Abstract
Ethanol extracts of brown seaweeds from Pakistan and China were isolated and compared for their antiallergenic activities. They included Sargassum tennerimum (ST) and Sargassum cervicorne (SC) from Pakistan, and Sargassum graminifolium turn (SG), Sargassum thunbergii (STH), and Laminaria japonica (LJ) from China. The ethanol extracts of these brown seaweeds were optimized at 85% (v/v) ethanol for the maximum yield of phlorotannin, an inhibitor against hyaluronidase. Total phlorotannins contained in the crude extracts were measured as 1.71% (SG), 0.74% (STH), 0.97% (LJ), 3.30% (SC), and 5.06% (ST). The 50% inhibitory concentrations (IC50) of Pakistani SC and ST were 109.5 and 21 μg/ml, respectively, lower than those of Chinese SG, STH, and LJ (134, 269, and 148 μg/ml, respectively). An antiallergic drug, disodium cromoglycate (DSCG), had an IC50=39 μg/ml, and a natural inhibitor of hyaluronidase, catechin, had an IC50=20 μg/ml. The IC50 of ST extract was found similar to that of catechin (21 vs 20 μg/ml) and lower than that of DSCG (21 vs 39 μg/ml). This suggests that ST is a potent inhibitor of hyaluronidase, indicating a promising future development of natural antiallergic medicines or functional foods.
Keywords: Anti-allergic activity, Brown seaweed, Ethanol extracts, Hyaluronidase, Phlorotannin
INTRODUCTION
Food allergy is common in most part of the world. It has been frequently reported that the allergy induced by ingestion of food can cause severe hypersensitive reaction in humans (Daul et al., 1990; 1993). Food allergy is considered as type I allergy out of four general categories, based on mechanism of immunological involvement. The pathological mechanism of the type I allergy has been examined as the degranulation of mast cells and the release of chemical mediators such as histamine, leucotrienes, and prostaglandins from these cells. Mast cell degranulation occurs in response to immunological stimuli in which the antigen-IgE antibody reaction predominates on the cell membrane (Metcalfe et al., 1997; Lorentz et al., 2000; Borish, 2003; Liu et al., 2007). Hyaluronidase (HAase, EC 3.2.1.35), an enzyme which cleaves the polysaccharide hyaluronic acid in the extracellular matrix of connective tissue, is mainly known to be involved in allergic reaction (Kakegawa et al., 1988; He et al., 2001).
It was reported that antiallergic agent had a strong inhibitory effect on the activation of hyaluronidase (Fujitani et al., 2001). Some metals, metallic salts, polyphenols, flavonoids, polysaccharides, and clinical drugs were reported as antiallergic agents or inhibitors of hyaluronidase (Kakegawa et al., 1992; Tung et al., 1994; Facino et al., 1995; Asada et al., 1997; Jeong et al., 1999; 2000; Akhtar and Bhakuni, 2003). Apart from these inhibitors, some food materials tested so far demonstrated to be antiallergic (Sano et al., 1999; Sanbongi et al., 2004; Yamamoto et al., 2004). Marine algal polyphenols, which contain phlorotannin that is only present in brown algae and restricted to polymers phloroglucinols (1,3,5-tri-hydroxibenzene) (Ragan and Glombitza, 1986), were tested for their antihyaluronidase activity (Shibata et al., 2002). However, the study was confined to a few species. A wide variety of other seaweeds in different regions still remain to be investigated for their antihyaluronidase activity.
Along the coast lines of Pakistan and China, exist a large number of bathetic algae and seaweed species. These seaweeds are usually found lying on the beaches during the ebb of sea waters either as drift or entangled with rocks, or growing submerged in the water pools. In the coastal belt of Pakistan alone, about 70 species and 27 genera of brown seaweeds are available. Besides, the uses of seaweeds as human food, as industrial materials for their bioactivity, and as new bioactive components have emerged as a challenge worldwide. Moreover, the activity of phlorotannin is region-specific. Temperate and tropical species of seaweeds may have their own significance for the activities.
In the present study, we examined brown seaweeds for the inhibition of hyaluronidase for the development of natural antiallergic components and some functional foods. Phlorotannin contents of the ethanol extracts were assessed followed by antihyaluronidase activity in order to establish a relationship for comparison of the seaweeds from different regions. To evaluate the effectiveness of the antiallergic activity, the 50% inhibitory concentrations (IC50) of ethanol extracts were also compared with the well-known antiallergic drug, disodium cromoglycate (DSCG), and a terrestrial plant polyphenol, catechin.
MATERIALS AND METHODS
Algae
Sargassum tennerimum (ST) and Sargassum cervicorne (SC) were collected from the coastal area of Karachi, Pakistan, and Sargassum graminifolium turn (SG), Sargassum thunbergii (STH) and Laminaria japonica (LJ) were collected from the coastal area of Qingdao, China, in January 2007. Samples were washed three times with tap water to remove salt, epiphytes, and sand attached to the surface of the samples, and were then air-dried within a shade protecting from direct sunlight. The dried seaweeds were crushed and ground into a powder form to pass through a 40-mesh sieve and stored at room temperature.
Chemicals
Folin-Ciocalteu’s phenol reagent (2 mol/L), hyaluronidase, type IV from bovine testes (1060 U/mg solid), DSCG (95%, purity), +(−) catechin hydrate (98% purity), and dimethyl sulfoxide (DMSO) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). 4-Dimethylaminobenzaldehyde (99% purity) American Chemical Society (ACS) reagent was purchased from Sigma-Aldrich, USA, and hyaluronic acid sodium salt from Streptococcus equi sp. was purchased from Fluka, Germany. All other chemicals were ACS and spectrophotometric grade. Water was purified by milli-Q system.
Extraction
Crude extracts of brown seaweeds were prepared using the modified method of Kim et al.(2006). 10 g of dried samples of various brown seaweeds were immersed in 100 ml optimized 85% (v/v) ethanol and stirred for 24 h at 25 °C, followed by centrifuging the extract to collect the supernatant. The residues were transferred to a conical flask and washed with 85% ethanol. The procedure was repeated twice to obtain the supernatants. The combined supernatant was filtered and then concentrated to about 50 ml under vacuum using a rotary evaporator (Buchi R-200 V, Brinkmann Instruments, Mississauga, ON, USA) at 40 °C, as described by Yuan et al.(2005). The concentrated sample was then washed three times with the equal volume of chloroform to remove fats and pigments in a separatory funnel. The upper layer, corresponding to the non-lipid fraction (Folch et al., 1957), was extracted with ethyl acetate (50 ml, thrice). The ethyl acetate fraction was dried under reduced pressure (crude phlorotannins). The residues were added with 10 ml DMSO.
Total phlorotannin content
Total phlorotannin content in the ethanolic extracts was determined according to a modified version of Folin-Ciocalteu method (Waterman and Mole, 1994) using phloroglucinol as the standard. Samples were diluted taking into account the measurable range of the spectrophotometer (e.g., a 0.025~0.1 ml aliquot of extracts of soluble phenolics was mixed with 0.4~0.475 ml water). A 0.1 ml aliquot of the diluted sample was mixed in a test tube with 0.5 ml of 2 mol/L Folin-Ciocalteu reagent and 0.5 ml of water. The mixture was allowed to stand for 3 min following addition of 2.0 ml of 20% (w/v) Na2CO3. Samples were incubated in the dark at room temperature for 45 min and centrifuged at 1600×g for 8 min. Absorbance of the supernatant was measured at 730 nm using spectrophotometer. Total phlorotannin content was calculated using the standard graph plotted and expressed as a percentage.
Antihyaluronidase activity
Antihyaluronidase activity was conducted using a modified version of Morgan-Elson method (Ingo et al., 1998). The following reagents and solutions were used. A stock solution of 5 mg/ml hyaluronic acid was prepared in water and stored at 4 °C. 50 μl of the hyaluronic acid stock solution and 100 μl of buffer were added to 250 μl of water. The buffer containing 0.2 mol/L sodium formate, 0.1 mol/L NaCl, and 0.2 mg/ml bovine serum albumin (BSA) was adjusted to the appropriate pH (2.0~5.0) with formic acid. Incubation mixtures were equilibrated at 37 °C for 10 min before the reaction was started by the addition of 50 μl of hyaluronidase along with 100 μl standard or sample. The enzymatic reaction was stopped by the addition of 110 μl of alkaline borate solution and subsequent heating for 4.5 min in a boiling water bath. The borate solution was prepared by dissolving 17.3 g H3BO3 and 7.8 g KOH in 100 ml water. To 10 ml of the alkaline solution, 1 ml of 0.8 g/ml K2CO3 solution was added before use. The test tubes were then placed in ice water for 20 min and then 1.5 ml of p-dimethylaminobenzaldehyde solution was added. To prepare this reagent, 20 g p-dimethylaminobenzaldehyde was dissolved in a mixture of 25 ml of concentrated hydrochloric acid and 75 ml of glacial acetic acid; the solution was diluted with 400 ml of glacial acetic acid immediately before use. To produce maximum coloration of the reaction mixture, the tubes were incubated at 37 °C for 20 min. After centrifugation at 4 °C and 6000 r/min for 10 min, the clear supernatant was transferred to cuvettes and the absorbance of the colored product was measured with an UV-2550 spectrophotometer (Shimadzu, Japan) at 586 nm. Test samples were replaced by the buffer solution for the control, while the enzyme solution was replaced by buffer solution for the blank. Percent inhibition was calculated as follows:
| Inhibition (%)=[(A−B)−(C−D)]/(A−B)×100, |
where A is the control absorbance; B is the control blank absorbance; C is the sample absorbance; and D is the sample blank absorbance.
IC50 was calculated using the mean of three observations from each of the five concentrations (Kobayashi et al., 2004) for all the samples including the two positive controls, DSCG and catechin.
Statistic analysis
The data was analyzed using SPSS 11.0 software. One way analysis of variance (ANOVA) was performed and was followed by Duncan’s multiple range test. A value of P<0.05 was used to indicate significant differences.
RESULTS
The ethanol extracts of two Pakistani and three Chinese brown seaweeds were tested and compared for their inhibitory effects on hyaluronidase activity. Antiallergic drug DSCG and a natural polyphenol catechin were also examined as positive controls. Previously, DSCG was proved to be an effective inhibitor of hyaluronidase (Kakegawa et al., 1985). The extraction method of the seaweeds was first optimized for the maximum yield of crude phlorotannin. The maximum crude phlorotannin was obtained at 85% (v/v) ethanol concentration. The optimized crude phlorotannin contents of all the seaweeds are shown in Table 1.
Table 1.
Crude phlorotannin contents extracted from dried powder of marine algae at different concentrations of ethanol*
| Marine algae | Crude phlorotannin content (%) |
|||
| 60% ethanol | 70% ethanol | 85% ethanol | 95% ethanol | |
| Sargassum graminifolium turn | 0.481±0.08 | 0.484±0.08 | 0.569±0.03 | 0.544±0.09 |
| Sargassum thunbergii | 1.054±0.09 | 1.058±0.08 | 1.083±0.06 | 1.069±0.08 |
| Laminaria japonica | 0.802±0.05 | 0.814±0.03 | 0.831±0.02 | 0.829±0.03 |
| Sargassum cervicorne | 0.896±0.02 | 0.910±0.03 | 0.925±0.04 | 0.918±0.03 |
| Sargassum tennerimum | 0.283±0.02 | 0.287±0.01 | 0.295±0.01 | 0.289±0.01 |
Values (mean±SD) are the means of three determinations
Total phlorotannin contents of SC and ST extracts were evaluated as 3.30% and 5.06%, respectively, higher than those of SG (1.71%), STH (0.74%), and LJ (0.97%) extracts (Table 2). Total phlorotannin contents were calculated against the standard curve of phloroglucinol (R 2=0.99) (Fig.1). Extracts from both Pakistani seaweeds SC and ST had higher values of total phlorotannin contents than the seaweed extracts from SG, STH, and LJ of China. Among all, ST was found to possess the highest value of total phlorotannin contents.
Table 2.
Total phlorotannin contents and the antihyaluronidase activity of the crude extracts along with the positive controls*
| Marine algae | C (%)a | IC50 (μg/ml)b |
| Sargassum graminifolium turn | 1.71±0.16 | 133.70±5.40 |
| Sargassum thunbergii | 0.74±0.19 | 268.66±7.05 |
| Laminaria japonica | 0.97±0.19 | 147.91±2.73 |
| Sargassum cervicorne | 3.30±0.20 | 109.49±1.75 |
| Sargassum tennerimum | 5.06±0.24 | 20.97±0.16 |
| DSCG (positive control)c | − | 38.60±0.49 |
| Catechin (positive control)d | − | 19.98±0.14 |
All the analytical data are the means (±SD) of three determination
Total phlorotannin content of the residual extract on dry weight basis determined by Folin-Ceocalteu’s method;
IC50 was calculated using the mean of three observations from each of the five concentrations for all the samples including the positive controls DSCG and catechin;
The antiallergic drug used as positive control;
The natural polyphenol used as positive control;
Fig. 1.
Standard curve of phloroglucinol absorbance at 730 nm
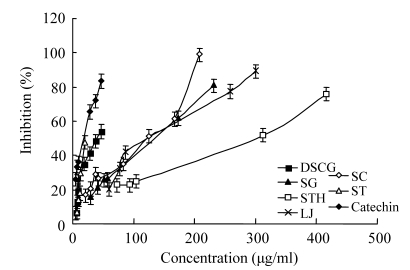
Meanwhile, the IC50 values were 134 μg/ml for SG, 269 μg/ml for STH, 148 μg/ml for LJ, 109.5 μg/ml for SC, and 21 μg/ml for ST (Table 2), whereas the IC50 values for DSCG and catechin, the two positive controls for antihyaluronidase activity, were 38.6 and 20 μg/ml, respectively (Table 2). Among all the five seaweeds tested, ST had the lower IC50 than DSCG and nearly equal to catechin, indicating that the crude extract of ST was the most effective and potent inhibitor against hyaluronidase activity among all the seaweeds tested.
DISCUSSION
In the present study, we evaluated the antihyaluronidase activity of crude extracts from the brown seaweeds of two different countries for the first time. Antihyaluronidase activity of brown seaweeds was compared with the well-known antiallergic drug DSCG and a natural tea polyphenol catechin. DSCG, together with tranilast, was previously reported to have strong antihyaluronidase activity (Kakegawa et al., 1985). Among natural hyaluronidase inhibitors, tea polyphenol catechin and epigallocatechin-3-gallate (EGCG) have been reported to possess strong antihyaluronidase activity (Tamagawa et al., 1999) and anti-allergic activity (Matsuo et al., 1996).
In order to evaluate the activities of hyaluronidase inhibitors from marine resources, we collected two species from Karachi coastal area of Pakistan and three from Qingdao coastal area of China. Ethanol extraction was designed to obtain the crude phlorotannin. Four concentrations of ethanol (60%, 70%, 85%, and 95%) were tested to optimize the extraction procedure, and 85% ethanol was found to be the optimum concentration for the maximum yield of crude phlorotannin (Table 1). In the experiments, the use of chloroform reduced the pigments and fat contents of the crude extracts, which might affect the antihyaluronidase activity.
Furthermore, the total phlorotannin content was determined using phloroglucinol as a standard (Fig.1), since phlorotannin is the polymers of phloroglucinol (1,3,5-trihydroxibenzene) (Ragan and Glombitza, 1986). The ethanol extracts including the positive controls were then subjected to the analysis of antihyaluronidase activity. The inhibitory effects on hyaluronidase can be usually measured to evaluate antiallergic activity (Maeda et al., 1991; Sawabe et al., 1992; Asada et al., 1997; Ito et al., 1998; Ippoushi et al., 2000; Fujitani et al., 2001). The inhibitory effects of all the samples including the controls were measured in different concentrations (Fig.2). Samples of higher activity were measured at lower concentration for their inhibition effect. The IC50 values of ST, SC, SG, LJ, STH extracts were 21<109.5<134<148<269 μg/ml, respectively (Table 2), meanwhile the total phlorotannin contents for the same order of samples were 5.06%>3.30%>1.71%>0.97%>0.74% respectively (Table 2). A linear relation was found between the IC50 values of the crude extracts and the total phlorotannin contents. The IC50 values decreased with the increase in total phlorotannin contents of the crude extracts. But the relation of the IC50 values of the crude extracts and the total phlorotannin contents does not reflect that phlorotannin is the only compound for the antiallergenicity. As the percentage of phlorotannin in the crude extract was very low, some other bioactive components might play a role for the inhibition of hyaluronidase, like porphyran of red algae, Porphyra tenera and P. yezoensis (Ishihara et al., 2005).
Fig. 2.
Comparison of inhibition among all the samples including DSCG and catechin
DSCG: disodium chromoglycate; SG: Sargassum graminifolium turn; STH: Sargassum thunbergii; LJ: Laminaria japonica; SC: Sargassum cervicorne; ST: Sargassum tennerimum. All values of inhibition (%) are the means (±SD) of three determinations
From our results, it was found that phlorotannin might be one of the compounds responsible for the antihyaluronidase activity. However, the inhibitory effect on hyaluronidase of different samples from different regions might be affected by various factors including high molecular weight phlorotannin or degree of sulphation of the compounds present in the crude extract. Asada et al.(1997) reported that the inhibition of hyaluronidase by sodium alginate was dependent on molecular weight; the higher the molecular weight, the stronger the inhibition. Another investigation (Toida et al., 1999) was made on O-sulphated glucosaminoglycan as a fully sulphated compound inhibitor. Therefore, further study is needed to identify these factors.
In conclusion, we examined five brown seaweeds from Pakistan and China for their antiallergic activities, and found that Sargassum tennerimum is as potent as catechin, the natural hyaluronidase inhibitor, and more potent than DSCG, a clinically used antiallergic medicine. This may suggest a promising future development of natural antiallergic medicines or functional foods.
Acknowledgments
We are thankful to Dr. Alia, University of Karachi, Pakistan and Prof. Dr. Xiang-zhong Gong, Ocean University of China for the identification of marine algae. An appreciation also goes to Pakistan Council of Scientific and Industrial Research (PCSIR) laboratories complex, Karachi for the help to collect the samples in Pakistan.
Footnotes
Project supported by the Hi-Tech Research and Development Program (863) of China (No. 2006AA09Z427), and the National Natural Science Foundation of China (Nos. 30800859 and 30871948)
References
- 1.Akhtar MS, Bhakuni V. Streptococcus pneumoniae hyaluronate lyase contains two noncooperative independent folding/unfolding structural domains: characterization of functional domain and inhibitors of enzyme. J Biol Chem. 2003;278(28):25509–25516. doi: 10.1074/jbc.M301894200. [DOI] [PubMed] [Google Scholar]
- 2.Asada M, Sugie M, Inoue M, Nakagomi K, Hongo S, Murata K, Irie S, Takeuchi T, Tomizuka N, Oka S. Inhibitory effect of alginic acids on hyaluronidase and on histamine release from mast cells. Biosci Biotechnol Biochem. 1997;61(6):1030–1032. doi: 10.1271/bbb.61.1030. [DOI] [PubMed] [Google Scholar]
- 3.Borish L. Allergic rhinitis: systematic inflammation and implications for management. J Allergy Clin Immunol. 2003;112(6):1021–1031. doi: 10.1016/j.jaci.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Daul CB, Morgan JE, Lehrer SB. The natural history of shrimp hypersensitivity. J Allergy Clin Immunol. 1990;86(1):88–93. doi: 10.1016/S0091-6749(05)80127-7. [DOI] [PubMed] [Google Scholar]
- 5.Daul CB, Morgan JE, Lehrer SB. Hypersensitivity reaction to crustacean and mollusks. Clin Rev Allergy. 1993;11(2):201–222. doi: 10.1007/BF02914471. [DOI] [PubMed] [Google Scholar]
- 6.Facino R, Carini M, Stefani R, Aldini G, Saibene L. Anti-elastase and anti-hyaluronidase activities of saponins and sapogenins from Hedera helix, Aesculus hippocastanum, and Ruscus aculeatus: factors contributing to their efficacy in the treatment of venous insufficiency. Arch Pharm. 1995;328(10):720–724. doi: 10.1002/ardp.19953281006. [DOI] [PubMed] [Google Scholar]
- 7.Folch J, Less M, Sloane Stanley GH. A simple method for the isolation and purification of total lipid from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 8.Fujitani N, Sakaki S, Yamaguchi Y, Takenaka H. Inhibitory effects of microalgae on the activation of hyaluronidase. J Appl Phycol. 2001;13(6):489–492. doi: 10.1023/A:1012592620347. [DOI] [Google Scholar]
- 9.He D, Zhou A, Wei W, Nie L, Yao S. A new study of the degradation of hyaluronic acid by hyaluronidase using quartz crystal impedance technique. Talanta. 2001;53(5):1021–1029. doi: 10.1016/S0039-9140(00)00599-3. [DOI] [PubMed] [Google Scholar]
- 10.Ingo M, Günther B, Thilo S, Barbara D, Armin B. Quantization of hyaluronidase by the Morgan-Elson reaction: comparison of the enzyme activities in the plasma of tumor patients and healthy volunteers. Cancer Letters. 1998;131(1):13–20. doi: 10.1016/S0304-3835(98)00196-7. [DOI] [PubMed] [Google Scholar]
- 11.Ippoushi K, Yamaguchi Y, Itou H, Azuma K, Higashio H. Evaluation of inhibitory effects of vegetables and herbs on hyaluronidase and identification of rosmarinic acid as a hyaluronidase inhibitor in lemon balm (Melissa officinalis L.) Food Sci Technol Res. 2000;6(1):74–77. doi: 10.3136/fstr.6.74. [DOI] [Google Scholar]
- 12.Ishihara K, Oyamada C, Matsushima R, Murata M, Muraoka T. Inhibitory effect of porphyran, prepared from dried “Nori”, on contact hypersensitivity in mice. Biosci Biotechnol Biochem. 2005;69(10):1824–1830. doi: 10.1271/bbb.69.1824. [DOI] [PubMed] [Google Scholar]
- 13.Ito H, Miyazaki T, Ono M, Sakurai H. Antiallergic activities of rabdosiin and its related compounds: chemical and biochemical evaluations. Bioorg Med Chem. 1998;6(7):1051–1056. doi: 10.1016/S0968-0896(98)00063-7. [DOI] [PubMed] [Google Scholar]
- 14.Jeong SJ, Ahn N, Kim Y, Inagaki M, Miyamato T, Higuchi R. Norlignans with hyaluronidase inhibitory activity from Anemarrhena asphodeloides . Planta Med. 1999;65(4):367–368. doi: 10.1055/s-2006-960789. [DOI] [PubMed] [Google Scholar]
- 15.Jeong SJ, Kim NY, Kim DH, Kang TH, Ahn NH, Miyamoto T, Higuchi R, Kim YC. Hyaluronidase inhibitory active 6H-dibenzo[b,d]pyran-6-ones from the feces of Trogopterus xanthipes . Planta Med. 2000;66(1):76–77. doi: 10.1055/s-0029-1243114. [DOI] [PubMed] [Google Scholar]
- 16.Kakegawa H, Matsumoto H, Satoh T. Activation of hyaluronidase by metallic salts and compound 48/80, and inhibitory effect of antiallergic agents on hyaluronidase. Chem Pharm Bull. 1985;33(2):642–646. doi: 10.1248/cpb.33.642. [DOI] [PubMed] [Google Scholar]
- 17.Kakegawa H, Matsumoto H, Satoh T. Inhibitory effects of hydrangenol derivatives on the activation of hyaluronidase and their antiallergic activities. Planta Medica. 1988;54(5):385–389. doi: 10.1055/s-2006-962477. [DOI] [PubMed] [Google Scholar]
- 18.Kakegawa H, Matsumoto H, Satoh T. Inhibitory effects of some natural products on the activation of hyaluronidase and their antiallergic actions. Chem Pharm Bull. 1992;40(6):1439–1442. doi: 10.1248/cpb.40.1439. [DOI] [PubMed] [Google Scholar]
- 19.Kim MM, Ta QV, Mendis E, Rajapakse N, Jung WK, Byun HG, Jeon YJ, Kim SK. Phlorotannin in Ecklonia cava extract inhibit matrix metalloproteinase activity. Life Sciences. 2006;79(15):1436–1443. doi: 10.1016/j.lfs.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi M, Hasimoto Y, Taniyuchi S, Tanabe S. Degradation of wheat allergen in Japanese soy sauce. Int J Mol Med. 2004;13(6):821–827. [PubMed] [Google Scholar]
- 21.Liu T, Bai ZT, Pang XY, Chai ZF, Jiang F, Ji YH. Degranulation of mast cells and histamine release involved in rat pain-related behaviors and edema induced by scorpion Buthus martensi Karch venom. European Journal of Pharmacology. 2007;575(1-3):46–56. doi: 10.1016/j.ejphar.2007.07.057. [DOI] [PubMed] [Google Scholar]
- 22.Lorentz A, Schwengberg S, Sellge G, Manns MP, Bischoff SC. Human intestinal mast cells are capable of producing different cytokine profiles. Role of IgE receptor cross linking and IL-4. J Immunol. 2000;164(1):43–48. doi: 10.4049/jimmunol.164.1.43. [DOI] [PubMed] [Google Scholar]
- 23.Maeda Y, Yamamoto M, Masui T, Sugiyama K, Yokota M, Okada N, Sugiyama K, Katayama H, Nakagomi K. Hyaluronidase inhibitor in the fruit of Citrus reticulata Blanco. Eisei Kagaku. 1991;37(3):205–210. (In Japanese) [Google Scholar]
- 24.Matsuo N, Yamada K, Yamashita K, Shoji K, Mori M, Sugano M. Inhibitory effect of tea polyphenols on histamine and leukotriene B4 release from rat potential exudates cells. In Vitro Cellular and Developmental Biology-Animal. 1996;32(6):340–344. doi: 10.1007/BF02722960. [DOI] [PubMed] [Google Scholar]
- 25.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77(4):1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 26.Ragan MA, Glombitza KW. Phlorotannin, brown algal polyphenols. Progress in Physiological Research. 1986;4:129–241. [Google Scholar]
- 27.Sanbongi C, Takano H, Osakabe N, Sasa N, Natsume M, Yamagisawa T. Rosmarinic acid in perilla extract inhibits allergic inflammation induced by mite allergen, in a mouse model. Clin Exp Allergy. 2004;34(6):971–977. doi: 10.1111/j.1365-2222.2004.01979.x. [DOI] [PubMed] [Google Scholar]
- 28.Sano M, Suzuki M, Miyase T, Yoshino K, Maeda-Yamamoto M. Novel anti-allergic catechin derivatives isolated from oolong tea. J Agric Food Chem. 1999;47(5):1906–1910. doi: 10.1021/jf981114l. [DOI] [PubMed] [Google Scholar]
- 29.Sawabe Y, Nakagomi K, Iwagami S, Suzuki S, Nakazawa H. Inhibitory effects of pectic substances on activated hyaluronidase and histamine release from mast cells. Biochim Biophys Acta. 1992;1137(3):274–278. doi: 10.1016/0167-4889(92)90147-4. [DOI] [PubMed] [Google Scholar]
- 30.Shibata T, Fujimoto K, Kohki N, Nagayama K, Yamaguchi K, Nakmura T. Inhibitory activity of brown algal phlorotannin against hyaluronidase. Int J Food Sci Tech. 2002;37(6):703–709. doi: 10.1046/j.1365-2621.2002.00603.x. [DOI] [Google Scholar]
- 31.Tamagawa K, Lizuka S, Ikeda A, Koike H, Naganuma K, Komiyama Y. Inhibitory effect of proanthocyanidins isolated from barley bran on hyaluronidase activity, soybean lipoxigenase activity and complimentry activity. Nippon Shokuhin Kagaku Kogaku Kaishi. 1999;46:521–527. [Google Scholar]
- 32.Toida T, Ogita Y, Suzuki A, Toyoda H, Imanari T. Inhibition of hyaluronidase by fully O-sulfonated glycosaminoglycans. Arch Biochem Biophys. 1999;370(2):176–182. doi: 10.1006/abbi.1999.1395. [DOI] [PubMed] [Google Scholar]
- 33.Tung JS, Mark GE, Hollis GF. A microplate assay for hyaluronidase and hyaluronidase inhibitors. Anal Biochem. 1994;223(1):149–152. doi: 10.1006/abio.1994.1560. [DOI] [PubMed] [Google Scholar]
- 34.Waterman PG, Mole S, editors. Analysis of Phenolic Plant Metabolites. Oxford, England: Blackwell Scientific Publications; 1994. [Google Scholar]
- 35.Yamamoto T, Yoshimura M, Yamaguchi F, Kaochi T, Tsuji R, Saito M, Obata A, Kikuchi M. Anti-allergic activity of naringenin chaicone from a tomato skin extract. Biosci Biotechnol Biochem. 2004;68(8):1706–1711. doi: 10.1271/bbb.68.1706. [DOI] [PubMed] [Google Scholar]
- 36.Yuan YV, Bone DE, Carrington MF. Antioxidant activity of pulse (Palmaria palmata) extract evaluated in vitro. Food Chemistry. 2005;91(3):485–494. doi: 10.1016/j.foodchem.2004.04.039. [DOI] [Google Scholar]