Abstract
In a recent paper Diogo (2008) reported the results of the first part of an investigation of the comparative anatomy, homologies and evolution of the head and neck muscles of osteichthyans (bony fish + tetrapods). That report mainly focused on actinopterygian fish, but also compared these fish with certain non-mammalian sarcopterygians. The present paper focuses mainly on sarcopterygians, and particularly on how the head and neck muscles have evolved during the transitions from sarcopterygian fish and non-mammalian tetrapods to monotreme and therian mammals, including modern humans. The data obtained from our dissections of the head and neck muscles of representative members of sarcopterygian fish, amphibians, reptiles, monotremes and therian mammals, such as rodents, tree-shrews, colugos and primates, including modern humans, are compared with the information available in the literature. Our observations and comparisons indicate that the number of mandibular and true branchial muscles (sensu this work) present in modern humans is smaller than that found in mammals such as tree-shrews, rats and monotremes, as well as in reptiles such as lizards. Regarding the pharyngeal musculature, there is an increase in the number of muscles at the time of the evolutionary transition leading to therian mammals, but there was no significant increase during the transition leading to the emergence of higher primates and modern humans. The number of hypobranchial muscles is relatively constant within the therian mammals we examined, although in this case modern humans have more muscles than other mammals. The number of laryngeal and facial muscles in modern humans is greater than that found in most other therian taxa. Interestingly, modern humans possess peculiar laryngeal and facial muscles that are not present in the majority of the other mammalian taxa; this seems to corroborate the crucial role played by vocal communication and by facial expressions in primate and especially in human evolution. It is hoped that by compiling, in one paper, data about the head and neck muscles of a wide range of sarcopterygians, the present work could be useful to comparative anatomists, evolutionary biologists and functional morphologists and to researchers working in other fields such as developmental biology, genetics and/or evolutionary developmental biology.
Keywords: anatomy, bony fish, evolution, homologies, mammals, modern humans, muscles, Sarcopterygii, tetrapods
Introduction
In a recent paper Diogo (2008) reported the results of the first part of a long-term study of the comparative anatomy, homologies and evolution of the head and neck muscles of osteichthyans (the group comprising the bony fish and the tetrapods). That paper concentrated on the results relevant to the actinopterygians (the group that includes extant cladistians, chondrosteans, ginglymods, halecomorphs and teleosts), but it also compared the head and neck muscles of those fish with the musculature of a sample of non-mammalian sarcopterygians. This paper reports the results of the second part of the study, which focuses on sarcopterygians (the group that includes extant actinistians, dipnoans and tetrapods), and particularly on how the head and neck muscles evolved during the transitions from sarcopterygian fish and non-mammalian tetrapods to monotreme and therian mammals, including modern humans.
Several studies have provided information on the head and neck musculature of osteichthyans, but many concentrated on a single taxon (Diogo 2007, 2008; Diogo et al. 2008). The few more inclusive comparative analyses that were actually based on dissections of taxa representing sarcopterygian fish, amphibians, reptiles, monotremes and therian mammals, including modern humans, were published at least half a century ago, and some much earlier than that (e.g. Humphry, 1872; Edgeworth, 1902, 1935; Luther, 1913, 1914; Huber, 1930a,b, 1931; Brock, 1938; Kesteven, 1942–1945). These authors did not have access to information that is now available about, for example, the muscles of the coelacanth Latimeria chalumnae (discovered only in 1938), the role played by neural crest cells in the development and patterning of the head and neck muscles, or about the molecular and other evidence that has accumulated about phylogenetic relationships of sarcopterygians (e.g. Millot & Anthony, 1958; Jarvik, 1963, 1980; Alexander, 1973; Le Lièvre & Le Douarin, 1975; Anthony, 1980; Lauder, 1980c; Rosen et al. 1981; Noden, 1983, 1984, 1986; Hatta et al. 1990, 1991; Adamicka & Ahnelt, 1992; Couly et al. 1992; Miyake et al. 1992; Köntges & Lumsden, 1996; Pough et al. 1996; Schilling & Kimmel, 1997; Kardong & Zalisko, 1998; McGonnell, 2001; Olsson et al. 2001; Hunter & Prince, 2002; Kardong, 2002; West-Eberhard, 2003; Diogo, 2004a,b, 2007, 2008; Ericsson & Olsson, 2004; Ericsson et al. 2004; Carroll et al. 2005; Thorsen & Hale, 2005; Kisia & Onyango, 2005; Noden & Schneider, 2006; Diogo & Abdala, 2007). The aims of the present study were to dissect the head and neck muscles of representative members of sarcopterygian fish, amphibians, reptiles, monotremes and therian mammals (including modern humans), to compare this new evidence with the information available in the literature, and then to collate and synthesize all of the new and existing data. The results of this synthesis are summarized in Tables 1–4, which present the best supported hypotheses of homology for the head and neck muscles for the sarcopterygian taxa listed in those tables.
Table 1.
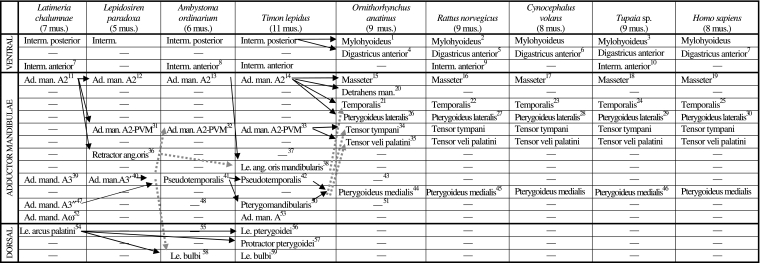
Scheme illustrating the authors’ hypotheses regarding the homologies of the mandibular muscles of adults of representative sarcopterygian taxa. The nomenclature of the muscles follows that used in the text; in order to facilitate comparisons, in some cases names often used by other authors to designate a certain muscle/bundle are given, between round brackets, in the text below the table; additional comments are given between square brackets. Data from evidence provided by our own dissections and comparisons and by a review of the literature (see text and Figs 3–17). Data from evidence provided by our own dissections and comparisons and by a review of the literature. The black arrows indicate the hypotheses that are most strongly supported by the evidence available; the grey arrows indicate alternative hypotheses that are supported by some of the data, but overall they are not as strongly supported by the evidence available as are the hypotheses indicated by black arrows (e.g. the overall analysis of the data available indicates that the urodele levator bulbi is a dorsal mandibular muscle, but the possibility that it derives from the adductor mandibulae cannot be completely ruled out: see text, Table 1, and Figs 3–17; VENTRAL, DORSAL = Ventral musculature and dorsal constrictor musculature sensuEdgeworth, 1935; ad. = adductor; ang. = anguli; interm. = intermandibularis; le. = levator; man. = mandibulae; mus. = muscles)
1[as described by e.g. Lightoller 1942, there is a mylohyoideus profundus, a mylohyoideus superficialis and, superficially to the latter, a digastricus anterior; Saban, 1971, states that these three structures come from the same embryological structure, i.e. that they seem to correspond to the intermandibularis posterior of other vertebrates: this is also supported by e.g. Jarvik 1963, 1980]
2[the mylohyoideus and digastricus anterior of rats clearly seem to correspond to the posterior, not the anterior, intermandibularis of other sarcopterygians, because the transversus mandibularis of rats corresponds to the intermandibularis anterior of other sarcopterygians; this is also supported by e.g. Bryant 1945]
3(posterior part of mylohyoid sensuLe Gros Clark 1924)
4[the correspondence between the mammalian digastricus anterior and part of the intermandibularis of other sarcopterygians is strongly corroborated by e.g. innervation (the intermandibularis and digastricus anterior are usually innervated by the ramus ventralis of CN5), ontogeny (e.g. the development of the marsupial Dasyrurus), and comparative anatomy of adults: see e.g. Edgeworth 1935]
5 (anterior belly of digastricus sensuGreene 1935)
6 (part of biventer sensuLeche 1886)
7 (anterior belly of biventer mandibulae sensuHuber 1930a)
8 (submentalis sensuIordansky 1992)
9 (transversus mandibularis sensuGreene 1935)
10 (anterior part of mylohyoid sensuLe Gros Clark 1924, and Sprague 1944a)
11 (adductor mandidulae ‘superficiel’sensuMillot & Anthony 1958)
12 (part of adductor mandidulae posterior sensuBemis & Lauder 1986)
13 (adductor mandibulae externus sensuIordansky 1992)
14 (adductor mandibulae externus sensuAbdala and Moro 2003)
15 (corresponds to the masseter + zygomatico-mandibularis, and possibly to the maxillo-mandibularis, sensuSaban 1971) [as shown in e.g. Saban's 1971 fig. 569, in the platypus specimens dissected by us the masseter is mainly divided into a deep part with anterior and posterior bundles and a superficial part with anterior and posterior bundles]
16[as described by Greene 1935, in the Norwegian rats dissected by us the masseter is mainly divided into a deep part with anterior and posterior bundles and a superficial part with anterior and posterior bundles]
17 (masseter + zygomatico-mandibularis sensuStafford & Szalay 2000) [in the colugo specimens dissected by us the masseter is subdivided into a superficial bundle, a deep bundle, and a zygomatico-mandibular bundle; the latter is sometimes considered as an independent muscle, but at least in the case of Cynocephalus, it is deeply mixed with the other masseter bundles]
18[as described by e.g. Le Gros Clark, 1924, in the Tupaia specimens dissected by us the masseter is mainly divided into deep, intermediate and superficial bundles]
19[in modern humans the masseter is usually mainly divided into deep and superficial bundles]11
20[some authors consider that the detrahens mandibulae is homologous to the digastricus anterior of other mammals, but this does not seem to be the case: see Saban 1968, p. 264; as stressed by e.g. Saban 1971, the detrahens mandibulae clearly seems to correspond to part of the adductor mandibulae A2 of non-mammalian tetrapods]
21[corresponds to part of the A2 of non-mammalian tetrapods but may possibly also include part of other adductor mandibulae structures such as the pseudotemporalis: see Barghusen 1968]
22[Greene 1935, describes the temporalis of rats as an undivided muscle, but as stated by Walker and Homberger, 1997, in the specimens dissected by us this muscle is divided into two bundles, one more superficial and anterior and the other more deep and posterior]
23[in the Cynocephalus specimens dissected by us the temporalis is not clearly divided into superficial and deep bundles, and there is no distinct pars suprazygomatica such as that found in Tupaia]
24[in the Tupaia specimens dissected by us the temporalis is mainly divided into a superficial bundle, a deep bundle, and a pars suprazygomatica sensuSaban 1971]
25[the temporalis of modern humans is usually described as an undivided muscle, but various authors, as e.g. Gorniak 1985, consider that it is in fact often divided into superficial and deep bundles]
26[in some parts of Edgeworth's 1935 work he seems to suggest that the pterygoideus lateralis and medialis are both included in the ’pterygoideus medialis’ of monotremes and that the pterygoideus lateralis only becomes separated in other extant mammals; however in other parts of Edgeworth's 1935 work he clearly states that the pterygoideus lateralis corresponds to part of the adductor mandibulae externus (= A2) of reptiles; more recent works, e.g. Barghusen 1968 and Jouffroy 1971, support this latter hypothesis; developmental data also indicate that the pterygoideus lateralis and pterygoideus medialis do not develop from the same anlage (e.g. Smith 1994); the platypus specimens dissected by us have both a pterygoideus lateralis and a pterygoideus medialis]
27 (pterygoideus externus sensuGreene 1935) [in the Norwegian rats dissected by us the pterygoideus lateralis is constituted by a single bundle]
28[in the Cynocephalus specimens dissected by us the pterygoideus lateralis is constituted by a single bundle]
29 (pterygoideus externus sensu Le Gros Clark 1924, 1926) [as described by e.g. Le Gros Clark 1924, in the Tupaia specimens dissected by us the pterygoideus lateralis is constituted by a single bundle]
30[in modern humans the pterygoideus lateralis is usually divided into superior and inferior heads: see e.g. Birou et al. 1991; Aziz et al. 1998; El Haddioui et al. 2005]
31 (part of adductor mandidulae posterior sensuBemis & Lauder 1986)
32 (adductor mandibulae posterior sensuIordansky 1992; levator mandibulae posterior sensuEdgeworth 1935 and Piatt 1938) [authors such as Piatt 1938 suggest that the A2-PVM of tetrapods as e.g. urodeles derives ontogenetically from the A3’ and/or A3’’, but the developmental work of Ericsson and Olsson 2004 strongly supports that it derives instead from the A2, as suggested by Diogo 2007, 2008 and Diogo et al. 2008]
33 (adductor mandibulae posterior sensuAbdala and Moro, 2003, and Holliday and Witmer, 2007)
34[there is some confusion regarding the origin of the tensor tympani and the tensor veli palatini; authors such as Brocks 1938, Barghusen 1986, and Smith 1992, state that it comes from the ‘pterygoideus posterior’ of reptiles; according to Edgeworth 1935, and Saban 1971 the mammalian tensor tympani and tensor veli palatini clearly correspond to the levator mandibulae posterior (= A2-PVM) of reptiles; our dissections and comparisons strongly support this latter view]
35[as described by e.g. Saban 1971, in the platypus specimens dissected by us the tensor veli palatini is present as an independent muscle]
36[seemingly derived from lateral portion of adductor mandibulae: e.g. Diogo 2007, 2008]
37[seemingly absent, but see 38]
38 (levator anguli oris sensuDiogo 2007, 2008) [present, somewhat mixed with A2; it may correspond to, or be derived/modified from, the retractor anguli oris of other sarcopterygians; we use the name ‘mandibularis’ to distinguish this muscle from the levator anguli oris facialis of certain mammals, which is a facial (hyoid), and not a mandibular, muscle]
39 (adductor mandidulae ‘moyen’sensuMillot and Anthony 1958)
40 (adductor mandidulae anterior sensuBemis & Lauder 1986)
41 (pseudotemporalis posterior and anterior sensuIordansky 1992; superficial and deep levator mandibulae anterior sensuEdgeworth 1935, and Piatt 1938; adductor mandibulae A3’ and A3’’sensuDiogo 2007, 2008)
42 (pseudotemporalis superficialis and profundus sensuAbdala and Moro, 2003, and Holliday and Witmer, 2007; adductor mandibulae A3’ and A3’’sensuDiogo, 2007, 2008)
43[the pseudotemporalis of non-mammalian tetrapods seems to correspond to part of the pterygoideus medialis, and possibly also to part of the temporalis, of extant mammals: see 21]
44[the pterygoideus medialis seems to correspond to the pseudotemporalis of amphibians such as Ambystoma, and, thus, to both the pseudotemporalis and pterygomandibularis of some other urodeles and some caecilians and of reptiles such as Timon: see also 49, 50, 51]
45 (pterygoideus internus sensuGreene 1935)
46 (pterygoideus internus sensuLe Gros Clark 1924, 1926)
47 (adductor mandidulae ‘profond’sensuMillot and Anthony 1958)
48[both the adductor A3’ and A3’’ seem to be included in the pseudotemporalis and/or pterygomandibularis of extant amphibians and reptiles: see Diogo 2007, 2008]
49[at least some caecilian and urodele amphibians have an independent muscle ‘pterygoideus’, which, according to Kleinteich and Haas 2007, probably corresponds to the pterygomandibularis of reptiles; in the Ambystoma ordinarium specimens dissected by us this ’pterygoideus’ is poorly differentiated from the pseudotemporalis]
50 (pterygoideus sensuHolliday & Witmer 2007) [seemingly derived from mesial portion of adductor mandibulae]
51[the pterygomandibularis of reptiles such as Timon seems to correspond to part of the pterygoideus medialis, and possibly also to part of the tensor tympani and/or tensor veli palatini, of extant mammals: see 34]
52 (intramandibular adductor sensuLauder 1980b)
53[in Timon the adductor mandibulae has a large and distinct anteroventral division that is lodged in the ‘adductor fossa’ of Lauder 1980b, and that is very similar to the Aω of other osteichthyans; similar adductor mandibulae structures are also found in other reptiles such as crocodilians, turtles and Aves: Edgeworth 1935; Holliday & Witmer 2007; according to Iordansky 2008, at least some of these ’Aω’ structures were acquired independently in evolution]
54[Edgeworth 1935 suggested that the dorsal mandibular musculature was probably acquired independently within gnathostomes, but the presence of this musculature is very likely plesiomorphic for this group, and perhaps for vertebrates as a whole: e.g. Holland et al. 1993; Diogo 2007, 2008]
55[the only dorsal mandibular muscle present in urodeles such as Ambystoma is the levator bulbi; amphibians such as caecilians have a ’levator quadrati’: see e.g. Kleinteich & Haas 2007; according to authors such as Edgeworth 1935, this latter muscle is derived from the adductor mandibulae, but authors such as Brocks 1938 argue that it is a dorsal mandibular muscle]
56[it is derived from the constrictor dorsalis, so it probably corresponds to part of the levator arcus palatini of Latimeria: Brocks 1938; Holliday & Witmer 2007; Diogo 2007, 2008]
57[it is derived from the constrictor dorsalis, so it probably corresponds to part of the levator arcus palatini or of e.g. Latimeria: see e.g. Brocks 1938; Holliday & Witmer 2007; Diogo 2007, 2008]
58[according to e.g. Edgeworth 1935 this muscle is derived from the adductor mandibulae; however, our dissections and comparisons support Brocks’ 1938 hypothesis, i.e. that the levator bulbi, as well as the ’levator quadrati’ of caecilians, are the remains of the constrictor dorsalis group in amphibians; according to Brocks 1938 the constrictor dorsalis group is conserved in many reptiles because of their kinetic skull]
59 (the levator bulbi sensu Frazzeta 1962, Haas 1997, and Schumacher 1973 seemingly corresponds to the tensor periorbitae sensuHolliday & Witmer 2007)
Table 4.
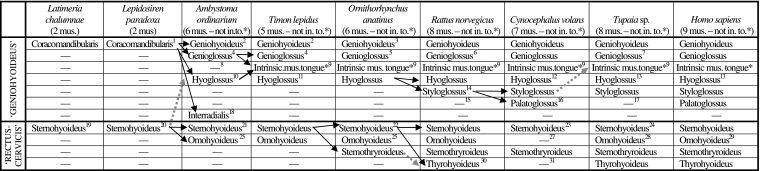
Scheme illustrating the authors’ hypotheses regarding the homologies of the hypobranchial muscles of adults of representative sarcopterygian taxa (see caption of Table 1, text, and Figs 3–17; ’GENIOHYOIDEUS’, ‘RECTUS CERVICIS’=‘geniohyoideus’ and ‘rectus cervicis’ groups sensu Edgeworth, 1935; in. to. = intrinsic muscles of the tongue; mus. = muscles)
1 (geniothoracis sensuBemis & Lauder 1986)
2 (coracomandibularis sensuDiogo 2007, 2008)
3[according to Edgeworth 1935 the geniohyoideus, genioglossus and hyoglossus of mammals develop ontogenetically at the same time; according to him, the two former muscles develop internally (more medially), while the latter develops externally (more laterally)]
4[as explained by e.g. Edgeworth 1935 and Piatt 1938 the genioglossus of salamanders such as Ambystoma and of reptiles such as lizards corresponds to part of the coracomandibularis of sarcopterygian fish]
5[as explained by e.g. Piekarski & Olsson 2007 in mammals such as dogs the tongue muscles are sometimes innervated by both the hypoglossal (CNXII) and the facial (VII) nerves, thus indicating that at least in some cases these muscles may have a dual origin]
6[not described by e.g. Greene 1935, but it is present as an independent structure in the rats dissected by us; authors such as Bryant 1945 support the claim that this muscle is effectively often present in rodents]
7 (geniohyoglossus sensuLe Gros Clark 1924, 1926)
8[according to e.g. Piatt 1938 and Saban 1968, 1971, extant amphibians such as salamanders do not have well-developed, independent intrinsic muscles of the tongue like those found in extant amniotes]
9[according to Saban 1968 the intrinsic muscles of the tongue of amniotes derive from both the genioglossus and hyoglossus; examples of these muscles are e.g. the longitudinalis superior, longitudinalis inferior, transversus linguae and/or verticalis linguae: e.g. Anderson 1881; Edgeworth 1935; Jarvik 1963; Saban 1968, 1971; Smith 1988, 1992; Sokoloff 2000; Herrel et al. 2005]
10[the statements of Edgeworth 1935 concerning the hyoglossus of salamanders such as Ambystoma are confusing: on page 196 he states that it derives from the sternohyoideus (= his ’rectus cervicis’) but on page 211 he suggests that as in other amphibians, as well as in reptiles and mammals, it derives from the coracomandibularis (= his ’geniohyoideus’); the results of the developmental work of Piatt 1938 support this latter hypothesis]
11[according to Edgeworth 1935 the hyoglossus of lizards such as Timon corresponds to part of the coracomandibularis of other amphibians and sarcopterygian fish]
12[as described by e.g. Edgeworth 1935, in colugos the hyoglossus and thyrohyoideus are seemingly fused]
13 (hyoglossus + chondroglossus sensuLe Gros Clark 1926) [according to Saban 1968, in primates and tree-shrews the hyoglossus is divided into a chondroglossus and a ceratoglossus; this is supported by e.g. Le Gros Clark 1926, and Sprague 1944a, although this latter author erroneously states that the chondroglossus is part of the genioglossus and not of the hyoglossus]
14[our dissections and comparisons support Edgeworth's 1935 suggestion that the styloglossus and palatoglossus of therian mammals likely correspond to part of the hyoglossus of monotremes]
15 (seemingly not present as an independent muscle in the rats dissected; this is supported by authors such as Barrow & Capecchi 1999)
16[seemingly present as an independent muscle in the colugos dissected by us, being formed by a group of fibers running from the soft palate and/or the lateral wall of the oropharynx to the posterolateral surface of the tongue]
17[seemingly not present as a separate muscle in the Tupaia specimens dissected by us; it is also not described by authors such as Le Gros Clark 1924, 1926]
18[according to Piatt 1938 in at least some adult Ambystoma there is a hypobranchial muscle interradialis, which derives ontogenetically from the genioglossus]
19 (rectus cervicis sensuBemis & Lauder 1986)
20 (rectus cervicis sensuLauder & Shaffer 1988)
21 (rectus cervicis sensuKardong 2002; episternohyoideus sensuEdgeworth 1935)
22[in the platypus specimens examined by us this muscle is deeply mixed posteriorly with the sternothryroideus, as stated by Saban 1971]
23[as described by e.g. Leche 1886, and Saban 1968, in Cynocephalus the sternohyoideus has two bundles: the posterior one extends anteriorly in order to reach the posterior region of the thyroid cartilage and then contacts, via a broad but thin tendon, the anterior one that extends anteriorly to attach to the lesser cornu of the hyoid]
24[deeply mixed with the sternothyroideus]
25[the omohyoideus, sternothyroideus and thyrohyoideus of tetrapods clearly correspond to part of the sternohyoideus of sarcopterygian fish: e.g. Edgeworth 1935; Saban 1968, 1971; Diogo 2007, 2008; this work]
26[as stated by e.g. Saban 1971 in the platypus specimens dissected by us the omohyoideus is anteriorly divided into superficial and deep bundles]
27[not present as an independent structure in the colugos dissected by us as well as by authors such as Gunnell & Simmons 2005]
28[George 1977 states that this muscle has no distinct tendinous intersection, but Le Gros Clark 1924, 1926, and Sprague 1944a, describe such an intersection in tree-shrews as e.g. Tupaia and Ptilocercus]
29[it has superior and inferior bellies, which are separated by a distinct tendon]
30[as explained above, the thyrohyoideus of therian mammals clearly corresponds to part of the sternohyoideus of non-mammalian tetrapods; however, it is not clear if it corresponds to part of the monotreme sternohyoideus or, instead, to part of the monotreme sternothyroideus: e.g. Edgeworth 1935; Saban 1968]
31[not present as an independent structure in the colugos dissected by us; it is seemingly fused with the hyoglossus: see 12]
Materials and methods
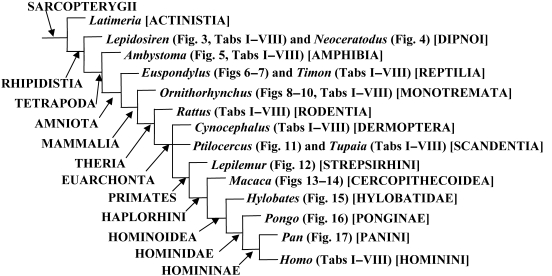
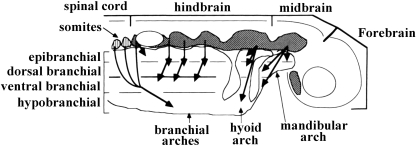
The phylogenetic framework for the discussion provided in the present paper and the comparison between the head and neck muscles of the genera listed in Tables 1–4 and shown in Figs 3–17 is set out in Fig. 1. As noted above, one of the main aims of the present work is to investigate how the head and neck muscles have changed during the evolutionary transitions from non-mammalian sarcopterygians to monotreme and therian mammals, and then to higher primates as exemplified by modern humans. We therefore dissected the head and neck muscles of representative members of sarcopterygian fish, amphibians, reptiles, and mammals and carefully chose to include in Tables 1–4: 1–2) the non-mammalian sarcopterygians included in the tables of Diogo (2008), i.e. the coelacanth Latimeria chalumnae, the dipnoan Lepidosiren paradoxa, the salamander Ambystoma ordinarium and the lizard Timon lepidus; 5) a member of the phylogenetically most plesiomorphic extant mammal clade, the monotremes (Ornithorhynchus anatinus, or ‘platypus’); 6) a member of the rodents, the Norwegian rat (Rattus norvegicus), because rats are often considered ‘anatomically generalized’ therian mammals, but at the same time are somewhat closely related to primates (see Fig. 1); 7 and 8) a member of the colugos (or ‘flying lemurs’ (Cynocephalus volans) and a member of the tree-shrews (Tupaia sp.), i.e. of the two groups that are usually considered the closest living relatives of primates (see above); 9) a member of the Primates, Homo sapiens. The dissected specimens are from the Museo Nacional de Ciencias Naturales de Madrid (MNCN), the Centro Nacional Patagónico de Argentina (CONICET), Macquarie University of Australia (MU), the Colección Mamíferos Lillo of the Universidad Nacional de Tucumán (CML), the herpetological collection of Diamante-CONICET-Argentina (DIAMR), the Fundación Miguel Lillo of Argentina (FML), the Primate Foundation of Arizona (PFA), San Diego State University (SDSU), the Department of Anatomy (GWU-ANA) and the Department of Anthropology (GWU-ANT) of the George Washington University, the Department of Anatomy of Howard University (HU-ANA), and the Smithsonian Institution's National Museum of Natural History (USNM). The list of specimens examined for the present work is given in the Appendix 1; the number of specimens examined is followed by an abbreviation that refers to the state of the specimen (alc = alcohol-fixed; fre = fresh; for = formalin-embalmed). The dissections were undertaken using a Wild M5 dissecting microscope. In our dissections, there were no notable differences regarding the attachments, overall configuration and general appearance of the muscles of fresh, alcohol-fixed, and formalin-embalmed specimens, other than their color. The nomenclature Diogo (2008) used for the bony fish and non-mammalian sarcopterygians was reconciled with the nomenclature used by researchers working with mammals (for a review see e.g. Saban, 1968, 1971; Jouffroy & Saban, 1971) and with modern humans (e.g. Terminologia Anatomica, 1998). For the sake of uniformity, and to make it easier for the reader to compare the different taxa shown in Figs 3–17, the illustrations of non-mammalian sarcopterygians were adapted from Diogo (2008), whereas those of mammals were modified from Saban (1968, 1971) and Jouffroy & Saban (1971). When cited papers use a nomenclature that differs from that followed here, the respective synonymy is given in Tables 1, 2, 3 and 4. It should be noted that the Homo sapiens muscles listed in these Tables are the ones that are usually present in modern humans. Thus, we do not list all the muscles that occasionally appear as variants and/or abnormalities in modern humans (for instance, some modern human adults have a platysma cervicale, but in most commentaries of the head and neck muscles of modern humans. this muscle is absent as an independent structure: e.g. Huber, 1931; see also e.g. Wood, 1866, 1867a,b, 1868, 1870; Anderson, 1880; Shattock, 1882; Parsons, 1898; Taylor, 1925; Wells & Thomas, 1927; Chi, 1937; Pettersen et al. 1979; Aziz, 1981; Gibbs, 1999). When we refer to the anterior, posterior, dorsal and ventral regions of the body, we do so in the sense the terms are used for pronograde tetrapods (e.g. the eye is anterior to the ear and dorsal to the mandible). Although there is obviously some subjectivity concerning the identification of separate muscles, we followed as strictly as possible Edgeworth's (1935) criteria for analysing the evidence acquired by others and ourselves. This includes criteria such as the degree of separation of the fibers, differences in origin and/or insertion, differences in function, orientation of the fibers and/or differences in innervation. For example, it is sometimes considered that colugos have a ‘zygomatico-mandibularis’ muscle. However, as explained in Table 1, in the colugo specimens we dissected the ‘zygomatico-mandibularis’ fibers were indistinguishable from the remaining fibers of the masseter. Therefore, in this case, one of the above criteria (the separation of the fibers) is not consistent with identifying the ‘zygomatico-mandibularis’ as a ‘separate, independent’ muscle sensuEdgeworth (1935) (see Table 1). It should, however, be noted that, in general, there are no major problems in identifying separate muscles within the head and neck musculature of sarcopterygian fish and tetrapods (Diogo, 2008; e.g. it is usually more problematic to identify separate muscles within the hand musculature of these vertebrates, as they often have a series of small muscles that are blended with each other, such as the interossei or the lumbricales).
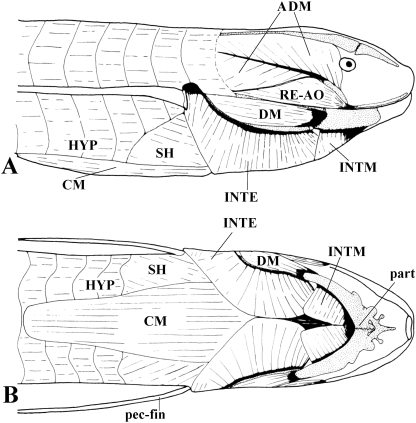
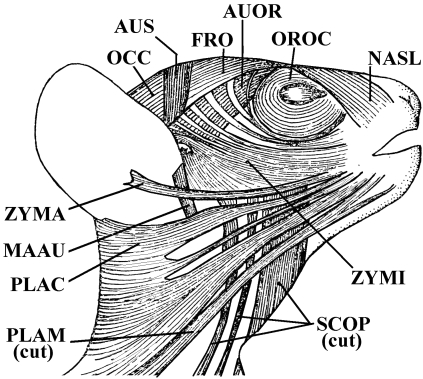
Fig. 3.
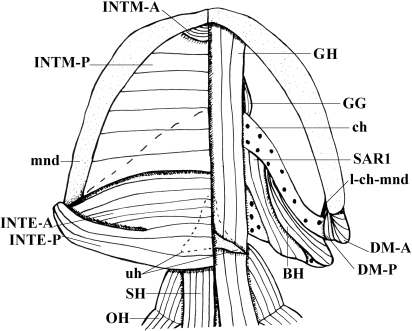
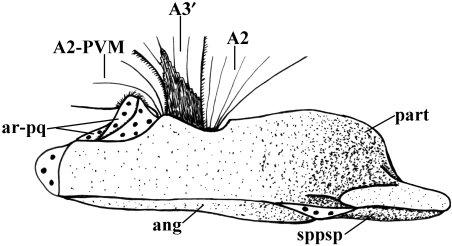
Lepidosiren paradoxa (Dipnoi): A) lateral view of the cephalic musculature; B) ventral view of the cephalic musculature (modified from Bemis & Lauder, 1986 and Diogo, 2008; the nomenclature of the structures illustrated follows that used in the present work; anterior is to the right). ADM, adductor mandibulae complex; CM, coracomandibularis; DM, depressor mandibulae; HYP, hypaxialis; INTE, interhyoideus; INTM, intermandibularis; part, prearticular; RE-AO, retractor anguli oris; SH, sternohyoideus.
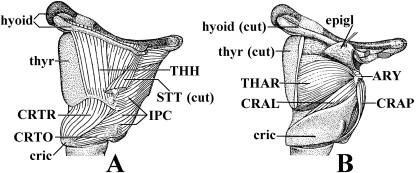
Fig. 17.
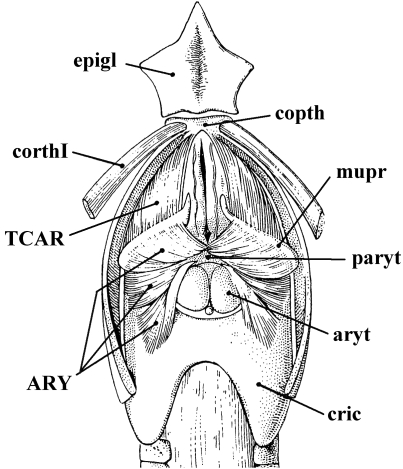
Pan troglodytes(Mammalia, Primates): A) lateral view of the laryngeal musculature; B) same view, but the thyrohyoideus, sternothyroideus, constrictor pharyngis inferior and cricothyroideus were removed and the lateral portions of the thyroid cartilage and hyoid bone were partially cut [modified from Starck & Schneider (1960) and Saban (1968); the nomenclature of the structures illustrated basically follows that used in the present work; anterior is to the top, dorsal is to the right: see text]. ARY, arytenoideus; CRAL, cricoarytenoideus lateralis; CRAP, cricoarytenoideus posterior; cric, cricoid cartilage; CRTO, CRTR, pars obliqua and pars recta of cricothyroideus; epigl, epiglottis; IPC, constrictor pharyngis inferior; STT, sternothyroideus; THAR, thyroarytenoideus; THH, thyrohyoideus; thyr, thyroid cartilage.
Fig. 1.
Phylogenetic framework for the discussion provided in the present paper and the comparison between the head and neck muscles of the genera listed in Tables 1–4 and shown in Figs 3–17, based on Shoshani et al. (1996), Kardong (2002), Sargis (2002a,b, 2004), Dawkins (2004), Kemp (2005), Marivaux et al. (2006), Diogo (2007), Janeka et al. (2007), and Silcox et al. (2007). N.B. When we use the term reptiles we refer to the group including taxa such as turtles, tuataras, lizards, snakes, crocodiles, and Aves, which, despite some controversy, continues to be considered a monophyletic taxon by most taxonomists and in most general textbooks (e.g. Kardong, 2002; Dawkins, 2004; Diogo, 2007); the Primates, Dermoptera (including colugos or ‘flying lemurs’) and Scandentia (including tree-shrews) are placed in an unresolved trichotomy, because the relationships between these three groups remains mainly unresolved (some authors continuing to group colugos with tree-shrews, others group tree-shrews with primates, and yet others group colugos with primates: e.g. Sargis, 2002a,b, 2004; Dawkins, 2004; Marivaux et al. 2006; Janeka et al. 2007; Silcox et al. 2007).
Table 2.
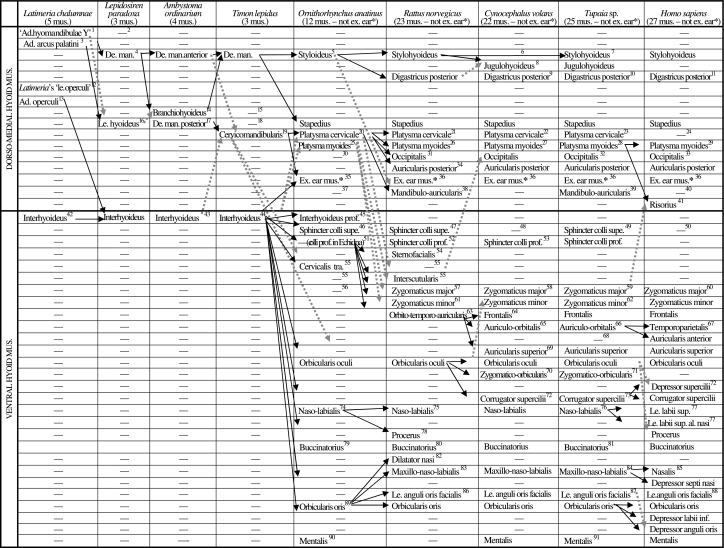
Scheme illustrating the authors’ hypotheses regarding the homologies of the hyoid muscles of adults of representative sarcopterygian taxa (see caption of Table 2, text, and Figs 3–17; ad. = adductor; al. = alaeque; de. man. = depressor mandibulae; ex. = extrinsic; inf. = inferioris; le. = levator; mus. = muscles; prof. = profundus; sup. = superioris; supe. = superficialis; tra. = transversus)
1[seemingly not homologous with the adductor hyomandibulae of actinopterygians such as teleosts: Diogo 2007, 2008]
2[seemingly absent in dipnoans and tetrapods, although it may possibly be included in the levator hyoideus/depressor mandibulae: Diogo 2007, 2008]
3[the portion of the hyoid muscle anlage that gives rise to the levator hyoideus/depressor mandibulae of non-actinistian sarcopterygians probably corresponds to that giving rise to the adductor arcus palatini in other osteichthyans: Diogo 2007, 2008]
4[according to Forey 1986 the depressor mandibulae and levator hyoideus of extant dipnoans develop from the same ontogenetic anlage]
5 (the styloideus sensuHuber 1930a corresponds to the interhyoideus sensuEdgeworth 1935 and to the posterior digastric sensuParsons 1898) [Edgeworth 1935 suggested that the styloideus of monotremes and the stylohyoideus of other mammals derive from the interhyoideus; our observations and comparisons strongly support the interpretations of e.g. Huber 1930a and Saban 1968, 1971, i.e. that the monotreme styloideus and stapedius and the therian stylohyoideus, digastricus posterior, jugulohyoideus, stapedius and possibly mandibulo-auricularis correspond to the depressor mandibulae of reptiles such as Timon; Gasser's 1967 developmental study indicates that in modern humans the digastricus posterior, stapedius and stylohyoideus derive from a same anlage]
6[Saban 1968 states that the stylohyoideus is present in Cynocephalus but Gunnell & Simmons 2005 consider that this muscle is missing in this taxon; our dissections clearly indicate that the stylohyoideus is not present as an independent structure in adult colugos]
7[Sprague 1944a states that the styloglossus of certain tree-shrews is innervated by the hypoglossal nerve, but Le Gros Clark 1926 and Lightoller 1934 claim that this muscle is innervated by the facial nerve, as in other mammals]
8 (mastoideostyloideus sensuSaban 1968) [seemingly corresponds to part of the stylohyoideus and/or possibly of the digastricus posterior of mammals such as rats; see e.g. Huber 1930a, 1931; Saban 1968]
9 (part of biventer sensuLeche 1886) [our dissections indicate that the digastricus posterior and digastricus anterior of colugos are joined by a tendinous intersection, as described by e.g. Saban 1968]
10[our dissections indicate that the digastricus posterior and digastricus anterior of Tupaia are joined by a well-developed tendon, as described by e.g. Sprague 1944a]
11[the digastricus posterior and digastricus anterior of modern humans are usually joined by a well-developed tendon]
12[seemingly not homologous with the levator operculi of halecomorph and teleostean actinopterygians: Diogo, 2007, 2008]
13[its fibers are seemingly deeply mixed with those of the adductor arcus palatini: Diogo, 2007, 2008]
14[according to Edgeworth 1935 and Ericsson & Olsson 2004 the branchiohyoideus, interhyoideus and levator hyoideus appear at about the same time in urodele embryos, thus being difficult to infer if the branchiohyoideus is ontogenetically derived from the ventral or from the dorso-medial hyoid musculature; however, the developmental study of Piatt 1938 indicates that this muscle is in fact part of the dorso-medial hyoid musculature; it should be noted that Ambystoma ordinarium, as many other urodeles, has a muscle ceratomandibularis, which seems to derive from the same anlage that gives rise to the branchiohyoideus, but that, contrary to this latter muscle, is not present as an independent structure in the adult Ambystoma ordinarium specimens dissected by us; this muscle ceratomandibularis seems to correspond to the caecilian muscle ’hyomandibularis’ sensu Kleinteich & Haas 2007]
15[ the ’branchiohyoideus’ of reptiles is a branchial muscle that seemingly corresponds to the subarcualis rectus 1, and not to the hyoid muscle branchiohyoideus, of amphibians: e.g. Edgeworth 1935; Diogo 2007, 2008]
16[the portion corresponding to the levator hyoideus of dipnoans becomes attached on the mandible, forming the depressor mandibulae posterior; the depressor mandibulae anterior thus seemingly corresponds to the depressor mandibulae of dipnoans: Diogo 2007, 2008]
17[the Timon specimens dissected do not have an independent depressor mandibulae posterior nor an independent levator hyoideus but according to e.g. Edgeworth 1935 some adult reptiles do have a levator hyoideus]
18 (levator hyoideus sensuEdgeworth 1935) [the mammalian stapedius clearly derives from the levator hyoideus/depressor mandibulae of other tetrapods: e.g. Huber 1930a,b, 1931; Edgeworth 1935; Brocks 1938; Saban 1968, 1971; Kardong 2002]
19[our dissections and comparisons support the view of e.g. Huber 1930a and Edgeworth 1935, i.e. that this muscle corresponds to part of the depressor mandibulae/levator hyoideus of amphibians such as urodeles; see also the recent work of Tsuihiji 2007]
20 (the platysma cervicale sensuJouffroy and Saban 1971 corresponds to the pars nuchalis of the platysma sensuSaban 1971 and to part of the platysma sensuLightoller 1942) [our dissections and comparisons support the suggestion of authors such as Lightoller 1942 and Saban 1971, i.e. it seems likely that the reptilian sphincter colli (= interhyoideus) corresponds to the mammalian sphincter colli superficialis + sphincter colli profundus + their derivatives, and that the reptilian cervicomandibularis, which is more oblique than, and deep to, the interhyoideus, corresponds to the mammalian platysma + its derivatives; although the hypothesis that the facial musculature of mammals corresponds exclusively to the ventral (superficial) hyoid musculature of other tetrapods cannot be completely ruled out, it seems likely that at least some facial muscles may in fact be part of the dorso-medial, and not only of the ventral, hyoid musculature]
21[the cranial panniculus of Greene 1935 corresponds to our platysma cervicale + auriculolabialis inferior (= zygomaticus major); his superficial portion of the cervical panniculus corresponds to our sphincter colli profundus + superficialis; his deep cervical panniculus corresponds to our sternofacialis, which he describes as an upper limb muscle but, as noted by Jouffroy & Saban 1971, it is in fact a facial muscle that is probably derived from the sphincter colli profundus: e.g. Jouffroy & Saban 1971; Ryan 1986, 1989; this work]
22 (part of platysma sensuLeche 1886)
23 (part of platysma sensuLe Gros Clark 1924 and of notoplatysma sensuLightoller 1934)
24[according to e.g. Gasser 1967 the platysma cervicale (=his nuchal platysma) is present in early developmental stages of modern humans, disappearing in later stages; Aziz 1981 considers that the transversus nuchae found in some humans is a remnant of the platysma cervicale, but Gasser 1967 describes both a platysma cervicale and a transversus nuchae in early human embryos]
25 (seemingly corresponds to pars omoidea sensuSaban 1971)
26 (mixed with platysma cervicale)
27 (platysma myoides superior +’jugalis propatagii’sensuLeche 1886; dorsal sheet of propatagial complex sensuThewissen & Babcock 1991, 1993) [mixed with platysma cervicale; Leche 1886 stated that the propatagial complex of dermopterans has a dorsal muscle formed by the platysma myoides and the ‘jugalis propatagii’ and a ventral muscle; Thewissen & Babcock 1991, 1993, studied the configuration and innervation of these muscles and concluded that the dorsal one is innervated by the facial nerve and the ventral one by cervical spinal nerves; the dorsal and ventral muscles therefore seem to correspond respectively to the platysma myoides and to part of the panniculus carnosus of other mammals]
28[mixed with platysma cervicale]
29 (platysma sensuNetter 2006; tracheoplatysma sensuLightoller 1940a)
30[as described by e.g. Lightoller 1942 in the platypus specimens dissected by us there is a bundle of the platysma that is somewhat similar to the occipitalis of the mammals listed on the right, but this bundle is clearly part of the platysma, i.e. it does not constitute an independent muscle]
31 (cranial part of levator auris longus sensuGreene 1935) [the occipitalis of Rattus is similar to that of Tupaia and Cynocephalus, i.e. it has a medial portion (=occipitalis sensuLightoller 1934) that extends anteriorly to mix with the frontalis and a lateral portion (=cervico-auriculo-occipitalis sensuLightoller 1934) that runs anteroventrolaterally to attach on the posterior surface of the ear; these two portions are deeply mixed posteriorly, attaching to the dorsal region of the neck, just medially to the posterior attachment of the auricularis posterior]
32 (occipitalis + cervico-auriculo-occipitalis sensuLightoller 1934: see 31)
33[Gasser's 1967 developmental study in modern humans indicates that the occipitalis, auricularis posterior and transversus nuchae develop from a same anlage]
34 (caudal part of levator auris longus sensuGreene 1935: see 31)
35[our dissections and comparisons indicate that the platypus has at least some extrinsic muscles of the ear, as suggested by Lightoller 1942; according to Huber 1930a,b, 1931, and Jouffroy & Saban 1971, some of the extrinsic muscles of the ear derive from the platysma while others derive from the sphincter colli profundus]
36[examples of extrinsic, facial muscles of the ear present in therian mammals are the obliquus auriculae, transversus auriculae, helicis, tragicus and/or antitragicus: see e.g. Jouffroy & Saban 1971]
37[although authors such as Adams et al. 1929 suggest that the therian mandibulo-auricularis is a ’preauricular’ muscle and thus derives from the sphincter colli profundus, most researchers consider that it is instead a ’post-auricular’ muscle derived from the platysma (e.g. Huber 1930a,b, 1931; Ryan 1986, 1989); however, a few authors, such as Lightoller 1934 and Jouffroy & Saban 1971, have suggested that the mandibulo-auricularis may in fact be ontogenetically and phylogenetically more related to deeper dorso-median muscles such as the stylohyoideus, digastricus posterior, stapedius and/or jugulohyoideus than to the facial muscles; although we tentatively follow here the most consensual view, we consider that Lightoller's hypothesis should not be completely ruled out, because the mandibulo-auricularis does usually lie deeper to all the other facial muscles and also because its topology, the orientation of its fibers, and its attachments (e.g. on the mandible and/or near the ear region) are in fact similar to those of the deeper dorsomedial hyoid muscles of mammals and to the depressor mandibulae/levator hyoideus of other tetrapods; also, Seiler's 1980 developmental studies of tree-shrews and primates seem to suggest that the mandibulo-auricularis does not develop from the anlages that give rise to most other facial muscles, but instead from a different, deeper anlage]
38[as stated by Lightoller 1934, contrary to lemurs and e.g. Tupaia, in primates as e.g. Tarsius and marmosets the mandibulo-auricularis probably corresponds to a strong sheet connecting the posterior edge of the mandible to the bony external auditory meatus, which might well correspond to the stylo-mandibular ligament of modern humans; such a configuration is found in the colugos dissected, i.e. there is no fleshy muscle mandibulo-auricularis, but instead a strong fascia running from the posterior edge of the mandible to the bony external auditory meatus]
39 (auriculo-mandibularis sensuLightoller 1934)
40[seemingly corresponds to the stylo-mandibular ligament, which is usually present in modern humans: see 38; according to Jouffroy & Saban 1971 it may possibly also correspond to the stylo-auricularis muscle abnormally present in a few modern humans]
41[authors as e.g. Huber 1930a,b, 1931, suggest that the risorius derives from the sphincter colli profundus; our dissections and comparisons support the conclusions of Jouffroy & Saban's 1971 review, i.e. that the risorius derives instead from the platysma myoides; this latter hypothesis is also supported by the developmental data of Gasser 1937: see e.g. his fig. 10]
42 (’géniohyoïdien’+‘hyohyoïdien’sensuMillot & Anthony 1958, which, as is shown in the illustrations of these authors, are effectively deeply mixed]
43 (interhyoideus anterior + interhyoideus posterior sensu Piatt 1938, Bauer 1992, 1997, and Ericsson & Olsson 2004) [as stated by Piekarski & Olsson 2007, recent developmental works indicate that the interhyoideus of sarcopterygians as e.g. Ambystoma might be derived ontogenetically not only from the hyoid region but also possibly from anterior somites]
44 (constrictor colli sensuHerrel et al. 2005)
45 (sphincter colli profundus sensuLightoller, 1942; hyomandibularis sensuEdgeworth, 1935) [Edgeworth, 1935, claimed that none of the mammalian facial muscles are derived from the interhyoideus because all of them are de novo structures; however this view has been abandoned and it is now commonly accepted that the mammalian muscles correspond to the interhyoideus and possibly dorso-medial muscles such as the cervicomandibularis of reptiles such as the lizards Timon: see 20 and also text]
46 (corresponds to Huber's 1930a sphincter colli externus – of platypus – and sphincter colli – of echidna) [corresponds to part of the interhyoideus of non-mammalian tetrapods: e.g. Huber 1930a; Jouffroy & Saban 1971; Lightoller 1940a states that Huber's 1930a sphincter colli superficialis (of e.g. marsupials and rodents) corresponds to his transitus, i.e. to a part of the sphincter colli profundus that passes superficial to the platysma but that originally was deep to it: Lightoller claims that the rest of the sphincter colli profundus (i.e. everything except the transitus) is absent in all primates, and that it is thus this transitus that gives the tracheo-platysma of primates; however, our dissections and comparisons strongly indicate that the configuration of the platysma of primates such as lemurs is in fact similar to that found in e.g. colugos and tree-shrews, i.e. these latter mammals have both a platysma cervicale and a platysma myoides, although these two latter muscles are blended; in primates such as modern humans, the platysma cervicale is usually missing, i.e. the platysma sensuNetter 2006 corresponds to the platysma myoides of other mammals; if we accept, as it is nowadays commonly accepted, that the sphincter colli of mammals derives from the interhyoideus of other tetrapods, it makes sense to suppose that plesiomorphically the sphincter colli was a superficial muscle, as is the non-mammalian interhyoideus, and not a deep muscle: see text]
47 (transitus sensuLightoller 1942) [as explained by e.g. Lightoller 1940a, 1942, although much reduced, in rodents such as rats the sphincter colli does have a component that is superficial to the platysma – i.e. a sphincter colli superficialis]
48[Jouffroy & Saban 1971, p. 484, state that colugos have a sphincter colli superficialis, but as they explain in their p. 496, this is because they consider that the ventral sheet of the propatagial muscle complex of colugos probably corresponds to the sphincter colli superficialis of other mammals; Thewissen & Badcock 1991, 1993, have however shown that this ventral sheet is in fact innervated by cervical spinal nerves and not by the facial nerve, as is the sphincter colli superficialis; moreover, as shown in fig. 1 of these latter authors, the position and the orientation of the fibers of that ventral sheet are not similar to those of the sphincter colli superficialis of other mammals (e.g. in a lateral view it appears deep, not superficial, to the platysma); our dissections indicate that colugos do not have a fleshy, distinct muscle sphincter colli superficialis]
49 (seems to correspond to the occipito-cervicalis sensuLightoller 1934, and might correspond to the cervico-mandibularis sensuLe Gros Clark 1926, which was originally described as part of the platysma of Ptilocercus but seems rather to correspond to the sphincter colli superficialis of Tupaia and other mammals)
50[it is commonly accepted that primates such as modern humans and chimpanzees do not have a sphincter colli superficialis, but according to Burrows et al. 2006 this muscle may be found in some chimpanzees and perhaps even in some modern humans; in the modern human cadavers we dissected the sphincter colli superficialis was not present as an independent muscle]
51[absent as an independent muscle in the platypus, although part of it might have given rise to deep facial muscles such as the orbicularis oris, orbicularis oculi, mentalis and/or naso-maxillo-labialis (which clearly seem to correspond to the muscles that are designated by the same names in other mammals) and possibly to the 'sphincter bursae buccalis’sensu Huber 1930a (which, contrary to what was stated by Huber 1930a, seems to correspond to the ’buccinatorius’ of the echidna and to the buccinatorius of other mammals); in the echidna part of the sphincter colli passes deep to other facial muscles, forming the sphincter colli profundus: e.g. Lightoller 1942; Jouffroy & Saban 1971]
52 (superficial portion of cervical platysma sensuGreene 1935; sphincter colli profundus +’primitive sphincter colli’ of fig. 6 of Huber 1930a; transitus sensuLightoller 1940a) [deeply mixed with the sphincter colli superficialis]
53[absent according to Jouffroy & Saban 1971, but this muscle is clearly present in the specimens dissected: dorsally its runs deep to the platysma myoides while ventrally it meets its counterpart in the ventral midline of the head]
54 (deep cervical panniculus sensuGreene 1935: see 21)
55[its position and the orientation of its fibers are similar to those of the interscutularis of non-monotreme mammals such as rats; Lightoller 1940a seems to support the homology of these muscles, because he states that there is a cervicalis transversus in rodents; however, according to Jouffroy & Saban 1971, the interscutularis is derived from the pars intermedia of the sphincter colli profundus, while the cervicalis transversus is derived from the pars cervicalis of this latter muscle]
56[our dissections and comparisons support Jouffroy & Saban's 1971 hypothesis, i.e.: 1) the zygomaticus major and minor are absent in mammals such as monotremes; 2) in placentals, the zygomaticus is plesiomorphically attached to the zygomatic arch, but in some cases it extends posteriorly to attach to the ear (that is why it is sometimes named auriculolabialis); 3) in a few mammals, such as some ungulates, pinnipedes, bats, rodents, tree-shrews and primates, the zygomaticus is divided into superficial (= auriculolabialis inferior and zygomaticus major sensuJouffroy & Saban 1971) and deep (= auriculolabialis superior and zygomaticus minor sensuJouffroy & Saban 1971) portions, the former originating ventrally and/or posteriorly to the latter, thus usually lying nearer the ear and being more associated with the platysma (that is why some authors argue that it might derive from the platysma, although its innervation seems to indicate the contrary); Jouffroy & Saban 1971 explicitly state that these superficial and deep portions correspond very likely to the zygomaticus major and minor of modern humans, respectively; 4) according to them, in mammals such as tree-shrews and primates as lemurs the zygomatic muscles, and particularly the zygomaticus major, often extends posteriorly in order to attach to the ear (Fig. 12), but this ‘trend’ is reverted in higher primates: for example, in modern humans both the zygomaticus major and zygomaticus minor usually originate relatively far from the ear, although in a few cases at least one of these muscles might originate on the ear region]
57 (part or the totality of auriculolabialis sensuGreene 1935; zygomatico-labialis superficialis and or auriculolabialis inferior sensuJouffroy & Saban 1971) [our dissections indicate that the ’auriculolabialis’ of the Norwegian rat is deeply mixed with the platysma; does this mean that it is really part of and/or derived from the platysma? Probably not, because as stated by Greene 1935 in other rats and other rodents this ’auriculolabialis’ is much more distinct from the platysma, being seemingly a derivative of the sphincter colli profundus; however, it is possible that some of the mammalian structures that are designated as ’zygomaticus major and minor’ and/or ’auriculolabialis inferior and superior’ in the literature are really part of and/or derive from the platysma, i.e. that they are not really homologous to the zygomaticus major and minor sensu this work: e.g. Boas & Paulli 1908; Huber 1930a; Edgeworth 1935; Jouffroy & Saban 1971; it cannot be completely ruled out, however, that at least in some cases the ‘zygomaticus major’ and/or ‘auriculolabialis inferior’ derive from the platysma, while the ‘zygomaticus minor’ and/or ‘auriculolabialis superior’ derive from e.g. the orbicularis oculi]
58[in Huber's 1930a fig. 27 of the ’primate ground plan of superficial facial musculature’, he suggests that the auriculabialis inferior (= zygomaticus major) derives from the platysma, but at least in the case of colugos the former muscle is well distinguished from the latter, because these muscles are in fact perpendicular to each other; there is a significant difference between Cynocephalus, Lemur and Tupaia: in Cynocephalus the auriculolabialis inferior (= zygomaticus major) is superficial to the platysma cervicale; in Lemur these two muscles lie in the same plane; in Tupaia the auricularis inferior is deep to the platysma cervicale: e.g. Lightoller 1934; this work]
59 (auriculolabialis inferior or zygomatico-labialis sensuJouffroy & Saban 1971, Le Gros Clark 1926, and Lightoller 1934)
60[Gasser's 1967 developmental study indicate that the zygomaticus major and zygomaticus minor of modern humans derive from his ’infraorbital lamina’, and not from his ‘mandibular lamina’, i.e. they seem to be ontogenetically more related with the facial muscles of the orbit region than with those of the mouth region; interestingly, in earlier stages of human development these two muscles are more separated from each other than in later stages, i.e. in this respect the configuration seen in those early stages is more similar to that seen in adult mammals such as colugos, tree-shrews and lower primates: see e.g. Fig. 12]
61 (zygomaticus sensuGreene 1935; zygomatico-labialis profundus and or auriculolabialis superior sensuouffroy & Saban 1971)
62 (auriculolabialis superior sensuJouffroy & Saban 1971, Le Gros Clark 1926, and Lightoller 1934)
63 (the frontalis sensuGreene 1935 corresponds to the orbito-temporo-auricularis sensuEdgeworth 1935)
64[our dissections and comparisons indicate that the frontalis and auriculo-orbitalis of Cynocephalus and Tupaia and the frontalis, temporoparietalis and auricularis anterior of modern humans, correspond to the orbito-temporo-auricularis of mammals such as rats]
65[this muscle usually runs from the auricular region to the orbital region, being inferior and/or deep to the frontalis: e.g. Fig. 12]
66 (auriculo-orbitalis or orbito-auricularis sensuLightoller 1934; it might correspond to Tupaia's attrahens aurem sensuLe Gros Clark 1924, and/or to the Ptilocercus’ scutularis + portio transiens sensuLe Gros Clark 1926)
67[according to Jouffroy & Saban 1971, this muscle is related to, but different from, the auricularis superior; they state that it corresponds to the temporal part of the frontalis, which is also named epicranio-temporal or orbito-temporalis, and which has a longitudinal orientation and covers the temporal aponeurosis, being often fused in primates with the auriculares anterior and superior and also to part of the galea aponeurotica]
68[contrary to Ptilocercus, Tupaia seems to only have an auriculo-orbitalis sensuLightoller 1934, i.e. it does not have a separate temporoparietalis and a separate auricularis anterior: see 66]
69 (auricularis anterior superior of e.g. fig. 409 of Jouffroy & Saban 1971)
70[there is a very thin group of fibers attaching to the medial margin of the posterior portion of the orbicularis oculi, anteriorly, and to the dorsal surface of the zygomatic arch, posteriorly; this thin group of fibers lies deep to most fibers of the orbicularis oculi and auriculo-orbitalis, being in fact deeply mixed with the temporal fascia covering the temporalis in lateral view; however, our dissections indicate that there are in fact some fleshy fibers, which thus should be considered a zygomatico-orbicularis sensuLe Gros Clark 1924, 1926, even if this is a poorly developed muscle; the lemur shown in fig. 4 of Lightoller 1934 also seems to have such a group of fleshy fibers]
71[Lightoller 1934 states that in Tupaia javanica there is a group of fibers running from the region lying posterodorsally to the eye to the dorsal margin of the zygomatic arch, but that it cannot correspond to the zygomatico-orbicularis sensuLe Gros Clark 1924 because it lies deep to the orbicularis oculi; however, Le Gros Clark 1924 stated that his zygomatico-orbicularis lies deep to at least some fibers of the orbicularis oculi; the group of fibers described by Lightoller thus seems to correspond to the zygomatico-orbicularis sensu Le Gros Clark]
72[the depressor supercillii and corrugator supercilii are seemingly derived from the orbicularis oris]
73 (superciliaris sensuJouffroy & Saban 1971)
74[Lightoller 1942 and Saban 1971 state that deep to the cranial (anterior) portion of the orbicularis oculi of the echidna lies a small naso-labialis; according to Lightoller 1942 in the platypus there is a somewhat similar structure, but it is not as differentiated from the other facial musculature as in the echidna; in the platypus dissected by us the naso-labialis does seem to be an independent muscle, being in fact very similar to the naso-labialis of the echidna - see e.g. fig. 4 of Lightoller 1942, which suggests that the naso-labialis derives from a part of the sphincter colli superficialis (transitus sensu Lightoller): Huber 1930a; Jouffroy & Saban 1971]
75 (levator labii superioris sensuParsons 1898 and Greene 1935; pars jugularis of superficial maxillo-naso-labialis sensuLightoller 1940b)
76 (levator labii superioris sensuLe Gros Clark 1924) [not described by Le Gros Clark, 1926 but his fig. 49 seems to suggest that it may also be present in Ptilocercus]
77[the levator labii superioris and levator labii superioris alaeque nasi clearly seem to correspond to the naso-labialis of the other therian mammals listed on this Table; Gasser's 1967 developmental study suggests that at least in modern humans these two muscles appear ontogenetically in the orbital region]
78 (nasolabialis superficialis sensuRyan 1989) [not described by Greene 1935 but seems to be present in the rats dissected by us; see also e.g. Ryan 1989]
79 (the buccinatorius sensuLightoller 1942 corresponds to the sphincter bursae buccalis sensu Huber 1930a) [according to Huber 1930a there is no buccinatorius in the platypus, and the ‘buccinatorius’ of echidna may well not be homologous with that of other mammals, because it may well be derived from the platysma and not from the sphincter colli profundus; however, as noted in later works as e.g. Lightoller 1942 and Jouffroy & Saban 1971, the ‘sphincter bursae buccalis’ of platypus might well correspond to the ‘buccinatorius’ of echidna and/or to the buccinatorius of other mammals]
80[not described by Greene 1935 but is clearly present in the rats dissected by us, being in fact subdivided into various sections; see also e.g. Ryan 1989]
81[not described by Le Gros Clark 1924, but it is clearly present in the Tupaia specimens we dissected; it is also present in Ptilocercus, see e.g. Le Gros Clark 1926]
82 (dilatator naris sensuGreene 1935 and Peterka 1936) [we prefer to use the name dilatator nasi because the name dilatator naris is often used to designate the pars alaris of the nasalis: see e.g. Jouffroy & Saban 1971]
83 (seemingly incorporates the maxillo-labialis and naso-labiais profundus sensuJouffroy & Saban 1971 and Ryan 1989) [not described by Greene 1935, but it is clearly present in the rats dissected by us; see also e.g. Ryan 1989]
84 (maxillo-nasalis sensuJouffroy & Saban 1971; it might correspond to the dilator naris, zygomatici and/or erector vibrissae sensuLe Gros Clark 1924, and thus might possibly be included in the orbicularis oculi (sensue Gros Clark 1926) of Ptilocercus)
85[the nasalis and depressor septi nasi of modern humans seem to correspond to the maxillo-naso-labialis of e.g. rats, colugos and tree-shrews: see e.g. Jouffroy & Saban 1971]
86 (levator anguli oris or caninus sensuLightoller 1934; bucco-naso-labialis sensuRyan 1986; buccinatorius sensuGreene 1935, and Bryant 1945; pars profunda of maxillo-naso-labialis sensuLightoller 1940b) [we use the name levator anguli facialis in order to distinguish this muscle from the levator anguli oris mandibularis of some non-mammalian tetrapods, which is a mandibular, and not a hyoid, muscle: see Table 1]
87 (levator anguli oris sensuLe Gros Clark 1926; incisivus superior + caninus sensuLightoller 1934) [as noted by Lightoller 1934, it is deeply mixed with the orbicularis oris]
88[Gasser's 1967 study of human development supports the claim that the levator anguli oris facialis, orbicularis oris, depressor labii inferioris, depressor anguli oris and mentalis have a common ontogenetic origin, being derived from his ‘mandibular lamina’; see also e.g. Sullivan & Osgood 1927, and Jouffroy & Saban 1971]
89 (plicae anguli oris sensu Huber 1930a)
90[present in the platypus, but seemingly not in echidna, according to e.g. Lightoller 1942 and Saban 1971]
91 (labiorum profundi inferioris sensuLightoller 1934)
Table 3.
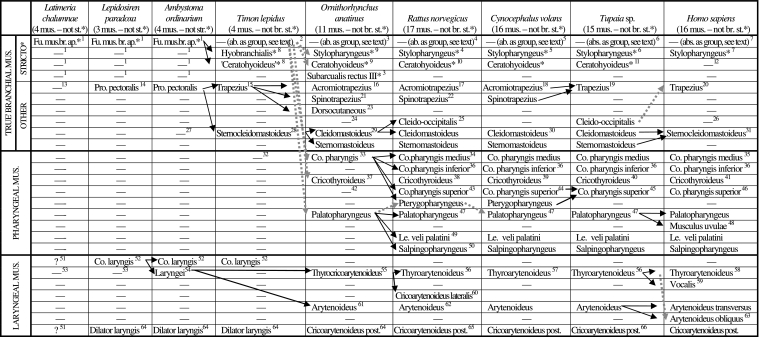
Scheme illustrating the authors’ hypotheses regarding the homologies of the branchial, pharyngeal and laryngeal muscles of adults of representative sarcopterygian taxa (see caption of Table 1, text, and Figs 3–17; ab. = absent; ap. =apparatus; br. = branchial; co. = constrictor; fu. = functional; le. = levator; mus. = muscles; post. = posterior; pre. = present; pro. = protractor; st =sensu stricto)
1[adult bony fish and amphibians often have various branchial muscles sensu stricto, e.g. the constrictores branchiales, levatores arcuum branchialium, transversi ventrales and/or subarcuales recti, among others: e.g. Edgeworth 1935; Kesteven 1942–1945]
2[absent as a group; adult lizards such as Timon lack all the branchial muscles sensu stricto except the hyobranchialis and ’ceratohyoideus’: these two muscles are seemingly the result of a subdivision of the subarcualis rectus I: see text]
3[absent as a group; the only branchial muscles sensu stricto that are present as independent structures in adult monotremes such as the platypus are the subarcualis rectus III, the ceratohyoideus, and seemingly the stylopharyngeus: see text; these two latter muscles seem to be the result of a subdivision of the subarcualis rectus I; it should be noted that a subarcualis rectus II is present in extant mammals such as marsupials: e.g. Edgeworth 1935]
4[absent as a group; the only branchial muscles sensu stricto that are present as independent structures in adult rodents such as the Norwegian rat are the ceratohyoideus and seemingly the stylopharyngeus: see text]
5[absent as a group; the only branchial muscles sensu stricto that are present as independent structures in adult colugos are the ceratohyoideus and seemingly the stylopharyngeus: see text]
6[absent as a group; the only branchial muscles sensu stricto that are present as independent structures in adult tree-shrews as Tupaia are the ceratohyoideus and seemingly the stylopharyngeus: see text]
7[absent as a group; the only branchial muscle sensu stricto that is present as an independent structure in adult modern humans is seemingly the stylopharyngeus: see text]
8 (part or totality of subarcualis rectus I or of branchiohyoideus sensuEdgeworth 1935 and Herrel et al. 2005) [see text]
9[the data now available on innervation, development, topology and comparative anatomy indicate that the mammalian stylopharyngeus is probably not a de novo pharyngeal muscle sensu Edgeworth, but instead a derivative of the branchial muscles sensu stricto: see text]
10 (the ceratohyoideus sensuHouse 1953 corresponds to the branchiohyoideus sensuSprague 1943 and to the hyoideus latus, keratohyoideus brevis and intercornualis sensuSaban 1968) [see text]
11 (interhyoideus sensuLe Gros Clark 1926) [see text]
12[absent as an independent muscle in modern humans, but present in other primates: e.g. Sprague 1944b; Saban 1968]
13[the protractor pectoralis is not present as an independent structure in Latimeria, but it is present in numerous sarcopterygians and actinopterygians and was very likely present in the common ancestor of these two groups: e.g. Edgeworth 1935; Straus & Howell 1936; Diogo 2007]
14 (cucullaris sensuEdgeworth 1935)
15 (capitodorsoclavicularis sensuTsuihiji 2007)
16 (anterior trapezius sensuSaban 1971) [the anterior and posterior trapezius sensuSaban 1971, are well separate in platypus and clearly correspond to the acromiotrapezius and spinotrapezius of other mammals; according to Edgeworth 1935 in monotremes the subarcualis I derives from the branchial arch 1, while the subarcualis III, trapezius and sternocleidomastoideus derive from the branchial arch 3; but see text]
17 (dorsoscapularis superior, anterior trapezius or trapezius superior sensuGreene 1935) [it is somewhat mixed with the spinotrapezius, but is considered as a separate muscle by many authors, see e.g. Greene 1935; according to Edgeworth 1935 in placentals the subarcualis I usually derives from the branchial arch 1 (in some cases it may atrophy during development as e.g. in Manis and seemingly in most anthropoids, including modern humans), while the acromiotrapezius, spinotrapezius, cleido-occipitalis, cleidomastoideus, sternomastoideus and sternocleidomastoideus derive from the branchial arch 2; also according to Edgeworth 1935 in certain adult placentals as e.g. Sus there is a single branchial arch, which gives rise to all the muscles listed above; but see text]
18[contrary to what is stated by Macalister 1872 and Gunnell & Simmons 2005 in the colugos dissected by us both the spinotrapezius and the acromiotrapezius are present as independent structures: the former mainly inserts on the scapular spine, while the latter mainly inserts on the acromion; this is also the case in the specimens examined by e.g. Leche 1886: see his fig. 8; as stated by Macalister 1872 in colugos the trapezius complex (= acromiotrapezius + spinotrapezius of the present work) does not reach the cranium anteriorly and does not attach on the clavicle posteriorly; i.e. this trapezius complex does not include a ’cleido-trapezius’sensuKardong 2002 and does not seem to include the cleido-occipitalis sensu the present work]
19[in both Tupaia and Ptilocercus, it is a single, continuous muscle, which seems to correspond to the acromiotrapezius + spinotrapezius of other mammals: e.g. Le Gros Clark 1924, 1926; George 1977; this work]
20[it has 3 parts, i.e. the acromiotrapezius, claviculotrapezius and spinotrapezius sensuKardong 2002, which are not differentiated into separate muscles, as is the case in various other mammals; the human ’claviculotrapezius’ probably corresponds to part of the trapezius of e.g. Tupaia, although it may possibly correspond to the cleido-occipitalis of this latter taxon: e.g. Jouffroy 1971]
21 (posterior trapezius sensuSaban 1971: see 16)
22 (dorsoscapularis, inferior posterior trapezius or trapezius inferior superior sensuGreene 1935) [see 17]
23[present in monotremes as well as in some other extant mammals; seemingly corresponds to part of the trapezius of tetrapods such as lizards: e.g. Jouffroy 1971; Jouffroy & Lessertisseur 1971]
24[according to Edgeworth 1935 the cleido-occipitalis of mammals as e.g. Tatusia seems to correspond to part of the reptilian trapezius, but the ’cleido-occipitalis’ of e.g. the placental carnivores may well correspond to the part of the reptilian sternocleidomastoideus]
25 (the cleido-occipitalis sensu Wood 1870 and Edgeworth 1935 corresponds to the clavotrapezius and cleido-occipitalis cervicalis sensuGreene 1935) [the position and orientation of the fibers of the cleido-occipitalis of e.g. Rattus and Tupaia are more similar to those of the monotreme sternocleidomastoideus than to those of the monotreme trapezius; also, according to Greene 1935 in e.g. rats the cleido-occipitalis, sternomastoideus and cleidomastoideus are all innervated by the ‘spinal accessory and third and fourth cervical nerves through the subtrapezial plexus’, while the spinotrapezius and acromiotrapezius are innervated by the ‘spinal accessory and second and third cervical nerves through the subtrapezial plexus’]
26[usually absent as an independent muscle, but may be found in a few modern humans: e.g. Wood 1870]
27[according to Howell 1933–1937, the sternocleidomastoideus is only present as a separate muscle in reptiles and mammals, being derived from the protractor pectoralis]
28 (episternocleido-mastoideus sensuHerrel et al. 2005; capiticleidoepisternalis sensuTsuihiji 2007)
29[as suggested by Howell 1937a and Saban 1971, in the platypus specimens dissected by us both the sternomastoideus and cleidomastoideus are present as independent structures]
30[contrary to what is suggested in Leche's 1986 fig. 4, the colugos dissected have both a sternomastoideus and a cleidomastoideus, which are well separated; each of these muscles attaches anteriorly on the mastoid process by a thin and long tendon]
31[including sternal and clavicular heads, which clearly seem to correspond to the sternomastoideus and cleidomastoideus of other mammals, but are not really differentiated into independent muscles]
32[plesiomorphically reptiles have no muscular pharynx; reptiles such as crocodilians do possess a secondary palate and a means to constrict the pharynx, but this constrictor is a derivative of an hyoid muscle, the interhyoideus: e.g. Schumacher 1973; Smith 1992]
33[there is only one constrictor of the pharynx in monotremes, but the cricothyroideus and the palatopharyngeus are already differentiated in these mammals; some authors consider that amphibians may have ’pharyngeal muscles’ lying between the hyoid apparatus and the pharyngeal wall: e.g. Piatt 1938; Smith 1992; however, these ’pharyngeal muscles’ seem in fact to be branchial muscles sensu stricto as e.g. the levatores arcuum branchialium and/or the transversi ventrales sensu Edgeworth, 1935: see e.g. Saban, 1971, p. 708]
34 (ceratopharyngeus and/or hyopharyngeus sensuHouse 1953)
35[including the pars ceratopharyngea and the pars chondropharyngea sensuTerminologia Anatomica 1998, which insert on the hyoid bone and on the thyroid cartilage, respectively]
36[as described by Saban 1968, the constrictor pharyngis inferior of therian mammals is often divided into a pars thryropharyngea attaching on the thyroid cartilage, a pars cricopharyngea attaching on the cricoid cartilage, and a pars intermedia lying between these two myological structures; the pars intermedia is often reduced in mammals as e.g. primates and is often absent in mammals as e.g. rodents]
37[in the platypus specimens dissected by us the cricothyroideus is seemingly not divided into a pars obliqua and a pars recta; it should be noted that in terms of both its ontogeny and phylogeny the mammalian cricothyroideus is clearly a pharyngeal muscle, and not a laryngeal muscle as it is sometimes suggested in the literature: e.g. Edgeworth 1935; Negus, 1949; DuBrul 1958; Starck & Schneider 1960; Saban 1968; Wind 1970; Crelin 1987; Harrison 1995]
38[including a pars obliqua and a pars recta: see 39]
39[including a pars obliqua and a pars recta, which are more separated than in Rattus and Tupaia but are not as separated as in modern humans]
40[including a pars obliqua and a pars recta: see 39]
41[including a pars obliqua and a pars recta: see 39]
42[according to Edgeworth 1935 the constrictor pharyngis superior is missing in monotremes and was very likely poorly developed in the first placentals, being probably similar to the ’glossopharyngeus ’of e.g. rats; according to that author the constrictor pharyngis superior only became a broad muscle as that found in e.g. modern humans later in evolution; House 1953 and Smith 1992 suggest that the pterygopharyngeus of e.g. rats probably correspond to part of the constrictor pharyngis superior of modern humans; in our opinion it is more plausible to assume that the pterygopharyngeus became part of the human constrictor pharyngis superior (see e.g. Fig. 15) than to assume that a muscle such as the ’glossopharyngeus’ of rats migrated superiorly in order to attach on the hard palate; however, until more data are available, one cannot completely discard the hypothesis that the pterygopharyngeus of e.g. rats and colugos might be simply missing or deeply mixed with the palatopharyngeus in mammals such as Tupaia and Homo]
43 (glossopharyngeus sensuHouse 1953) [the constrictor pharyngis superior of rats seemingly includes only a pars glossopharyngea: see 42]
44[our dissections indicate that it includes a pars glossopharyngea and possibly a pars buccopharyngea]
45[seemingly includes a pars buccopharyngea, a pars pterygopharyngea (corresponding to the pterygopharyngeus of e.g. rats and colugos? see 42), and possibly a pars glossopharyngea: e.g. Sprague 1944a; this work]
46[includes a pars buccopharyngea, a pars pterygopharyngea (corresponding to the pterygopharyngeus of e.g. rats and colugos? see 42), a pars mylopharyngea, and a pars glossopharyngea]
47[more mixed with the salpingopharyngeus than in modern humans]
48[according to Edgeworth 1935 this muscle is only found in a few mammals such as primates, corresponding to part of the palatopharyngeus of other mammals]
49[corresponds to part of the palatopharyngeus of monotremes: e.g. Edgeworth 1935; Saban 1968; this work]
50[corresponds to part of the palatopharyngeus of monotremes: e.g. Edgeworth 1935; Saban 1968; this work]
51 Present? Some non-sarcopterygian vertebrates as e.g. Polypterus have a ’constrictor laryngis’ and/or a ‘dilatator laryngis’, but it is not clear if these muscles actually correspond to the constrictor laryngis and dilatator laryngis of sarcopterygians and thus if these latter muscles are plesiomorphically present in osteichthyans: e.g. Edgeworth 1935; the few descriptions of the laryngeal region of Latimeria chalumnae do not allow us to appropriately discern if these muscles are, or are not, present in this taxon: e.g. Millot & Anthony 1958]
52[recent developmental works indicate that in amphibians as e.g. salamanders and reptiles as e.g. chickens laryngeal muscles such as the dilatator laryngis are at least partially derived ontogenetically from somites and possibly also from branchial mesoderm: e.g. Piekarski & Olsson 2007; the ontogenetic derivation of these muscles is thus actually similar to that of muscles such as the protractor pectoralis of amphibians and the trapezius/sternocleidomastoideus of reptiles: see text; according to e.g. Piekarski & Olsson, 2007 in some cases the constrictor oesophagus might also be at least partially derived ontogenetically from somites]
53[the laryngei of tetrapods does not seem to be plesiomorphically found in sarcopterygians, because it is absent in sarcopterygian fish as dipnoans; a detailed study of the laryngeal region of Latimeria is however needed in order to support, or to contradict, this hypothesis: see 51]
54[the laryngei and constrictor laryngis of amphibians derive ontogenetically from the same anlage: e.g. Edgeworth 1935]
55 (the thyrocricoarytenoideus sensuSaban 1968 corresponds to the thyroarytenoideus sensuEdgeworth 1935; it has two bundles, which seemingly correspond to the thyroarytenoideus and cricoarytenoideus lateralis of other mammals: that is why we prefer to use the name thyrocricoarytenoideus for the monotreme muscle) [Smith 1992, p. 340, states, that ‘the laryngeal muscles of mammals and amphibians are innervated by two homologous branches of cranial nerve X, the superior and inferior (or recurrent) laryngeal nerves; in contrast in reptiles (except in Aves) the innervation of the larynx is via a single laryngeal nerve that is a branch of cranial nerve IX’; this supports Edgeworth's 1935 view that the ’laryngei’ of reptiles is not homologous to that of amphibians and thus to the thyrocricoarytenoideus + arytenoideus of monotremes]
56[mainly divided into superficial and deep bundles]
57[divided into a posterior, medial bundle, and an anterior, lateral part, which seem to correspond respectively to the pars intermedia and pars superioris of fig. 69 of Starck & Schneider 1960; the latter bundle is in turn subdivided into a medial bundle and a lateral bundle, the latter being fused with the cricoarytenoideus posterior and thus seemingly corresponding to the ceratoarytenoideus lateralis sensuHarrison 1995]
58[often includes a pars thyroepiglottica, a pars aryepiglottica, a pars superioris, a pars ventricularis and/or a ceratoarytenoideus lateralis sensuSaban 1968 and Harrison 1995]
59 (thyroarytenoideus inferior sensuSaban 1968) [according to e.g. Edgeworth 1935 the vocalis is only found in a few taxa such as some primates, and corresponds to the medial portion of the thyroarytenoideus of other mammals]
60 (cricoarytenoideus ventralis sensuWhidden 2000) [see 55]
61 (interarytenoideus sensuSaban 1971 which is divided into crico-pro-arytenoideus and ary-pro-arytenoideus)
62 (interarytenoideus sensuEdgeworth 1935)
63[it is commonly accepted that the arytenoideus transversus and arytenoideus obliquus derive from the arytenoideus of other mammals; however, as the pars aryepiglottica is seemingly derived from the thyroarytenoideus, the possibility that the arytenoideus obliquus also derives from this latter muscle, and not from the arytenoideus cannot be ruled out: e.g. Saban 1968]
64 (cerato-crico-arytenoideus sensuSaban 1971) [the cricoarytenoideus posterior of mammals corresponds to the dilatator laryngis of reptiles, amphibians and dipnoans, which is not homologous to the ‘dilatator laryngis’ of the actinopterygians Amia and Lepisosteus nor to the ‘dilatator laryngis’ of the actinopterygian Polypterus, according to Edgeworth 1935]
65 (cricoarytenoideus dorsalis sensuWhidden 2000)
66[including the ceratocricoideus sensuHarrison 1995, which, according to this author, is found in about 63% of modern humans]
Results and Discussion
The results of our observations and comparisons are summarized in Tables 1–4. Hypotheses about the homologies and evolution of each muscle are based on a detailed analysis of all the lines of evidence obtained either from our dissections or gleaned from the literature (e.g. innervation; relationships with other muscular structures; relationships with hard tissues; configuration/orientation of the fibers; development; function; phylogeny; expression domains for homeobox genes; presence/absence/configuration in fossils, etc.). This is because, as pointed out by Edgeworth (1935), no single criterion is sufficient. For instance, although the innervation of a muscle generally remains constant and corresponds to its segment of origin, there are cases in which the same muscle has different innervations in different taxa (e.g. although wholly of mandibular origin, the intermandibularis of dipnoans is innervated by the Vth and/or the VIIth nerve). Also, there are cases in which the same muscle may be ontogenetically derived from different regions and/or segments of the body in different taxa (e.g. the trapezius of Ornithorhynchus is derived from the 3rd branchial muscle-plate, that of Talusia from the 2nd branchial muscle-plate and that of Sus from the 1st branchial muscle-plate). In the sections that follow we comment on the information presented in Tables 1–4, paying special attention to topics that remain particularly controversial among comparative morphologists.
Edgeworth (1935) divided the cephalic muscles of vertebrates into five main groups: mandibular, hyoid, branchial, epibranchial, and hypobranchial (Fig. 2). He viewed the development of these muscles in the light of developmental pathways leading from presumptive premyogenic condensations to different states in each cranial arch (the condensations of the first and second arches corresponding respectively to Edgeworth's ‘mandibular and hyoid muscle plates’, and those of the more posterior, ‘branchial’ arches corresponding to his ‘branchial muscle plates’). According to him these developmental pathways involve the migration of premyogenic cells, the differentiation of myofibers, directional growth of myofibers and possibly interactions with surrounding structures, all occurring in specific locations, such as the dorsal, medial or ventral areas of each cranial arch (Fig. 2). According to Edgeworth, although exceptions may occur, the mandibular muscles are generally innervated by the Vth nerve, the hyoid muscles by the VIIth nerve and the branchial muscles by the IXth and Xth nerves. Also according to this author, the epibranchial and hypobranchial muscles are developed from the anterior myotomes of the body and thus are intrusive elements of the head; they retain a spinal innervation and may also be innervated by the XIth and XIIth nerves, but usually do not receive any branches from the Vth, VIIth, IXth and Xth nerves. We will not discuss here the epibranchial muscles sensu Edgeworth, which are absent in extant osteichthyans, nor the ocular muscles sensu this author, which are usually innervated by nerves III, IV and/or VI.
Fig. 2.
Schematic presentation of embryonic origin of cranial muscles in gnathostomes based on Edgeworth's (1902, 1911, 1923, 1926a,b,c, 1928, 1935) works: premyogenic cells originate from the paraxial mesoderm (hatched areas) and several somites (areas with vertical bars); large arrows indicate a contribution of cells in segments of the mesoderm to muscle formation of different cranial arches. For more details, see text (modified from Miyake et al., 1992; the nomenclature of the structures illustrated follows that of these authors).
Mandibular, hyoid, branchial, pharyngeal, laryngeal and hypobranchial muscles
Some aspects of Edgeworth's scheme have been contradicted by subsequent phylogenetic, comparative and/or developmental studies (e.g. that the actinopterygians and tetrapods are derived from an ‘early dipnoan stock’, that the first gnathostomes lacked dorsal mandibular musculature and that the latter was thus independently acquired in some gnathostome subgroups: see e.g. Holland et al. 1993; Diogo, 2007). However, as stressed by Miyake et al. (1992) and Diogo (2007), important aspects of Edgeworth's scheme did receive strong support from these studies. For example, Noden (1983, 1984, 1986) elegantly demonstrated with quail-chick chimeras that most cranial muscles are embryologically of paraxial mesoderm origin, and not, as was commonly thought, of lateral plate origin (Miyake et al. 1992). Also, molecular developmental studies such as those of Hatta et al. (1990, 1991) have corroborated one of Edgeworth's findings: the existence of one premyogenic condensation (the constrictor dorsalis) in the cranial region of teleost fish. Another of Edgeworth's ideas accepted by researchers and followed in general textbooks is the recognition of the five main groups – mandibular, hyoid, branchial, epibranchial, and hypobranchial – of head and neck muscles (Diogo, 2007), and this is the nomenclature we use in this study.
Mandibular muscles (Table 1)
The plesiomorphic condition for sarcopterygians is that two ventral mandibular muscles, the intermandibularis anterior and intermandibularis posterior (Diogo, 2007, 2008), connect the hemimandibles (Figs 3,5 and Table 1). The mylohyoideus and digastricus anterior of mammals (Figs 9, 16) correspond to the intermandibularis posterior of other sarcopterygians (e.g. Bryant, 1945; Jarvik, 1963, 1980; Saban, 1971; this work) (Table 1). Contrary to the condition in monotremes (Fig. 9), in most marsupials and placentals, including modern humans, the digastricus anterior and the digastricus posterior (a dorso-medial hyoid muscle: see below) form a compound structure (the ‘digastricus’) that is often related to the depression of the mandible. Interestingly, in teleostean fish (which are actinopterygians, not sarcopterygians) the intermandibularis posterior and a ventral hyoid muscle (the interhyoideus) combine to form the protractor hyoidei, which is also often related to the opening of the mouth (e.g. Greenwood, 1971; Winterbottom, 1974; Lauder, 1980a; Lauder & Liem, 1983; Stiassny, 2000; Diogo, 2007, 2008). According to Edgeworth (1935) various tetrapod groups have independently acquired different mechanisms for depressing the mandible (i.e. to open the mouth) that use muscles other than the hypobranchial ones: amphibians and reptiles usually have a depressor mandibulae (which is a modified dorso-medial hyoid muscle: Table 2 and Figs 3, 5, 6; see below), monotremes have a detrahens mandibulae (which is a new division of the adductor mandibulae: Table 1 and Fig. 9; see below), and marsupial and placental mammals usually have the ‘digastricus’, i.e. the compound structure formed from the digastricus anterior and digastricus posterior.
Fig. 5.
Ambystoma ordinarium (Amphibia): ventral view of the cephalic musculature; on the right side the most ventral muscles were removed (modified from Diogo, 2008; the nomenclature of the structures illustrated follows that used in the present work; anterior is to the top). BH, branchiohyoideus; ch, ceratohyal; DM-A, DM-P, depressor mandibulae anterior and posterior; GG, genioglossus; GH, geniohyoideus; INTE-A, INTE-P, anterior and posterior bundles of interhyoideus; INTM-A, INTM-P, intermandibularis anterior and posterior; l-ch-mand, ligament between ceratohyal and mandible; mnd, mandible; OH, omohyoideus; SAR1, subarcualis rectus 1; SH, sternohyoideus; uh, urohyal.
Fig. 9.
Ornithorhynchus anatinus(Mammalia, Monotremata): A) ventral view of the head and neck musculature, muscles such as the geniohyoideus and sternohyoideus are not shown; B) same view, but the digastricus anterior, superficial part of the mylohyoideus, sternomastoideus, and cleidomastoideus were removed and the anterior portion of the sternohyoideus and of the superficial part of the omohyoideus were partially cut (modified from Edgeworth, 1935 and Saban, 1971; the nomenclature of the structures illustrated basically follows that used in the present work; anterior is to the top). CMA, cleidomastoideus; DETR, detrahens mandibulae; DIA, digastricus anterior; GEH, geniohyoideus; MA, masseter; mnd, mandible; MYHPR, MYHSU, pars profunda and pars superficialis of mylohyoideus; OM, omohyoideus; OMPR, OMSU, pars profunda and pars superficialis of omohyoideus; SMA, sternomastoideus; STEH, sternohyoideus; STT, sternothyroideus; STYH, styloideus.
Fig. 16.
Pongo pygmaeus(Mammalia, Primates): ventral view of the head musculature; on the right side the superficial portion of the masseter was removed (modified from Edgeworth, 1935 and Saban, 1968; the nomenclature of the structures illustrated basically follows that used in the present work; anterior is to the top). DIP, digastricus posterior; mnd, mandible; MA, masseter; MPT, pterygoideus medialis; MYH, mylohyoideus; STG, styloglossus; STH, stylohyoideus.
Fig. 6.
Euspondylus acutirostris (Reptilia): lateral view of the cephalic musculature; the adductor mandibulae A2-PVM is not shown (modified (2002 and Diogo, 2008; the nomenclature of the structures illustrated follows that used in the present work; anterior is to the right). A2, adductor mandibulae A2; CERV, cervicomandibularis; DM, depressor mandibulae; LEV-AO-M, levator anguli oris mandibularis; PSEU, pseudotemporalis.
The plesiomorphic condition for the sarcopterygian adductor mandibular muscles is seemingly that in which there is an adductor mandibulae A2, an adductor mandibulae Aω, an adductor mandibulae A3′, and possibly an adductor mandibulae A3″ (sensuDiogo, 2007, 2008) (Table 1). The adductor mandibulae Aω was not present as an independent muscle in any of the mammals we dissected, and to our knowledge it has also not been found in any extant mammals described in the literature (Table 1). The adductor mandibulae A2-PVM, retractor anguli oris and levator anguli oris mandibularis of extant dipnoans and non-mammalian tetrapods correspond to part of the A2 of bony fishes such as Latimeria (Diogo, 2007, 2008) (Table 1 and Figs 3,4,6). The masseter, temporalis, pterygoideus lateralis and detrahens mandibulae of monotremes and the masseter, temporalis and pterygoideus lateralis of other extant mammals apparently correspond to the A2 of reptiles such as Timon (Table 1 and Figs 4,6,9,13,16). However, it should be noted that although the mammalian temporalis seemingly corresponds to part of the A2 of other tetrapods, it may also include part of other adductor mandibulae structures such as the pseudotemporalis (see e.g. Barghusen, 1968). The tensor tympani and tensor veli palatini of mammals are most likely equivalent to the adductor mandibulae A2-PVM of reptiles such as Timon; the pterygoideus medialis of the former seems to correspond to the pseudotemporalis and pterygomandibularis of the latter (Table 1 and Figs 6, 7, 16; see also e.g. Edgeworth, 1935; Saban, 1968, 1971). Unusually among vertebrates, extant mammals lack any dorsal mandibular muscles sensuEdgeworth (1935) (e.g. Saban, 1968, 1971; Kardong, 2002; Diogo, 2007, 2008) (Table 1). Interestingly, engrailed immunoreactivity has been detected in tetrapod mandibular muscles that are derived from the ‘adductor mandibulae’ plate sensu Edgeworth, such as the masseter, temporalis, pterygoideus medialis and pterygoideus lateralis of mice (Knight et al. 2008). As explained above, within teleost fish such as the zebrafish, engrailed immunoreactivity has only been detected in dorsal mandibular muscles sensu Edgeworth, i.e. in the levator arcus palatini and dilatator operculi. This means that the muscles that arise from cells expressing the same gene in two different vertebrate taxa are not necessarily homologous among those taxa, thus supporting the idea that no single criterion (including the expression of genes such as engrailed) is enough to establish myological homologies (see above).
Fig. 4.
Neoceratodus forsteri (Dipnoi): mesial view of adductor mandibulae and mandible; the mandibular tooth-plates are not illustrated (modified from Diogo, 2008; the nomenclature of the structures illustrated follows that used in the present work; anterior is to the right, dorsal is to the top). A2, A2-PVM, A3′, adductor mandibulae A2, A2-PVM and A3′; ang, angular; ar-pq, articulatory facet for palatoquadrate; part, prearticular; sppsp, splenio-postsplenial bone.
Fig. 13.
Macaca mullata(Mammalia, Primates): lateral view of the masseter and temporalis (modified from Schumacher, 1961 and Saban, 1968; the nomenclature of the structures illustrated basically follows that used in the present work; anterior is to the right). MA, masseter; TEMP, temporalis; zyar, zygomatic arch.
Fig. 7.
Euspondylus acutirostris(Reptilia): ventral view of the deep ventral cephalic musculature; muscles such as the intermandibularis, interhyoideus, geniohyoideus, genioglossus and hyoglossus are not shown (modified from Montero et al. 2002 and Diogo, 2008; the nomenclature of the structures illustrated follows that used in the present work; anterior is to the top). cb1, ceratobranchial 1; CEH, ceratohyoideus; ehy, epihyal; hc, hyoid cornu; HYOB, hyobranchilais; mnd, mandible; OH, omohyoideus; pte, pterygoid; PTM, pterygomandibularis; SH, sternohyoideus.
Hyoid muscles (Table 2)
Edgeworth (1935) and Huber (1930a,b, 1931) divided the hyoid muscles into two main groups: dorso-medial and ventral (Table 2). The plesiomorphic configuration for sarcopterygians is a single ventral hyoid muscle, the interhyoideus, and two dorso-medial hyoid muscles, the adductor arcus palatini and the adductor operculi (note that the ‘adductor hyomandibulae Y’ and ‘levator operculi’ of Latimeria are not homologues of the adductor hyomandibulae and levator operculi of actinopterygians such as teleosts: Diogo, 2007, 2008) (Table 2). The depressor mandibulae, levator hyoidei, branchiohyoideus and cervicomandibularis of extant dipnoans, amphibians and reptiles seem to develop from the anlage that gives rise to the adductor arcus palatini in other osteichthyans (Diogo, 2007, 2008) (Table 2 and Figs 3,5,6). The adductor operculi is not present as an independent muscle in extant dipnoans, amphibians and reptiles; at least in dipnoans it is most likely fused with the ventral hyoid muscle interhyoideus (Diogo, 2007, 2008) (Table 2 and Fig. 3). The number of hyoid muscles found in extant mammals, and particularly in therians (placentals + marsupials), is much greater than that found in extant non-mammalian tetrapods (Table 2). Also, in non-mammalian vertebrates the hyoid muscles are mainly restricted to the region of the second branchial arch and occasionally to the mandibular and/or neck regions (Figs 3,5,6), whereas in extant mammals these muscles extend more anteriorly, covering much of the anterior region of the head (Figs 8,12,14). With the exception of styloideus, stylohyoideus, digastricus posterior, jugulohyoideus and stapedius, all the mammalian hyoid muscles listed in Table 2 are usually designated as facial muscles because they attach to freely movable skin and are associated with the display of facial expressions (e.g. Ruge, 1885, 1897, 1910; Boas & Paulli, 1908; Lightoller, 1928a,b, 1934, 1940a,b, 1942; Huber, 1930a,b, 1931; Edgeworth, 1935; Andrew, 1963; Gasser, 1967; Jouffroy & Saban, 1971; Saban, 1971; Seiler, 1971a,b,c,d,e, 1974a,b, 1975, 1979, 1980; Minkoff et al. 1979; Preuschoft, 2000; Schmidt & Cohn, 2001; Burrows & Smith, 2003; Burrows et al. 2006; Burrows, 2008). Some researchers have suggested that the mammalian facial muscles derive exclusively from the interhyoideus of non-mammalian tetrapods (e.g. Huber, 1930a,b, 1931), but our dissections and comparisons support authors such as Lightoller (1942) and Jouffroy & Saban (1971), who claim that at least some of these muscles (e.g. platysma cervicale, platysma myoides, mandibulo-auricularis) correspond to part of the dorso-medial hyoid musculature (e.g. cervicomandibularis) of other tetrapods (Table 2 and Figs 6, 8, 12, 14). The evolution and homologies of the mammalian facial muscles have been, and continue to be, controversial. In light of the overall analysis of the data obtained by our dissections and comparisons and by a review of the literature, it can be said that some of the hypotheses proposed and shown in Table 2 (black arrows) are in fact well supported by the data that are now available. For instance, the data available on topology, functional morphology, development and innervation strongly suggest that the platysma cervicale, platysma myoides, occipitalis, auricularis posterior and some of the extrinsic muscles of the ear (e.g. antitragicus, helicis and/or transversus and obliquus auriculae) of mammals have a common phylogenetic and ontogenetic origin (e.g. Boas & Paulli, 1908; Huber, 1930a,b, 1931; Gasser, 1967; Jouffroy & Saban, 1971; Saban, 1971; this work) (Figs 8, 12, 14 and Table 2). These same lines of evidence also suggest that the interhyoideus profundus, sphincter colli superficialis, sphincter colli profundus, naso-labialis, levator labii superioris, levator labii superioris alaeque nasi, buccinatorius, dilatator nasi, maxillo-naso-labialis, nasalis, depressor septi nasi, levator anguli oris facialis, orbicularis oris, depressor labii inferioris, depressor anguli oris and/or mentalis of mammals derive from the interhyoideus (Table 2; see also e.g. Gasser, 1967; Jouffroy & Saban, 1971; Saban, 1971; Seiler, 1971a,b,c,d,e, 1974a,b, 1975, 1979, 1980). However, it is still not clear, for instance, if the therian mandibulo-auricularis (a muscle that is usually deep to all the other mammalian facial muscles) is phylogenetically more closely related to the other facial muscles than to deeper dorso-median muscles such as the stylohyoideus, digastricus posterior, jugulohyoideus and stapedius (e.g. Lightoller, 1934; Jouffroy & Saban, 1971; Seiler, 1971a,b,c,d,e, 1974a,b, 1975, 1979, 1980; this work) (Table 2 and Fig. 12). Also, it is commonly accepted that muscles such as the zygomaticus major, zygomaticus minor, orbito-temporo-auricularis, frontalis, auriculo-orbitalis, temporoparietalis, auricularis anterior and auricularis superior derive from the sphincter colli profundus and/or superficialis, but Seiler (1971a,b,c,d,e, 1974a,b, 1975, 1979, 1980), based on his comparative and developmental studies, argues that at least some of these muscles may derive from the platysma cervicale and/or myoides (Table 2). However, some of Seiler's methods and interpretations are questionable. For example, in his 1980 developmental study of primates and tree-shrews, he argues that the facial muscles that are more superficial in early developmental stages are necessarily part of a ‘platysma anlage’ and thus derived phylogenetically from an ‘ancestral platysma’, whereas the majority of the other facial muscles are part of a ‘sphincter colli profundus’ anlage and thus are derived phylogenetically from a ‘primitive sphincter colli profundus’. This contrasts with Gasser's (1967) study of the ontogeny of the facial muscles of modern humans, in which various other anlages are recognized in early developmental stages. Also, it should be stressed that in adult mammals, including monotremes, at least some portions of the platysma (cervicale and/or myoides) lie deep to facial muscles such as the sphincter colli superficialis and even to facial muscles that Seiler categorizes as ‘sphincter colli profundus derivatives’ (e.g. part of the orbicularis oris and/or levator labii superioris) (e.g. Boas & Paulli, 1908; Lightoller, 1928a,b, 1934, 1940a,b, 1942; Huber, 1930a,b, 1931; Andrew, 1963; Jouffroy & Saban, 1971; Saban, 1971; Minkoff et al. 1979; Preuschoft, 2000; Schmidt & Cohn, 2001; Burrows & Smith, 2003; Burrows et al. 2006; this work) (Table 2 and Fig. 8). The majority of researchers consider that the sphincter colli of mammals derives from the interhyoideus of other tetrapods, so it is likely that the mammalian sphincter colli was plesiomorphically mainly superficial, and not deep, to the other hyoid muscles (the interhyoideus of other tetrapods is usually superficial not only to the other hyoid muscles, but to all the other muscles of the head). Monotremes are plesiomorphic mammals, and both the platypus and the echidna have a well-developed, broad sphincter colli superficialis that is superficial to most of the other facial muscles (the platypus actually lacks a sphincter colli profundus, although it has an interhyoideus profundus that seems to be derived from the deeper part of the interhyoideus; in the echidna most of the sphincter colli is superficial to the other facial muscles, but part of it passes deep to these muscles, forming a sphincter colli profundus: e.g. Huber, 1930a; Lightoller, 1942; Jouffroy & Saban, 1971; this work) (Table 2 and Fig. 8). A more detailed comparative analysis of the development and innervation of the hyoid group of muscles in vertebrates, including various key mammalian groups such as monotremes, is planned to clarify these and other controversial issues regarding the origin, homologies and evolution of the mammalian facial muscles and to test the hypotheses proposed in Table 2.
Fig. 8.
Ornithorhynchus anatinus(Mammalia, Monotremata): lateral view of the deep facial musculature; muscles such as the interhyoideus profundus, buccinatorius, orbicularis oris and mentalis are not shown (modified from Lightoller, 1942 and Saban, 1971; the nomenclature of the structures illustrated basically follows that used in the present work; anterior is to the right). CETR, cervicalis transversus; OROC, orbicularis oculi; PLAC, PLAM, platysma cervicale and platysma myoides; SCOS, sphincter colli superficialis.
Fig. 12.
Lepilemur sp. (Mammalia, Primates): lateral view of the facial musculature; muscles such as the buccinatorius, orbicularis oris and mentalis are not shown (modified from Jouffroy & Saban, 1971; the nomenclature of the structures illustrated basically follows that used in the present work; anterior is to the right). AUOR, auriculo-orbitalis; AUS, auricularis superior; FRO, frontalis; MAAU, mandibulo-auricularis; NASL, nasolabialis; OCC, occipitalis; OROC, orbicularis oculi; PLAC, PLAM, platysma cervicale and platysma myoides; SCOP, sphincter colli profundus; ZYMA, ZYMI, zygomaticus major and zygomaticus minor.
Fig. 14.
Macaca cyclopis(Mammalia, Primates): anterior view of the facial musculature; muscles such as the buccinatorius, platysma, frontalis, and occipitalis are not shown; on the left side the depressor supercilii was removed and the orbicularis oculi, zygomaticus minor, zygomaticus major, levator labii superioris, levator labii superioris alaeque nasi were partially cut (modified from Shibata, 1959 and Jouffroy & Saban, 1971; the nomenclature of the structures illustrated basically follows that used in the present work). COS, corrugator supercilii; DES, depressor supercilii; DLI, depressor labii inferioris; DSN, depressor septi nasi; LAO, levator anguli oris facialis; LELS, levator labii superioris; LELSA, levator labii superioris alaeque nasi; MENT, mentalis; NAS, nasalis; OROC, orbicularis oculi; OROR, orbicularis oris; PRO, procerus; ZYMA, ZYMI, zygomaticus major and zygomaticus minor.
Branchial, pharyngeal and laryngeal muscles (Table 3)
The muscles listed in Table 3 correspond to the branchial muscles sensu lato of Edgeworth (1935). They can be divided into three groups. The first comprises the ‘true’ branchial muscles, which are subdivided into 1) the branchial muscles sensu stricto that are directly associated with the movements of the branchial arches and that in mammals are usually innervated by the glossopharyngeal nerve (CNIX); 2) the protractor pectoralis and its derivatives, which are instead mainly associated with the pectoral girdle and are primarily innervated by the spinal accessory nerve (CNXI) (N.B., the cranial accessory nerve usually joins the vagus nerve, and modern descriptions often consider this cranial component part of the vagus nerve and not part of the ‘accessory nerve proper’: see e.g. Edgeworth, 1935; Straus & Howell, 1936; Netter, 2006). The second group consists of the pharyngeal muscles, which are only present as independent structures in extant mammals. They are considered to be derived from arches 4–6, and they are usually innervated by the vagus nerve (CNX). The mammalian stylopharyngeus, which is considered to be derived from the third arch and is primarily innervated by the glossopharyngeal nerve, is grouped here with the ‘true’ branchial muscles, and not with the pharyngeal muscles (see below). The third group is made up of the laryngeal muscles, which are considered to be derived from arches 4–6 and are usually innervated by the vagus nerve (CNX).
Sarcopterygians such as coelacanths, dipnoans and many amphibians retain various branchial muscles sensu stricto (e.g. Bischoff, 1840; Owen, 1841; Cuvier & Laurillard, 1849; Pollard, 1892; Gaupp, 1896; Allis, 1897, 1922; Danforth, 1913; Lubosch, 1914; Sewertzoff, 1928; Edgeworth, 1935; Brock, 1938; Piatt, 1938; Millot & Anthony, 1958; Osse, 1969; Larsen & Guthrie, 1975; Greenwood, 1977; Wiley, 1979a,b; Jollie, 1982; Bemis et al. 1983, 1997; Lauder & Shaffer, 1985, 1988; Bemis, 1986; Reilly & Lauder, 1989, 1990, 1991; Miyake et al. 1992; Wilga et al. 2000; Kardong, 2002; Carroll & Wainwright, 2003; Johanson, 2003; Kleinteich & Haas, 2007). Most authors agree that the branchial muscles sensu stricto are not present as a group in extant reptiles and extant mammals (Table 3). For instance, many adult reptiles have only one branchial muscle sensu stricto, the subarcualis rectus I (which is often named ‘branchiohyoideus’, although it is not homologous with the hyoid muscle branchiohyoideus found in, for example, salamanders: Table 2). The two branchial muscles sensu stricto seen in adult reptiles such as the lizard Euspondylus, i.e. the hyobranchialis and ‘ceratohyoideus’, seem to be the result of a subdivision of the subarcualis rectus I (Table 3 and Fig. 7). Adult extant mammals lack all the branchial muscles sensu stricto except the subarcualis rectus I (present in most adult mammals), the subarcualis rectus II (usually present only in adult marsupials) and the subarcualis rectus III (usually present only in adult monotremes) (e.g. Edgeworth, 1935; Smith, 1992).
Edgeworth (1935) claimed that the pharyngeal muscles of mammals are not derived from branchial muscle plates, but from a separate de novo condensation of myoblasts surrounding the pharyngeal epithelium. He did not consider the mammalian pharyngeal muscles to be homologous with the ‘pharyngeal muscles’ of some amphibians (which probably correspond to branchial muscles sensu stricto, such as the levatores arcuum branchialum and/or the transversus ventralis) and some reptiles (which are seemingly derived from the hyoid musculature) (e.g. Piatt, 1938; Schumacher, 1973; Smith, 1992). However, our dissections and literature research support Smith's (1992) and Noden & Francis-West's (2006) claims that one of the mammalian muscles included in Edgeworth's pharyngeal group, the stylopharyngeus, is not a de novo structure, but is instead a derivative of the branchial musculature sensu stricto and namely of the subarcualis rectus I (Table 3). The mammalian stylopharyngeus and the reptilian subarcualis rectus I are among the few muscles in either taxon innervated by the glossopharyngeal nerve (CNIX): most of the mammalian pharyngeal muscles are innervated, instead, by the vagus nerve (CNX). In fact, in many mammals, including primates such as Macaca, the ceratohyoideus and stylopharyngeus are closely related and are innervated by the same ramus of the glossopharyngeal nerve (buccal ramus sensuSprague, 1944a,b; see also Saban, 1968). Developmental data from monotremes and marsupials show that early in development the stylopharyngeus is similar to the non-mammalian subarcualis rectus I in position, function, and connections. As stressed by Smith (1992), although Edgeworth (1935) did not accept that the stylopharyngeus was derived from the branchial musculature sensu stricto, he did state that it develops from a muscle primordium that differs from the one that gives rise to the other pharyngeal muscles. The homology between the mammalian stylopharyngeus and part of the subarcualis rectus I of other tetrapods is further supported by the results of a comparison between adult mammals and adult non-mammalian tetrapods. The stylopharyngeus of mammals usually originates from the styloid process, which is derived from a portion of the second (hyoid) arch; the subarcualis rectus I of non-mammalian taxa is usually associated with this arch (Saban, 1971; Smith, 1992). Also, some reptiles, e.g. lizards, have two branchial muscles sensu stricto, which apparently are the result of a subdivision of the subarcualis rectus I (Table 3 and Fig. 7). The more anterior of these muscles (sometimes called the ‘branchiohyoideus’), usually originates from the hyoid arch (as does the mammalian stylopharyngeus, see above), and connects the hyoid cornu to the epihyal (Fig. 7). We refer to this muscle as the hyobranchialis because it is not homologous to the hyoid muscle branchiohyoideus of, for example, salamanders (see above). The most posteriorly situated muscle in lizards, often named the ‘ceratohyoideus’, usually connects the hyoid arch to other (more posterior) branchial arches, as does the mammalian ceratohyoideus (Table 3 and Figs 7 and 11). It should be noted that in various mammals, such as colugos and tree-shrews, the stylopharyngeus does not reach the styloid process, i.e. it may originate from more distal hyoid structures such as the epihyal (as does the reptilian hyobranchialis; e.g. Sprague, 1942, 1943, 1944a,b; Saban, 1968; this work) (Fig. 7). This observation, together with the other data available (see above), suggest that the combination of stylopharyngeus and the ceratohyoideus in mammals, and the combination of hyobranchialis and the ‘ceratohyoideus’ in reptiles, are both the result of the subdivision of the subarcualis rectus I (Table 3) However, this does not mean that the stylopharyngeus of mammals is necessarily the homologue of the reptilian hyobranchialis, for one cannot refute the hypothesis that the subdivision of the subarcualis rectus I into two muscles occurred more than once within the amniotes, resulting in the hyobranchialis and ‘ceratohyoideus’ of lizards and in the stylopharyngeus and ceratohyoideus of mammals. A detailed comparative and phylogenetic analysis, including several extant amniotes and ideally taking into account the data obtained from fossils, is needed to clarify whether such a subdivision was already present in the last common ancestor of reptiles and mammals and, thus, if the mammalian stylopharyngeus and ceratohyoideus are directly homologous to the reptilian hyobranchialis and ‘ceratohyoideus’, respectively.
Fig. 11.
Ptilocercus lowii(Mammalia, Scandentia): ventral view of the musculature of the hyoid region of the right side of the body; muscles such as the geniohyoideus, sternothyroideus and thyrohyoideus are not shown (modified from Le Gros Clark, 1926 and Saban, 1968; the nomenclature of the structures illustrated basically follows that used in the present work; anterior is to the right). aub, auditory bulla; CEH, ceratohyoideus; CNVII, X, XII, cranial nerves VII, X and XII; cric, cricoid cartilage; CRT, cricothyroideus; DIP, digastricus posterior; GEG, genioglossus; HYG, hyoglossus; OM, omohyoideus; SMA, sternomastoideus; STEH, sternohyoideus; STG, styloglossus; STH, stylohyoideus; STP, stylopharyngeus; thyr, thyroid cartilage.
The mammalian acromiotrapezius, spinotrapezius, dorsocutaneous, cleido-occipitalis, sternocleidomastoideus, cleidomastoideus and sternomastoideus correspond to the reptilian trapezius and sternocleidomastoideus and thus to the protractor pectoralis of amphibians, dipnoans and other osteichthyans. The protractor pectoralis is not a branchial muscle sensu stricto because it is mainly involved in the movements of the pectoral girdle and not of the branchial arches (Table 3 and Fig. 9; see above). The results of recent developmental studies indicate that the protractor pectoralis of Ambystoma and the trapezius of chickens and mice may be at least partially derived from somites (Piekarski & Olsson, 2007). It is thus not certain whether the protractor pectoralis was primarily derived from the paraxial mesoderm as suggested by Edgeworth (1935) and only later became ontogenetically also derived from the cranialmost somites, or whether it was instead primarily derived from somites. Interestingly, the results of Piekarski & Olsson (2007) suggest that at least in certain cases even branchial muscles sensu stricto, such as the levatores arcuum branchialium, may also be partially derived from somites. If this is the case, then the fact that muscles such as the protractor pectoralis have a partial somitic origin does not necessarily mean that they cannot be considered to be part of the branchial musculature (N.B. Piekarski & Olsson 2007 claim that hyoid muscles such as the interhyoideus might be also partially derived ontogenetically from somites). Matsuoka et al. (2005) recognize that the trapezius of at least some tetrapods is partially derived ontogenetically from somites, but they argue that the sum of the data available (i.e. innervation, topology, development and phylogeny) provides more support for grouping it with the branchial muscles sensu strictothan for its inclusion in the postcranial axial muscles sensuJouffroy (1971). In fact, it is important to stress that lineage tracing analyses in transgenic mice support the idea that the trapezius is essentially a branchial muscle: these analyses reveal that neural crest cells from a caudal pharyngeal arch travel with the trapezius myoblasts and form tendinous and skeletal cells within the spine of the scapula (Noden & Schneider, 2006). According to Noden & Schneider (2006: p. 14), ‘this excursion seemingly recapitulates movements established ancestrally, when parts of the pectoral girdle abutted caudal portions of the skull’. The innervation of the trapezius by not only XI, but also, in many cases, C3 and 4 spinal cord segments adds weight to the argument that the muscle is derived from both the paraxial mesoderm and somites (see Table 3). Support for a branchial component also comes from the position of the accessory nucleus in the ventral horn of the spinal cord, which is in line with the more cranial branchiomotor nuclei (see e.g. Wilson-Pauwels et al. 2002; Butler & Hodos, 2005).
The detailed analysis of the data obtained from our dissections, combined with the information provided in the literature, have allowed us to develop robust hypotheses of homology for most of the pharyngeal and laryngeal muscles (Table 3). The monotreme pharyngeal muscle constrictor pharyngis corresponds to the constrictor pharyngis medius + constrictor pharyngis inferior + constrictor pharyngis superior + pterygopharyngeus of therian mammals, although it is possible that at least part of this latter muscle is derived from the palatopharyngeus (Table 3 and Fig. 15). The pharyngeal muscles salpingopharyngeus + levator veli palatini + musculus uvulae + palatopharyngeus of therian mammals clearly seem to correspond to the palatopharyngeus of monotremes (Table 3 and Fig. 15). With respect to the laryngeal muscles, the thyroarytenoideus, vocalis, cricoarytenoideus lateralis and arytenoideus of therian mammals correspond to the thyrocricoarytenoideus and arytenoideus of monotremes and to the laryngeus of non-mammalian tetrapods such as salamanders. The mammalian cricoarytenoideus posterior corresponds to the dilator laryngis of other tetrapods (Table 3 and Figs 10, 17). It should be noted that in terms of both its ontogeny and phylogeny the mammalian cricothyroideus is clearly a pharyngeal, and not a laryngeal, muscle as is sometimes suggested in the literature (e.g. Terminologia Anatomica, 1998) (Table 3 and Figs 11, 15, 17). It should also be noted that according to authors such as Smith (1994) marsupials have no levator veli palatini. Their ‘functional superior constrictor’ is formed by an expansion of the stylopharyngeus, and not from the same mass of muscles that give rise to the middle and inferior constrictors, as is the case in placental mammals.
Fig. 15.
Hylobates hoolock(Mammalia, Primates): lateral view of the pharyngeal musculature (modified from Kanagasuntheram, 1952–1954 and Saban, 1968; the nomenclature of the structures illustrated basically follows that used in the present work; anterior is to the top, dorsal is to the left: see text). CRT, cricothyroideus; GEG, genioglossus; HYG, hyoglossus; IPC, constrictor pharyngis inferiori; LVP, levator veli palatini; MGP, mylo-glossopharyngeus; MPC, constrictor pharyngis medius; PAP, palatopharyngeus; PTEP, pterygopharyngeus; STG, styloglossus; STP, stylopharyngeus.
Fig. 10.
Ornithorhynchus anatinus(Mammalia, Monotremata): dorsal view of the laryngeal musculature; the cricoarytenoideus posterior is not shown (modified from Göppert, 1937 and Saban, 1971; the nomenclature of the structures illustrated basically follows that used in the present work; anterior is to the top). ARY, arytenoideus; aryt, arytenoid cartilage; copth, copula thyroidea; corthI, cornua thyroidea I; cric, cricoid cartilage; epigl, epiglottis; mupr, muscular process; paryt, proarytenoid cartilage; TCAR, thyrocricoarytenoideus.
Hypobranchial muscles (Table 4)
According to Edgeworth (1935), the hypobranchial muscles are divided into a ‘geniohyoideus’ group and a ‘rectus cervicis’ group (Table 4). However, it is not clear whether Edgeworth's groups represent separate premyogenic condensations, or whether they only become apparent at the later stages of muscle development (Miyake et al. 1992; Diogo, 2007, 2008). The plesiomorphic condition for sarcopterygians is seemingly that found in extant actinistians and dipnoans: there are two hypobranchial muscles that are mainly related to the opening of the mouth, the coracomandibularis and the sternohyoideus (Edgeworth, 1935; Kesteven, 1942–1945; Wiley, 1979a,b; Jollie, 1982; Mallat, 1997; Wilga et al. 2000; Johanson, 2003; Diogo, 2007, 2008) (Table 4 and Fig. 3). Amphibians and reptiles have various hypobranchial muscles (e.g. the omohyoideus and the specialized glossal muscles related to tongue movements) that are not present in sarcopterygian fish (Table 4 and Figs 5, 7). The geniohyoideus, genioglossus, hyoglossus and intrinsic muscles of the tongue of non-mammalian tetrapods very likely correspond to the coracomandibularis of sarcopterygian fish, although it is possible that the ‘hyoglossus’ of salamanders, for example, is at least partially derived from the sternohyoideus (Edgeworth, 1935; Jarvik, 1963; Diogo, 2007, 2008) (Table 4 and Fig. 5). The styloglossus and palatoglossus of therian mammals seem to correspond to part of the hyoglossus of monotremes (Table 4 and Figs 11, 15, 16). The mammalian thyrohyoideus and sternothyroideus mammals correspond to part of the sternohyoideus of reptiles such as lizards (Table 4 and Fig. 9).
General remarks
As can be seen in Tables 1, 2, 3 and 4, the evolution of the sarcopterygian head and neck muscles apparently involved more events during which a muscle became subdivided (diverging arrows) than events that involve the fusion of muscles (converging arrows). However, contrary to what is often stated in general textbooks (e.g. Kisia & Onyango, 2005), this does not mean that ‘higher’ primates, and namely modern humans, have more muscles than other mammals. For example, modern humans have fewer mandibular muscles than reptiles such as lizards, and fewer than mammals such as tree-shrews, rats and monotremes (Table 1). In our opinion, there are three possible reasons for this. First, in this case most of the evolutionary splittings occurred during the transitions that led to the origin of amniotes and of mammals, and not during the transitions that have subsequently led to the emergence of higher primates, including modern humans (Table 1). Secondly, some of the muscles present in at least some of our ancestors were lost at various stages in our evolution (e.g. the intermandibularis anterior was probably present in the common ancestor of sarcopterygians and/or of tetrapods, but it is missing in modern humans) (Table 1). Thirdly, some of the muscles that are found in other sarcopterygian taxa (e.g. the detrahens mandibulae of monotremes), are peculiar, apomorphic, features of those taxa (Table 2). This stresses that, as is now well known in theory but unfortunately often neglected in general textbooks and even in specialized papers, evolution is not directed ‘towards’ a goal, and surely not ‘towards’ modern humans. Each taxon has its own particular mix of primitive and derived anatomical structures, which is the result of its unique evolutionary history. That is why monotremes, for instance, have peculiar muscles such as detrahens mandibulae, which are not found in any other extant tetrapods, including mammals. And that is why in this paper we prefer to use the term correspond, because muscles such as the detrahens mandibulae are not ‘ancestral’ to the muscles of therian mammals. The monotreme detrahens mandibulae simply corresponds to a part of the adductor mandibulae of non-mammalian tetrapods that, in monotremes, became sufficiently differentiated to deserve being recognized as a separate muscle.
The number of true branchial muscles (Table 3) found in modern humans is also smaller than that found in most other mammals. Actually, in the case of these muscles if there is a ‘trend’ at the time of the evolutionary transitions that led to the origin of primates and subsequently to modern humans, then it is to reduce, and not increase, the total number of muscles (e.g. monotremes such as the platypus usually have 8 muscles, rodents such as rats 7, colugos 6, three-shrews such as Tupaia 6, and modern humans 3: Table 3). With respect to the pharyngeal musculature, there is a clear increase in the number of muscles at the time of the evolutionary transition leading to therian mammals, but then no increase at the time of the transition leading to the emergence of higher primates and modern humans (e.g. monotremes such as the platypus usually have 3 muscles, rodents such as rats 8, colugos 8, tree-shrews such as Tupaia 7, and modern humans 8: Table 3). The number of hypobranchial muscles (not including the intrinsic muscles of the tongue) is relatively constant within the therian mammals listed in Table 4, although in this case modern humans do have more muscles than the other taxa (e.g. rodents such as rats usually have 8 muscles, colugos 7, three-shrews such as Tupaia 8, and modern humans 9: Table 4).
Interestingly, the number of facial and laryngeal muscles found in modern humans is clearly greater than that found in most other mammalian taxa. Modern humans usually have 24 facial muscles (not including the extrinsic muscles of the ear); monotremes such as the platypus usually have 10, rodents such as rats 20, colugos 19, and three-shrews such as Tupaia 21 (Table 2). Examples of facial muscles present in modern humans and which are lacking in most other mammals are the risorius, depressor supercilii, levator labii superioris alaeque nasi, depressor septi nasi, depressor labii inferioris and depressor anguli oris (e.g. Ruge, 1885, 1897, 1910; Boas & Paulli, 1908; Lightoller, 1928a,b, 1934, 1940a,b, 1942; Huber, 1930a,b, 1931; Edgeworth, 1935; Andrew, 1963; Gasser, 1967; Jouffroy & Saban, 1971; Saban, 1971; Seiler, 1971a,b,c,d,e, 1974a,b, 1975, 1979, 1980; Minkoff et al. 1979; Preuschoft, 2000; Schmidt & Cohn, 2001; Burrows & Smith, 2003; Burrows et al. 2006; Burrows, 2008; this work) (Table 2). With respect to the laryngeal muscles, there are usually 6 present in modern humans, while there are 4 in Tupaia, colugos and rats, and 3 in monotremes (Table 3). The vocalis and arytenoideus obliquus are examples of laryngeal muscles that are usually present in modern humans, but which are lacking in most other mammalian and non-mammalian taxa (e.g. Edgeworth, 1935; Negus, 1949; DuBrul, 1958; Starck & Schneider, 1960; Saban, 1968; Wind, 1970; Crelin, 1987; Harrison, 1995) (Table 3). These data are consistent with the important role played by facial expression and by vocal communication in primates in general, and in modern humans in particular.
Before ending this paper, we would like to stress that the suggested homologies summarized in Tables 1–4 are simply scientific hypotheses that need to, and hopefully will, be tested by data obtained in the future. What we have provided are the most strongly supported hypotheses based on a painstaking analysis of the results of our own dissections combined with the existing data. As explained in the text and shown by the grey arrows of Tables 1, 2, 3 and 4, some of the hypotheses are still problematic and need to be addressed in future studies. For example, detailed comparative ontogenetic studies of key taxa such as the monotremes as well as a range of other mammalian and non-mammalian tetrapods, are badly needed. We plan to undertake such studies in the future, but in the meantime we hope that the information presented in this paper will serve as a provisional statement about the comparative anatomy, development, homologies, and evolution of the head and neck muscles of mammalian and non-mammalian sarcopterygians.
To our knowledge the type of information provided in Tables 1–4 and in the text has never been integrated in a single publication, and the homologies of these muscles have not previously been explored in this way. In addition to its value for anatomists and functional morphologists, we hope this comparative anatomical information will be useful to researchers working in developmental biology, genetics and/or evolutionary developmental biology to help them determine the wider homologies of these structures in mammalian and non-mammalian model organisms.
Acknowledgments
We would like to thank M. Carleton, D. Schmidt and L. Gordon (National Museum of Natural History), R. Etheridge (San Diego State University), J. Daza (University of Puerto Rico), B. Sacham and Y. Werner (The Hebrew University of Jerusalem), S. Ktretschmar, M. Cánepa, J. C. Stazonelli and G. Scrocchi (Fundación Miguel Lillo), A. Manzano (CCyTTP, Diamante), R. Montero (Universidad Nacional de Tucumán), J. Joss (Macquarie University), A. Gosztonyi (Centro Nacional Patagónico), J. Fernández, I. Doadrio and K. Tots (Museo Nacional de Ciencias Naturales de Madrid), R. Walsh and F. Slaby (Department of Anatomy, George Washington University), B. Richmond (Department of Anthropology, George Washington University), M. Aziz (Department of Anatomy, Howard University) and J. Fritz and J. Murphy (Primate Foundation of Arizona) for kindly providing a large part of the specimens analysed for this study. We specially thank J. Clarke and two anonymous reviewers for providing numerous helpful suggestions. R.D. was supported by a George Washington University Presidential Merit Fellowship. N.L. was supported by a George Washington University Academic Excellence Graduate Fellowship. V.A. was supported by the Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET), Argentina PIP 6347, PICT 12418 ‘Agencia-Argentina’, and CIUNT G218. B.W.'s involvement was made possible by a George Washington University Professorship in Human Origins, and by support from the GWU VPAA and from the GW Academic Excellence Program.
Appendix 1 – List of specimens dissected
Amphibia:Ambystoma texanum: FML 03402, 1 (alc). Ambystoma ordinarium: MNCN, uncatalogued, 2 (alc). Ambystoma mexicanum: MNCN, uncatalogued, 2 (alc). Bufo arenarum: FML 01352-1, 1 (alc). Dipnoi:Lepidosiren paradoxa: CONICET, uncatalogued, 1 (alc). Neoceratodus forsteri: MU, uncatalogued, 2 (alc). Mammalia:Cynocephalus volans: USNM, 144941, 1 (alc); USNM, uncatalogued, 1 (alc). Didelphis albiventris: CML 5971, 1 (alc). Homo sapiens: GWU-ANA, 1-16, 16 (for). Lutreolina crassicaudata: CML 4114, 1 (alc). Monodelphis dimidiata: CML 4118, 1 (alc). Ornithorhynchus anatinus: USNM, 13678, 1 (alc); USNM, uncatalogued, 1 (alc). Pan troglodytes: PFA, 1016, 1 (fre); PFA, 1009, 1 (fre); PFA, 1051, 1 (alc.); HU-ANA, C104, 1 (for); GWU-ANT, 01, 1 (for); GWU-ANT, 02, 1 (for). Pongo pygmaeus: HU-ANA, O01, 1 (for); GWU-ANT, 01, 1 (for). Rattus norvegicus: USNM, uncatalogued, 2 (alc). Thylamys venustus: CML 5586, 1 (alc). Tupaia sp.: UNSM, 87244, 1 (alc), USNM, uncatalogued, 1 (alc). Reptilia:Anas sp.: FML, uncatalogued, 1 (alc). Anisolepis longicaudus: FML, uncatalogued, 1 (alc). Anolis allogus: SDSU 2136, 1 (alc). Anolis lineatopus: SDSU 2157, 1 (alc). Anolis macrolepis: SDSU 2183, 1 (alc). Anolis notopholis: SDSU 2188, 1 (alc). Anolis sagrei: SDSU 2175, 1 (alc). Caiman latirostris: DIAMR, uncatalogued, 4 (alc); FML, uncatalogued, 1 (alc). Gallus domesticus: FML, uncatalogued, 4 (alc). Homonota fasciata: FML, uncatalogued, 1 (alc). Liolaemus cuyanus: FML 13891, 1 (alc). Liolaemus donosobarrosi: FML 02871, 1 (alc). Liolaemus riojanus: FML 02876, 1 (alc). Phymaturus sp.: FML 13834 1(alc). Polychrus acutirostris: FML 00140, 1 (alc). Pristidactylus valeriae: FML 9592, 1 (alc). Teius suquiensis: FML 03628, 1 (alc). Testudo graeca: HUJ-R 22843, 1 (alc); HUJ-R 22845, 1 (alc). Teyous ocelatus: FML 03633, 1 (alc). Timon lepidus: MNCN, 32544, 1 (alc), MNCN, uncatalogued, 1 (alc). Tropidurus plica: FML 6660, 1 (alc).
References
- Abdala V, Moro S. A cladistic analysis of ten lizard families (Reptilia: Squamata) based on cranial musculature. Russ J Herpetol. 2003;10:53–78. [Google Scholar]
- Adamicka P, Ahnelt H. Two jaw articulations in Latimeria chalumnae(Actinistia, Coelacanthidae) Zool Jb Anat. 1992;122:107–112. [Google Scholar]
- Adams SB, Wheeler JFG, Edgeworth FH. On the innervation of the platysma and the mandibulo-auricularis. J Anat. 1929;63:242–252. [PMC free article] [PubMed] [Google Scholar]
- Alexander RMcN. Jaw mechanisms of the coelacanth Latimeria. Copeia. 1973;1973:156–158. [Google Scholar]
- Allis EP. The cranial muscles and cranial nerves of Amia calva. J Morphol. 1897;12:487–737. [Google Scholar]
- Allis EP. The cranial anatomy of Polypterus, with special reference to Polypterus bichir. J Anat. 1922;56:180–294. [PMC free article] [PubMed] [Google Scholar]
- Anderson RJ. A variety of the mylopharyngeus and other unusual muscular abnormalities. J Anat Physiol. 1880;14:357–359. [PMC free article] [PubMed] [Google Scholar]
- Anderson RJ. The morphology of the muscles of the tongue and pharynx. J Anat Physiol. 1881;15:382–391. [PMC free article] [PubMed] [Google Scholar]
- Andrew RJ. Evolution of facial expression. Science. 1963;142:1034–1041. doi: 10.1126/science.142.3595.1034. [DOI] [PubMed] [Google Scholar]
- Anthony J. Évocation des travaux français sur Latimerianotamment depuis 1972. Proc R Soc Lond B. 1980;208:349–367. doi: 10.1098/rspb.1980.0055. [DOI] [PubMed] [Google Scholar]
- Aziz MA. Possible ‘atavistic’ structures in human aneuploids. Am J Phys Anthropol. 1981;54:347–353. doi: 10.1002/ajpa.1330540308. [DOI] [PubMed] [Google Scholar]
- Aziz MA, Cowie RJ, Skinner CE, Abdi TS, Orzame G. Are the two heads of the human lateral pterygoid separate muscles? A perspective based on their nerve supply. J Orofac Pain. 1998;2:226–239. [PubMed] [Google Scholar]
- Barghusen HR. The lower jaw of cynodonts (Reptilia, Therapsida) and the evolutionary origin of mammal-like adductor jaw musculature. Postilla. 1968;116:1–49. [Google Scholar]
- Barghusen HR. On the evolutionary origin of the therian tensor veli palatini and tensor tympani muscles. In: Hotton N, MacLean PD, Roth JJ, Roth EC, editors. The Ecology and Biology of Mammal-like Reptiles. Washington DC: Smithsonian Institution Press; 1986. pp. 253–262. [Google Scholar]
- Barrow JR, Capecchi MR. Compensatory defects associated with mutations in Hoxa1 restore normal palatogenesis to Hoxa2 mutants. Development. 1999;126:5011–5026. doi: 10.1242/dev.126.22.5011. [DOI] [PubMed] [Google Scholar]
- Bauer WJ. A contribution to the morphology of the m. interhyoideus posterior (VII) of urodele Amphibia. Zool Jb Anat. 1992;122:129–139. [Google Scholar]
- Bauer WJ. A contribution to the morphology of visceral jaw-opening muscles of urodeles (Amphibia: Caudata) J Morphol. 1997;233:77–97. doi: 10.1002/(SICI)1097-4687(199707)233:1<77::AID-JMOR7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Bemis WE. Feeding mechanisms of living Dipnoi: anatomy and function. J Morphol Suppl. 1986;1:249–275. [Google Scholar]
- Bemis WE, Lauder CV. Morphology and function of the feeding apparatus of the lungfish, Lepidosiren paradoxa(Dipnoi) J Morphol. 1986;187:81–108. doi: 10.1002/jmor.1051870108. [DOI] [PubMed] [Google Scholar]
- Bemis WE, Schwenk K, Wake MH. Morphology and function of the feeding apparatus in Dermophis mexicanus. Zool J Linn Soc. 1983;77:75–96. [Google Scholar]
- Bemis WE, Findeis EK, Grande L. An overview of Acipenseriformes. Environ Biol Fish. 1997;48:25–71. [Google Scholar]
- Birou G, Garcier JM, Guillot M, Vanneuville G, Chazal J. A study of the lateral pterygoid muscle: anatomic sections and CT appearances. Surg Radiol Anat. 1991;13:307–311. doi: 10.1007/BF01627763. [DOI] [PubMed] [Google Scholar]
- Bischoff TLW. Description anatomique du Lepidosiren paradoxa. Ann Sci Nat, ser 2. 1840;14:116–159. [Google Scholar]
- Boas JEV, Paulli S. The Elephant Head – Studies in the Comparative Anatomy of the Organs of the Head of the Indian Elephant and Other Mammals, 1: The Facial Muscles and the Proboscis. Jena: Gustav Fischer; 1908. [Google Scholar]
- Brock GT. The cranial muscles of the Gecko– a general account with a comparison of muscles in other gnathostomes. Proc Zool Soc Lond, ser B. 1938;108:735–761. [Google Scholar]
- Bryant MD. Phylogeny of Nearctic Sciuridae. Am Midland Nat. 1945;33:257–390. [Google Scholar]
- Burrows AM. The facial expression musculature in primates and its evolutionary significance. Bioessays. 2008;30:212–225. doi: 10.1002/bies.20719. [DOI] [PubMed] [Google Scholar]
- Burrows AM, Smith TD. Muscles of facial expression in Otolemur with a comparison to Lemuroidea. Anat Rec. 2003;274:827–836. doi: 10.1002/ar.a.10093. [DOI] [PubMed] [Google Scholar]
- Burrows AM, Waller BM, Parr LA, Bonar CJ. Muscles of facial expression in the chimpanzee (Pan troglodytes): descriptive, comparative and phylogenetic contexts. J Anat. 2006;208:153–167. doi: 10.1111/j.1469-7580.2006.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AB, Hodos W. Comparative Vertebrate Neuroanatomy: Evolution and Adaptation. Hoboken: Wiley Interscience; 2005. [Google Scholar]
- Carroll AM, Wainwright PC. Functional morphology of feeding in the sturgeon, Scaphirhyncus albus. J Morphol. 2003;256:270–284. doi: 10.1002/jmor.10095. [DOI] [PubMed] [Google Scholar]
- Carroll SB, Grenier JK, Weathrbee SD. From DNA to Diversity – Molecular Genetics and the Evolution of Animal Design. 2nd edn. Malden: Blackwell Science; 2005. [Google Scholar]
- Chi TK. An unusual variation of the pharyngeal muscles. J Anat. 1937;72:134–135. [PMC free article] [PubMed] [Google Scholar]
- Couly GF, Coltey PM, LeDouarin NM. The developmental fate of the cephalic mesoderm in quail-chick chimeras. Development. 1992;114:1–15. doi: 10.1242/dev.114.1.1. [DOI] [PubMed] [Google Scholar]
- Crelin ES. The Human Vocal Tract: Anatomy, Function, Development, and Evolution. New York: Vantage Press Inc; 1987. [Google Scholar]
- Cuvier G, Laurillard L. Recueil de Planches de Myologie. Paris: Anatomie Comparée, 3; 1849. [Google Scholar]
- Danforth CH. The myology of Polyodon. J Morphol. 1913;24:107–146. [Google Scholar]
- Dawkins R. The Ancestor's Tale – A Pilgrimage to the Dawn of Life. Boston: Houghton Mifflin; 2004. [Google Scholar]
- Diogo R. Morphological Evolution, Aptations, Homoplasies, Constraints, and Evolutionary Trends: Catfishes as a Case Study on General Phylogeny and Macroevolution. Enfield: Science Publishers; 2004a. [Google Scholar]
- Diogo R. Muscles versus bones: catfishes as a case study for an analysis on the contribution of myological and osteological structures in phylogenetic reconstructions. Anim Biol. 2004b;54:373–391. [Google Scholar]
- Diogo R. On the Origin and Evolution of Higher-Clades: Osteology, Myology, Phylogeny and Macroevolution of Bony Fishes and the Rise of Tetrapods. Enfield: Science Publishers; 2007. [Google Scholar]
- Diogo R. Comparative anatomy, homologies and evolution of the mandibular, hyoid and hypobranchial muscles of bony fish and tetrapods: a new insight. Anim Biol. 2008;58:123–172. [Google Scholar]
- Diogo R, Abdala V. Comparative anatomy, homologies and evolution of the pectoral muscles of bony fish and tetrapods: a new insight. J Morphol. 2007;268:504–517. doi: 10.1002/jmor.10531. [DOI] [PubMed] [Google Scholar]
- Diogo R, Hinits Y, Hughes SM. Development of mandibular, hyoid and hypobranchial muscles in the zebrafish: homologies and evolution of these muscles within bony fishes and tetrapods. BMC Dev Biol. 2008 doi: 10.1186/1471-213X-8-24. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBrul EL. Evolution of the Speech Apparatus. Springfield: Thomas; 1958. [Google Scholar]
- Edgeworth FH. The development of the head muscles in Scyllium canicula. J Anat Physiol. 1902;37:73–88. [PMC free article] [PubMed] [Google Scholar]
- Edgeworth FH. On the morphology of the cranial muscles in some vertebrates. Q J Microsc Sci N S. 1911;56:167–316. [Google Scholar]
- Edgeworth FH. On the development of the hypobranchial, branchial and laryngeal muscles of Ceratodus, with a note on the development of the quadrate and epihyal. Q J Microsc Sci N S. 1923;67:325–368. [Google Scholar]
- Edgeworth FH. On the hyomandibula of Selachii, Teleostomi and Ceratodus. J Anat Physiol. 1926a;60:173–193. [PMC free article] [PubMed] [Google Scholar]
- Edgeworth FH. On the development of the coraco-branchialis and cucullaris in Scyllium canicula. J Anat Physiol. 1926b;60:298–308. [PMC free article] [PubMed] [Google Scholar]
- Edgeworth FH. On the development of the cranial muscles in Protopterus and Lepidosiren. Trans R Soc Edinb Earth Sci. 1926c;54:719–734. [Google Scholar]
- Edgeworth FH. The development of some of the cranial muscles of ganoid fishes. Philos Trans R Soc Lond B Biol Sci. 1928;217:39–89. [Google Scholar]
- Edgeworth FH. The Cranial Muscles of Vertebrates. Cambridge: University Press; 1935. [Google Scholar]
- El Haddioui AL, Zouaoui A, B, ravetti P, Gaudy JF. Functional anatomy of the human lateral pterygoid muscle. Surg Radiol Anat. 2005;27:271–286. doi: 10.1007/s00276-005-0324-9. [DOI] [PubMed] [Google Scholar]
- Ericsson R, Olsson L. Patterns of spatial and temporal visceral arch muscle development in the Mexican axolotl (Ambystoma mexicanum. J Morphol. 2004;261:131–140. doi: 10.1002/jmor.10151. [DOI] [PubMed] [Google Scholar]
- Ericsson R, Cerny R, Falck P, Olsson L. Role of cranial neural crest cells in visceral arch muscle positioning and morphogenesis in the Mexican axolotl (Ambystoma mexicanum. Dev Dynam. 2004;231:237–247. doi: 10.1002/dvdy.20127. [DOI] [PubMed] [Google Scholar]
- Forey PL. Relationships of lungfishes. J Morphol Suppl. 1986;1:75–91. [Google Scholar]
- Frazzetta TH. A functional consideration of cranial kinesis in lizards. J Morphol. 1962;111:287–320. doi: 10.1002/jmor.1051110306. [DOI] [PubMed] [Google Scholar]
- Gasser RF. The development of the facial muscles in man. Am J Anat. 1967;120:357–376. [Google Scholar]
- Gaupp E. A. Ecker's und R. Wiedersheim's Anatomie des Frosches, Part 1. Braunschweig: Friedrich Vieweg und Sohn; 1896. [Google Scholar]
- George RM. The limb musculature of the Tupaiidae. Primates. 1977;18:1–34. [Google Scholar]
- Gibbs S. Comparative Soft Tissue Morphology of the Extant Hominoidea, Including Man. Liverpool: The University of Liverpool; 1999. Unpublished PhD Thesis. [Google Scholar]
- Göppert E. Kehlkopf und Trachea. In: Bolk L, Göppert E, Kallius E, Lubosch W, editors. Handbuch der Vergleichenden, Anatomie der Wirbeltiere, Vol. 3. Berlin: Urban & Schwarzenberg; 1937. pp. 797–866. [Google Scholar]
- Gorniak GC. Trends in the action of mammalian masticatory muscles. Am Zool. 1985;25:331–337. [Google Scholar]
- Greene EC. Anatomy of the Rat. New York: Hafner Publishing Co; 1935. [Google Scholar]
- Greenwood PH. Hyoid and ventral gill arch musculature in osteoglossomorph fishes. Bull Br Mus Nat Hist (Zool) 1971;22:1–55. [Google Scholar]
- Greenwood PH. Notes on the anatomy and classification of elopomorph fishes. Bull Br Mus Nat Hist (Zool) 1977;32:65–103. [Google Scholar]
- Gunnell GF, Simmons NB. Fossil evidence and the origin of bats. J Mammal Evol. 2005;12:209–246. [Google Scholar]
- Haas G. Muscles of the jaws and associated structures in the Rhynchocephalia and Squamata. In: Gans C, Parsons TS, editors. Biology of the Reptilia. Vol. 14. New York: Academic Press; 1973. pp. 285–490. [Google Scholar]
- Harrison DFN. The Anatomy and Physiology of the Mammalian Larynx. Cambridge: University Press; 1995. [Google Scholar]
- Hatta K, Schilling TF, Bremiller R, Kimmel CB. Specification of jaw muscle identity in zebrafish: correlation with engrailed-homeoprotein expression. Science. 1990;250:802–805. doi: 10.1126/science.1978412. [DOI] [PubMed] [Google Scholar]
- Hatta K, Bremiller R, Westerfield M, Kimmel CB. Diversity of expression of engrailed-like antigens in zebrafish. Development. 1991;112:821–832. doi: 10.1242/dev.112.3.821. [DOI] [PubMed] [Google Scholar]
- Herrel A, Canbek M, Özelmas Ü, Uyanoglu M, Karakaya M. Comparative functional analysis of the hyolingual anatomy in lacertid lizards. Anat Rec A. 2005;284:561–573. doi: 10.1002/ar.a.20195. [DOI] [PubMed] [Google Scholar]
- Holland ND, Holland LZ, Honma Y, Fudjii T. Engrailed expression during development of a lamprey, Lampetra japonica: a possible clue to homologies between agnathan and gnathostome muscles of the mandibular arch. Dev Growth Diff. 1993;35:153–160. doi: 10.1111/j.1440-169X.1993.00153.x. [DOI] [PubMed] [Google Scholar]
- Holliday CM, Witmer LM. Archosaur adductor chamber evolution: integration of musculoskeletal and topological criteria in jaw muscle homology. J Morphol. 2007;268:457–484. doi: 10.1002/jmor.10524. [DOI] [PubMed] [Google Scholar]
- House EL. A myology of the pharyngeal region of the albino rat. Anat Rec. 1953;116:363–381. doi: 10.1002/ar.1091160311. [DOI] [PubMed] [Google Scholar]
- Howell AB. Morphogenesis of the shoulder architecture, Part I – General considerations. Q Rev Biol. 1933a;8:247–259. [Google Scholar]
- Howell AB. Morphogenesis of the shoulder architecture, Part II – Pisces. Q Rev Biol. 1933b;8:434–456. [Google Scholar]
- Howell AB. Morphogenesis of the shoulder architecture, Part III – Amphibia. Q Rev Biol. 1935;10:397–431. [Google Scholar]
- Howell AB. Morphogenesis of the shoulder architecture, Part IV – Reptilia. Q Rev Biol. 1936a;11:183–208. [Google Scholar]
- Howell AB. Phylogeny of the distal musculature of the pectoral appendage. J Morphol. 1936b;60:287–315. [Google Scholar]
- Howell AB. The phylogenetic arrangement of the muscular system. Anat Rec. 1936c;66:295–316. [Google Scholar]
- Howell AB. Morphogenesis of the shoulder architecture, Part V – Monotremata. Q Rev Biol. 1937a;12:191–205. [Google Scholar]
- Howell AB. Morphogenesis of the shoulder architecture, Part IV – Therian Mammalia. Q Rev Biol. 1937b;12:440–463. [Google Scholar]
- Huber E. Evolution of facial musculature and cutaneous field of trigeminus – Part I. Q Rev Biol. 1930a;5:133–188. [Google Scholar]
- Huber E. Evolution of facial musculature and cutaneous field of trigeminus – Part II. Q Rev Biol. 1930b;5:389–437. [Google Scholar]
- Huber E. Evolution of facial musculature and expression. Baltimore: The Johns Hopkins University Press; 1931. [Google Scholar]
- Humphry GM. The disposition of muscles in Vertebrate animals. J Anat Physiol. 1872;6:293–376. [PMC free article] [PubMed] [Google Scholar]
- Hunter MP, Prince VE. Zebrafish Hox Paralogue group 2 genes function redundantly as selector genes to pattern the second pharyngeal arch. Dev Biol. 2002;247:367–389. doi: 10.1006/dbio.2002.0701. [DOI] [PubMed] [Google Scholar]
- Iordansky NN. Jaw muscles of the Urodela and Anura: some features of development, functions, and homology. Zool Jb Anat. 1992;122:225–232. [Google Scholar]
- Iordansky NN. Intramandibular muscles and some problems of evolution of the jaw apparatus in vertebrates. Zool Zhurnal. 2008;87:49–61. [Google Scholar]
- Janeka JE, Miller W, Pringle TH, et al. Molecular and genomic data identify the closest living relative of Primates. Science. 2007;318:792–794. doi: 10.1126/science.1147555. [DOI] [PubMed] [Google Scholar]
- Jarvik E. The composition of the intermandibular division of the head in fishes and tetrapods and the diphyletic origin of the tetrapod tongue. K Sven Vetenskab Handl. 1963;9:1–74. [Google Scholar]
- Jarvik E. Basic Structure and Evolution of Vertebrates. London: Academic Press; 1980. [Google Scholar]
- Johanson Z. Placoderm branchial and hypobranchial muscles and origins in jawed vertebrates. J Vert Paleont. 2003;23:735–749. [Google Scholar]
- Jollie M. Ventral branchial musculature and synapomorphies questioned. Zool J Linn Soc. 1982;75:35–47. [Google Scholar]
- Jouffroy FK. Musculature des membres. In: Grassé PP, editor. Traité de Zoologie, XVI: 3 (Mammifères) Paris: Masson et Cie; 1971. pp. 1–475. [Google Scholar]
- Jouffroy FK, Lessertisseur J. Particularités musculaires des Monotrémes – musculature post-craniénne. In: Grassé PP, editor. Traité de Zoologie, XVI: 3 (Mammifères) Paris: Masson et Cie; 1971. pp. 679–836. [Google Scholar]
- Jouffroy FK, Saban R. Musculature peaucière. In: Grassé PP, editor. Traité de Zoologie, XVI: 3 (Mammifères) Paris: Masson et Cie; 1971. pp. 477–611. [Google Scholar]
- Kanagasuntheram R. Observations on the anatomy of the hoolock gibbon. Ceylon J Sci Sect G. 1952–1954;5:11–64+69. [Google Scholar]
- Kardong KV. Vertebrates: Comparative Anatomy, Function, Evolution. 3rd edn. New York: McGraw-Hill; 2002. [Google Scholar]
- Kardong KV, Zalisko EJ. Comparative Vertebrate Anatomy – A Laboratory Dissection Guide. New York: McGraw-Hill; 1998. [Google Scholar]
- Kemp TS. The Origin and Evolution of Mammals. New York: Oxford University Press; 2005. [Google Scholar]
- Kesteven HL. The evolution of the skull and the cephalic muscles. Mem Aust Mus. 1942–1945;8:1–361. [Google Scholar]
- Kisia SM, Onyango DW. Muscular System of Vertebrates. Enfield: Science Publishers; 2005. [Google Scholar]
- Kleinteich T, Haas A. Cranial musculature in the larva of the caecilian, Ichthyophis kohtaoensis(Lissamphibia: Gymnophiona) J Morphol. 2007;268:74–88. doi: 10.1002/jmor.10503. [DOI] [PubMed] [Google Scholar]
- Knight RD, Mebus K, Roehl HH. Mandibular arch muscle identity is regulated by a conserved molecular process during vertebrate development. J Exp Zool (Mol Dev Evol) 2008;310B:355–369. doi: 10.1002/jez.b.21215. [DOI] [PubMed] [Google Scholar]
- Köntges G, Lumsden A. Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development. 1996;122:3229–3242. doi: 10.1242/dev.122.10.3229. [DOI] [PubMed] [Google Scholar]
- Larsen JH, Guthrie DJ. The feeding system of terrestrial tiger salamanders (Ambystoma tigrinum melanostictum Baird) J Morphol. 1975;147:137–154. doi: 10.1002/jmor.1051470203. [DOI] [PubMed] [Google Scholar]
- Lauder GV. Evolution of the feeding mechanisms in primitive actinopterygian fishes: a functional anatomical analysis of Polypterus, Lepisosteus, and Amia. J Morphol. 1980a;163:283–317. doi: 10.1002/jmor.1051630305. [DOI] [PubMed] [Google Scholar]
- Lauder GV. On the evolution of the jaw adductor musculature in primitive gnathostome fishes. Breviora. 1980b;460:1–10. [Google Scholar]
- Lauder GV. The role of the hyoid apparatus in the feeding mechanism of the coelacanth Latimeria chalumnae. Copeia. 1980c;1980:1–9. [Google Scholar]
- Lauder GV, Liem KF. The evolution and interrelationships of the actinopterygian fishes. Bull Mus Comp Zool. 1983;150:95–197. [Google Scholar]
- Lauder GV, Shaffer HB. Functional morphology of the feeding mechanism in aquatic ambystomatid salamanders. J Morphol. 1985;185:297–326. doi: 10.1002/jmor.1051850304. [DOI] [PubMed] [Google Scholar]
- Lauder GV, Shaffer HB. Ontogeny of functional design in tiger salamanders (Ambystoma tigrinum): are motor patterns conserved during major morphological transformations? J Morphol. 1988;197:249–268. doi: 10.1002/jmor.1051970302. [DOI] [PubMed] [Google Scholar]
- Le Gros Clark WE. The myology of the tree-shrew (Tupaia minor. Proc Zool Soc Lond. 1924;1924:461–497. [Google Scholar]
- Le Gros Clark WE. On the anatomy of the pen-tailed tree-shrew (Ptilocercus lowii. Proc Zool Soc Lond. 1926;1926:1178–1309. [Google Scholar]
- Le Lièvre C, Le Douarin NM. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol. 1975;34:125–154. [PubMed] [Google Scholar]
- Leche W. Über die Säugethiergattung Galeopithecus: eine morphologische untersuchung. 1886;21:3–92. [Google Scholar]
- Lightoller GS. The action of the m. mentalis in the expression of the emotion of distress. J Anat. 1928a;62:319–332. [PMC free article] [PubMed] [Google Scholar]
- Lightoller GS. The facial muscles of three orang utans and two cercopithecidae. J Anat. 1928b;63:19–81. [PMC free article] [PubMed] [Google Scholar]
- Lightoller GS. The facial musculature of some lesser primates and a Tupaia. Proc Zool Soc Lond. 1934;1934:259–309. [Google Scholar]
- Lightoller GS. The comparative morphology of the platysma: a comparative study of the sphincter colli profundus and the trachelo-platysma. J Anat. 1940a;74:390–396. K Sven Vetenskap Akad Stockholm. [PMC free article] [PubMed] [Google Scholar]
- Lightoller GS. The comparative morphology of the m. caninus. J Anat. 1940b;74:397–402. [PMC free article] [PubMed] [Google Scholar]
- Lightoller GS. Matrices of the facialis musculature: homologization of the musculature in monotremes with that of marsupials and placentals. J Anat. 1942;76:258–269. [PMC free article] [PubMed] [Google Scholar]
- Lubosch W. Vergleischende Anatomie der Kaumusculatur der Wirbeltiere, in fünf Teilen: 1 – die Kausmukulatur der Amphibien. Jen Z Naturwiss. 1914;53:51–188. [Google Scholar]
- Luther A. Über die vom N trigeminus versorgte Muskulatur des Ganoiden and Dipneusten. Acta Soc Scient Fenn. 1913;41:1–72. [Google Scholar]
- Luther A. Über die vom N Trigeminus versorgte Muskulatur der Amphibien, mit einem Vergleichenden aublick über deu Adductor Mandibulae der Gnathostomen, und cinem Beitrag zum Verständnis der Organisation der Anurenlarven. Acta Soc Scient Fenn. 1914;44:1–151. [Google Scholar]
- Macalister A. The myology of the Cheiroptera. Philos Trans R Soc Lond. 1872;162:125–171. [Google Scholar]
- Mallat J. Shark pharyngeal muscles and early vertebrate evolution. Acta Zool-Stockholm. 1997;78:279–294. [Google Scholar]
- Marivaux L, Bocat L, Chaimanee Y, et al. Cynocephalid dermopterans from the Palaeogene of South Asia (Thailand, Myanmar and Pakistan): systematic, evolutionary and palaeobiogeographic implications. Zool Scripta. 2006;35:395–420. [Google Scholar]
- Matsuoka T, Ahlberg PE, Kessaris N, Iannarelli P, Dennehy U, Richardson WD, McMahon AP, Koentges G. Neural crest origins of the neck and shoulder. Nature. 2005;436:347–355. doi: 10.1038/nature03837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonnell IM. The evolution of the pectoral girdle. J Anat. 2001;199:189–194. doi: 10.1046/j.1469-7580.2001.19910189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millot J, Anthony J. Anatomie de Latimeria Chalumnae, I – Squelette, Muscles, et Formation de Soutiens. Paris: CNRS; 1958. [Google Scholar]
- Minkoff EC, Mikkelsen P, Cunningham WA, Taylor KW. The facial musculature of the opossum (Didelphis virginiana. J Mammal. 1979;60:46–57. [Google Scholar]
- Miyake T, McEachran JD, Hall BK. Edgeworth's legacy of cranial muscle development with an analysis of muscles in the ventral gill arch region of batoid fishes (Chondrichthyes: Batoidea) J Morphol. 1992;212:213–256. doi: 10.1002/jmor.1052120304. [DOI] [PubMed] [Google Scholar]
- Montero R, Moro SA, Abdala V. Cranial anatomy of Euspondylus acutirostris(Squamata: Gymnophthalmidae) and its placement in a modern phylogenetic hypothesis. Russ J Herpetol. 2002;9:215–228. [Google Scholar]
- Negus VE. The Comparative Anatomy and Physiology of the Larynx. New York: Hafner Publishing Company; 1949. [Google Scholar]
- Netter FH. Atlas of Human Anatomy. 4th edn. Philadelphia: Saunders; 2006. [Google Scholar]
- Noden DM. The embryonic origins of avian cephalic and cervical muscles and associated connective tissues. Am J Anat. 1983;168:257–276. doi: 10.1002/aja.1001680302. [DOI] [PubMed] [Google Scholar]
- Noden DM. Craniofacial development: new views on old problems. Anat Rec. 1984;208:1–13. doi: 10.1002/ar.1092080103. [DOI] [PubMed] [Google Scholar]
- Noden DM. Patterning of avian craniofacial muscles. Dev Biol. 1986;116:347–356. doi: 10.1016/0012-1606(86)90138-7. [DOI] [PubMed] [Google Scholar]
- Noden DM, Francis-West P. The differentiation and morphogenesis of craniofacial muscles. Dev Dyn. 2006;235:1194–1218. doi: 10.1002/dvdy.20697. [DOI] [PubMed] [Google Scholar]
- Noden DM, Schneider RA. Neural crest cells and the community of plan for craniofacial development: historical debates and current perspectives. In: Saint-Jeannet J, editor. Neural Crest Induction & Differentiation – Advances in Experimental Medicine and Biology. Vol. 589. Georgetown: Landes Bioscience; 2006. pp. 1–31. [DOI] [PubMed] [Google Scholar]
- Olsson L, Falck P, Lopez K, Cobb J, Hanken J. Cranial neural crest cells contribute to connective tissue in cranial muscles in the anuran amphibian, Bombina orientalis. Dev Biol. 2001;237:354–367. doi: 10.1006/dbio.2001.0377. [DOI] [PubMed] [Google Scholar]
- Osse JWM. Functional morphology of the head of the perch (Perca fluviatis L.): an electromyographic study. Neth J Zool. 1969;19:289–392. [Google Scholar]
- Owen R. Description of the Lepidosiren annectens. Trans Linn Soc Lond. 1841;18:327–361. [Google Scholar]
- Parsons FG. The muscles of mammals, with special relation to human myology: a course of lectures delivered at the Royal College of Surgeons of England – lecture I, the muscles of the head and neck. J Anat Physiol. 1898;32:428–450. [PMC free article] [PubMed] [Google Scholar]
- Peterka HE. A study of the myology and osteology of tree sciurids with regard to adaptation to arboreal, glissant and fossorial habits. Trans Kansas Acad Sci. 1936;39:313–332. [Google Scholar]
- Pettersen GC, Koltis GG, White MJ. An examination of the spectrum of anatomic defects and variations found in eight cases of trisomy 13. Am J Med Genet. 1979;3:183–210. doi: 10.1002/ajmg.1320030209. [DOI] [PubMed] [Google Scholar]
- Piatt J. Morphogenesis of the cranial muscles of Ambystoma punctatum. J Morphol. 1938;63:531–587. [Google Scholar]
- Piekarski N, Olsson L. Muscular derivatives of the cranialmost somites revealed by long-term fate mapping in the Mexican axolotl (Ambystoma mexicanum. Evol Dev. 2007;9:566–578. doi: 10.1111/j.1525-142X.2007.00197.x. [DOI] [PubMed] [Google Scholar]
- Pollard HB. On the anatomy and phylogenetic position of Polypterus. Zool Jb. 1892;5:387–428. [Google Scholar]
- Pough FH, Heiser JB, McFarland WN. Vertebrate Life. 4th edn. New Jersey: Prentice-Hall; 1996. [Google Scholar]
- Preuschoft S. Primate faces and facial expressions. Soc Res. 2000;67:245–271. [Google Scholar]
- Reilly S, Lauder GV. Kinetics of tongue projection in Ambystoma tigrinum: quantitative kinematics, muscle function and evolutionary hypotheses. J Morphol. 1989;199:223–243. doi: 10.1002/jmor.1051990208. [DOI] [PubMed] [Google Scholar]
- Reilly S, Lauder GV. The evolution of tetrapod feeding behavior: kinematic homologies in prey transport. Evolution. 1990;44:1542–1557. doi: 10.1111/j.1558-5646.1990.tb03845.x. [DOI] [PubMed] [Google Scholar]
- Reilly S, Lauder GV. Prey transport in the tiger salamander: quantitative electromyography and muscle function in tetrapods. J Exp Zool. 1991;260:1–17. [Google Scholar]
- Rosen DE, Forey PL, Gardiner BG, Patterson C. Lungfishes, tetrapods, paleontology and plesiomorphy. Bull Am Mus Nat Hist. 1981;167:159–276. [Google Scholar]
- Ruge G. Über die Gesichtsmuskulatur der Halbaffen. Morph Jb. 1885;11:243–315. [Google Scholar]
- Ruge G. Über das Peripherische Gebiet des Nervus Facialis boi Wirbelthieren. Leipzig: Festschr f Gegenbaur.; 1897. [Google Scholar]
- Ruge G. Verbindungen des Platysma mit der tiefen Muskulatur des Halses beim Menschen. Morph Jb. 1910;41:708–724. [Google Scholar]
- Ryan JM. Comparative morphology and evolution of cheek pouches in rodents. J Morphol. 1986;190:27–42. doi: 10.1002/jmor.1051900104. [DOI] [PubMed] [Google Scholar]
- Ryan JM. Evolution of cheek pouches in African pouched rats (Rodentia: Cricetomyinae) J Mammal. 1989;70:267–274. [Google Scholar]
- Saban R. Musculature de la tête. In: Grassé PP, editor. Traité de Zoologie, XVI: 3 (Mammifères) Paris: Masson et Cie; 1968. pp. 229–472. [Google Scholar]
- Saban R. Particularités musculaires des Monotrémes – musculature de la tête. In: Grassé PP, editor. Traité de Zoologie, XVI: 3 (Mammifères) Paris: Masson et Cie; 1971. pp. 681–732. [Google Scholar]
- Sargis EJ. The postcranial morphology of Ptilocercus lowii(Scandentia, Tupaiidae): an analysis of primatomorphan and volitantian characters. J Mammal Evol. 2002a;9:137–160. [Google Scholar]
- Sargis EJ. Functional morphology of the forelimb of tupaiids (Mammalia, Scandentia) and its phylogenetic implications. J Mammal. 2002b;253:10–42. doi: 10.1002/jmor.1110. [DOI] [PubMed] [Google Scholar]
- Sargis EJ. New views on tree shrews: the role of tupaiids in primate supraordinal relationships. Evol Anthropol. 2004;13:56–66. [Google Scholar]
- Schilling TF, Kimmel CB. Musculoskeletal patterning in the pharyngeal segments of the zebrafish. Development. 1997;124:2945–2960. doi: 10.1242/dev.124.15.2945. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, Cohn JF. Human facial expressions as adaptations: evolutionary questions in facial expression research. Yearb Phys Anthropol. 2001;44:3–24. doi: 10.1002/ajpa.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher GH. Funktionelle Morphologie der Kaumuskulatur. Jena: VEB Gustav Fischer Verlag; 1961. [Google Scholar]
- Schumacher GH. The head muscles and hyolaryngeal skeleton of turtles and crocodilians. In: Gans C, Parsons TS, editors. Biology of the Reptilia. Vol. 14. New York: Academic Press; 1973. pp. 101–199. [Google Scholar]
- Seiler R. Proc 3rd Int Congr Primat, Zürich 1970. Vol. 1. Basel: Karger; 1971a. A comparison between the facial muscles of Catarrhini with long and short muzzles; pp. 157–162. [Google Scholar]
- Seiler R. Facial musculature and its influence on the facial bones of catarrhinous Primates. I. Morphol Jb. 1971b;116:122–142. [PubMed] [Google Scholar]
- Seiler R. Facial musculature and its influence on the facial bones of catarrhine Primates. II. Morphol Jb. 1971c;116:147–185. [PubMed] [Google Scholar]
- Seiler R. Facial musculature and its influence on the facial bones of catarrhine Primates. III. Morphol Jb. 1971d;116:347–376. [PubMed] [Google Scholar]
- Seiler R. Facial musculature and its influence on the facial bones of catarrhine Primates. IV. Morphol Jb. 1971e;116:456–481. [PubMed] [Google Scholar]
- Seiler R. Muscles of the external ear and their function in man, chimpanzees and Macaca. Morphol Jb. 1974a;120:78–122. [PubMed] [Google Scholar]
- Seiler R. Particularities in facial muscles of Daubentonia madagascariensis(Gmelin 1788) Folia Primatol. 1974b;22:81–96. doi: 10.1159/000155620. [DOI] [PubMed] [Google Scholar]
- Seiler R. On the facial muscles in Perodicticus potto and Nycticebus coucang. Folia Primatol. 1975;23:275–289. doi: 10.1159/000155677. [DOI] [PubMed] [Google Scholar]
- Seiler R. Criteria of the homology and phylogeny of facial muscles in primates including man. I. Prosimia and Platyrrhina. Morphol Jb. 1979;125:191–217. [PubMed] [Google Scholar]
- Seiler R. Ontogenesis of facial muscles in primates. Morphol Jb. 1980;126:841–864. [PubMed] [Google Scholar]
- Sewertzoff AN. The head skeleton and muscles of Acipenser ruthenus. Acta Zool-Stockholm. 1928;9:193–319. [Google Scholar]
- Shattock SG. A Kerato-thyro-hyoid muscle as a variation in human anatomy. J Anat Physiol. 1882;171:124–125. [PMC free article] [PubMed] [Google Scholar]
- Shibata S. On the facial musculature of Macacus cyclopsis. OFAJ. 1959;34:159–176. [Google Scholar]
- Shoshani J, Groves CP, Simons EL, Gunnell GF. Primate phylogeny: morphological vs molecular results. Mol Phylogenet Evol. 1996;5:102–154. doi: 10.1006/mpev.1996.0009. [DOI] [PubMed] [Google Scholar]
- Silcox MT, Sargis EJ, Bloch JI, Boyer DM. Primate origins and supraordinal relationships: morphological evidence. In: Henke W, Tattersall I, editors. Handbook of Palaeoanthropology, Vol. 2: Primate Evolution and Human Origins. Springer Verlag: Heidelberg; 2007. pp. 831–859. [Google Scholar]
- Smith KK. Form and function of the tongue in agamid lizards with comments on its phylogenetic significance. J Morphol. 1988;196:157–171. doi: 10.1002/jmor.1051960205. [DOI] [PubMed] [Google Scholar]
- Smith KK. The evolution of the mammalian pharynx. Zool J Linn Soc. 1992;104:313–349. [Google Scholar]
- Smith KK. Development of craniofacial musculature in Monodelphis domestica(Marsupialia: Didelphidae) J Morphol. 1994;222:149–173. doi: 10.1002/jmor.1052220204. [DOI] [PubMed] [Google Scholar]
- Sokoloff AJ. Localization and contractile properties of intrinsic longitudinal motor units of the rat tongue. J Neurophysiol. 2000;84:827–835. doi: 10.1152/jn.2000.84.2.827. [DOI] [PubMed] [Google Scholar]
- Sprague JA. The hyoid region of placental mammals with special reference to the bats. Am J Anat. 1943;72:385–472. [Google Scholar]
- Sprague JA. The hyoid region in the insectivora. Am J Anat. 1944a;74:175–216. [Google Scholar]
- Sprague JA. The innervation of the pharynx in the rhesus monkey, and the formation of the pharyngeal plexus in primates. Anat Rec. 1944b;90:197–208. [Google Scholar]
- Sprague JM. The hyoid apparatus of Neotoma. J Mammal. 1942;23:405–411. [Google Scholar]
- Stafford BJ, Szalay FS. Craniodental functional morphology and taxonomy of dermopterans. J Mammal. 2000;81:360–385. [Google Scholar]
- Starck D, Schneider R. Respirationsorgane. In: Hofer H, Schultz AH, Starck D, editors. Primatologia III/2. Basel: S. Karger; 1960. pp. 423–587. [Google Scholar]
- Stiassny MLJ. Gross functional anatomy: muscular system. In: Bullock G, Bunton TE, editors. The Handbook of Experimental Animals. London: Academic Press; 2000. pp. 119–128. [Google Scholar]
- Straus WL, Howell AB. The spinal accessory nerve and its musculature. Q Rev Biol. 1936;11:387–405. [Google Scholar]
- Sullivan WE, Osgood CW. The musculature of the superior extremity of the orang-utan, Simia satyrus. Anat Rec. 1927;35:193–239. [Google Scholar]
- Taylor J. An unusual variation of the omo-hyoid muscle. J Anat. 1925;59:331–332. [PMC free article] [PubMed] [Google Scholar]
- Terminologia Anatomica. Federative Committee on Anatomical Terminology. Stuttgart: Georg Thieme; 1998. [Google Scholar]
- Thewissen JGM, Babcock SK. Distinctive cranial and cervical innervation of wing muscles: new evidence for bat monophyly. Science. 1991;251:934–936. doi: 10.1126/science.2000493. [DOI] [PubMed] [Google Scholar]
- Thewissen JGM, Babcock SK. The implications of the propatagial muscles of flying and gliding mammals for archontan systematics. In: MacPhee RDE, editor. Primates and Their Relatives in Phylogenetic Perspective. New York: Plenum Press; 1993. pp. 91–107. [Google Scholar]
- Thorsen DH, Hale ME. Development of zebrafish (Danio rerio) pectoral fin musculature. J Morphol. 2005;266:241–255. doi: 10.1002/jmor.10374. [DOI] [PubMed] [Google Scholar]
- Tsuihiji T. Homologies of the longissimus, iliocostalis, and hypaxial muscles in the anterior presacral region of extant diapsida. J Morphol. 2007;268:986–1020. doi: 10.1002/jmor.10565. [DOI] [PubMed] [Google Scholar]
- Walker WF, Homberger DG. Anatomy and Dissection of the Rat. 3rd edn. New York: Freeman Company; 1998. [Google Scholar]
- Wells LH, Thomas EA. A note on two abnormal laryngeal muscles in a Zulu. J Anat. 1927;61:340–343. [PMC free article] [PubMed] [Google Scholar]
- West-Eberhard MJ. Developmental Plasticity and Evolution. New York: Oxford University Press; 2003. [Google Scholar]
- Whidden HP. Comparative myology of moles and the phylogeny of the Talpidae (Mammalia, Lipotyphla) Am Mus Novitates. 2000;3294:1–53. [Google Scholar]
- Wiley EO. Ventral gill arch muscles and the interrelationships of gnathostomes, with a new classification of the Vertebrata. J Linn Soc (Zool) 1979a;67:149–179. [Google Scholar]
- Wiley EO. Ventral gill arch muscles and the phylogenetic interrelationships of Latimeria. Occas Pap Calif Acad Sci. 1979b;134:56–67. [Google Scholar]
- Wilga CD, Wainwright PC, Motta PJ. Evolution of jaw depression mechanisms in aquatic vertebrates: insights from Chondrichthyes. Biol J Linn Soc. 2000;71:165–185. [Google Scholar]
- Wilson-Pauwels L, Akesson EJ, Stewart PA, Spacey SD. Cranial Nerves in Health and Disease. Hamilton: B.C. Decker Inc; 2002. [Google Scholar]
- Wind J. On the Phylogeny and Ontogeny of the Human Larynx. Groningen: Wolters-Noordhoff; 1970. [Google Scholar]
- Winterbottom R. A descriptive synonymy of the striated muscles of the Teleostei. Proc Acad Nat Sci (Phil) 1974;125:225–317. [Google Scholar]
- Wood J. Variations in human myology observed during the Winter Session of 1865–1866 at King's College, London. Proc R Soc Lond B. 1866;15:229–244. [Google Scholar]
- Wood J. On human muscular variations and their relation to comparative anatomy. J Anat Physiol. 1867a;1867:44–59. [PMC free article] [PubMed] [Google Scholar]
- Wood J. Variations in human myology observed during the Winter Session of 1866–1867 at King's College London. Proc R Soc Lond B. 1867b;15:518–545. [Google Scholar]
- Wood J. Variations in human myology observed during the winter session of 1867–1868 at King's College, London. R Soc Lond. 1868;16:483–525. [Google Scholar]
- Wood J. On a group of varieties of the muscles of the human neck, shoulder, and chest, with their transitional forms and homologies in the Mammalia. Philos Trans R Soc Lond. 1870;160:83–116. [Google Scholar]