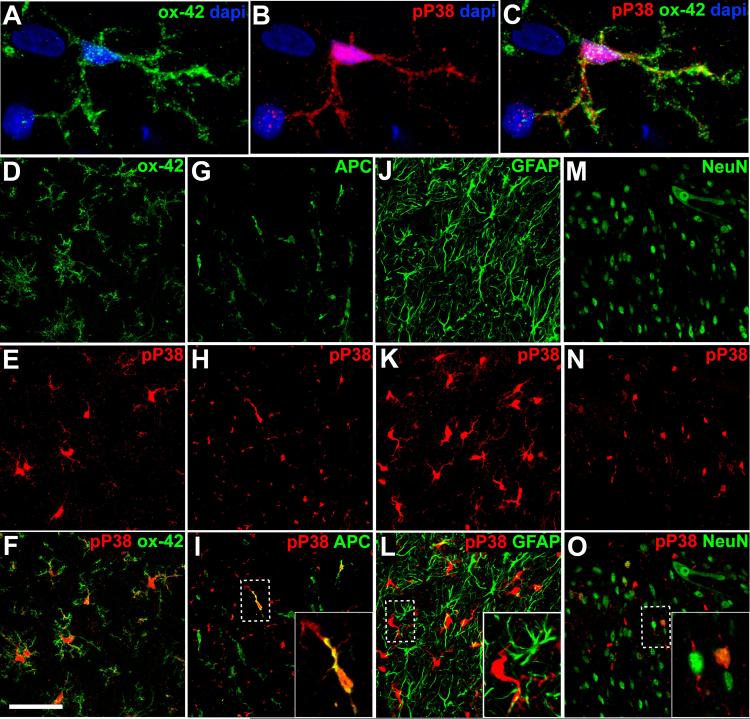
To determine the spinal cell type in which p38 MAPK is phosphorylated after first-degree burn, we performed dual label immunofluorescence microscopy. P-p38 MAPK co-localized predominantly with OX-42, a marker of microglia, indicating that p38 MAPK was activated in these cells (Fig. 3A-C). Microglia that were P-p38 MAPK-positive were observed throughout the grey matter. While P-p38 MAPK localized principally to microglia in the white matter as well, we also saw a small but significant level of staining in white matter oligodendrocytes. Interestingly, we observed no evidence of P-p38 MAPK in grey matter oligodendrocytes. Occasional co-localization with NeuN indicated P-p38 MAPK in a small subpopulation of lamina II neurons. By contrast, P-p38 MAPK and GFAP co-localization was not observed, indicating that p38 MAPK was not activated in astrocytes.
Figure 3.
Double labeling immunofluorescence of P-p38 MAPK and cell markers in spinal dorsal horn after burn-injury. Ipsilateral spinal cords were used; microphotographs were taken from medial superficial dorsal horn except for the APC image which was obtained from the dorsal column. Cell markers include OX-42 for microglia (A-F), APC for oligodendrocytes (G-I), GFAP for astrocytes J-L) and NeuN for neurons (M-O). Alexa 488 (green) or 564 (red) conjugated secondary antibodies were used. Note co-localization in yellow. Images are representative of 4 animals. Scale bar shown (F) is 10 μm for A-C and 50 μm for D-O.
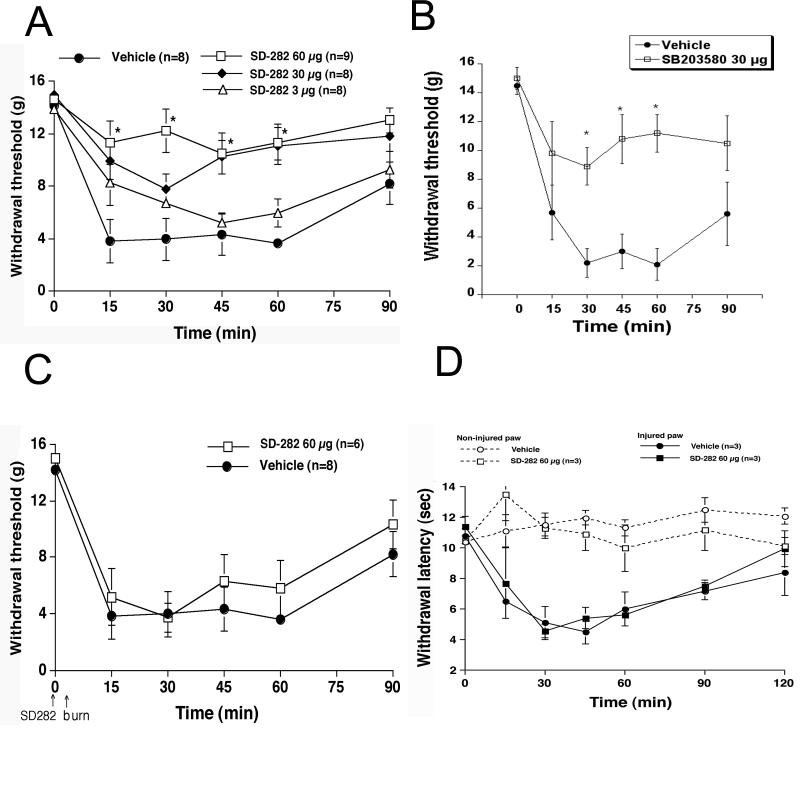
Next, we performed behavioral experiments to determine whether p38 MAPK was involved in spinal nociceptive signaling after peripheral burn-injury. We first tested for secondary mechanical allodynia. Basal withdrawal thresholds were between 14.0-15.0 g for all cohorts prior to drug injection. After the first-degree burn, there was a substantial decrease in thresholds in the burned paws of vehicle-treated rats (Fig. 4A). This increase in sensitivity was of the same magnitude as that seen in previous experiments in which rats did not have intrathecal catheters (Jones and Sorkin 1998).
Figure 4.
Intrathecal pretreatment, but not post treatment with p38 MAPK inhibitors reduced secondary mechanical allodynia elicited by first-degree burn. (A) Mechanical withdrawal thresholds (grams) in vehicle-treated rats (closed circle) and rats treated with 3 μg SD-282 (open triangle); 30 μg SD-282 (closed diamond); and 60 μg SD-282 (open square). (B) Mechanical withdrawal thresholds (grams) in vehicle-treated rats (closed circle) or those pre-treated with 30 μg SB203580 (open square). (C) Mechanical thresholds (grams) in rats after intrathecal post treatment with vehicle (closed circle) or 60 μg SD-282 (open square). For all behavior studies, (n = 8-9 rats/group) * indicates significance p<0.01. (D) Intrathecal pretreatment with p38 MAPK inhibitor did not reduce primary hyperalgesia (thermal) after first-degree burn. Withdrawal latencies (seconds) in uninjured rats that received vehicle (open circle) or 60 μg SD-282 (open square). Withdrawal latencies (seconds) in burned rats (B) after intrathecal pretreatment with vehicle (closed circle) or 60 μg SD-282, (closed square). (n= 3 rats/group).
Spinal SD-282 pre-treatment, to antagonize p38 MAPK, dose-dependently inhibited the injury-induced decreases in withdrawal threshold (Fig. 4A). Compared with vehicle-treated rats, both the 30 and 60 μg doses of SD-282 were effective (p ≤ 0.05). The 3 μg dose was ineffective. Similar anti-allodynia was observed using spinal SB203580 treatment (Fig. 4B). To test whether inhibiting p38 MAPK immediately after thermal injury is efficacious, we treated rats intrathecally with SD-282, 5 min after burn injury. Figure 4C shows that post-treatment with SD-282 was not effective at inhibiting the decrease in withdrawal thresholds.
Thermal escape experiments confirmed that the first-degree burn produced primary hyperalgesia (at the site of injury). Intrathecal pretreatment with the highest dose of SD-282, which was effective in the secondary hyperalgesia paradigm, did not block development of primary thermal hyperalgesia in the injured paw (Fig. 4D). Examination of latencies in the contralateral paws of these rats indicated that spinal SD-282 did not regulate acute processing of thermal stimuli.
Discussion
Following a first-degree burn of the paw, primary thermal hyperalgesia is observed; this is mediated at least in part by peripheral sensitization (Jun and Yaksh 1998). A marked secondary tactile allodynia, mediated at least in part by spinal sensitization, also develops (Nozaki-Taguchi and Yaksh 1998). Although the spinal pathways responsible for these changes in nociception remain incompletely understood, Ca2+ permeable AMPA/kainate receptors have been implicated (Jones and Sorkin 2005). This distinguishes this form of injury from other model systems in which spinal NMDA receptors are principally involved. Given the unique aspects of this model system, we undertook studies to determine the role of p38 MAPK in the development of allodynia and hyperalgesia.
Our results demonstrated robust activation of p38 MAPK in spinal microglia following first-degree burn to the hindpaw. Activation of microglial p38 MAPK appears to be a necessary component of spinal sensitization and the resultant pain behavior occurring in a number of animal models of pain including phase 2 of the formalin test and nerve injury (Jin et al. 2003; Kim et al. 2002; Schafers et al. 2003; Svensson et al. 2003b; Tsuda et al. 2004). Thus, p38 MAPK may serve as a common downstream mediator of events triggered by Ca2+ influx through activated NMDA or AMPA receptors. Indeed, intrathecal administration of NMDA by itself results in p38 MAPK phosphorylation in microglia, and to a lesser extent in lamina II neurons, as well as pain-related behavior (Svensson et al. 2003a). In addition, inhibition of p38 MAPK protects neurons from mild, but not severe NMDA-receptor mediated excitotoxic injury (Legos et al. 2002).
Two separate p38 MAPK pharmacological inhibitors were effective at inhibiting the development of burn-induced tactile allodynia when administered as intrathecal pretreatments. However, when p38 MAPK was administered shortly after thermal injury, no efficacy was observed. These results suggest that activated p38 MAPK impacts on downstream mediators very early after injury. Once the downstream mechanisms are initiated, subsequent p38 MAPK inhibition is ineffective. Similarly, the Ca2+ permeable AMPA/kainate receptor antagonists, joro spider toxin and philanthotoxin, also are effective only when administered prior to injury (Jones and Sorkin 2004). While this co-variance does not prove a causal link, it supports the hypothesis that, in this model, p38 MAPK activation occurs as a result of Ca2+ permeable AMPA/kainate receptor activation. Both events occur rapidly and trigger stable changes that impact on pain-related behavior long after antagonists become ineffective.
Our finding that p38 MAPK is activated in microglia after first-degree burn is consistent with studies in peripheral nerve injury (Jin et al. 2003; Tsuda et al. 2004) and peripheral inflammation (Svensson et al. 2003b). We did observe P-p38 MAPK in a small number of white matter oligodendrocytes and lamina II neurons after thermal injury, however, the significance of these findings remains unclear. Activation of p38 MAPK could occur by a number of pathways. Release of ATP, TNF-α and/or fractalkine or several other mediators secondary to neuronal activation could be responsible (Tsuda et al. 2003; Watkins et al. 2001). We also noted the presence of P-p38 MAPK in dorsal column microglia as a function of peripheral injury. Similar findings were observed after spinal nerve ligation (Terayama et al. 2008). The gracile nucleus has been suggested to be important in the pathogenesis of tactile allodynia (Bian et al. 1998; Sun et al. 2001). Whether P-p38 MAPK signaling at this location contributes in regulating secondary tactile allodynia remains unknown.
In summary, burn-induced tactile allodynia, which is mediated by spinal Ca2+ permeable AMPA/kainate receptors, results in activation of glial p38 MAPK. Activated p38 MAPK appears to serve as an essential mediator of pain-related behavior. This signaling pathway appears to be critical for the development of allodynia.
Acknowledgments
Grant information: This work was supported by NIH NINDS R01 NS41580 to L.S.S. and NIH NINDS R01 NS041983 to W.M.C.
References
- Bian D, Ossipov MH, Zhong C, Malan TP, Jr, Porreca F. Tactile allodynia, but not thermal hyperalgesia, of the hindlimbs is blocked by spinal transection in rats with nerve injury. Neurosci Lett. 1998;241(2-3):79–82. doi: 10.1016/s0304-3940(98)00051-2. [DOI] [PubMed] [Google Scholar]
- Campana WM, Hiraiwa M, Addison KC, O’Brien JS. Induction of MAPK phosphorylation by prosaposin and prosaptide in PC12 cells. Biochem Biophys Res Commun. 1996;229(3):706–712. doi: 10.1006/bbrc.1996.1869. [DOI] [PubMed] [Google Scholar]
- Campana WM, Hiraiwa M, O’Brien JS. Prosaptide activates the MAPK pathway by a G-protein-dependent mechanism essential for enhanced sulfatide synthesis by Schwann cells. Faseb J. 1998;12(3):307–314. doi: 10.1096/fasebj.12.3.307. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Cohen P. The search for physiological substrates of MAP and SAP kinases in mammalian cells. Trends Cell Biol. 1997;7(9):353–361. doi: 10.1016/S0962-8924(97)01105-7. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hefferan MP, Kucharova K, Kinjo K, Kakinohana O, Sekerkova G, Nakamura S, Fuchigami T, Tomori Z, Yaksh TL, Kurtz N, Marsala M. Spinal astrocyte glutamate receptor 1 overexpression after ischemic insult facilitates behavioral signs of spasticity and rigidity. J Neurosci. 2007;27(42):11179–11191. doi: 10.1523/JNEUROSCI.0989-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci. 1999;2(12):1114–1119. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- Ji RR, Rupp F. Phosphorylation of transcription factor CREB in rat spinal cord after formalin-induced hyperalgesia: relationship to c-fos induction. J Neurosci. 1997;17(5):1776–1785. doi: 10.1523/JNEUROSCI.17-05-01776.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S-X, Z-Y Z, Woolf C, Ji R. p38 mitogen-activated protein kinase Is activated after a spinal nerve ligation in spinal Cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23(10):4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL, Sorkin LS. Systemic gabapentin and S(+)-3-isobutyl-gamma-aminobutyric acid block secondary hyperalgesia. Brain Res. 1998;810(1-2):93–99. doi: 10.1016/s0006-8993(98)00890-7. [DOI] [PubMed] [Google Scholar]
- Jones TL, Sorkin LS. Calcium-permeable alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate receptors mediate development, but not maintenance, of secondary allodynia evoked by first-degree burn in the rat. J Pharmacol Exp Ther. 2004;310(1):223–229. doi: 10.1124/jpet.103.064741. [DOI] [PubMed] [Google Scholar]
- Jones TL, Sorkin LS. Activated PKA and PKC, but not CaMKIIalpha, are required for AMPA/Kainate-mediated pain behavior in the thermal stimulus model. Pain. 2005;117(3):259–270. doi: 10.1016/j.pain.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Jun JH, Yaksh TL. The effect of intrathecal gabapentin and 3-isobutyl gamma-aminobutyric acid on the hyperalgesia observed after thermal injury in the rat. Anesth Analg. 1998;86(2):348–354. doi: 10.1097/00000539-199802000-00025. [DOI] [PubMed] [Google Scholar]
- Kim SY, Bae JC, Kim JY, Lee HL, Lee KM, Kim DS, Cho HJ. Activation of p38 MAP kinase in the rat dorsal root ganglia and spinal cord following peripheral inflammation and nerve injury. Neuroreport. 2002;13(18):2483–2486. doi: 10.1097/00001756-200212200-00021. [DOI] [PubMed] [Google Scholar]
- Koppelman B, Webb HK, Medicherla S, Almirez R, Feng Y, Chavez JC, Mao CP, Nguyen A, Liu YW, Kapoun AM, Muiru G, Huang YA, Dugar S, Mavunkel BJ, Lim DW, Chakravarty S, Luedtke G, Protter AA, Higgins LS. Pharmacological Properties of SD-282 - An alpha-Isoform Selective Inhibitor for p38 MAP Kinase. Pharmacology. 2008;81(3):204–220. doi: 10.1159/000112865. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Thalhammer JG, Torebjork HE, Robinson CJ. Peripheral neural mechanisms of cutaneous hyperalgesia following mild injury by heat. J Neurosci. 1982;2(6):765–781. doi: 10.1523/JNEUROSCI.02-06-00765.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legos JJ, McLaughlin B, Skaper SD, Strijbos PJ, Parsons AA, Aizenman E, Herin GA, Barone FC, Erhardt JA. The selective p38 inhibitor SB-239063 protects primary neurons from mild to moderate excitotoxic injury. Eur J Pharmacol. 2002;447(1):37–42. doi: 10.1016/s0014-2999(02)01890-3. [DOI] [PubMed] [Google Scholar]
- Ma W, Quirion R. Partial sciatic nerve ligation induces increase in the phosphorylation of extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) in astrocytes in the lumbar spinal dorsal horn and the gracile nucleus. Pain. 2002;99(1-2):175–184. doi: 10.1016/s0304-3959(02)00097-0. [DOI] [PubMed] [Google Scholar]
- Milligan ED, O’Connor KA, Armstrong CB, Hansen MK, Martin D, Tracey KJ, Maier SF, Watkins LR. Systemic administration of CNI-1493, a p38 mitogen-activated protein kinase inhibitor, blocks intrathecal human immunodeficiency virus-1 gp120-induced enhanced pain states in rats. J Pain. 2001;2(6):326–333. doi: 10.1054/jpai.2001.26174. [DOI] [PubMed] [Google Scholar]
- Nozaki-Taguchi N, Yaksh TL. A novel model of primary and secondary hyperalgesia after mild thermal injury in the rat. Neurosci Lett. 1998;254(1):25–28. doi: 10.1016/s0304-3940(98)00648-x. [DOI] [PubMed] [Google Scholar]
- Nozaki-Taguchi N, Yaksh TL. Pharmacology of Spinal Glutamatergic Receptors in Post-Thermal Injury- evoked Tactile Allodynia and Thermal Hyperalgesia. Anesthesiology. 2002;96(3):617–626. doi: 10.1097/00000542-200203000-00018. [DOI] [PubMed] [Google Scholar]
- Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003;23(7):2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholtz J, Abele A, Marian C, Haussler A, Herbert TA, Woolf CJ, Tegeder I.Low-dose methotrexate reduces peripheral nerve injury-evoked spinal micoglia activation and neuropathic pain behavior in rats Pain 2008Jan 19 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone DA, Sorkin LS, Oh UT, Chung JM, Owens C, LaMotte RM, Willis WD. Neurogenic hyperalgesia: central correlates in reponses of spinothalamic tract neurons. J Neurophysiol. 1991;66:228–246. doi: 10.1152/jn.1991.66.1.228. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Doom CM, Maruyama KP, Nanigian DB. Secondary hyperalgesia in the rat first degree burn model is independent of spinal cyclooxygenase and nitric oxide synthase. Eur J Pharmacol. 2008;587:118–123. doi: 10.1016/j.ejphar.2008.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin LS, Yaksh TL, Doom CM. Mechanical allodynia in rats is blocked by a Ca2+ permeable AMPA receptor antagonist. Neuroreport. 1999;10(17):3523–3526. doi: 10.1097/00001756-199911260-00011. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Yaksh TL, Doom CM. Pain models display differential sensitivity to Ca2+-permeable non-NMDA glutamate receptor antagonists. Anesthesiology. 2001;95(4):965–973. doi: 10.1097/00000542-200110000-00028. [DOI] [PubMed] [Google Scholar]
- Sun H, Ren K, Zhong CM, Ossipov MH, Malan TP, Lai J, Porreca F. Nerve injury-induced tactile allodynia is mediated via ascending spinal dorsal column projections. Pain. 2001;90(1-2):105–111. doi: 10.1016/s0304-3959(00)00392-4. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Hua XY, Protter AA, Powell HC, Yaksh TL. Spinal p38 MAP kinase is necessary for NMDA-induced spinal PGE(2) release and thermal hyperalgesia. Neuroreport. 2003a;14(8):1153–1157. doi: 10.1097/00001756-200306110-00010. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, Catalano R, Feng Y, Protter AA, Scott B, Yaksh TL. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem. 2003b;86(6):1534–1544. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- Terayama R, Omura S, Fujisawa N, Yamaai T, Ichikawa H, Sugimoto T. Activation of microglia and p38 mitogen-activated protein kinase in the dorsal column nucleus contributes to tactile allodynia following peripheral nerve injury. Neuroscience. 2008;153(4):1245–1255. doi: 10.1016/j.neuroscience.2008.03.041. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia. 2004;45(1):89–95. doi: 10.1002/glia.10308. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2×4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424(6950):778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24(8):450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Fitzgerald M. Somatotropic organization of cutaneous afferent terminals and dorsal horn neuronal receptive fields in the superficial and deep laminae of the rat lumbar spinal cord. J Comp Neurol. 1986;251(4):517–31. doi: 10.1002/cne.902510407. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114(1-2):149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]