Abstract
The somatic mutation hypothesis of cancer predicts that reducing the frequency of mutations induced by carcinogens will reduce the incidence of cancer. To examine this, we developed an antimutator strategy based on the manipulation of the level of a protein required for mutagenic bypass of DNA damage induced by the ubiquitous carcinogen benzo[a]pyrene. The expression of this protein, REV1, was reduced in mouse cells using a vector encoding a gene-specific targeting ribozyme. In the latter cells, mutagenesis induced by the activated form of benzo[a]pyrene was reduced over 90%. To examine if REV1 transcripts could be lowered in vivo, the plasmid was complexed with polyethyleneimine, a non-viral cationic polymer, and delivered to the lung via aerosol. The endogenous REV1 transcript in the bronchial epithelium as determined by quantitative real-time PCR in laser capture microdissected cells was reduced by 60%. There was a significant decrease in the multiplicity of carcinogen induced lung tumors from 6.4 tumors/ mouse to 3.7 tumors/ mouse. Additionally, REV1 inhibition completely abolished tumor formation in 27% of the carcinogen-exposed mice. These data support the central role of the translesion synthesis pathway in the development of lung cancer. Further, the selective modulation of members of this pathway presents novel potential targets for cancer prevention.
Keywords: REV1, benzo[a]pyrene, mutagenesis, lung cancer, translesion synthesis
Introduction
Genotoxic carcinogens in tobacco smoke increase an individual’s risk of developing lung cancer. It is generally recognized that point mutations in critical growth control genes are centrally involved in the development of genetic instability characteristic of cells derived from these and other cancers(1;2). Most mutations induced by genotoxic carcinogens occur when DNA containing unrepaired damage is replicated during S-phase of the cell cycle (3;4). Recently, insights have been gained into the mechanisms used by cells to complete the replication of damaged genomes and the induction of mutations(5–7). These mechanisms have been studied most intensively in the budding yeast, Saccharomyces cerevisiae (8). In this organism, virtually all mutations induced by bulky DNA adducts are dependent on the activity of DNA polymerase ζ(pol ζ ) acting in concert with another protein encoded by the REV1 gene(9) This gene encodes a protein with homology to Y-family DNA polymerases, and is required for mutagenic translesion DNA synthesis (TLS) past adducts induced in the DNA by a variety of mutagens(10;11). Although REV1 has deoxycytidyl transferase activity, data indicate that this activity of the protein is not required for TLS, since a mutant that retains polymerase activity is deficient in mutagenesis(12). Since the protein interacts with homologous Y-family DNA polymerases and the REV7 subunit of pol ζ, REV1 is presumed to have a scaffolding function that serves to tether error-prone polymerases to the site of stalled DNA replication forks(13). These data support the conclusion that REV1 is an integral part of the mutagenic lesion bypass mechanism.
The strategies used by yeast cells to complete the replication of damaged genomes appear to have been appreciably conserved in higher eukaryotes. The human homologs REV1 (14), REV3 (15;16) and REV7 (17) have been cloned, and the human REV1 protein purified and studied in vitro (10). These data indicate that the function of REV1 in higher eukaryotes has been conserved. Further, the predicted sequence of mouse REV1 is 86% identical to that of the human protein. These similarities support the overall hypothesis that lowering the level of REV1 in mouse cells will alter the mutagenic responses after exposure to carcinogens such as benzo[a]pyrene (B[a]P), although this has not been systematically studied to date. It follows that if strategies can be devised to reduce the level of mutagenesis in vivo, then the incidence of carcinogen-induced cancer may be similarly reduced.
To examine this question we constructed a plasmid that expresses a ribozyme directed against murine REV1, and which incorporates structural elements that are expected to direct the ribozyme to the nucleus. Expression of this construct in vitro resulted in reduced levels of REV1 mRNA and in greatly reduced mutant frequencies induced by the activated form of B[a]P. To determine the efficacy of REV1 inhibitory ribozymes against the target in vivo, we complexed the expression plasmid with the cationic polymer polyethyleneimine and delivered it via non-invasive aerosol therapy. Laser capture microdissection was used to isolate bronchial epithelial cells from lung tissue, and quantitative real-time PCR analysis revealed a ~50–60% decrease in the REV1 transcript. This delivery methodology was used before or after injecting A/J mice with B[a]P to induce lung tumors(18;19). Mice were sacrificed 28 weeks later, and tumor burden, individual tumor size, and tumor location were elucidated. There was a statistically significant (p <.02) reduction in tumor multiplicity in animals that received the aerosolized expression plasmid before carcinogen treatment. In addition to having a lower overall tumor multiplicity, REV1 transcript inhibition prevented tumor formation in 27% of the carcinogen treatment group. The reduction in incidence was also statistically significant (p<0.05. All tumors were histopathologically classified as adenomas, and this was not affected by treatment. Collectively, the data presented here support the use of non-invasive aerosolized PEI gene therapy and the use of gene-specific ribozyme inhibitory RNAs in the chemoprevention of carcinogen induced lung cancer.
Materials and Methods
Design of ribozymes and recombinant plasmid constructs
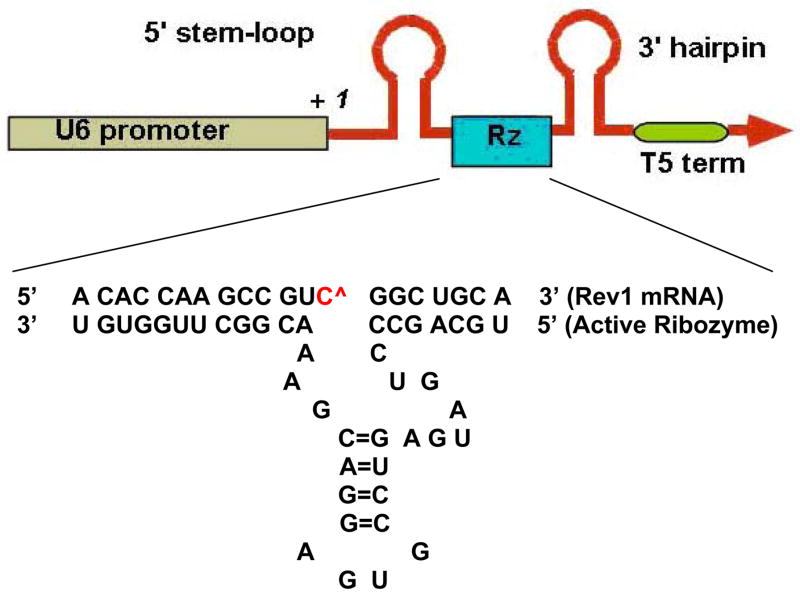
Hammerhead ribozymes were designed according to established structural and sequence principles, maintaining the conserved catalytic core and variable target-specific 5′- and 3′-arms. A GUC cleavage site at position 407 on the mouse Rev1 mRNA (homologous to position 618 on the human REV1 mRNA) was chosen based on potentially accessible loop structures of the mouse REV1 mRNA, as modeled with the MFOLD program(20). BLAST search determined that the targeted sequence for mouse REV1 around the GUC cleavage site has no homology to any other murine genomic sequence and is therefore unique to the mouse REV1 target gene. The ribozyme was cloned into pU6+27 vector containing a human U6 snrp RNA promoter/5′-stem-loop/3′-terminator element (21), a gift from Dr. Wolfgang Zacharias, University of Louisville. A schematic of the construct is presented in Fig. 1.
Figure 1.
Ribozyme expression cassettes designed to target REV1 transcripts i the nucleus. The expression cassette is driven by the murine U6 promoter and contains 5′ and 3′ stem-loop structures on either side of the cloning site. The ribozyme-coding sequence was inserted between the loop elements, replacing the native U6 RNA coding region. Sequence of the target mRNA (top sequence) and the catalytically active ribozyme (bottom sequence) are shown. The two sequence recognition arms are shown base paired with the mouse REV1 target, surrounding the cleavage site at base 407, marked as C^. The central catalytic core is invariant.
Custom-synthesized DNA oligonucleotides coding for the ribozyme were annealed and cloned between the SalI and XbaI sites of the expression cassette, generating the ribozyme clone Rz407pU6. Thus, the cloned ribozyme inserts are transcribed from the constitutive U6 promoter. The sequences coding for ribozymes were as follows; the underlined regions correspond to the catalytic ribozyme core; the bold and italic regions to restriction site ends (Fig. 1).
Rz407pU6 upper strand:
5′ TCGACTGCAGCCCTGATGAGTCCGTGAGGACGAAACGGCTTGGTGTT 3′
Rz407pU6 lower strand:
5′ CTAGAACACCAAGCCGTTTCGTCCTCACGGACTCATCAGGGCTGCAG 3′
The presence of the ribozyme insert in clones was confirmed by sequencing.
Cells and cell culture
Primary murine fibroblasts were established from 8–9 week old male A/J mice using standard techniques, as published (22). Briefly, using an autoclaved ear punch and forceps, small pieces of ear from anesthetized mice were removed and treated with collagenase (2000U/ ml) for 4h at 37°C with gentle shaking. The cells were dispersed with a sterile pipette, and pelleted by centrifugation at 200 X g for 10 min at 4°C. The cells were resuspended and plated in MEM-α medium (Gibco) supplemented with 10% fetal calf serum (Hyclone), 2mM glutamine, nonessential amino acids (Gibco), penicillin (100 U/ml), streptomycin (100 μg/ml) and Fungizone (Gibco, 1 μg/ml). The medium was changed within 48h, and the Fungizone was omitted. The cells were maintained in exponential growth, and were assayed for cytotoxic and mutagenic responses to benzo[a]pyrene-7,8-diol, 9,10-epoxide (BPDE, NCI Chemical Carcinogen Repository, Midwest Research Institute) at an early passage, typically within 6–8 doublings after the primary cultures were established. For selection of thioguanine-resistant (TGr) clones, cells were plated in DMEM medium with 5% fetal calf serum, 5% supplemented calf serum, penicillin and streptomycin as above), and 20 μM 6-TG. Cloning efficiency at the time of selection was determined by plating cells at cloning density in the same medium, but omitting 6-TG.
Electroporation of cells and qRT-PCR
To examine the effect of the expression constructs on endogenous mouse REV1 levels in cells in culture, the plasmids were electroporated into cells using a Nucleofector 1 device (Amaxa Inc, Gaithesrsburg, MD). Following the manufacturer’s instructions, 1x 106 cells were suspended in Nucleofector V ™ Solution (Amaxa Inc, Gaithesrsburg, MD) with 15μg of either R407pU6 or the empty plasmid. Electroporation was accomplished using programs V13 for mouse primary cells. Subsequently, the cell suspension was plated in complete medium without antibiotics. 48h later the cells were harvested and total RNA was isolated using RNEasy Mini Kit (Qiagen Inc, Valencia, CA) according to manufacturer’s instructions. The RNA was analyzed and quantified using a Bioanalyzer 2100 (Agilent Technologies) and reverse transcribed using reverse transcriptase (Promega), oligo (dT) primers and 2.5 μg of DNase I. The effect of the ribozyme on cellular levels of Rev1 mRNA was then determined by qRT-PCR, using TaqMan technology and a gene-specific probe. Briefly, qRT-PCR using TaqMan Universal PCR Mix (Applied Biosystems, Foster City, CA); gene specific MGB TaqMan Probe (Applied Biosystems); mouse REV1 primers; TaqMan rodent GAPDH control reagents (Rodent GAPDH internal control probe, forward and reverse primers; Applied Biosystems) was performed using an ABI PRISM 7900 Sequence Detection System. The TaqMan gene-specific probe and primers were designed using the ABI Prism Primer Express Software Version 1.5 (Applied Biosystems, Foster City, CA) and the sequence of the mouse REV1 primers are as follows. The product length is 67 bp.
mRev1Frt (forward) - 5′CCGGGAGTTGGACGTTCA3′
mRev1Rrt (reverse)- 5′GCAAATCTCCACAAGTCTTAATCC3′
The sequence of the mREV1 TaqMan Probe is as follows.
6FAM-AGCTTGCATCTTCG-MGBNFQ
Relative amounts of target RNA for duplicate sample runs were quantitated with the instrument software and normalized to the corresponding GAPDH values. Fold change values were calculated using the 2-(delta delta (CT)) method (23).
Exposure to BPDE
The culture medium was aspirated and the cells were washed with sterile phosphate buffered saline (PBS, pH 7.4) and replaced with serum free medium. Powdered BPDE was dissolved in anhydrous tetrahydrofuran (THF) at a concentration of 5mM, which was diluted to single-use aliquots of 150μM and stored under nitrogen at −80°C. The stock solution was added to the culture medium to a final concentration of 0.150μM. After 1 hr incubation at 37°C, the medium was replaced with complete medium. The control cells were exposed to solvent only.
PEI mediated cell transfections
Polyethyleneimine (PEI), high molecular weight (water free), was obtained from Sigma-Aldrich (CAS9002-98-6). Stock solutions were prepared at a concentration of 4.3mg/mL in 3xdH2O. Experiments were performed using gWiz™ luciferase (Aldeveron) complexed with PEI to optimize the charge ratio expressed as PEI nitrogen:DNA phosphorous. For cell culture assays, transfection reagents were prepared 20min before transfection using 2μg of DNA complexed with PEI at varying concentrations. DNA and PEI were both mixed with 100μl Opti-MEM®I before combining solutions in a drop-wise fashion under vigorous vortexing. The murine cells seeded at a density of 2x104 cm2 were exposed to appropriate dilutions of DNA:PEI complexes for 24h. Opti-MEM®I transfection media was removed and replaced with complete media for 24–48h before subsequent analysis with a luminometer (PharMingen Monolight™ 3010). The optimal DNA:PEI ratio determined for the luciferase expression system was confirmed with qRT- PCR as described above after PEI-mediated transfection of Rz407pU6.
In vivo nebulization and carcinogenesis procedures
Eight week-old A/J mice (Jackson Laboratories, Bar Harbor ME) were housed with 12h day/night cycles, and fed normal chow. Groups of five were exposed to PEI mixtures in a 22 x 9 x 10cm anesthesia chamber connected to an Aero-Mist nebulizer (CIS-US Bedford, MA). The mice were not restrained. The desired amount of DNA (2mg, endotoxin free) was mixed in 5ml of 3 x dH2O per nebulization experiment. PEI was also mixed with 5ml 3xdH2O. The DNA was slowly added to the PEI solution with vigorous vortexing after every 1 ml added. The solution was incubated at room temperature for 20 min prior to nebulization. Normal air supplemented with 5% CO2 at a 10 L/min flow rate was used to deliver 2mg of DNA complexed with PEI in each treatment. 4mg of DNA was delivered over 2 treatments 24h apart. 48hrs after the first PEI treatment all mice were administered 100mg of B[a]P or vehicle IP. The control groups received cornoil only and were exposed to diluted PEI alone or to PEI/pU6 during the nebulization procedure. The remaining mice, which received B[a}P, received by aerosol either Rz407pU6/PEI or pU6/PEI as appropriate. Animals were sacrificed ~28 wks post carcinogen injection.
Isolation and quantitation of lung REV1 mRNA
To measure REV1 levels after ribozyme exposure, lungs were dissected 24hrs after animals received 2mg of the plasmid. Animals were euthanized, lungs removed and flash frozen in O.C.T. Compound (Tissue-Tek, Sakura, Torrence, CA) using a 2-methylbutane (Sigma-Aldrich CAS 78-78-4) bath suspended in liquid nitrogen. The frozen lungs were immediately transported in dry ice and sectioned in RNase free conditions. Lung sections were stained with HistoGene™ LCM Frozen Section Staining Kit (Arcturus, Mt. View, CA) and cells captured on an Arcturus Pixcell® IIe Laser Capture Microdissection (LCM) System instrument using CapSure™ HS LCM Caps. RNA was isolated using PicoPure™ RNA Isolation Kit according to the manufacturer’s directions, and the quality and quantity of the isolated RNA confirmed with an Agilent Bioanalyzer. The RNA was stored at −80°C prior to performing qRT-PCR as described above.
Tumor burden/ Pathology
To determine tumor burden, lungs from sacrificed animals were placed in Tellyesniczky’s Solution (90% ethanol, 5% formalin <10%>, and 5% acetic acid) for 24h followed by immersion in 70% ethanol for a minimum of 24h. Individual lobes of the lungs were separately dissected and gross tumor counts in each lobe of the lungs determined using a light box. Pathology was performed on paraffin embedded sections stained with H&E.
Statistics
Statistical analyses consisted of a negative binomial regression and planned post hoc comparisons between individual groups for tumor multiplicity count data. One-way ANOVA and (t-tests) between individual groups were used elsewhere when appropriate. Tumor incidence data were analyzed with a Fisher’s exact one sided test. Data with a trend p value of 0.05 or less were considered significant.
Results and Discussion
Targeting gene-specific ribozymes to murine REV1 mRNA greatly suppresses endogenous REV1 expression
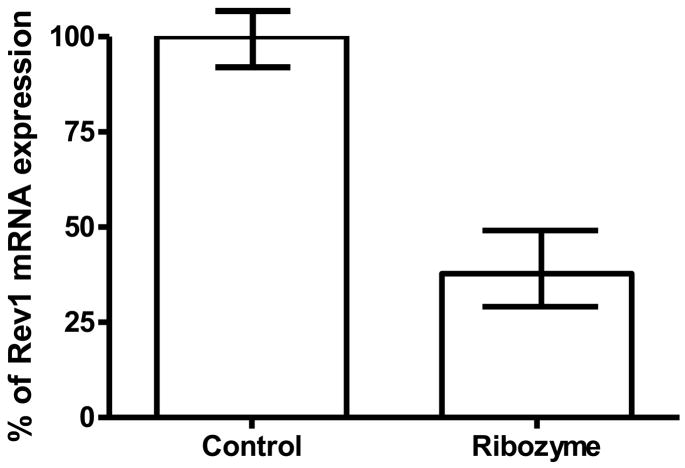
We chose to target a sequence of the mouse REV1 mRNA surrounding position 407 that is homologous to region 618 in human REV1 and has been shown to be readily cleaved by a ribozyme in vitro and in intact cells (24;25). Additionally, this sequence is predicted to be in an unpaired loop region by the MFOLD program (see Methods and Materials). The ribozyme sequence recognition arms and the invariant core are shown in Fig.1, and the predicted cleavage site is between C407 and G408. We cloned DNA encoding the ribozyme sequence into the vector pU6+27 (21) that contains a human U6 snrp RNA promoter/5′-stem-loop/3′-terminator element. The biological function of the natural U6 gene/promoter system is to transcribe high levels of U6 RNA, which is involved in the RNA splicing process. It is known to produce large amounts of short stable RNAs, and these will be localized in the cell nucleus due to the localization signal incorporated in the 5′ stem/loop structural element of the U6 transcript (26–28). Thus, this U6 expression cassette should yield high levels of ribozyme RNAs that will be retained in the nucleus. Expression of this ribozyme construct in mouse primary cells resulted in over 50% suppression of REV1 mRNA (Fig. 2). This reduced expression can be interpreted in light of the fact that for any given designed ribozyme element, its intracellular efficiency is mainly determined by the ratio of ribozyme to target copy numbers, their stabilities and turnover rates, the folded target mRNA structure and accessibilities of cleavage sites, and the presence of potentially interfering RNA-protein complexes. Although ribozyme and target RNA folding can be modeled in silico as guidance, as was done in this study, none of these intracellular factors can be measured nor predicted accurately.
Figure 2.
REV1 mRNA levels in mouse primary fibroblasts 48 h after electroporation of plasmids, determined by quantitative RT-PCR using GAPDH as an internal control and the (Δ−Δ(CT)) method as described in the text. The value represents the average fold change and standard deviation derived from three independent experiments. The real-time PCR was done in triplicate. Control, electroporation of empty vectors (no ribozyme coding sequence) pU6+27, set to 100%. Ribozyme, electroporation of Rz407pU6, resulting in 46± 10% of control, which was statistically significant (p < 0.05, two tailed Student’s T-test, two sample unequal variance).
Reduced expression of endogenous mouse REV1 mRNA greatly reduced BPDE-induced mutagenicity in mouse primary dermal fibroblasts
Our in vitro studies show that reducing the endogenous level of REV1 in human cells has a profound antimutator effect and also that targeting REV1 in cell culture does not enhance the cytotoxic sensitivity of the cells to genotoxic carcinogens (14;24;25). To examine if this is also true of mouse cells, we compared the frequency of mutations induced by BPDE in the HPRT gene of primary murine cells with normal and reduced levels of REV1. As summarized in Table I, at equicytotoxic doses the frequency of mutations induced in the HPRT gene was greatly reduced, from 195 induced mutants per 106 clonable cells in the wild-type background to 23 mutants per 106 clonable cells in the REV1 deficient background. This represents ~91% reduction in the induced mutant frequency and supports the central role of REV1 in mutagenesis.
The molecular role played by REV1 in carcinogen-induced mutagenesis has not been fully elucidated. The protein is a Y-family DNA polymerase that is associated with translesion replication and was first identified in budding yeast. In vitro, the purified protein is a dCMP transferase that catalyzes the insertion of dCMP when it encounters a template guanine, a guanine with a large adduct at the C8 or N2 position, or an abasic site. In contrast, it is completely unresponsive to thymine-thymine cyclobutane pyrimidine dimers or to 6-4 photoproducts (10). These are principal lesions induced in DNA by UV, yet REV1 is required for UV mutagenesis. Therefore, the role played by REV1 in TLS is considered to be independent of the polymerase activity. This is underscored by data that indicate a mutation in the N-terminal BRCT domain retains catalytic function but is deficient in mutagenesis (29). This is consistent with earlier observations in yeast (9,10). It has been postulated that REV1 acts as a scaffold through interactions with other Y-family polymerases and DNA polymerase ζ. The regions of REV1 that are important for these interactions have been variously reported to be the N-terminal BRCT domain (29) the extreme C-terminus (30), or a region in the more proximal C-terminus(31). Further, the protein forms foci in the nuclei of cells that enter S-phase with DNA damage (25). These foci colocalize with other Y-family DNA polymerases (32) as well as with the ubiquitin ligase RAD18. The latter protein is required for signaling TLS polymerases to stalled replication forks by directing the RAD6 ubiqutiin conjugase to PCNA (reviewed in (33)). Of particular interest in this regard is the role of DNA polymerase iota (pol ι) in lung carcinogenesis. The gene coding for this polymerase is linked to the pulmonary adenoma resistance 2 (PAR2) locus on mouse chromosome 18. This polymerase has been implicated in the high degree of susceptibility of the A/J strain used in these studies. This is because there are 10 amino acid changes in the enzyme derived from this strain compared with that from lung cancer-resistant BALB/c mice. The enzymes from both strains are active, but in vitro primer extension studies on an undamaged template indicate a higher efficiency of insertion of dG across from a template T (34). Data indicate that pol ι is error-prone when bypassing UV photoproducts, which is especially noted when pol eta is absent (35), and prelimnary data in mouse cells indicate that this is also true of BPDE-induced adducts.(Stallons and McGregor, unpublished).
In any case, our data indicate that REV1 is central to the process of mutagenic TLS in mouse cells as in human cells, and that the level of the protein can be reduced within the cells. Further, reducing REV1 greatly reduces their mutagenic responses without enhancing their sensitivity to carcinogens. This property is important when contemplating the use of chemopreventive agents in populations that have been exposed to a carcinogen but are otherwise healthy. Using these vectors to reduce REV1 in vivo in tissues that are exposed to carcinogens, such as the bronchoalveolar cells of B[a]P-exposed animals, should reduce the mutagenic load and the incidence of cancer. To examine this hypothesis we optimized gene delivery to the murine lung using PEI and reporter plasmids.
Optimization of PEI-reporter genes and PEI-ribozyme transfection in pulmonary fibroblasts and lung tissue
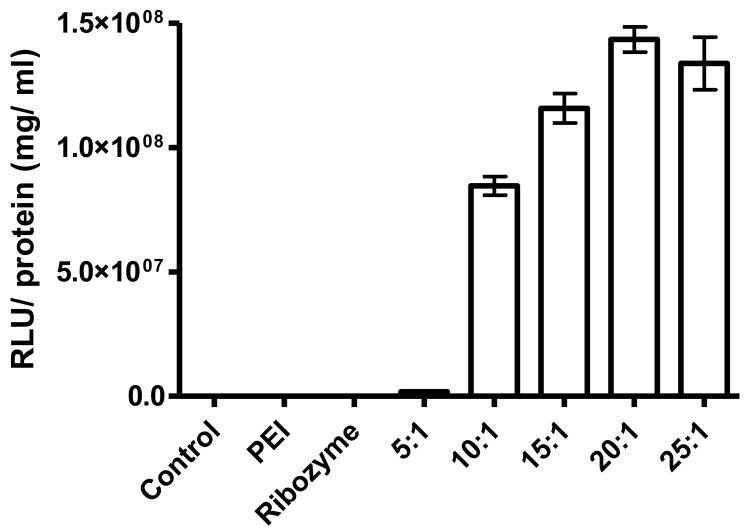
Polyethylenimine (PEI)-mediated gene therapy is an attractive means to deliver a variety of transgene products to the lung(36;37). One major use of the cationic polymer PEI is to complex with DNA, rendering the complex electrostatically neutral. The PEI nitrogen to DNA phosphorous (N:P) ratio has a significant impact on transfection efficiency and must be optimized. As shown in Fig. 3, a N:P ratio of 20:1 was effective in delivering the luciferase reporter plasmid into cells. The aerosolized mixture can successfully deliver transgenes to the lung, and the mixture is being used clinically (38;39). In particular, PEI does not induce cellular protective responses that are characteristic of viral gene delivery. PEI holds therapeutic promise in many pulmonary diseases that are sensitive to exogenous particles and viruses. One important consideration supporting PEI gene therapy in carcinogen-induced mouse models is that it does not appear to be inflammatory to the murine lung (40). This feature is noteworthy since inflammatory reactions have been shown to increase both tumor multiplicity and size in mice of lungs exposed to B[a]P (41). PEI based gene delivery to the lung has been shown to be successful in multiple animal models of human disease, including reestablishment of wild-type p53 function and reduction of the metastatic spread of lung cancers in mice (42–44). Because of these studies, the use of non-viral gene delivery is currently gaining promise in the fields of gene therapy and was used in these studies to aid in the delivery of the REV1 targeting ribozyme plasmid Rz407pU6.
Figure 3.
In vitro expression of PEI/g wiz luciferase in mouse primary fibroblasts 24 hours after PEI-mediated transfection. Primary murine fibroblasts were seeded at a density of 2x104 cm2 in six well plates, the growth media removed and the cells were rinsed in sterile PBS (pH 7.4). Appropriate dilutions of PEI/ Luciferase complex were then added to each well to allow sufficient coverage. The cells were maintained at 37°C for at least 24 hours before lysis and analysis using a luminometer. Graph illustrates significant luciferase expression in the cells using 10–25:1 N:P ratios.
We utilized the whole body exposure approach to deliver aerosolized PEI/ DNA complexes to the murine lung. This exposure method remains a fast reliable method to deliver and sustain transgene expression in the lung(45). REV1 is expressed at low levels, which is a common feature of proteins required for TLS and presumably reflects the deleterious effects of high expression of these proteins on overall genomic integrity. We have determined that REV1 transcript levels are reduced within 48 h of pulmonary exposure to the ribozyme expression construct and remain low for at least 7 days. Plasmids delivered in this way have been reported to have sustained expression for weeks after treatment (46). It should be noted that a plasmid that expressed shRNA that was effective in lowering REV1 in cell culture was not effective in vivo. The reason for this is unknown, but it is possible that the structural elements surrounding the ribozyme stabilize it.
REV1 expression in mouse lung following aerosol delivery of PEI-Rz407pU6 complexes
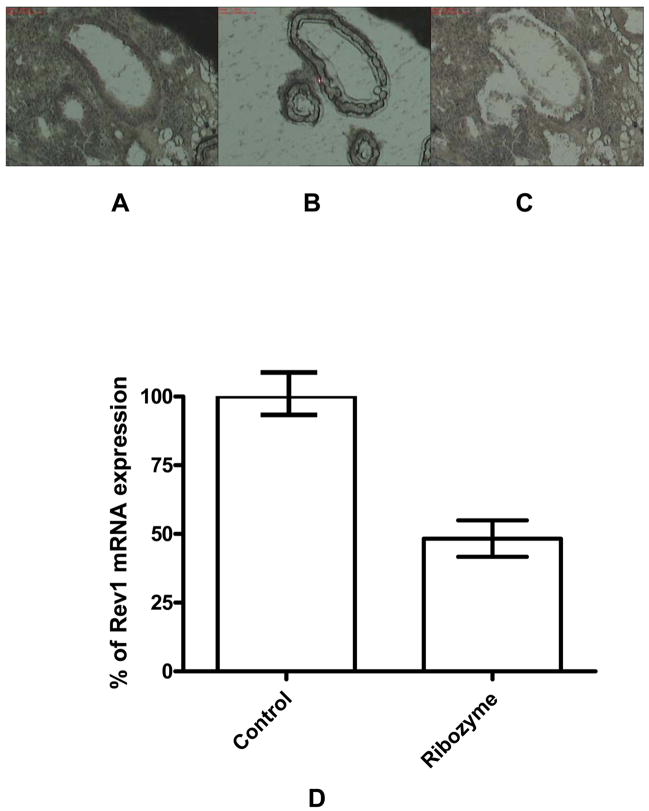
To determine the degree of reduction of REV1 mRNA in the murine lung we treated mice with aerosolized PEI/ Rz407pU6 and analyzed REV1 expression with qRT-PCR. In these kinetic studies, mice were exposed to a single two milligram aerosolized dose of ribozyme plasmid complexed with PEI (20:1 N:P ratio) or PEI /pU6 aerosol alone for 30 min in a whole body exposure chamber. REV1 expression in LCM-isolated bronchial epithelium was examined using qRT-PCR. This methodology is appropriate since PEI has been shown to mediate significant expression of plasmids in bronchial epithelium but not necessarily in lung parenchyma. Using LCM we were able to obtain cells from the bronchial epithelium and analyze REV1 expression (Fig. 4). This tissue has been reported to express plasmids delivered with aerosolized PEI/DNA, and the bronchioalveolar junction harbors stem cells that may be responsible for adenocarcinomas(47;48). We report here a ~50% reduction in REV1 mRNA transcripts in the bronchial epithelium relative to control, using 2 mg of Rz407pU6 delivered over a 30 min exposure (Fig.4D). Together these data support the use of PEI/ DNA based aerosol therapy as a safe and effective method to deliver genes to bronchial epithelium. The reduction in REV1 mRNA after Rz407pU6 transgene expression in murine cells in culture confirms that such a reduction in this transcript greatly lowers the total number of mutations induced in cells after either BPDE (Table 1) or UV (data not shown) exposure. These data support the potential of hammerhead ribozymes to effectively knockdown the REV1 transcript in vivo.
Figure 4.
Relative REV1 expression in bronchial epithelial cells 48 h after inhalation of Rz407pU6 or the corresponding empty vector, complexed with PEI as described in the text. Lungs were dissected from the mice and flash frozen in liquid nitrogen-cooled isobutane. Lung sections were stained with HistoGene™ LCM Frozen Section Staining Kit (Arcturus, Mt. View, CA) and cells captured on an Arcturus Pixcell® LCM instrument using CapSure™ HS LCM Caps. RNA was isolated using PicoPure™ RNA Isolation Kit. A, 10x magnification of tissue before capture. B. 10x view of cells captured. C. 10x view of tissue section after capture. D. REV1 mRNA expression in cells derived from the LCP captures Tissue samples were taken from three separate locations from three mice in each group, and qRT-PCR was done on each sample in triplicate Values presented are an average of these data, plus and minus SEM. The treatment with ribozyme Rz407pU6 lead to an approximate 50% decrease in REV1 transcript in the bronchial epithelium.
Table 1.
Representative BPDE-Induced Cytotoxicity and Mutagenicity Data for Primary Dermal Fibroblasts Derived from A/J Mice
| Cell Line | REV1 expression | BPDE (μM) | Percent Survival | Thioguanine-resistant Clones | No. of Cells Selected X 10−6 | Mutants /106 Clonable Cells2 |
|---|---|---|---|---|---|---|
| B-1 | 100% | 0 | 100 | 0 | 1 | <10 |
| 0.15 | 36 | 86 | 2 | 195 | ||
| B-2 | 15% | 0 | 100 | 0 | 1 | <10 |
| 0.15 | 28 | 12 | 2 | 23 |
Corrected for cloning efficiency on the day of selection ( B-1, 22%; B-2, 26%)
Inhibition of lung tumor incidence and multiplicity after aerosol delivery of PEI-Ribozyme complexes
It would seem inherently obvious that antimutator strategies based on inhibition of mutagenic TLS would reduce the incidence of carcinogen-induced cancer. However, the interplay of the Y-family polymerases with other processes involved in the cellular responses to DNA damage is poorly understood. The most striking example of this to date is the unexpected role of the Y-family pol ι in tumor suppression. Despite dramatic reductions in mutant frequencies observed in the cells of mice that are deficient in pol ηwhen pol ι is also deficient, the pol ι deficiency causes a highly significant decrease in the latency and an increase in the multiplicity of aggressive UV-induced skin cancers (35;49). Therefore, it is of biological and translational interest to determine if inhibition of REV1 would affect carcinogenesis. Analysis of the data reported here indicate that such inhibition is effective, resulting in a significant reduction in the multiplicity of tumors and in a significant percentage of mice that do not develop tumors at all. Although a matter of speculation, it is likely that reducing the overall degree of TLS by limiting the availability of the scaffolding protein (REV1) forces the cell to resolve blocked replication forks by error-free homologous recombination. In this scenario, inhibition of individual polymerases results in the bypass function being assumed by an alternate in the Y-family. The most well-documented case is that of the deficiency of pol η, which underlies the cancer prone XP variant syndrome.
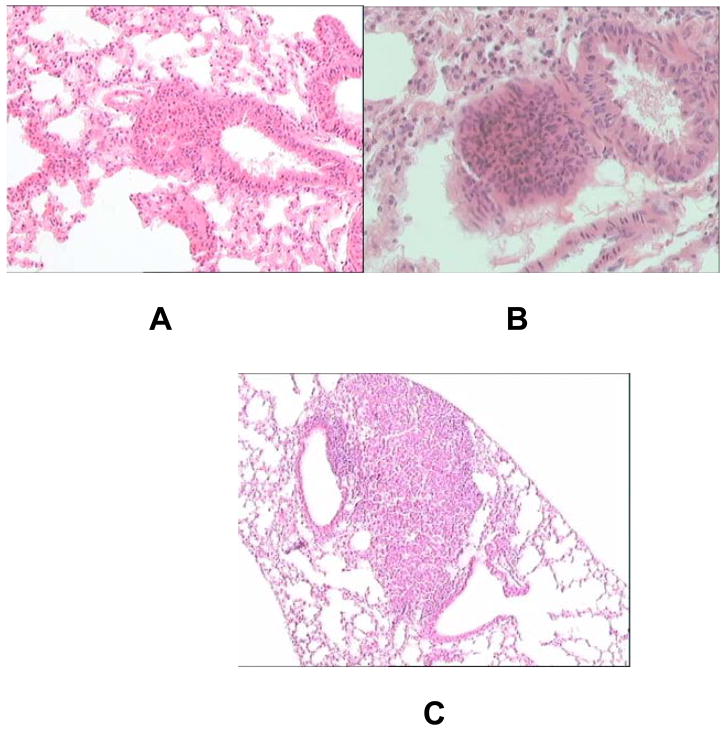
Pathological examination of lungs derived from mice in all five groups showed areas of bronchial epithelial hyperplasia. Homogeneous spherical masses were also observed in all groups that were classified as adenomas, as shown in Fig.5. Additionally, extremely large adenomas with no apparent loss of border or metastasis were observed in all groups. These results indicated that Rz406pU6 had no observable effects the types of tumors formed and suggest further that inhibition occurred during the initiation phase of carcinogenesis.
Figure 5.
Hyperplasia/adenoma formation in the murine lung. Lungs from sacrificed animals were placed in Tellyesniczky’s Solution for 24 hours followed by emersion in 70% ethanol for a minimum of 24 hours. Lungs were paraffin embedded and sectioned at 8 μm. Sections were stained with hematoxylin and eosin (H&E) stain and analyzed at 10X magnification. Tumors from all groups exhibited the same morphology. A. Severe hyperplasia/ small adenoma. B. moderately small adenoma. C. large adenoma with dysplastic features.
The overall hypothesis underlying this study is that mutagenic bypass of B[a]P-induced adducts in bronchial epithelial cells is responsible for the increased incidence of tumors after carcinogen exposure. If so, then REV1 inhibition would have no effect after the adducts had been repaired by nucleotide excision repair. Benzo[a]pyrene adducts are cleared from lung cells within one week of exposure, and we determined what effect, if any, on tumor development would be observed when REV1 inhibition occurred after adducts were cleared. To address this we included one treatment group given Rz407pU6 ~4 weeks post carcinogen (Fig 6, B[a]P/ ribozyme). As expected, there were no differences in mouse mortality or tumor development and morphology. These data suggest noninvasive REV1 inhibition may still be used in tissue previously exposed to a carcinogen with no ill effects and support REV1 inhibition as a useful strategy in the chemoprevention of lung cancer. Such a strategy would envision reducing the accumulation of mutations despite the continued exposure to carcinogens in the form of cigarette smoke.
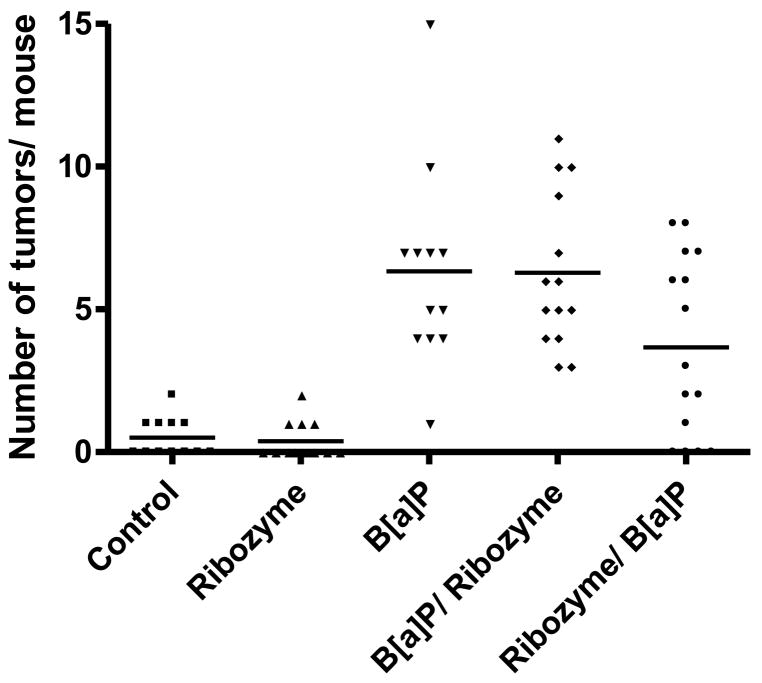
Figure 6.
Tumor multiplicity 28 weeks after exposure to B[a]P. To determine tumor burden individual lobes of the lungs were separately dissected and gross tumor counts and diameter measurements were performed using a light box. Control, mice received no treatment; Ribozyme, mice inhaled ribozyme Rz407pU6 without B[a]P exposure; B[a]P, mice inhaled the empty plasmid, then received 100mg/kg B[a]P IP. B[a]P/Ribozyme, mice received 100 mg/kg B[a]P, then inhaled Rz407pU6 8 weeks later; Ribozyme/B[a]P, mice inhaled Rz407pU6 then were injected with 100mg/kg 48 h after the last inhalation treatment. Please see text for experimental details. Mice that did not receive B[a]P had a low incidence of spontaneous tumors with 5/12 (42%) developing 1 or 2 tumors. This incidence was not affected by the inhalation of Rz407pU6. However, all 13 mice that received B[a]P and the empty vector developed tumors with a multiplicity that ranged from 2 to 15 tumors per lung, with an average multiplicity of 6.4. As expected exposure to the plasmid expressing the active ribozyme 4 weeks after B[a]P exposure had no effect on these parameters. In contrast, inhalation of the active ribozyme before carcinogen exposure reduced the average multiplicity to 3.7 tumors per mouse. This reduction is statistically significant (p<0.02) as analyzed by negative binomial regression and planned post hoc comparisons between individual groups. It is noteworthy that 4 of the 15 mice (27%) treated in this way did not develop tumors, and this reduction in incidence is statistically significant (p<0.05) by Fisher’s exact one-tailed test. Histological examination of tumors from all groups revealed that they were adenomas and there were no histopathological characteristics that distinguished one group from another.
Acknowledgments
The authors thank Dr. A. Bennett Jenson for interpretation of histopathological sections. We also thank Drs. Wolfgang Zacharias and Charles Densmore for helpful discussions. This work was supported by the Kentucky Lung Cancer Research Board and NIH CA112664 and CA112197 (WGM).
Reference List
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000 Jan 7;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Estensen RD, Jordan MM, Wiedmann TS, Galbraith AR, Steele VE, Wattenberg LW. Effect of chemopreventive agents on separate stages of progression of benzo[alpha]pyrene induced lung tumors in A/J mice. Carcinogenesis. 2004 Feb;25(2):197–201. doi: 10.1093/carcin/bgg196. [DOI] [PubMed] [Google Scholar]
- 3.McGregor WG, Chen RH, Lukash L, Maher VM, McCormick JJ. Cell cycle-dependent strand bias for UV-induced mutations in the transcribed strand of excision repair-proficient human fibroblasts but not in repair-deficient cells. Mol Cell Biol. 1991 Apr;11(4):1927–34. doi: 10.1128/mcb.11.4.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen RH, Maher VM, McCormick JJ. Lack of a cell cycle-dependent strand bias for mutations induced in the HPRT gene by (+/−)-7 beta,8 alpha-dihydroxy-9 alpha,10 alpha-epoxy-7,8,9,10-tetrahydrobenzo(a)pyrene in excision repair-deficient human cells. Cancer Res. 1991 May 15;51(10):2587–92. [PubMed] [Google Scholar]
- 5.Friedberg EC, Lehmann AR, Fuchs RP. Trading places: how do DNA polymerases switch during translesion DNA synthesis? Mol Cell. 2005 May 27;18(5):499–505. doi: 10.1016/j.molcel.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 6.Friedberg EC, Wagner R, Radman M. Specialized DNA polymerases, cellular survival, and the genesis of mutations. Science. 2002 May 31;296(5573):1627–30. doi: 10.1126/science.1070236. [DOI] [PubMed] [Google Scholar]
- 7.Watson NB, Mukhopadhyay S, McGregor WG. Translesion DNA replication proteins as molecular targets for cancer prevention. Cancer Lett. 2005 Nov 19; doi: 10.1016/j.canlet.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Broomfield S, Hryciw T, Xiao W. DNA postreplication repair and mutagenesis in Saccharomyces cerevisiae. Mutat Res. 2001 Aug 9;486(3):167–84. doi: 10.1016/s0921-8777(01)00091-x. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence CW. Cellular roles of DNA polymerase zeta and Rev1 protein. DNA Repair (Amst) 2002 Jun 21;1(6):425–35. doi: 10.1016/s1568-7864(02)00038-1. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Wu X, Rechkoblit O, Geacintov NE, Taylor JS, Wang Z. Response of human REV1 to different DNA damage: preferential dCMP insertion opposite the lesion. Nucleic Acids Res. 2002 Apr 1;30(7):1630–8. doi: 10.1093/nar/30.7.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson JR, Lawrence CW, Hinkle DC. Deoxycytidyl transferase activity of yeast REV1 protein. Nature. 1996 Aug 22;382(6593):729–31. doi: 10.1038/382729a0. [DOI] [PubMed] [Google Scholar]
- 12.Nelson JR, Gibbs PE, Nowicka AM, Hinkle DC, Lawrence CW. Evidence for a second function for Saccharomyces cerevisiae Rev1p. Mol Microbiol. 2000 Aug;37(3):549–54. doi: 10.1046/j.1365-2958.2000.01997.x. [DOI] [PubMed] [Google Scholar]
- 13.Guo C, Fischhaber PL, Luk-Paszyc MJ, Masuda Y, Zhou J, Kamiya K, et al. Mouse Rev1 protein interacts with multiple DNA polymerases involved in translesion DNA synthesis. EMBO J. 2003 Dec 15;22(24):6621–30. doi: 10.1093/emboj/cdg626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibbs PE, Wang XD, Li Z, McManus TP, McGregor WG, Lawrence CW, et al. The function of the human homolog of Saccharomyces cerevisiae REV1 is required for mutagenesis induced by UV light. Proc Natl Acad Sci U S A. 2000 Apr 11;97(8):4186–91. doi: 10.1073/pnas.97.8.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibbs PE, McGregor WG, Maher VM, Nisson P, Lawrence CW. A human homolog of the Saccharomyces cerevisiae REV3 gene, which encodes the catalytic subunit of DNA polymerase zeta. Proc Natl Acad Sci U S A. 1998 Jun 9;95(12):6876–80. doi: 10.1073/pnas.95.12.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao W, Lechler T, Chow BL, Fontanie T, Agustus M, Carter KC, et al. Identification, chromosomal mapping and tissue-specific expression of hREV3 encoding a putative human DNA polymerase zeta. Carcinogenesis. 1998 May;19(5):945–9. doi: 10.1093/carcin/19.5.945. [DOI] [PubMed] [Google Scholar]
- 17.Murakumo Y, Roth T, Ishii H, Rasio D, Numata S, Croce CM, et al. A human REV7 homolog that interacts with the polymerase zeta catalytic subunit hREV3 and the spindle assembly checkpoint protein hMAD2. J Biol Chem. 2000 Feb 11;275(6):4391–7. doi: 10.1074/jbc.275.6.4391. [DOI] [PubMed] [Google Scholar]
- 18.Wattenberg LW, Wiedmann TS, Estensen RD, Zimmerman CL, Steele VE, Kelloff GJ. Chemoprevention of pulmonary carcinogenesis by aerosolized budesonide in female A/J mice. Cancer Res. 1997 Dec 15;57(24):5489–92. [PubMed] [Google Scholar]
- 19.Wattenberg LW, Estensen RD. Studies of chemopreventive effects of budenoside on benzo[a]pyrene-induced neoplasia of the lung of female A/J mice. Carcinogenesis. 1997 Oct;18(10):2015–7. doi: 10.1093/carcin/18.10.2015. [DOI] [PubMed] [Google Scholar]
- 20.Zuker M, Jacobson AB. Well-determined” regions in RNA secondary structure prediction: analysis of small subunit ribosomal RNA. Nucleic Acids Res. 1995 Jul 25;23(14):2791–8. doi: 10.1093/nar/23.14.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Good PD, Krikos AJ, Li SX, Bertrand E, Lee NS, Giver L, et al. Expression of small, therapeutic RNAs in human cell nuclei. Gene Ther. 1997 Jan;4(1):45–54. doi: 10.1038/sj.gt.3300354. [DOI] [PubMed] [Google Scholar]
- 22.Diaz M, Watson NB, Turkington G, Verkoczy LK, Klinman NR, McGregor WG. Decreased frequency and highly aberrant spectrum of ultraviolet-induced mutations in the hprt gene of mouse fibroblasts expressing antisense RNA to DNA polymerase zeta. Mol Cancer Res. 2003 Sep;1(11):836–47. [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001 Dec;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Clark DR, Zacharias W, Panaitescu L, McGregor WG. Ribozyme-mediated REV1 inhibition reduces the frequency of UV-induced mutations in the human HPRT gene. Nucleic Acids Res. 2003 Sep 1;31(17):4981–8. doi: 10.1093/nar/gkg725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukhopadhyay S, Clark DR, Watson NB, Zacharias W, McGregor WG. REV1 accumulates in DNA damage-induced nuclear foci in human cells and is implicated in mutagenesis by benzo[a]pyrenediolepoxide. Nucleic Acids Res. 2004;32(19):5820–6. doi: 10.1093/nar/gkh903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Good PD, Krikos AJ, Li SX, Bertrand E, Lee NS, Giver L, et al. Expression of small, therapeutic RNAs in human cell nuclei. Gene Ther. 1997 Jan;4(1):45–54. doi: 10.1038/sj.gt.3300354. [DOI] [PubMed] [Google Scholar]
- 27.Luukkonen BG, Seraphin B. Construction of an in vivo-regulated U6 snRNA transcription unit as a tool to study U6 function. RNA. 1998 Feb;4(2):231–8. [PMC free article] [PubMed] [Google Scholar]
- 28.Lai D, Fu L, Weng S, Qi L, Yu C, Yu T, et al. Blocking caspase-3 activity with a U6 SnRNA promoter-driven ribozyme enhances survivability of CHO cells cultured in low serum medium and production of interferon-beta. Biotechnol Bioeng. 2004 Jan 5;85(1):20–8. doi: 10.1002/bit.10769. [DOI] [PubMed] [Google Scholar]
- 29.Jansen JG, Tsaalbi-Shtylik A, Langerak P, Calleja F, Meijers CM, Jacobs H, et al. The BRCT domain of mammalian Rev1 is involved in regulating DNA translesion synthesis. Nucleic Acids Res. 2005;33(1):356–65. doi: 10.1093/nar/gki189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo C, Fischhaber PL, Luk-Paszyc MJ, Masuda Y, Zhou J, Kamiya K, et al. Mouse Rev1 protein interacts with multiple DNA polymerases involved in translesion DNA synthesis. EMBO J. 2003 Dec 15;22(24):6621–30. doi: 10.1093/emboj/cdg626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross AL, Simpson LJ, Sale JE. Vertebrate DNA damage tolerance requires the C-terminus but not BRCT or transferase domains of REV1. Nucleic Acids Res. 2005;33(4):1280–9. doi: 10.1093/nar/gki279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tissier A, Kannouche P, Reck MP, Lehmann AR, Fuchs RP, Cordonnier A. Co-localization in replication foci and interaction of human Y-family members, DNA polymerase pol eta and REVl protein. DNA Repair (Amst) 2004 Nov 2;3(11):1503–14. doi: 10.1016/j.dnarep.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 33.Jansen JG, Fousteri MI, de WN. Send in the clamps: control of DNA translesion synthesis in eukaryotes. Mol Cell. 2007 Nov 30;28(4):522–9. doi: 10.1016/j.molcel.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Wang M, Devereux TR, Vikis HG, McCulloch SD, Holliday W, Anna C, et al. Pol iota is a candidate for the mouse pulmonary adenoma resistance 2 locus, a major modifier of chemically induced lung neoplasia. Cancer Res. 2004 Mar 15;64(6):1924–31. doi: 10.1158/0008-5472.can-03-3080. [DOI] [PubMed] [Google Scholar]
- 35.Dumstorf CA, Clark AB, Lin Q, Kissling GE, Yuan T, Kucherlapati R, et al. Participation of mouse DNA polymerase iota in strand-biased mutagenic bypass of UV photoproducts and suppression of skin cancer. Proc Natl Acad Sci U S A. 2006 Nov 28;103(48):18083–8. doi: 10.1073/pnas.0605247103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Densmore CL. Polyethyleneimine-based gene therapy by inhalation. Expert Opin Biol Ther. 2003 Oct;3(7):1083–92. doi: 10.1517/14712598.3.7.1083. [DOI] [PubMed] [Google Scholar]
- 37.Densmore CL. Advances in noninvasive pulmonary gene therapy. Curr Drug Deliv. 2006 Jan;3(1):55–63. doi: 10.2174/156720106775197547. [DOI] [PubMed] [Google Scholar]
- 38.Klink D, Schindelhauer D, Laner A, Tucker T, Bebok Z, Schwiebert EM, et al. Gene delivery systems--gene therapy vectors for cystic fibrosis. J Cyst Fibros. 2004 Aug;3( Suppl 2):203–12. doi: 10.1016/j.jcf.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 39.Schwiebert LM. Cystic fibrosis, gene therapy, and lung inflammation: for better or worse? Am J Physiol Lung Cell Mol Physiol. 2004 Apr;286(4):L715–L716. doi: 10.1152/ajplung.00363.2003. [DOI] [PubMed] [Google Scholar]
- 40.Gautam A, Densmore CL, Waldrep JC. Pulmonary cytokine responses associated with PEI-DNA aerosol gene therapy. Gene Ther. 2001 Feb;8(3):254–7. doi: 10.1038/sj.gt.3301369. [DOI] [PubMed] [Google Scholar]
- 41.Bauer AK, Dwyer-Nield LD, Hankin JA, Murphy RC, Malkinson AM. The lung tumor promoter, butylated hydroxytoluene (BHT), causes chronic inflammation in promotion-sensitive BALB/cByJ mice but not in promotion-resistant CXB4 mice. Toxicology. 2001 Dec 1;169(1):1–15. doi: 10.1016/s0300-483x(01)00475-9. [DOI] [PubMed] [Google Scholar]
- 42.Gautam A, Waldrep JC, Kleinerman ES, Xu B, Fung YK, T’Ang A, et al. Aerosol gene therapy for metastatic lung cancer using PEI-p53 complexes. Methods Mol Med. 2003;75:607–18. doi: 10.1385/1-59259-324-0:607. [DOI] [PubMed] [Google Scholar]
- 43.Gautam A, Waldrep JC, Densmore CL, Koshkina N, Melton S, Roberts L, et al. Growth inhibition of established B16-F10 lung metastases by sequential aerosol delivery of p53 gene and 9-nitrocamptothecin. Gene Ther. 2002 Mar;9(5):353–7. doi: 10.1038/sj.gt.3301662. [DOI] [PubMed] [Google Scholar]
- 44.Gautam A, Densmore CL, Waldrep JC. Inhibition of experimental lung metastasis by aerosol delivery of PEI-p53 complexes. Mol Ther. 2000 Oct;2(4):318–23. doi: 10.1006/mthe.2000.0138. [DOI] [PubMed] [Google Scholar]
- 45.Rudolph C, Ortiz A, Schillinger U, Jauernig J, Plank C, Rosenecker J. Methodological optimization of polyethylenimine (PEI)-based gene delivery to the lungs of mice via aerosol application. J Gene Med. 2005 Jan;7(1):59–66. doi: 10.1002/jgm.646. [DOI] [PubMed] [Google Scholar]
- 46.Gautam A, Densmore CL, Xu B, Waldrep JC. Enhanced gene expression in mouse lung after PEI-DNA aerosol delivery. Mol Ther. 2000 Jul;2(1):63–70. doi: 10.1006/mthe.2000.0087. [DOI] [PubMed] [Google Scholar]
- 47.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005 Jun 17;121(6):823–35. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 48.Rudolph C, Schillinger U, Ortiz A, Plank C, Golas MM, Sander B, et al. Aerosolized nanogram quantities of plasmid DNA mediate highly efficient gene delivery to mouse airway epithelium. Mol Ther. 2005 Sep;12(3):493–501. doi: 10.1016/j.ymthe.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 49.Ohkumo T, Kondo Y, Yokoi M, Tsukamoto T, Yamada A, Sugimoto T, et al. UV-B radiation induces epithelial tumors in mice lacking DNA polymerase eta and mesenchymal tumors in mice deficient for DNA polymerase iota. Mol Cell Biol. 2006 Oct;26(20):7696–706. doi: 10.1128/MCB.01076-06. [DOI] [PMC free article] [PubMed] [Google Scholar]