Abstract
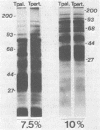
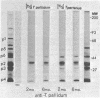
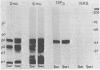
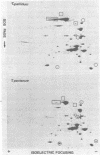
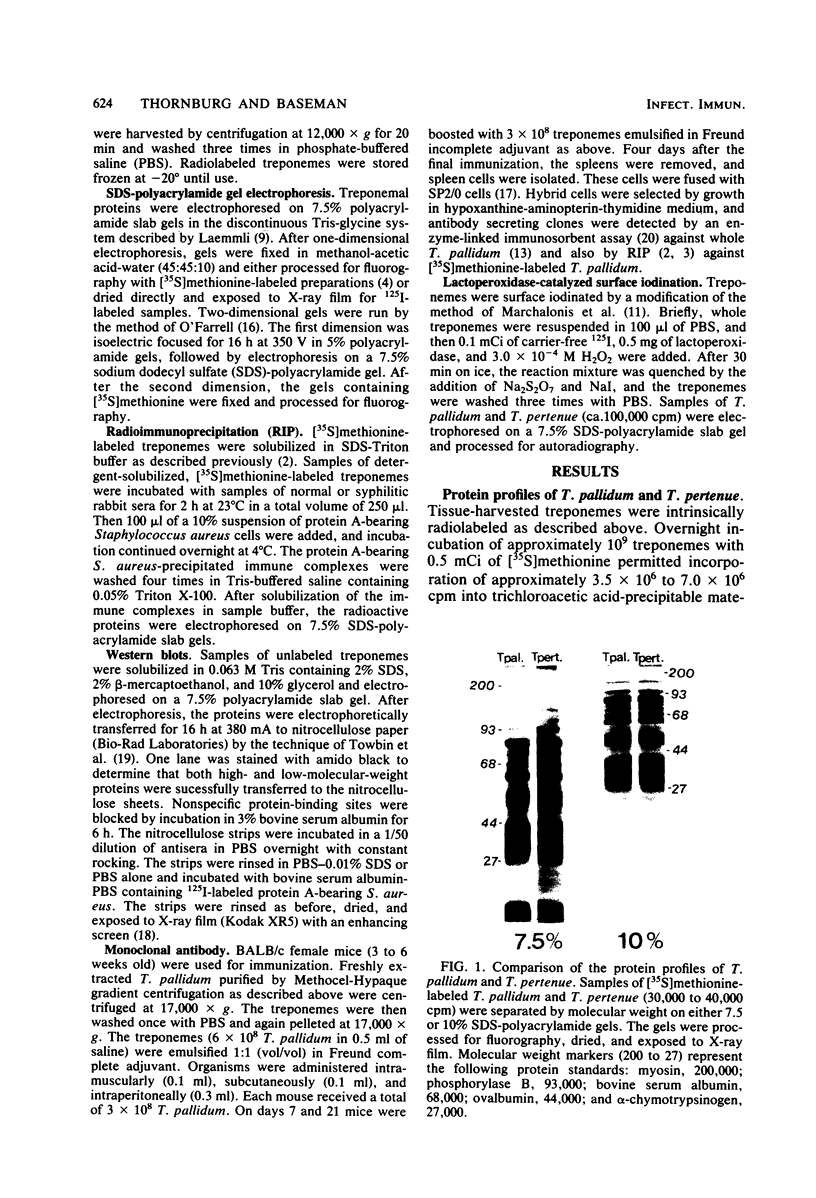
The protein profiles of Treponema pallidum and Treponema pertenue, the causative agents of syphilis and yaws, respectively, were compared by one- and two-dimensional gel electrophoresis. One-dimensional gels showed essentially no differences in the protein patterns of these treponemes. On two-dimensional gels most radiolabeled protein species were shared; however, variations were noticed in several minor protein species. Antigenic comparison by radioimmunoprecipitation and Western blotting also demonstrated similarities between these spirochetes. However, lactoperoxidase-catalyzed iodination of T. pallidum and T. pertenue suggested differences in their surface proteins.
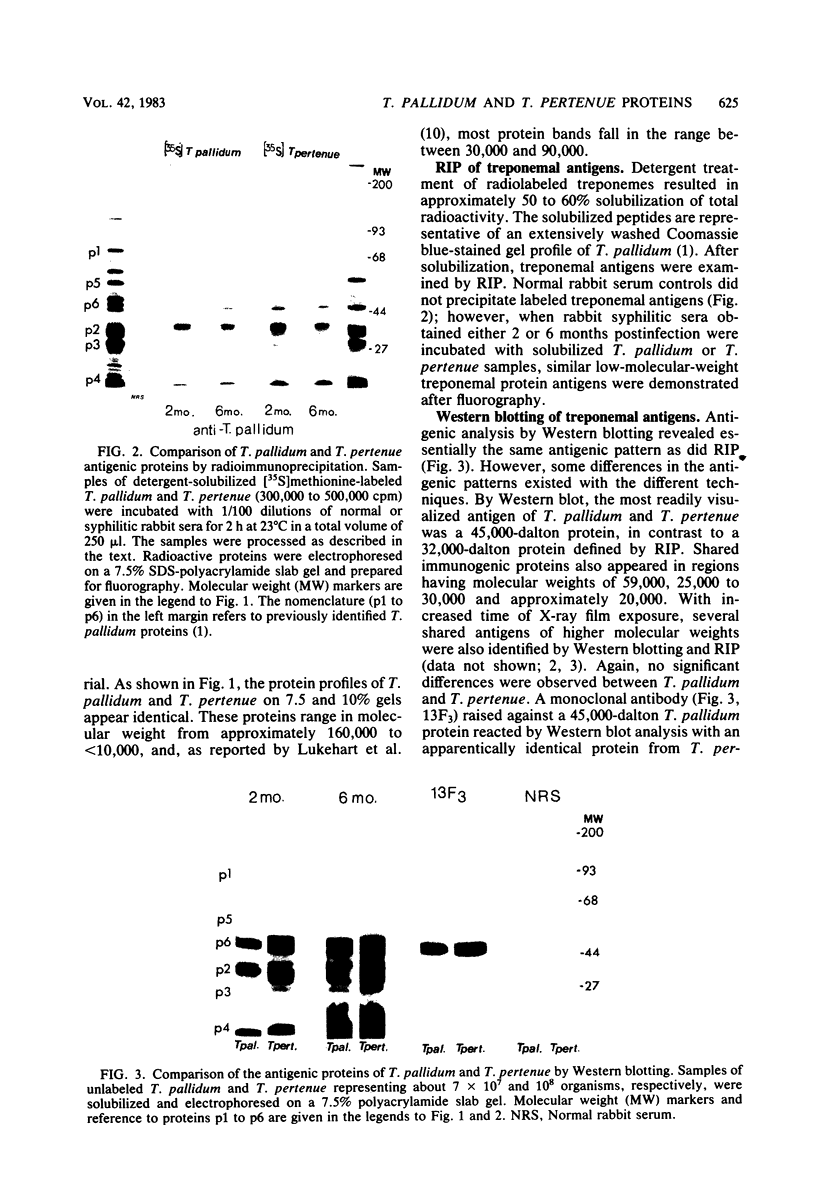
Full text
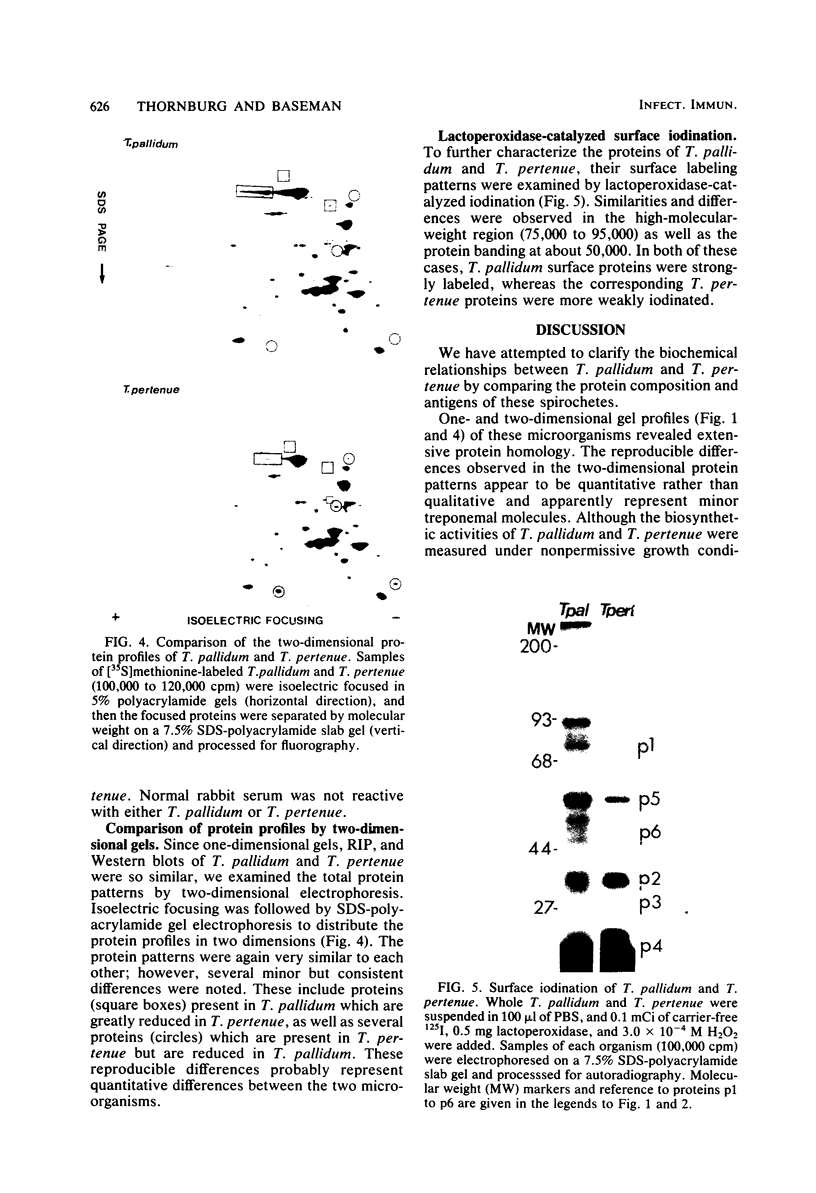
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Baseman J. B. Analysis of serum IgG against Treponema pallidum protein antigens in experimentally infected rabbits. Br J Vener Dis. 1981 Oct;57(5):302–308. doi: 10.1136/sti.57.5.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Baseman J. B. Surface-associated host proteins on virulent Treponema pallidum. Infect Immun. 1979 Dec;26(3):1048–1056. doi: 10.1128/iai.26.3.1048-1056.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Hayes E. C. Molecular characterization of receptor binding proteins and immunogens of virulent Treponema pallidum. J Exp Med. 1980 Mar 1;151(3):573–586. doi: 10.1084/jem.151.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Fieldsteel A. H., Cox D. L., Moeckli R. A. Cultivation of virulent Treponema pallidum in tissue culture. Infect Immun. 1981 May;32(2):908–915. doi: 10.1128/iai.32.2.908-915.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. F., Backhouse J. L., Daskalopoulos G., Walsh J. L. Use of T. pertenue in the fluorescent and immobilization tests. Investigation of sera from yaws areas found reactive only in the TPHA test. Br J Vener Dis. 1974 Aug;50(4):264–266. doi: 10.1136/sti.50.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HACKETT C. J. ON THE ORIGIN OF THE HUMAN TREPONEMATOSES (PINTA, YAWS, ENDEMIC SYPHILIS AND VENEREAL SYPHILIS). Bull World Health Organ. 1963;29:7–41. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Gubish E. R., Jr Identification of Treponema pallidum antigens: comparison with a nonpathogenic treponeme. J Immunol. 1982 Aug;129(2):833–838. [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao R. M., Fieldsteel A. H. Genetic relationship between Treponema pallidum and Treponema pertenue, two noncultivable human pathogens. J Bacteriol. 1980 Jan;141(1):427–429. doi: 10.1128/jb.141.1.427-429.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison-Plummer J., Alderete J. F., Baseman J. B. Enzyme-linked immunosorbent assay for the detection of serum antibody to outer membrane proteins of Treponema pallidum. Br J Vener Dis. 1983 Apr;59(2):75–79. doi: 10.1136/sti.59.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nell E. E., Hardy P. H., Jr The use of freeze-preserved treponemes in the Treponema pallidum immobilization test. Cryobiology. 1972 Oct;9(5):404–410. doi: 10.1016/0011-2240(72)90157-5. [DOI] [PubMed] [Google Scholar]
- Norris S. J. In vitro cultivation of Treponema pallidum: independent confirmation. Infect Immun. 1982 Apr;36(1):437–439. doi: 10.1128/iai.36.1.437-439.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Swanstrom R., Shank P. R. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. doi: 10.1016/0003-2697(78)90333-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]