Abstract
According to their feeding habits, ruminants can be classified as grazers, concentrate selectors and those of intermediate type. The different feeding types are reflected in distinct anatomical properties of the forestomachs. The present study was designed to investigate whether the intrinsic innervation patterns of the rumen (the main part of the forestomach) differ between intermediate types and grazers. Myenteric plexus preparations from the rumen of goats (intermediate type), fallow deer (intermediate type), cattle (grazer) and sheep (grazer) were analysed by immunohistochemical detection of the following antigens: Hu-protein (HuC/D), choline acetyltransferase (ChAT), nitric oxide synthase (NOS), vasoactive intestinal peptide (VIP), neuropeptide Y (NPY), substance P (SP), calbindin (CALB) and somatostatin (SOM). Myenteric ganglia of cattle contained 73 ± 6 neurons per ganglion, whereas the ganglia of sheep were significantly smaller (45 ± 18 neurons per ganglion). The ganglion density of the myenteric plexus was highest in fallow deer (15 ± 3 ganglia per cm2) and lowest in cattle (6 ± 1 ganglia per cm2). All myenteric neurons were either ChAT or NOS positive. The proportion of NOS-positive neurons was significantly lower in sheep (29.5 ± 8.2% of all neurons) than in goats (44.2 ± 9.8%). In all species, additional analysis of the different neuropeptides revealed the following subpopulations in descending order of percentile appearance: ChAT/SP > NOS/VIP/NPY > ChAT/– > NOS/NPY. Expression of CALB was detected in a minority of the ChAT-positive neurons in all species. Somatostatin immunoreactive somata were found only in preparations obtained from fallow deer and sheep. These data suggest that the rumen of grazers is under stronger cholinergic control than the rumen of species belonging to the intermediate type, although most subpopulations of neurons are present in all species. However, whether the strong mixing patterns of low quality roughage during digestion are enabled by the prominent excitatory input of the rumen of grazers requires elucidation in further studies.
Keywords: choline acetyltransferase, enteric nervous system, forestomach, neuropeptide Y, nitric oxide synthase, somatostatin, substance P, vasoactive intestinal peptide
Introduction
All ruminant species are able to digest herbaceous feed via microbial fermentation in their forestomachs. Despite this common digestive principle, feeding habits differ and ruminants can be classified according to their preferred feed. With regard to feeding preferences, Hofmann (1989) described three large groups: grazers, concentrate selectors and animals of an intermediate type. All domesticated ruminants belong to grazers or to the intermediate type. Grazers are able to digest low quality roughage, in particular different kinds of grass. The intermediate type, in contrast, select leaves and young plants with high energy content, if available (Hofmann, 1989). The different feeding habits are accompanied by different passage rates of digesta through the gastrointestinal tract, with the longest retention times found in the group of grazers (Huston et al. 1986). These different particle retention times are mainly caused by anatomical properties of the forestomachs. Grazers possess a larger rumen and a smaller omasal orifice than intermediate types and concentrate selectors (Hofmann, 1989). The two latter groups can use their reticular groove to bypass the forestomach and to lead the food directly into the abomasum (Hofmann, 1989). These findings imply that the motility patterns in the forestomachs show adaptations depending on the feeding type.
In general, forestomach motility is controlled by both intrinsic and extrinsic neural control mechanisms. The intrinsic neural control of motility is provided by neurons of the enteric nervous system (Ruckebusch, 1989). Only sparse data are available on the control of forestomach motility by enteric neurons in different ruminant species. However, it is a general rule in both monogastric animals and ruminants that intrinsic neurons controlling motility are located in the myenteric plexus and express specific combinations of excitatory and inhibitory neurotransmitters (Kitamura et al. 1986; Groenewald, 1994; Brookes, 2001; Pfannkuche et al. 2002; Franco et al. 2004; Pfannkuche et al. 2004b). The combination of neurotransmitters synthesized by one neuron is named the neurochemical code of the neuron. In monogastric species, the neurochemical codes of enteric neurons are strongly related to their functions (Brookes, 2001). In small laboratory animals, for example, choline acetyltransferase (ChAT) and substance P (SP) are colocalized in excitatory motor neurons of the myenteric plexus (Brookes, 2001). In contrast, the codes ChAT/SP/ calbindin and ChAT/SP/SOM have been described for intrinsic primary afferent neurons and interneurons, respectively (Brookes, 2001; Brehmer et al. 2004).
In the sheep forestomach, region- and age-related neurochemical codes have been described for the myenteric plexus (Pfannkuche et al. 2003a,b). The findings obtained in these studies point to a correlation between neurochemical codes and the functions of the forestomach regions. Therefore, we hypothesized that the neurochemical codes of myenteric neurons might also differ between ruminants belonging to different feeding types. Thus we analysed the expression of ChAT, SP, calbindin, somatostatin and the putative inhibitory transmitters nitric oxide (NO), vasoactive intestinal peptide (VIP) and neuropeptide Y (NPY) in myenteric plexus preparations from the rumens of grazers and intermediate types.
Materials and methods
Tissue preparation
Specimens were taken from 26 grazers (15 Merino sheep and 11 Holstein–Friesian cattle) and 24 intermediate type ruminants (12 goats: Weiße Deutsche Edelziege, Bunte Deutsche Edelziege, and 12 fallow deer). Animals of both sexes with an age of 12–48 months were used. The experiments described in this report comply with the current legislation covering the protection of animals. The sheep had been housed in a stable of the faculty and had free access to hay and water. Additionally they received concentrate feed (200 g per sheep) once daily. The sheep were slaughtered at the slaughterhouse of the veterinary faculty. Tissues from cattle (dairy cows or fattened bulls) were obtained at the local commercial abattoir. Detailed information regarding their housing and feeding was not available. Goats came from a local producer and were home slaughtered. They had been fed with straw and water ad libitum and twice daily with hay (400 g day−1) and concentrate for dairy goats (2000 g day−1). Fallow deer were housed in large enclosures where they had free access to pasture. Deer were slaughtered at the slaughterhouse of the veterinary faculty.
From all animals, the forestomach was removed and pieces of the ventral rumen sac were dissected. The forestomach contents were rinsed off by washes using ice-cold Krebs–Ringer solution of the following composition (in mm): 117 NaCl, 4.7 KCl, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, 2.5 CaCl2, 11.5 glucose and 1 nifedipine, pH 7.4, gassed with 5% CO2/95% O2. Some of the tissues were pinned flat in a Sylgard-covered petri dish and fixed for 24 h at 4 °C in 0.1 m phosphate buffer containing 4% paraformaldehyde and 0.2% picric acid. The fixed tissues were washed in 0.1 m phosphate buffer and stored in phosphate-buffered saline (PBS 0.1 m) containing 0.1% NaN3.
To enhance immunoreactivity of the neuropeptides SP, NPY and VIP in myenteric neurons, tissues were incubated with colchicine. For that purpose, tissues were pinned out in a Sylgard-covered petri dish and the mucosa and submucosa were completely dissected off. After several washes in sterile Krebs–Ringer solution, specimens were transferred to a tissue culture petri dish and cultured for 24 h at 37 °C in culture medium (Dulbecco's modified Eagle medium F12) supplemented with 40 µm colchicine, 10% heat-inactivated fetal calf serum, 100 IU mL−1 penicillin, 100 µg mL−1 streptomycin, 1.24 µg mL−1 amphotericin B, 20 µg mL−1 gentamicin, 2.1 mg mL−1 NaHCO3 and 1 µm nifedipine, pH 7.4 (all chemicals from CC-Pro, Neustadt, Germany, or Sigma, Deisenhofen, Germany). During the culture period, the tissues were continuously agitated using a rocking tray. After organotypic culture, the specimens were fixed for 24 h at 4 °C in 0.1 m phosphate buffer containing 4% paraformaldehyde and 0.2% picric acid. During the fixation process, the specimens were pinned maximally stretched. The fixed tissues were washed in 0.1 m phosphate buffer and stored in PBS (0.1 m) containing 0.1% NaN3.
Immunohistochemistry
For immunohistochemistry of the myenteric plexus, whole mount preparations were obtained by dissecting off circular and longitudinal muscle layers. After dissecting, the tissues were preincubated and permeabilized in PBS containing 4% horse serum and 0.5% Triton X-100. The primary antibodies were diluted in the same solution. The tissues were incubated for 40 h at room temperature in the solution containing the primary antibodies. The following antisera were used in the respective dilutions: rabbit anti-choline acetyltransferase (ChAT, 1 : 1000, P3YEB) (Schemann et al. 1993), mouse anti-nitric oxide synthase (NOS, 1 : 40, N31020, Transduction Laboratories, USA), rat anti-substance P (SP, 1 : 1000, 10-S015, Fitzgerald, USA), guinea-pig anti-vasoactive intestinal polypeptide (VIP, 1 : 1000, GHC7161, Peninsula, USA), rabbit anti-neuropeptide Y (NPY, 1 : 1000, T-4453 Peninsula, USA), rabbit anti-calbindin (CALB, 1 : 2000, CB-38, Swant, Bellinzona, Switzerland), rabbit anti-somatostatin (SOM, 1 : 1000, T-4102, Peninsula, USA) and mouse anti-HU neuronal protein (HuC/D, 1 : 200, A-21271, MoBiTec, Göttingen, Germany). Two to three primary antibodies raised in different host species were simultaneously used for each tissue.
After incubation with the primary antibodies, the specimens were washed three times and were incubated for 5 h in buffer solution containing the secondary antibodies. Affinity-purified secondary anti-rabbit, anti-mouse, anti-rat and anti-guinea-pig antibodies raised in donkeys, conjugated to carbocyanine (Cy2), indocarbocyanine (Cy3), biotin or indodicarbocyanine (Cy5) were used (all purchased from Dianova, Hamburg, Germany). The final dilutions of secondary antibodies were 1 : 200 (Cy2 conjugates), 1 : 500 (Cy3 and Cy5 conjugates) and 1 : 50 (biotin conjugates). The biotin-conjugated secondary antibodies were visualized using streptavidin conjugated with aminomethylcoumarin acetate (AMCA) in a dilution of 1 : 50. Finally, the specimens were washed in PBS, mounted on poly-l-lysine-covered slides and coverslipped with a solution of NaHCO3/Na2CO3 (0.5 m, pH 7.0) containing 0.1 NaN3 and 80% glycerol.
Although all myenteric neurons in the rumen of adult sheep are either ChAT-positive or NOS-positive neurons (Pfannkuche et al. 2002), it is not known whether this is also true for other ruminant species. Therefore some specimens initially labelled against ChAT and NOS were restained to visualize the general neuronal marker HuC/D. The ChAT and NOS immunoreactive neurons had been visualized using secondary antibodies labelled with Cy2 and Cy3, respectively. The primary mouse-anti-HuC/D antibodies were labelled with secondary antibodies conjugated with biotin, which was visualized using streptavidin-AMCA conjugates.
The preparations were examined using an epifluorescence microscope (IX50, Olympus, Japan). Appropriate filters were applied to visualize the fluorophores separately (Pfannkuche et al. 1998). Pictures were acquired with a black-and-white video camera (Mod. 4910, Cohu, San Diego, CA, USA) controlled by image analysis processing software (Olympus Hamburg, Germany).
NADPH staining
In some preparations (10 preparations from goats, 7 from fallow deer, 10 from cattle, 8 from sheep) nitric oxide synthase was detected by the NADPH-diaphorase reaction. To visualize NADPH-diaphorase, tissues were incubated at 37 °C in 0.1 m phosphate buffer (pH 8) containing 0.5% Triton X-100, β-NADPH-D (0.5 mg 10 mL−1, N-1630, Sigma) and 4-nitrotetrazoliumblue (1 mg 10 mL−1, N-6876, Sigma). The reaction was stopped after 30 min to 3 h by several rinses with PBS.
The histochemical reaction product quenched the signals from the fluorescent dyes. Therefore, photomicrographs of the immunohistochemical results were taken prior to the NADPH staining.
Specificity of antibodies and antisera
The specificity of the antibodies against ChAT, SP, VIP, NPY and SOM was tested for all species. Adsorption of the respective diluted antibodies with 1 µm of ChAT, SP, VIP, NPY or SOM for 24 h at 4 °C prior to application to the tissue resulted in no positive staining of the preparations.
Specificity of the CALB antibodies has been published previously (Reiche et al. 1999). We assume that these antibodies recognize the appropriate antigens in ruminants. However, this has not been directly tested in this study.
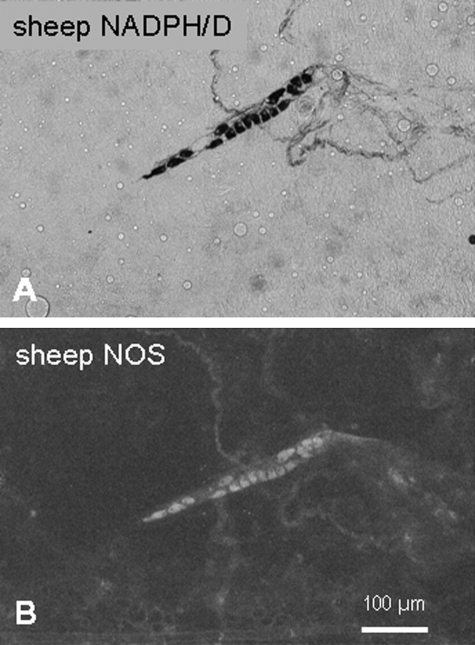
Specificity of the mouse anti-NOS antibody was determined by detecting NADPH-diaphorase reaction in tissues stained first against NOS. The mouse anti-NOS antibody and the NADPH-diaphorase reaction marked identical myenteric neurons.
To prove specificity of secondary antisera, they were applied without use of primary antibodies. No staining was seen after omitting the primary antibodies.
Data analysis and statistics
The number of myenteric ganglia per cm2 were counted in a 1 × 1 cm square of the whole mount preparations. All ganglia included in this square or touching the left or bottom margin were counted. The mean value was calculated from all preparations counted in one species.
For all markers, the number of cells per ganglion was counted using 20 ganglia in each preparation. For each preparation, median values of the number of neuronal somata per ganglion were calculated. The proportion of the different neurochemical subpopulations was calculated for each species by computing the mean from corresponding median values.
To analyse whether all myenteric neurons express either immunoreactivity for ChAT or NOS, 300 neurons per preparation were analysed for their immunoreactivity for ChAT and NOS in tissues restained with HuC/D.
Results are expressed as means ± standard deviation (SD; n = number of specimens; N = number of animals).
The number of ganglia per cm2 and the number of neurons per ganglion were compared between the species by using a one-way anova with subsequent multiple comparisons (Student–Newman–Keuls test). Two-way anova with subsequent multiple comparisons (Student–Newman–Keuls test) was used to compare the size of neuronal subpopulations with a distinct neurochemical code between the different species.
Differences were considered to be statistically significant when P < 0.05.
Results
General innervation pattern
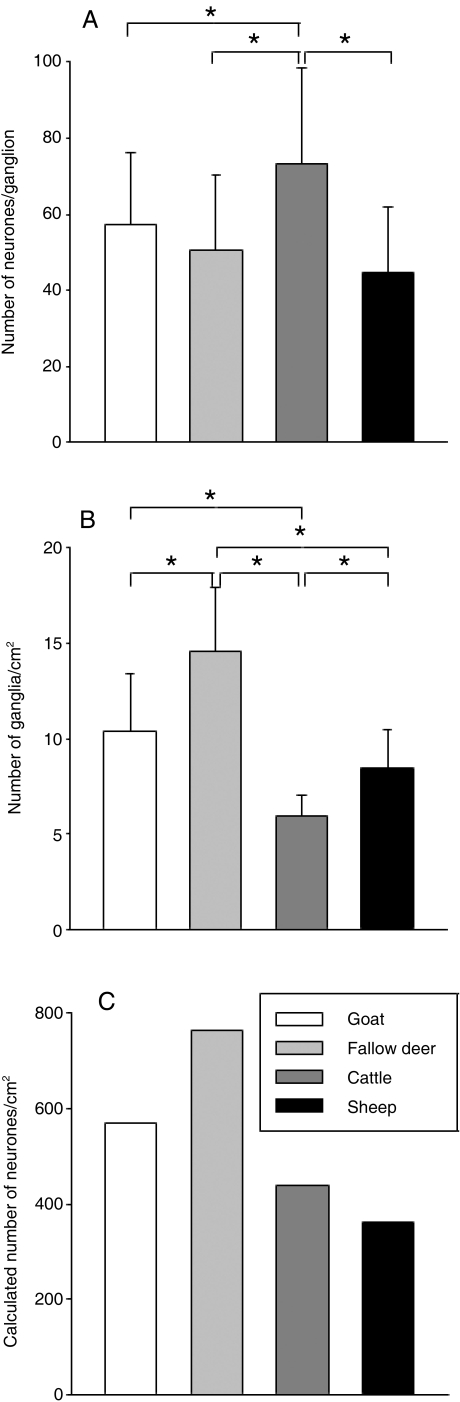
The antibodies used in this study strongly labelled the myenteric neurons and/or nerve fibres in all preparations analysed. Staining against HuC/D revealed a ganglionated myenteric plexus in all ruminant species examined. Ganglia of cattle contained more neurons than ganglia of the other species (Fig. 1). The number of ganglia per cm2 of myenteric plexus also differed between the species (Fig. 1). The density was highest in fallow deer and lowest in cattle. From the values for ganglion size and for number of ganglia per cm2, the innervation density (neurons per cm2) was calculated. The innervation density was highest in fallow deer and lowest in sheep (Fig. 1).
Fig. 1.
Ganglion size (A) and ganglion density (B) in the rumen of different species. *Asterisks in (A) and (B) indicate significant (P < 0.05) differences between species (one-way anova with subsequent Student–Newman–Keuls test). Data were obtained from 12–24 preparations from 8–15 animals. Columns in (C) are calculated from the respective mean values of columns in (A) and (B).
Cholinergic and nitrergic neurons
All myenteric neurons examined could be divided in two large populations (Fig. 2). One population was immunoreactive for ChAT. In the following sections, neurons immunoreactive for ChAT are referred to as cholinergic neurons. The other population was immunoreactive for NOS. Neurons immunoreactive for NOS could also be marked by NADPH-diaphorase staining (Fig. 3). The neurons that were immunoreactive for NOS or NADPH-diaphorase positive are referred to hereafter as nitrergic neurons.
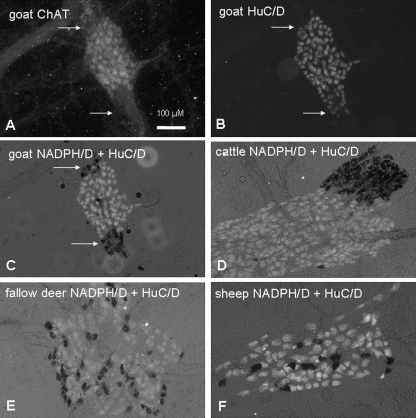
Fig. 2.
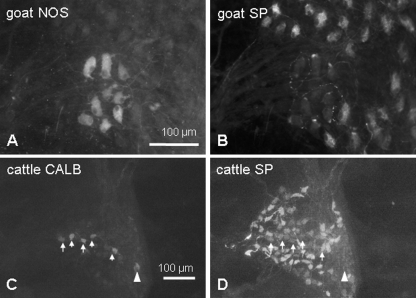
Cholinergic and nitrergic neurons in the rumen. Immunohistochemical detection of ChAT (A) revealed a subpopulation of all myenteric neurons in goat rumen stained against HuC/D. Neurons that were ChAT negative and HuC/D positive were labelled by NADPH-diaphorase reaction [dark stained neurons in (C), indicated by arrows]. NADPH/D-positive neurons were clustered at the margins of ganglia in goat (C) and cattle (D) or were equally distributed in the ganglia of fallow deer (E) and sheep (F).
Fig. 3.
NADPH-diaphorase reaction (A) and immunohistochemical staining against NOS (B) marked the same neurons.
In all species, the proportion of nitrergic neurons was significantly smaller than the proportion of cholinergic neurons (Table 1). In fallow deer, cattle and sheep the absolute number of nitrergic neurons per ganglion was also smaller than the number of cholinergic neurons per ganglion (Table 1).
Table 1.
Absolute number and relative proportion of cholinergic and nitrergic neurons in the rumen of different species
| Goats (n = 15; N = 9) | Fallow deer (n = 13; N = 8) | Cattle (n = 13; N = 9) | Sheep (n = 14; N = 9) | |
|---|---|---|---|---|
| Number of cholinergic neurons per ganglion | 28.8 ± 10.4a | 33.6 ± 13.3ab | 41.3 ± 15.5b | 32.6 ± 11.6ab |
| Number of nitrergic neurons per ganglion | 23.4 ± 5.3a | 20.4 ± 8.4ab | 23.7 ± 8.5a | 13.4 ± 5.3b |
| Proportion of cholinergic neurons (%) | 52.4 ± 11.3a | 61.6 ± 10.9b | 62.1 ± 13.3b | 71.5 ± 9.2c |
| Proportion of nitrergic neurons (%) | 44.2 ± 9.8a | 38.4 ± 10.2ab | 36.9 ± 11.2ab | 29.5 ± 8.2b |
Values with different letters in one row are significantly (P < 0.05) different (two-way anova with subsequent Student–Newman–Keuls test).
In fallow deer, cattle and sheep, the number of cholinergic neurons per ganglion was significantly (P < 0.05) larger than the number of nitrergic neurons per ganglion. In all species, the proportion of cholinergic neurons was significantly larger than the proportion of nitrergic neurons (two-way anova with subsequent Student–Newman–Keuls test).
n = number of tissues, N = number of animals.
Although in general there was a greater proportion of nitrergic neurons than cholinergic neurons, the ratio of the populations differed between the species. The largest amount of nitrergic neurons was found in goats (44.2 ± 9.8% of all myenteric neurons) and the smallest amount in sheep (29.5 ± 8.2% of all myenteric neurons, Table 1). Consequently, the relative amount of cholinergic neurons was largest in sheep and smallest in goats.
Not only the proportion but also the distribution of nitrergic neurons in the myenteric ganglia differed between the species. In goats and cattle, nitrergic neurons were clustered in groups at the margins of the ganglia. In contrast, in sheep and fallow deer the nitrergic neurons were distributed over the surface of the ganglia (Fig. 2).
Expression of neuropeptides
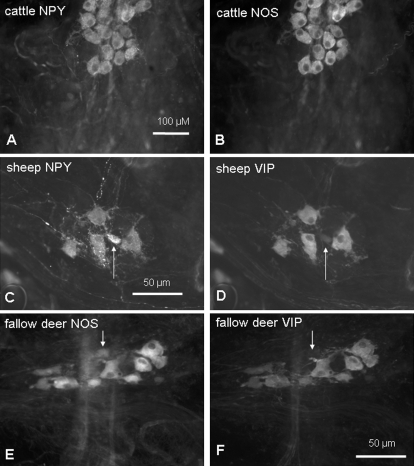
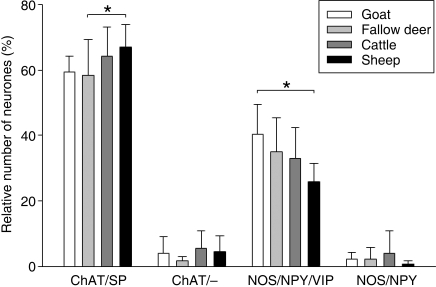
More than 99% of nitrergic neurons colocalized with NPY in all species (Fig. 4). The vast majority (> 97%) of the NOS/NPY immunoreactive neurons also expressed VIP immunoreactivity (Fig. 4). Therefore, two nitrergic populations could be defined: a large population referred to as NOS/NPY/VIP, and a very small population encoding NOS/NPY (Fig. 5). The relative number of neurons encoding NOS/NPY/VIP was larger in goats than in sheep, reflecting the differences found in the overall nitrergic population. The proportion of the NOS/NPY immunoreactive populations did not differ between the species (Fig. 5).
Fig. 4.
Complete colocalization of NOS and NPY in the rumen is shown in (A) and (B). The majority of NPY (C) and NOS (E) immunoreactive neurons colocalized with VIP (D,F). Only a small proportion of neurons [indicated by arrows in (C–F)] expressed immunoreactivity for NPY (C) or NOS (E), respectively, but not for VIP (D,F).
Fig. 5.
Proportion of neurochemically defined subpopulations in the rumen of different species. Within all four species, the proportion of the subpopulation was in descending order: ChAT/SP > NOS/NPY/VIP > ChAT/– = NOS/NPY (the significant differences between the relative sizes of the subpopulations are not indicated in the figure). *Asterisks indicate significant (P < 0.05) differences within one subpopulation between species (two-way anova with subsequent Student–Newman–Keuls test). Data were obtained from 5–11 preparations from 4–10 animals.
Cholinergic neurons did not express immunoreactivity for NPY or VIP, but the majority of cholinergic neurons were immunoreactive for SP (Figs 5, 6). Consequently, the largest population in all species had the code ChAT/SP (Fig. 5). The proportion of ChAT/SP immunoreactive neurons was larger in sheep than in fallow deer (Fig. 5). Some neurons were immunoreactive for ChAT but not for SP. The proportion of these ChAT/– encoded neurons did not differ between the species (Fig. 5).
Fig. 6.
Neurons immunoreactive for NOS (A) did not show immunoreactivity for SP (B) and vice versa. Consequently, SP immunoreactive neurons had a cholinergic phenotype. In cattle, the majority of CALB immunoreactive neurons [arrows in (C)] expressed SP [arrows in (D)]. One neuron immunoreactive for CALB but not for SP is marked by an arrowhead.
Expression of somatostatin and calbindin
Besides analysing the cholinergic or nitrergic phenotype and the expression of distinct neuropeptides, the immunoreactivity for somatostatin (SOM) and calbindin (CALB) in the myenteric neurons was evaluated.
CALB immunoreactivity of myenteric neurons could be detected in similar amounts in sheep (0.91 ± 0.52 neurons per ganglion), goats (1.04 ± 0.17 neurons per ganglion) and fallow deer (1.80 ± 0.61 neurons per ganglion). In contrast, the number of CALB-immunoreactive neurons in the myenteric ganglia of cattle was significantly higher (7.51 ± 4.20 neurons per ganglion) than in the other species examined. In all species, the vast majority of CALB-positive neurons expressed a cholinergic phenotype (goats: 93.0 ± 12.2% of CALB-positive neurons; fallow deer: 79.0 ± 20.8%; cattle: 96.7 ± 5.8%; sheep: 97.9 ± 3.6%). Immunoreactivity for SP was also detected (goats: 41.9 ± 10.3% of the ChAT/CALB population; fallow deer: 29.3 ± 18.4%; cattle: 62.1 ± 24.8%; sheep: 18.3 ± 20.2%, Fig. 6) in a subpopulation of the ChAT/CALB immunoreactive neurons.
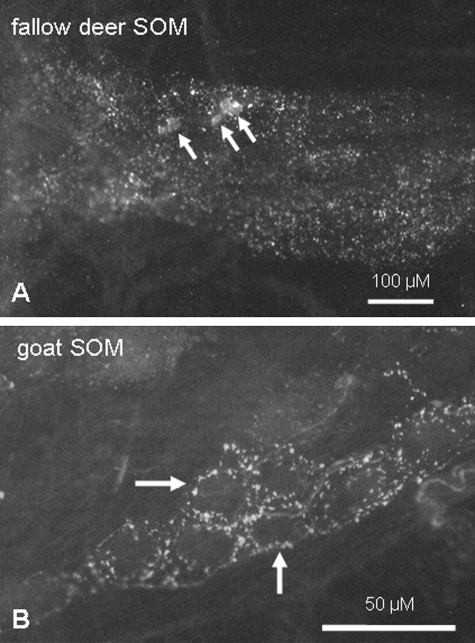
Somatostatin immunoreactive nerve fibres were found in myenteric plexus preparations of all species examined. In contrast, SOM-positive somata could only be detected in tissues from sheep (11.0 ± 5.0 neurons per ganglion) and fallow deer (4.1 ± 5.9 neurons per ganglion) but not in preparations from goats and cattle (Fig. 7). SOM-immunoreactive somata expressed a cholinergic phenotype (100% of SOM immunoreactive neurons in sheep; 99.2 ± 1.4% in fallow deer). Of the ChAT/SOM-positive neurons, most were also immunoreactive for SP (sheep: 86.1 ± 5.6% of the ChAT/SOM population; fallow deer: 79.4 ± 16.7%).
Fig. 7.
Immunoreactivity for SOM in the ruminal myenteric plexus of fallow deer (A) and goat (B). In fallow deer not only fibres but also neuronal somata were immunoreactive for SOM [arrows in (A)]. In goats, no SOM-positive neuronal somata but various fibres [marked by arrows in (B)] could be detected.
Discussion
The aim of the present study was to investigate whether the feeding type of ruminants is associated with distinct patterns of intrinsic innervation of the rumen. Our study revealed specific innervation patterns in the ruminal myenteric plexus in the four ruminant species examined. Most differences between the species appeared to be correlated with the feeding types and/or with body size of the different species.
The general innervation patterns described by the parameters (1) number of neurons per ganglion, (2) ganglia per cm2 and (3) calculated number of neurons per cm2 seem to depend on both body size and feeding type.
A positive correlation with body size was found particularly for the average number of neurons per ganglion. This value was higher in cattle than in the other ruminant species. This size dependency of ganglion properties confirms the results of Gabella (1987), who demonstrated a positive correlation between body size and the number of neurons in myenteric ganglia by comparing small intestines of mouse, guinea pig and sheep.
Innervation density is not only determined by size of ganglia but also by other factors such as the number of ganglia per cm2 or axonal sprouting. Regarding the number of ganglia per cm2, the study by Gabella (1987) showed a negative correlation between body size and the number of myenteric ganglia per cm2. Comparing cattle with the three smaller ruminant species we could confirm the observation of Gabella (1987). The number of myenteric ganglia per cm2 in the rumen of cattle was lower than in the rumen of the three other ruminant species examined.
From the mean values of (1) the number of neurons per ganglion and (2) ganglia per cm2, the innervation density (= number of neurons per cm2) was calculated. The innervation density was not correlated with body size but was correlated with the feeding type of the different species. The number of neurons per cm2 was highest in the rumen of both intermediate-type species. This high innervation density might be related to lower retention times for particles and higher turnover rates of undigested residues in intermediate types compared with grazers (Huston et al. 1986). Huston et al. (1986) showed that the reticuloruminal turnover of undigested particles was nearly twice as high in goats compared with sheep. A high turnover, together with a low retention time, requires effective transit through the reticulorumen, possibly facilitated by a dense intrinsic innervation.
In addition to the innervation density, the expression of excitatory and inhibitory neurotransmitters in the myenteric neurons was correlated with feeding type. Regarding the control of ruminal smooth muscle activity, acetylcholine and SP are considered to be neurotransmitters that act in an excitatory manner. This assumption is supported by the observations that (1) neurons expressing ChAT and SP directly innervate the forestomach muscle layers and not the epithelium (Pfannkuche et al. 2002, 2004b) and (2) both acetylcholine and SP evoke contractions of the isolated ruminal muscle (Vassileva et al. 1978; Veenendaal et al. 1982; Wong & McLeay, 1988).
Not only neurons encoding ChAT/SP but also neurons immunoreactive for NOS and VIP have been found to project to the circular and longitudinal muscle layers in the sheep ruminal wall (Pfannkuche et al. 2002, 2004b). In contrast to ACh and SP, NOS and VIP have been found to relax the smooth muscle layers in different parts of the ovine forestomach (Denac et al. 1987; Schneider & Eades, 1998). Therefore a function of NOS/VIP/NPY-positive neurons as inhibitory motor neurons can be suggested for the ruminant forestomach.
With regard to the relative amount of (presumably) excitatory ChAT/SP and (presumably) inhibitory NOS/VIP/NPY immunoreactive neurons, we found the most obvious differences between sheep (grazers) and goats (belonging to the intermediate type). In sheep, the ChAT/SP immunoreactive neurons outnumbered the NOS/VIP/NPY-positive neurons by 2.6 to 1, whereas the proportion was 1.5 to 1 in goats. Consequently, the ovine ruminal muscle may be under stronger excitatory control than the caprine ruminal muscle. Sheep are able to digest lower quality roughage than goats and fallow deer (Hofmann, 1989). Microbial fermentation of this feed requires strong mixing contractions. It can be hypothesized that these contractions have to be stronger in sheep than in intermediate-type species such as goats because of the different mechanical properties of their feed.
Fallow deer belong to the intermediate type but they show tendencies similar to those of grazers (Hofmann, 1989). Consequently, in this species the ratio of ChAT/SP immunoreactive neurons to NOS/VIP/NPY immunoreactive neurons (1.7 : 1) was between those of sheep and goats, as expected.
In cattle, one would expect the relative amounts of ChAT/SP and NOS/VIP/NPY neurons to be comparable to those in sheep because their physiological and anatomical properties are similar. However, in cattle we found a ratio of ChAT/SP immunoreactive neurons to NOS/VIP/NPY immunoreactive neurons of 2.0 : 1. This unexpected proportion may be attributable to the fact that tissues were taken from Holstein–Friesian cattle in this study. This breed is primarily used for milk production, and high-yielding dairy cows require a high energy intake relative to concentrate selectors or intermediate types. Intake of high dietary energy levels may modify the proportion of cholinergic and nitrergic neural populations in the myenteric plexus in such a way that the relative amount of nitrergic neurons increases because of a selective loss of cholinergic neurons. Such a diet-related loss of cholinergic neurons has already been shown in monogastric animals (Cowen et al. 2000; Phillips & Powley, 2007).
In all species examined, a small subpopulation of cholinergic neurons expressed immunoreactivity for CALB. The expression of CALB was not related to the feeding type, but to the body size, with the highest expression found in cattle. Not only the number of CALB immunoreactive neurons but also the co-expression of SP in these neurons differed between the species. In the sheep rumen, more than 80% of CALB immunoreactive neurons were SP negative. This finding is in accordance with results from previous studies in sheep (Pfannkuche et al. 2004a). In the rumen of goats and fallow deer, the majority of CALB-positive neurons were also SP negative. Only in cattle was the population of ChAT/CALB/SP immunoreactive neurons larger than the population immunoreactive for CALB and ChAT alone.
Regarding the putative function of CALB immunoreactive neurons, variations between regions and species have been demonstrated repeatedly. In the guinea pig stomach and rabbit ileum, CALB-positive neurons belong to the functional population of interneurons (Reiche et al. 1999; Dénes & Gábriel, 2004). In contrast, CALB-positive neurons are classified as intrinsic primary afferent neurons in the guinea pig small and large intestine (Furness et al. 1998; Neunlist et al. 1999; Brookes, 2001).
In ruminants the function of the CALB immunoreactive neurons has not yet been clearly demonstrated. Some studies point to afferent innervation of the ruminal epithelium by CALB-expressing neurons. In this regard, Lee & Nam (2006) suggested that CALB immunoreactive neurons were intrinsic primary afferent neurons in the goat forestomach. In cattle, SP immunoreactive neurons were thought to have intrinsic afferent functions (Kitamura et al. 1993). This is consistent with our finding that in cattle the majority of CALB immunoreactive neurons co-localize with SP. We have previously suggested that CALB-positive neurons may project to the ovine ruminal epithelium, although these neurons did not have morphological characteristics of intrinsic afferent neurons (Pfannkuche et al. 2004a). However, it has to be kept in mind that in monogastric animals no population of gastric myenteric neurons resembles the immunohistochemical, morphological and electrophysiological properties of intrinsic afferent neurons in the guinea pig intestine (Schemann & Wood, 1989a,b; Brookes et al. 1998; Van Nassauw et al. 2005). Therefore it is possible that intrinsic afferent neurons of the forestomach express CALB but show unknown, forestomach-specific, morphological and electrophysiological characteristics.
In contrast to all findings discussed in the previous sections, the expression of SOM seemed not to be related either to feeding type or to body size.
SOM immunoreactive myenteric neurons were found in sheep and fallow deer. These neurons had the code ChAT/SP/SOM. In goats and cattle, SOM immunoreactive fibres, but no neuronal somata, were detected.
The function of the SOM immunoreactive neurons remains speculative. Vergara-Esteras et al. (1990) demonstrated SOM immunoreactive fibres in both the myenteric plexus and the muscle layers of the ovine rumen. The findings imply that ChAT/SP/SOM encoded neurons project to the ruminal muscles. Supporting this view, ChAT/SP/SOM immunoreactive neurons have been suggested to be excitatory motoneurons in the guinea pig stomach (Schemann et al. 1995). However, the code ChAT/SP/SOM is not highly conserved between region and species. This combination of neurotransmitters is absent from the guinea pig ileum, for example. In the human small intestine, ChAT/SP/SOM encoded neurons can be detected, but this neuronal population has been classified as sensory neurons (Brehmer et al. 2004).
In our study, we found neurochemically different populations of myenteric neurons in the rumen of goats, fallow deer, cattle and sheep. Apart from SOM immunoreactivity, all populations could be detected in all ruminant species. This leads to the conclusion that the basal circuits in the myenteric plexus controlling ruminal functions are conserved between ruminants of different feeding types. However, the relative amounts of putative inhibitory and excitatory myenteric neurons differed between the species. From this finding it is suggested that the rumen of grazers is under a stronger excitatory control than the rumen of intermediate type species. Whether the strong excitatory input to the ruminal muscle of grazers is a prerequisite for the control of strong mixing patterns during ruminal fermentation remains speculative. The functional significance of our findings should be analysed in further studies.
Acknowledgments
We thank Prof. Dr Michael Schemann for kindly providing us with the anti-ChAT-antibodies. We also thank Greg Penner for linguistic corrections.
The skilful technical assistance of Petra Philipp is gratefully acknowledged.
References
- Brehmer A, Croner R, Dimmler A, Papadopoulos T, Schrodl F, Neuhuber W. Immunohistochemical characterization of putative primary afferent (sensory) myenteric neurons in human small intestine. Auton Neurosci. 2004;112:49–59. doi: 10.1016/j.autneu.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Brookes SJ. Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec. 2001;262:58–70. doi: 10.1002/1097-0185(20010101)262:1<58::AID-AR1011>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Brookes SJ, Hennig G, Schemann M. Identification of motor neurons to the circular muscle of the guinea pig gastric corpus. J Comp Neurol. 1998;397:268–280. doi: 10.1002/(sici)1096-9861(19980727)397:2<268::aid-cne8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Cowen T, Johnson RJ, Soubeyre V, Santer RM. Restricted diet rescues rat enteric motor neurones from age related cell death. Gut. 2000;47:653–660. doi: 10.1136/gut.47.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denac M, Marti J, Scharrer E. Relaxation of ruminal smooth muscle by vasoactive intestinal polypeptide (VIP) Zentralbl Veterinarmed A. 1987;34:317–320. doi: 10.1111/j.1439-0442.1987.tb00287.x. [DOI] [PubMed] [Google Scholar]
- Dénes V, Gábriel R. Calbindin-immunopositive cells are cholinergic interneurons in the myenteric plexus of rabbit ileum. Cell Tissue Res. 2004;318:465–472. doi: 10.1007/s00441-004-0931-5. [DOI] [PubMed] [Google Scholar]
- Franco AJ, Masot AJ, Aguado MC, Gomez L, Redondo E. Morphometric and immunohistochemical study of the rumen of red deer during prenatal development. J Anat. 2004;204:501–513. doi: 10.1111/j.0021-8782.2004.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB, Kunze WA, Bertrand PP, Clerc N, Bornstein JC. Intrinsic primary afferent neurons of the intestine. Prog Neurobiol. 1998;54:1–18. doi: 10.1016/s0301-0082(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Gabella G. The number of neurons in the small intestine of mice, guinea-pigs and sheep. Neuroscience. 1987;22:737–752. doi: 10.1016/0306-4522(87)90369-1. [DOI] [PubMed] [Google Scholar]
- Groenewald HB. Neuropeptides in the myenteric ganglia and nerve fibres of the forestomach and abomasum of grey, white and black Karakul lambs. Onderstepoort J Vet Res. 1994;61:207–213. [PubMed] [Google Scholar]
- Hofmann RR. Evolutionary steps of ecophysiological adaptation and diversification of ruminants: a comparative view of their digestive system. Oecologia. 1989;78:443–457. doi: 10.1007/BF00378733. [DOI] [PubMed] [Google Scholar]
- Huston JE, Rector BS, Ellis WC, Allen ML. Dynamics of digestion in cattle, sheep, goats and deer. J Anim Sci. 1986;62:208–215. doi: 10.2527/jas1986.621208x. [DOI] [PubMed] [Google Scholar]
- Kitamura N, Yamada J, Yamamoto Y, Yamashita T. Substance P-immunoreactive neurons of the bovine forestomach mucosa: their presumptive role in a sensory mechanism. Arch Histol Cytol. 1993;56:399–410. doi: 10.1679/aohc.56.399. [DOI] [PubMed] [Google Scholar]
- Kitamura N, Yamada J, Yamashita T. Immunohistochemical study on the distribution of neuron-specific enolase- and peptide-containing nerves in the reticulorumen and the reticular groove of cattle. J Comp Neurol. 1986;248:223–234. doi: 10.1002/cne.902480205. [DOI] [PubMed] [Google Scholar]
- Lee HS, Nam YS. Immunohistochemical localization of calcium binding proteins and some neurotransmitters in myenteric plexus of goat stomach. J Vet Sci. 2006;7:315–319. doi: 10.4142/jvs.2006.7.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunlist M, Dobreva G, Schemann M. Characteristics of mucosally projecting myenteric neurones in the guinea-pig proximal colon. J Physiol. 1999;517:533–546. doi: 10.1111/j.1469-7793.1999.0533t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannkuche H, Reiche D, Sann H, Schemann M. Different subpopulations of cholinergic and nitrergic myenteric neurones project to mucosa and circular muscle of the guinea-pig gastric fundus. Cell Tissue Res. 1998;292:463–475. doi: 10.1007/s004410051075. [DOI] [PubMed] [Google Scholar]
- Pfannkuche H, Schellhorn C, Schemann M, Aschenbach JR, Gabel G. Age-associated plasticity in the intrinsic innervation of the ovine rumen. J Anat. 2003a;203:277–282. doi: 10.1046/j.1469-7580.2003.00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannkuche H, Schellhorn C, Schemann M, Gabel G. Reticular groove and reticulum are innervated by myenteric neurons with different neurochemical codes. Anat Rec A Discov Mol Cell Evol Biol. 2003b;274:917–922. doi: 10.1002/ar.a.10104. [DOI] [PubMed] [Google Scholar]
- Pfannkuche H, Schellhorn C, Schemann M, Gabel G. Calbindin-immunoreactive neurones in the ovine rumen. Anat Rec A Discov Mol Cell Evol Biol. 2004a;278:528–532. doi: 10.1002/ar.a.20048. [DOI] [PubMed] [Google Scholar]
- Pfannkuche H, Schellhorn C, Schemann M, Gabel G. Intrinsic innervation patterns of the smooth muscle in the rumen and reticulum of lambs. J Anat. 2004b;204:293–299. doi: 10.1111/j.0021-8782.2004.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannkuche H, Schemann M, Gabel G. Ruminal muscle of sheep is innervated by non-polarized pathways of cholinergic and nitrergic myenteric neurones. Cell Tissue Res. 2002;309:347–354. doi: 10.1007/s00441-002-0554-7. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Innervation of the gastrointestinal tract: patterns of aging. Auton Neurosci. 2007;136:1–19. doi: 10.1016/j.autneu.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiche D, Pfannkuche H, Michel K, Hoppe S, Schemann M. Immunohistochemical evidence for the presence of calbindin containing neurones in the myenteric plexus of the guinea-pig stomach. Neurosci Let. 1999;270:71–74. doi: 10.1016/s0304-3940(99)00471-1. [DOI] [PubMed] [Google Scholar]
- Ruckebusch Y. Gastrointestinal motor functions in ruminants. In: Schultz SG, Wood JD, Rauner BB, editors. Handbook of Physiology, vol. 1. The Gastrointestinal System. New York: Oxford University Press; 1989. pp. 1225–1283. [Google Scholar]
- Schemann M, Wood JD. Electrical behaviour of myenteric neurones in the gastric corpus of the guinea-pig. J Physiol. 1989a;417:501–518. doi: 10.1113/jphysiol.1989.sp017815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schemann M, Wood JD. Synaptic behaviour of myenteric neurones in the gastric corpus of the guinea-pig. J Physiol. 1989b;417:519–535. doi: 10.1113/jphysiol.1989.sp017816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schemann M, Sann H, Schaaf C, Mader M. Identification of cholinergic neurons in enteric nervous system by antibodies against choline acetyltransferase. Am J Physiol. 1993;265:G1005–G1009. doi: 10.1152/ajpgi.1993.265.5.G1005. [DOI] [PubMed] [Google Scholar]
- Schemann M, Schaaf C, Mader M. Neurochemical coding of enteric neurons in the guinea pig stomach. J Comp Neurol. 1995;353:161–178. doi: 10.1002/cne.903530202. [DOI] [PubMed] [Google Scholar]
- Schneider DA, Eades SC. Antagonist of nitric oxide synthesis inhibits nerve-mediated relaxation of isolated strips of rumen and reticulum. J Dairy Sci. 1998;81:2588–2594. doi: 10.3168/jds.S0022-0302(98)75816-3. [DOI] [PubMed] [Google Scholar]
- Van Nassauw L, Wu M, De Jonge F, Adriaensen D, Timmermans JP. Cytoplasmic, but not nuclear, expression of the neuronal nuclei (NeuN) antibody is an exclusive feature of Dogiel type II neurons in the guinea-pig gastrointestinal tract. Histochem Cell Biol. 2005;124:369–377. doi: 10.1007/s00418-005-0019-7. [DOI] [PubMed] [Google Scholar]
- Vassileva P, Stoyanov I, Loukanov Y. Neurotransmitted responses of smooth-muscle strips of complex sheep stomach after electrical field stimulation. Acta Physiol Pharmacol Bulg. 1978;4:11–18. [PubMed] [Google Scholar]
- Veenendaal GH, Woutersen-van Nijnanten FM, Van Miert AS. Responses of goat ruminal musculature to substance P in vitro and in vivo. Vet Res Commun. 1982;5:363–367. doi: 10.1007/BF02215006. [DOI] [PubMed] [Google Scholar]
- Vergara-Esteras P, Harrison FA, Brown D. The localization of somatostatin-like immunoreactivity in the alimentary tract of the sheep with observations on the effect of an infection with the parasite Haemonchus contortus. Exp Physiol. 1990;75:779–789. doi: 10.1113/expphysiol.1990.sp003460. [DOI] [PubMed] [Google Scholar]
- Wong MH, McLeay LM. In vitro spontaneous motility of gastric smooth muscles of the sheep. Q J Exp Physiol. 1988;73:521–531. doi: 10.1113/expphysiol.1988.sp003172. [DOI] [PubMed] [Google Scholar]