Abstract
We provide quantitative muscle–tendon architecture and geometry data for the racing greyhound thoracic limb. Muscle mass, belly length, fascicle lengths, pennation angles and moment arms were measured, as were tendon masses and lengths. Maximum isometric force and maximum power were estimated for muscles, and maximum stress and strain were estimated for tendons. Results are compared with other fast quadrupedal runners, and to previously published data in mixed-breed dogs. The implications of the functional adaptations of the greyhound thoracic limb for sprinting performance are discussed. The thoracic limb was found to benefit from a similar proportion of locomotor muscle mass to the pelvic limb, suggesting that it may be used to some extent in propulsion, or alternatively that stabilisation is very important in this animal. Extrinsic muscles, especially latissimus dorsi and pectoralis profundus, were predicted to be powerful and important for generating net positive work during accelerations. Proximal biarticular muscles show specialisation toward preventing collapse of the shoulder and elbow joints to enable strut-like limb function, or some form of dynamic control. Distal muscles did not appear specialised for elastic energy storage, a functional difference to pelvic limb muscles, and the equivalents in horse thoracic limbs. The greyhound thoracic limb appears to possess substantial differences from both that of more ‘sub-maximal specialist’ quadrupeds, and from the greyhound pelvic limb.
Keywords: architecture, biomechanics, greyhound, locomotion, moment arms, muscle, sprinting, tendon
Introduction
Quadrupeds exhibit differential leg function during high-speed gaits and non steady-state locomotion. Both fore- and hind limbs are capable of creating accelerating and decelerating fore–aft impulses (Lee et al. 2004; Walter & Carrier, 2007). During galloping and bounding, the forelimbs of dogs and horses are reported to produce primarily decelerating forces, whereas the hind limbs accelerate the body (Cavagna et al. 1977; Heglund et al. 1982; Gregersen et al. 1998). Equally, when accelerating, the hind limbs of dogs generate more acceleratory force than the forelimbs (Williams et al. under review).
Little is known about how the division of labour between fore- and hind limbs is split during high-speed unsteady locomotor tasks, such as maximal accelerations. Investigation into the muscle–tendon architecture of various quadrupeds has allowed insight into how the functional roles of the thoracic and pelvic limbs may differ (Payne et al. 2005a; Smith et al. 2006), and how any differences in limb function might be achieved. Studies in horses suggest the forelimb plays an important role in weight support, in decelerating the body and in elastic energy storage and release (Merkens et al. 1993; Payne et al. 2005a,b). Previously (Williams et al. 2007b) we have suggested that the forelimb of the European hare (Lepus europeus) is more ‘strut-like’, with little potential for elastic energy storage. It is likely its role is simply to support and deflect the body during high-speed running, whilst the pelvic limb produces the majority of the acceleratory impulse (Williams et al. 2007a). The hare appears to have maintained a far more generalised thoracic limb structure, presumably a reflection on its lifestyle and the need to use the thoracic limbs for non-locomotor functions such as digging and manipulation. The horse shows a far more extreme specialisation in terms of limb construction, with a marked reduction in distal limb elements and restricted motion in the sagittal plane. It remains to be seen whether the greyhound is an ‘intermediate’ in terms of limb anatomical specialisation, or whether as an animal selectively bred for high-speed sprinting, it shows specific adaptations/specializations for this role.
A previous study has quantified thoracic limb architecture in four mixed-breed dogs (Shahar & Milgram, 2005). This study aims to further that work by evaluating thoracic limb muscle–tendon architecture and geometry in the racing greyhound (an example of an animal ‘designed’ for high-speed galloping). This work illuminates the role of the thoracic limb in high-speed sprinting.
Materials and methods
The experimental protocol was identical to that which is described in full in elsewhere (Williams et al. 2008). In brief, one forelimb of each of seven mature (young) racing greyhounds (mass 31.4 ± 1.1 kg; all data means ± SD) was dissected to obtain thoracic limb muscle–tendon architectural measurements, including muscle and fascicle lengths, muscle and tendon mass, tendon length, and muscle pennation angle. From these measurements, muscle physiological cross-sectional area (PCSA) and tendon cross-sectional area (CSA) were calculated, and estimates were made for various muscle and tendon properties, including maximum isometric muscle force (Fmax), maximum instantaneous muscle power, tendon stress, strain and length change at Fmax. Architectural Index (AI) was calculated and compared with that found in mixed-breed dogs (Shahar & Milgram, 2005); however, muscle masses could not be compared as no animal masses were given in that study, and thus it was not possible to normalise for differences in body size. The contralateral forelimbs of four of the subjects were used to measure muscle moment arms of selected forelimb muscles via the tendon travel method (Spoor & van Leeuwen, 1992).
Results
Distribution of muscle mass
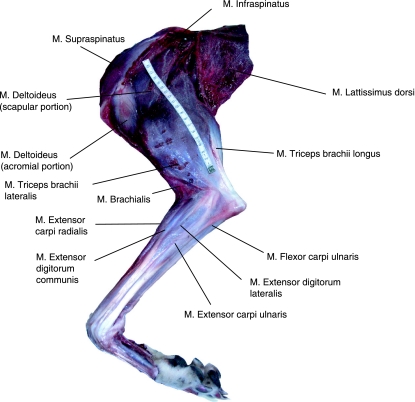
Thirty-six significant thoracic limb muscles were identified and assessed in this study; locations of superficial muscles (excluding extrinsic musculature) within the limb are indicated in Fig. 1. Abbreviations can be found in Table 1. The total muscle mass associated with the thoracic limb was 2913 ± 428 g, accounting for 9.3 ± 1.4% of total body mass. The heaviest muscle in the thoracic limb was pectoralis profundus (400 ± 27 g), followed by the long head of triceps brachii (341 ± 41 g). Distal muscles were lightest [e.g. extensor digitorum lateralis (EDLA) – 3.6 ± 6.0 g; pronator teres – 5.1 ± 1.6 g]. Muscle mass data can be found in Table 1.
Fig. 1.
Superficial musculature of the lateral aspect of the greyhound thoracic limb. M. trapezius has been removed.
Table 1.
Muscle data: muscle mass, belly length, fascicle length, physiological cross-sectional area (PCSA), pennation angle, and estimated maximum isometric force and power. Values obtained for muscle force and power were obtained as per methods described in (Williams et al. 2008). Values indicate mean and SD (n = 7)
| Muscle | Abbreviation | Muscle mass (g) | Belly length (cm) | Fascicle length (cm) | PCSA (cm2) | Pennation angle (°) | Fmax (N) | Corrected Fmax (N) | Power (W) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trapezius cervicis | TPC | 38.5 | (18.5) | 17.6 | (1.9) | 14.6 | (2.7) | 2.5 | 0 | (0) | 75 | 75 | 4 |
| Trapezius thoracis | TPT | 58.8 | (23.2) | 18.2 | (1.2) | 9.6 | (0.9) | 5.8 | 0 | (0) | 174 | 173 | 6 |
| Rhomboideus | RH | 90.5 | (13.3) | 33.4 | (2.4) | 24.3 | (3.3) | 3.5 | 0 | (0) | 105 | 105 | 10 |
| Serratus ventralis cervicis | SVC | 157 | (25.8) | 17.7 | (1.4) | 4.8 | (0.4) | 30.7 | 10 | (0) | 921 | 912 | 17 |
| Serratus ventralis thoracis | SVT | 120 | (8.0) | 13.2 | (2.7) | 4.6 | (0.4) | 24.6 | 10 | (0) | 737 | 727 | 13 |
| Brachiocephalicus | BCH | 91.7 | (11.1) | 46.5 | (2.4) | 35.7 | (2.2) | 2.4 | 0 | (0) | 73 | 73 | 5 |
| Omotransversarius | OMO | 56.8 | (3.8) | 26.1 | (0.6) | 24.1 | (1.2) | 2.2 | 0 | (0) | 67 | 67 | 6 |
| Lattisimus dorsi | LD | 325 | (31.7) | 39.5 | (3.9) | 40.3 | (1.4) | 7.6 | 0 | (0) | 228 | 228 | 35 |
| Pectoralis descedens | PD | 48.8 | (17.0) | 13.0 | (1.1) | 14.3 | (0.7) | 3.2 | 0 | (0) | 97 | 97 | 5 |
| Pectoralis profundus | PP | 400 | (26.7) | 40.5 | (4.9) | 31.8 | (1.1) | 11.8 | 0 | (0) | 355 | 356 | 43 |
| Pectoralis transversarius | PTV | 94.8 | (4.8) | 14.9 | (2.4) | 11.2 | (0.7) | 8.0 | 0 | (0) | 239 | 240 | 10 |
| Subscapularis | SS | 85.3 | (5.5) | 15.0 | (0.7) | 3.5 | (0.2) | 23.3 | 22 | (8) | 700 | 640 | 9 |
| Supraspinatous | SSP | 150 | (8.0) | 21.2 | (1.7) | 5.9 | (0.8) | 23.8 | 18 | (15) | 715 | 684 | 16 |
| Infraspinatus | ISP | 114 | (10.0) | 19.0 | (2.0) | 3.9 | (0.7) | 27.4 | 29 | (13) | 823 | 724 | 12 |
| Deltoideus | |||||||||||||
| – Acromial portion | DA | 28.7 | (3.5) | 8.7 | (1) | 8.3 | (1) | 3.3 | 38 | (15) | 98 | 77 | 3 |
| – Scapular portion | DS | 49.9 | (16.9) | 15.4 | (1.9) | 7.6 | (1.9) | 6.2 | 0 | (0) | 185 | 186 | 1 |
| Biceps brachii | BB | 54.1 | (15.7) | 18 | (2.4) | 1.8 | (0.3) | 28.4 | 41 | (12) | 853 | 642 | 3 |
| Brachialis | BRA | 23.9 | (4.9) | 16.6 | (1.8) | 4.2 | (1) | 5.4 | 15 | (7) | 161 | 156 | 10 |
| Corcobrachialis | CB | 43.7 | (8.7) | 9.3 | (0.4) | 2.7 | (0.5) | 15.3 | 30 | (15) | 458 | 397 | 3 |
| Teres major | TMJ | 56.2 | (8.8) | 15.9 | (0.7) | 14.8 | (0.5) | 3.6 | 9 | (4) | 107 | 106 | 6 |
| Teres minor | TMN | 41.7 | (12.4) | 8.80 | (1.5) | 1.9 | (0.2) | 21.2 | 32 | (23) | 637 | 527 | 5 |
| Triceps brachii | |||||||||||||
| – lateral head | TBLA | 129 | (14.5) | 20.9 | (1.6) | 10.4 | (0.3) | 11.7 | 10 | (10) | 289 | 246 | 9 |
| – long head | TBLO | 341 | (41.1) | 22.4 | (1.6) | 6.5 | (0.4) | 49.2 | 31 | (12) | 1475 | 1273 | 58 |
| – medial head | TBM | 81.9 | (30.5) | 21.0 | (2.8) | 8.0 | (2.8) | 9.6 | 28 | (16) | 104 | 102 | 5 |
| – accessory head | TBA | 42.1 | (16.5) | 19.5 | (2.9) | 11.5 | (0.7) | 3.5 | 11 | (13) | 255 | 255 | 2 |
| Anconeus | AN | 6.3 | (2.6) | 9.4 | (0.5) | 1.6 | (0.3) | 3.7 | 10 | (7) | 112 | 110 | 6 |
| Extensor carpi radialis | ECR | 33.3 | (6.6) | 13.5 | (1.3) | 3.2 | (0.4) | 9.7 | 37 | (14) | 292 | 235 | 4 |
| Extensor carpi ulnaris | ECU | 9.4 | (1.0) | 14.6 | (1.9) | 1.6 | (0.2) | 5.7 | 20 | (6) | 171 | 156 | 1 |
| Extensor digitorum communis | EDC | 11.9 | (2.3) | 16.2 | (1.5) | 2.7 | (0.3) | 4.2 | 37 | (17) | 126 | 100 | 1 |
| Extensor digitorum lateralis | EDLA | 3.8 | (6.0) | 16.1 | (0.9) | 0.9 | (0.2) | 3.8 | 41 | (12) | 114 | 90 | 0.4 |
| Abductor pollicis longus | APL | 19.5 | (1.4) | 14.6 | (0.7) | 2.2 | (0.4) | 8.5 | 30 | (12) | 255 | 217 | 1 |
| Pronator teres | PT | 5.1 | (1.6) | 8.70 | (1.1) | 1.3 | (0.1) | 3.6 | 20 | (5) | 109 | 104 | 0.6 |
| Flexor carpi radialis | FCR | 9.6 | (1.8) | 14.6 | (0.7) | 2.5 | (0.3) | 3.6 | 15 | (10) | 107 | 105 | 1 |
| Flexor carpi ulnaris | FCU | 26.4 | (6.9) | 22.3 | (4.8) | 1.3 | (0.3) | 18.8 | 55 | (12) | 564 | 330 | 3 |
| Flexor digitorum profundus | FDP | 46.9 | (14.5) | 21.0 | (1.2) | 1.3 | (0.2) | 34.5 | 29 | (12) | 1034 | 893 | 5 |
| Flexor digitorum superficialis | FDS | 18.3 | (2.9) | 20.5 | (1.6) | 1.2 | (0.2) | 14.6 | 41 | (14) | 439 | 326 | 2 |
Muscle–tendon architecture
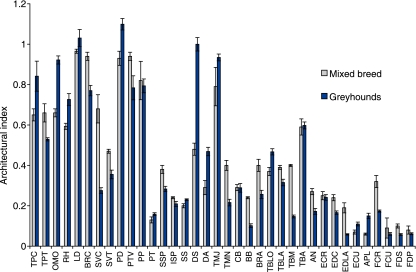
Muscle architecture data are given in Table 1 and tendon data in Table 2. Extrinsic muscles, with the exception of serratus ventralis cervicis (SVC) and thoracis (SVT) tended to have long parallel fascicles and high AIs (Fig. 2). These had little force generating capacity but some were predicted to be capable of producing large amounts of power (pectoralis profundus, 43 W; lattisimus dorsi, 35 W). Lowest AIs were found in the distal muscles [EDLA, flexor carpi ulnaris (FCU), flexor digitorum superficialis (FDS), flexor digitorum profundus (FDP)]; however, low AIs were also found in some relatively proximal muscles such as biceps brachii (0.1), infraspinatus (0.2) and medial head of triceps brachii (0.1). These muscles were able to produce large amounts of force. Figure 2 compares AIs from racing greyhounds with those from mixed-breed dogs (data taken directly from Shahar & Milgrim, 2005). Differences in AI are seen in most muscles. The longest tendons were the digital extensor tendons, EDLA and extensor digitorum communis (EDC) (23.7 ± 2.1 cm and 26.2 ± 3.2 cm). These also had the smallest cross-sectional areas (0.05 cm2 and 0.04 cm2), and undergo the largest estimated passive length changes (4.4 and 4.5 mm).
Table 2.
Tendon data: mass, volume, and resting length, and calculated cross-sectional area (CSA), stress, strain, and length change at Fmax of selected thoracic limb tendons. Estimated parameters calculated using methods described briefly in the text and in (Williams et al. 2008). Data are means (n = 7). Values in brackets are ± SD
| Muscle tendon unit | Mass (g) | Volume (cm3) | Rest length (cm) | CSA (cm2) | Stress (MPa) | Strain (%) | Length change (cm) | ||
|---|---|---|---|---|---|---|---|---|---|
| Infraspinatus | 0.1 | (0.38) | 0.1 | 1.3 | (0.8) | 0.09 | 91 | 6.1 | 0.8 |
| Triceps brachii (long head) | 3.1 | (0.22) | 2.6 | 5.5 | (0.4) | 0.47 | 6 | 0.4 | 0.2 |
| Biceps brachii | 0.9 | (0.23) | 0.8 | 2.6 | (0.4) | 0.28 | 30 | 2 | 0.5 |
| Extensor carpi radialis | 2 | (0.21) | 1.7 | 14.5 | (0.7) | 0.12 | 24 | 1.6 | 2.4 |
| Extensor carpi ulnaris | 0.7 | (0.26) | 0.6 | 10.4 | (0.8) | 0.06 | 29 | 1.9 | 2 |
| Extensor digitorum communis | 0.7 | (0.49) | 0.6 | 26.2 | (3.2) | 0.02 | 25 | 1.7 | 4.4 |
| Extensor digitorum lateralis | 1.3 | (0.49) | 1.1 | 23.7 | (2.1) | 0.04 | 29 | 1.9 | 4.5 |
| Abductor pollicis longus | 1 | (0.01) | 0.8 | 4.6 | (0.4) | 0.18 | 14 | 0.9 | 0.4 |
| Flexor carpi radialis | 2.2 | (0.23) | 1.7 | 15.5 | (0.4) | 0.11 | 10 | 0.6 | 1 |
| Flexor carpi ulnaris | 1.8 | (0.29) | 1.5 | 7.7 | (2.2) | 0.19 | 30 | 2 | 1.5 |
| Flexor digitorum profundus | 4.9 | (0.3) | 4 | 15.2 | (2.3) | 0.26 | 40 | 2.7 | 4 |
| Flexor digitorum superficialis | 1.5 | (1.04) | 1.2 | 11 | (0.6) | 0.11 | 40 | 2.7 | 2.9 |
Fig. 2.
Architectural Index (AI) for muscles of the thoracic limb. Bars represent mean ± SE. Dark bars indicate racing greyhound AI from this study (n = 7), pale bars show data from mixed-breed dogs (Shahar & Milgrim, 2004; n = 4).
Muscle moment arms at the shoulder joint
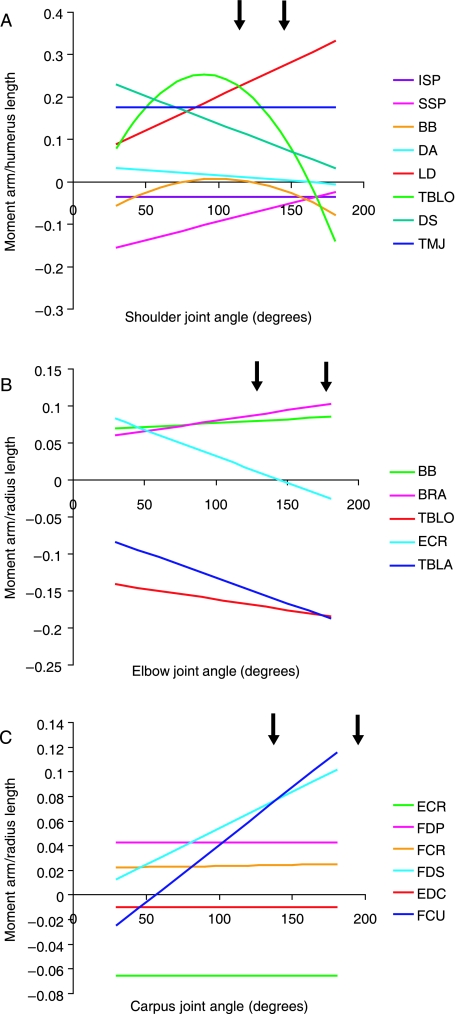
Moment arm data is given in Table 4 and displayed in Fig. 3 (scaled to thoracic limb segment lengths which are given in Table 3). The moment arms of supraspinatus and latissimus dorsi increased linearly with shoulder joint extension (Fig. 3A). Those of infraspinatus and teres major did not change with joint angle. The moment arm of biceps brachii decreased with joint extension, reaching its minimum at 100°, and then increasing again, reaching a second maximum at full joint extension. In contrast, that of the long head of triceps brachii increased, reaching a maximum moment arm at a shoulder angle of 90° and with two minima occurring at full joint flexion and extension. Moment arms of both heads of deltoideus decreased linearly with increased joint extension, with the scapular head showing greatest changes in moment arm with shoulder angle.
Table 4.
Mean maximum moment arms, maximum joint moments (calculated as described in Williams et al. 2008), and muscle fascicle length : moment arm (MFL : MA) ratios for greyhound thoracic limb muscles. Fascicle lengths for grouped muscles were calculated as weighted harmonic means (Alexander et al. 1981). Moment arms used for calculations are the mean of the maximum moment arms of each subject. n = 4
| Muscle–tendon unit | Joint of action | Joint angle of maximum moment arm (i.e. maximum flexion/extension) | Mean maximum muscle moment arm (cm) | Maximum joint moment of force (Ncm) | MFL : MA ratio |
|---|---|---|---|---|---|
| Infraspinatus | Shoulder | All | 0.7 | 576 | 1.85 |
| Supraspinatus | Shoulder | Max. flexion | 3.4 | 2431 | 0.71 |
| Deltoideus – acromial portion | Shoulder | Max. flexion | 0.8 | 78 | 4.92 |
| – scapular portion | Shoulder | Max. flexion | 5.3 | 981 | 0.22 |
| Lattisimus dorsi | Shoulder | Max. extension | 6.5 | 1482 | 5.46 |
| Teres major | Shoulder | All | 3.5 | 375 | 11.5 |
| Triceps brachii – long head | Shoulder | 90° | 4.9 | 7228 | 0.27 |
| Triceps brachii – lateral head | Elbow | Max. extension | 4.3 | 1509 | 5.66 |
| Triceps brachii – long head | Elbow | Max. extension | 3.6 | 5310 | 0.37 |
| Biceps brachii | Shoulder | Max. flexion | 2.4 | 2047 | 13.3 |
| Biceps brachii | Elbow | Max. extension | 1.9 | 1620 | 16.4 |
| Brachialis | Elbow | Max. extension | 2.3 | 370 | 4.82 |
| Extensor carpi radialis | Elbow | Max. flexion | 2.5 | 730 | 2.41 |
| Extensor carpi radialis | Carpus | Max. flexion | 1.5 | 438 | 3.93 |
| Extensor digitorum communis | Carpus | All | 0.23 | 29 | 64.5 |
| Flexor carpi radialis | Carpus | Max. extension | 0.57 | 61 | 18.4 |
| Flexor carpi ulnaris | Carpus | Max. extension | 2.6 | 1466 | 2.48 |
| Flexor digitorum profundus | Carpus | All | 1.0 | 1034 | 3.48 |
| Flexor digitorum superficialis | Carpus | Max. extension | 2.3 | 1010 | 12.0 |
Fig. 3.
Muscle moment arms (scaled to relevant segment length) for muscles at the shoulder (A), elbow (B) and carpus (C). Segment length data can be found in Table 3.
Table 3.
Thoracic limb segment lengths used to scale muscle moment arm measurements. Segment lengths are for total length of the appropriate bone
| Dog Number | Sex (M/F) | Mass (kg) | Humerus length (cm) | Radius length (cm) |
|---|---|---|---|---|
| 1 | F | 27 | 20 | 22 |
| 2 | M | 33 | 20 | 22 |
| 3 | M | 34 | 20 | 24 |
| 4 | M | 28 | 19 | 23 |
Muscle moment arms at the elbow joint
Moment arms of biceps brachii, brachiocephalicus and brachialis all increased linearly with increases in elbow joint angle (Fig. 3B). Moment arms of extensor carpi radialis (ECR), long and lateral heads of triceps brachii decreased with joint angle (also linearly). Moment arms of the two heads of triceps converged at full joint extension.
Muscle moment arms at the carpus joint
There was no change in moment arm with joint angle for many muscles at the carpus [FDP, flexor carpi radialis (FCR), EDC, ECR; Fig. 3C]. The moment arm of FDS increased linearly with increasing carpal joint angle, as did that of flexor carpi ulnaris (FCU).
Discussion
Distribution of muscle mass
Combined forelimb musculature (no differences exist between the muscle architecture of left and right thoracic limbs in racing greyhounds; Peckham, 2006) constitutes 18.6 ± 2.7% of the total body mass of the racing greyhound. The musculature of the hind limb accounts for 18.5 ± 0.3% of total body mass (Williams et al. 2008), thus both contribute approximately equally to the locomotor muscle mass of the greyhound. Previous studies have suggested that the hind limbs of quadrupedal cursors contain the bulk of locomotor muscle volume and thus are used predominantly for propulsion (Payne et al. 2005a,b; Williams et al. 2007a). However this appears not to be the case in the racing greyhound, where both hind and forelimbs have equal muscle masses. Arguably, the back should be considered a functional extension of the pelvic limb (Williams et al. 2007b), especially as it has been shown to play an important role in locomotion in greyhounds (Alexander et al. 1985). This has been shown to account for a further 12% of body mass (unpublished observations, D. Rodgers), meaning that ‘functional pelvic limb’ mass reaches 30.5% body mass. Nevertheless, this does not detract from the finding that a surprisingly large amount of muscle is situated in the thoracic limb.
It is not inconceivable that, as the greyhound is often likely to locomote at its physiological limits, when maximal work is being performed by the pelvic limb (for example during maximal accelerations) it may be possible and necessary to recruit thoracic limb musculature to achieve any further work production. It seems likely that in these short-burst, maximal exertions, an animal would utilize any mechanism available to achieve additional increases in mechanical work of the body so as to accelerate as quickly as possible. Any addition of further muscle mass to the pelvic limb could be detrimental to performance, as perhaps too much muscle bulk would add unnecessarily to body mass or limb mass (the distal limb needs to be light to be able to swing the limb quickly), and there must also be a limit to how much muscle can be packed around a joint before this adversely affects joint mobility.
Muscle architecture
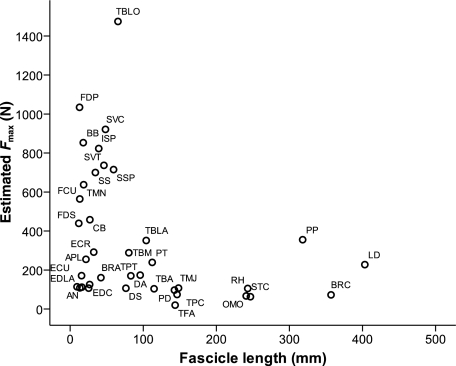
Within the thoracic limb the extrinsic musculature, in particular pectoralis profundus and latissimus dorsi, contributed the most to thoracic limb muscle mass. Both pectoralis profundus and latissimus dorsi had long fascicles and high AIs (Fig. 2), indicating that they are able to move limb segments through large ranges, and have a high velocity of contraction – this is ideally suited to a limb-moving function. Both muscles have indeed been suggested to play a major role in forelimb retraction during the swing phase of locomotion (Carrier et al. 2007). These extrinsic muscles should be able to produce large amounts of work (Fig. 4); in fact, pectoralis profundus is nearly as big as the largest and hence most powerful muscle in the pelvic limb, biceps femoris (Williams et al. under review). Thus it seems probable that the racing greyhound can use forelimb muscles, in particular latissimus dorsi and pectoralis profundus, to some extent in propulsion. A study of canine extrinsic muscle activity during trotting under varied locomotor conditions has shown that activity of latissimus dorsi and pectoralis profundus increase during stance when trotting uphill or pulling against a restraint (Carrier et al. 2007). This suggests that these extrinsic muscles produce net positive external work during accelerations (though not under steady state conditions). It seems fitting, then, that the racing greyhound – as an animal that is bred to, and which often undergoes, maximal accelerations – possesses large volumes of powerful extrinsic forelimb musculature.
Fig. 4.
Estimated maximum isometric force vs. muscle fascicle length for thoracic limb muscles. For abbreviations see Table 1.
The long head of triceps brachii was also large, but of a pennate nature. It has an AI of 0.47, demonstrating moderate length fascicles; however, the combination of pure size and shorter fascicles means that the long head of triceps brachii is able to generate extremely large amounts of force. It is the strongest muscle not only within the thoracic limb but is stronger than all muscles in the greyhound pelvic limb as well (Fig. 4). These high forces are likely to be crucial in stabilizing the elbow/shoulder joints during stance. The long head of triceps brachii is biarticular and thus may also play a role in dynamic control across both shoulder and elbow joints, for example in balancing the torques experienced at adjacent joints or allowing energy exchange between limb segments (Zajac et al. 2002). Stiffening of the elbow or shoulder joint during stance might be essential to resist high forces exerted on the thoracic limb during maximal acceleration or galloping. In addition, if the long head of triceps brachii were to facilitate energy transfer between segments, it would provide a mechanism by which work done by the more powerful extrinsic musculature can be transferred to more distal regions of the limb, and hence to the centre of mass.
Other muscles within the thoracic limb that also appear to show particular specialization are biceps brachii and FDP. Biceps brachii is also biarticular (elbow flexor, shoulder extensor), and is another high force generating muscle of the greyhound thoracic limb. It has a low AI and has relatively shorter fibres compared to mixed-breed dogs (Fig. 2), so it may be more extremely specialized for force production in the racing greyhound than in other generalized canids. Like the long head of triceps brachii, it may also have important functions in dynamic control and stiffening limb joints during stance. FDP is the strongest muscle of the distal limb – higher than the other digital flexor, FDS. This is the opposite of findings in horses (Brown et al. 2003) and hares (Williams et al. 2007b), where FDS is stronger and also capable of more elastic energy storage.
Whilst biceps brachii (discussed above) and other muscles (such as the lateral and medial heads of triceps brachii, and some carpal and digital flexors) have a lower AI in greyhounds (possibly reflecting a higher capacity for force generation), this is not the case for all muscles. This may reflect the different roles of muscles within the limb – for example, it might be an advantage for a powerful muscle, or one with a large range of motion to have relatively longer fibres (and hence a higher AI – see Williams et al. 2008). This is seen in the greyhound thoracic limb, with many extrinsic muscles having higher AIs than in their mixed-breed counterpart (including lattisimus dorsi, omotransversarius and pectoralis descedens).
Presence of elastic structures
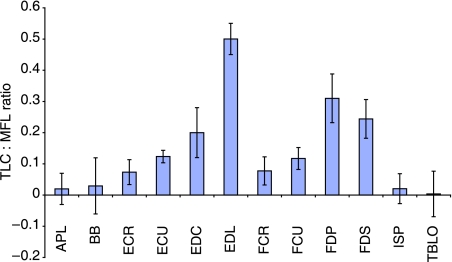
The ratio of estimated tendon length change at muscle Fmax to muscle fascicle length (TLC : MFL) was calculated for muscles with discernible distal limb tendons (Fig. 5). This ratio indicates the relative potential role of each muscle–tendon unit in elastic energy storage. A value greater than 0.4 indicates that passive stretching of the muscle tendon unit is likely to dominate active length change and hence there is high scope for elastic energy storage and release. Values below this indicate a more ‘stiff’ tendon design, able to cause large joint rotations directly. Only EDLA in the racing greyhound thoracic limb had a ratio larger than 0.4 (0.50), indicating length change within this muscle–tendon unit (MTU) may occur mostly via tendon elongation. However, EDLA is unlikely to be involved in energy storage and release as it is situated on the lateral aspect of the limb. Potentially, it may act as a stabilizing structure or as a ‘damper’, attenuating vibrations within the limb in a similar fashion to the equine FDS (Wilson et al. 2001). FDS and FDP – MTUs that might be expected to act as biological springs within the limb – had ratios substantially below that of EDLA, indicating that it is unlikely they would act as energy stores. This may account for the differences seen in architecture and estimated Fmax of the digital flexor muscles when compared to other quadrupeds (see above), as force generation is likely to be required for purposes other than facilitating storage and release of energy in the contractile component in these muscles. This finding suggests that whilst bred for high-speed running, efficiency and economy of locomotion via elastic storage and return may not be a specialist locomotor characteristic of the racing greyhound.
Fig. 5.
Tendon length change: Muscle fascicle length (TLC : MFL) ratios for selected thoracic limb muscle–tendon units.
Tendons as energy storing springs may be beneficial in terms of reducing energy usage and modulating limb force during high-speed running. However, a sprinter may need to perform without the locomotor constraints imposed by such an extreme specialization. Having a forelimb with mainly passive properties may serve as a disadvantage. Shorter muscle fibres in proportion to tendon length limit the shortening of the associated muscle and thus the tendon will stretch as opposed to causing movement at its insertion (Biewener & Roberts, 2000). There is hence less scope for control of movement in the limb. Greyhounds are originally bred to chase highly manoeuvrable prey and run around tightly curved tracks, thus further control is necessary for cornering. In addition, elastic elements in the limbs may be unfavourable for various non-locomotor movements. As the natural lifestyle and behaviour of the dog requires it to retain manipulative ability in the thoracic limb, more distally placed musculature will be necessary for finer manipulation tasks. Finally, high loads such as those experienced during these maximal activities impose extreme strains on structures within the limb including distal limb tendons. Animals that undergo frequent unsteady and maximal activities rather than steady-state sub-maximal locomotion may exert unnecessary, and ultimately damaging, forces on such elastic structures and thus it may be ‘safer’ to lose benefits of energy efficiency in favour of minimizing risk of injury.
Muscle moment arms at the shoulder
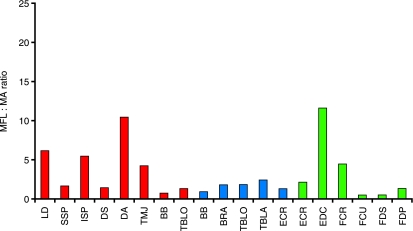
The moment arm of latissimus dorsi was small during shoulder joint flexion and became large in extended joint positions. Thus latissimus dorsi is able to create the largest joint moment at full shoulder extension, and is likely to play a role in initiating shoulder flexion and hence forelimb retraction during swing, and in pulling the trunk over the supporting limb during accelerations (Carrier et al. 2007). These data combine well with the muscle architecture data reported earlier, increasing the confidence in our suggestion that latissimus dorsi plays a propulsive role within the racing greyhound forelimb. Latissimus dorsi has long parallel fibres and a relatively high MFL : MA ratio (Fig. 6), and so is likely to be able to create large torques across a wide range of joint angles, and at relatively fast joint angular velocities. It might be beneficial to maintain these fast angular velocities during limb retraction, particularly during high-speed running. The thoracic limb attaches to the trunk via a synsarcosis, and so during locomotion the scapula is likely to move in its position on the thorax. It does not have a defined or fixed centre of rotation. In this study, we took measures of shoulder joint muscle moment arms with the scapula held static and in a ‘neutral’ position. We do not therefore know whether or how scapula motion affects shoulder joint muscle moment arms, as it was beyond the scope of this study, but this should be the focus of further work.
Fig. 6.
Muscle fascicle length : moment arm (MFL : MA) ratio for muscles of the thoracic limb. Red indicates muscles crossing the shoulder joint, blue across the elbow and green across the carpus joint.
Biceps and triceps brachii
The moment arm of the long head of triceps brachii is the largest at the most flexed (90°) of the common locomotor range of shoulder joint angles. This appears to be a paradox as, were the long head of triceps brachii involved in shoulder flexion (and thus propulsion), we would expect to see the converse (largest during extension). Equally, were the long head of triceps brachii involved in shoulder joint stability (such as its architecture might suggest), then a small MA would seem beneficial to avoid generating a large joint rotation. However, a large moment arm minimizes joint angular velocity for a given muscle contraction velocity. The long head of triceps brachii is biarticular and so a large moment arm at the shoulder might compensate if a high contraction velocity was required to elicit an effect at the elbow (one muscle cannot contract at multiple simultaneous velocities, even though it may have different locomotor roles at each joint it crosses). This highlights the large number of integrated factors that must be considered when discussing muscle function; for example, how elbow and shoulder joint angles interact during locomotion and how this might affect the action of the long head of triceps brachii. Biarticular muscle function and interaction within the locomotor system are interesting and complex issues, and cannot be fully understood through anatomical dissection alone. Detailed muscle architecture and geometry should be considered in conjunction with muscle activity patterns and length changes under varying locomotor conditions and at different stages of the stride cycle, which unfortunately is beyond the scope of this work.
Biceps brachii has classically been believed to play an active role in limb protraction (Goslow et al. 1981). Rapid limb protraction is essential for fast-running animals to achieve high speeds during locomotion. Thus, anatomical adaptations to reduce protraction time are highly beneficial. Cursorial animals do this well – in particular, reducing distal limb mass to decrease the angular inertia of the swinging limb, and hence reduce associated energetic costs. More recently, the lacertus fibrosis tendon of biceps brachii (and its continuation as the internal tendon of biceps) has been said to play an important role in limb protraction in the horse, via storage and quick release of elastic energy (Wilson et al. 2003). The dog does not possess such a defined elastic structure. Biceps brachii has a small MA at the shoulder and so neither is it likely to develop sufficient force to protract the limb via active means. At the elbow joint, however, the moment arm of biceps brachii increases with joint extension. Thus biceps brachii is able to create its greatest moment about the elbow in extended limb postures such as during stance. This may be important in allowing stability across elbow and shoulder joints when the limb is being loaded to prevent collapse of the limb. This arrangement would also mean that with biceps shortening there would be rapid elbow flexion and slower shoulder flexion, which is what is required for limb protraction at higher speed gaits.
Muscle moment arms at the carpus
ECR had the largest moment arm of the muscles crossing the extensor aspect of the carpal joint. ECR extends the carpus and thus a large moment arm means that it can generate a relatively large extensor moment here. It has a low MFL : MA ratio, however, and thus it appears not to be involved in moving the joint through large ranges. EDC (small moment arm) in contrast appears to have the opposite role, i.e. it is capable of creating minimal joint torques, but can act over a larger range of carpal joint angles. Of the carpal flexors, longest moment arms were found in full joint extension (FDS, FCU). The carpal joint is fully extended (and under high loads will hyper-extend) during stance. It is not surprising that the largest flexor moments are generated at this time, given that it is probably not of primary importance to rotate limb segments through large ranges at this stage of the stride cycle. The primary role for these muscles at this time is instead more likely to achieve stabilization and oppose the hyperextension that occurs at the carpus when the limb experiences large forces during high-speed locomotion (Fig. 7; Burn et al. 2006).
Fig. 7.
Still image from high-speed video of a racing greyhound during landing from a hurdle jump showing the large degree of carpal hyperextension experienced in high-load situations. Yellow lines indicate carpal joint angle.
Conclusion
We have detailed the muscle–tendon architecture and geometry of the racing greyhound thoracic limb. We compare the results to those found in other fast quadrupedal runners, and to those previously published in mixed-breed dogs and have discussed the implications of the functional adaptations of the greyhound thoracic limb for sprinting performance. Our main conclusions show the following.
The thoracic limb benefits from a similar proportion of locomotor muscle mass to the pelvic limb, suggesting that the thoracic limb may be used to some extent in propulsion, or alternatively that limb support is very important in this animal.
Extrinsic muscles, especially lattisimus dorsi and pectoralis profundus, are large and capable of producing high power outputs.
Distal muscles do not appear specialized for elastic energy storage, a functional difference to equivalent pelvic limb muscles and equivalent muscles in horse thoracic limbs (Brown et al. 2003).
Proximal limb biarticular muscles (long head of triceps brachii, biceps brachii) show specialization toward ‘stiffening’ and supporting the thoracic limb to enable strut-like limb function, or some form of dynamic control.
The greyhound thoracic limb hence appears to possess substantial differences from both that of more ‘sub-maximal specialist’ quadrupeds, and from the greyhound pelvic limb. These may be specializations for enhanced sprint or manoeuvring performance.
Acknowledgments
We would like to thank Professors Peter Aerts and Steven Harridge for their comments on early versions of this work, and the BBSRC for funding. S.B.W. was funded by a Royal Veterinary College studentship. A.M.W. is holder of a BBSRC research development fellowship and a Royal Society Wolfson Research Merit Award.
References
- Alexander RM, Dimery NJ, Ker RF. Elastic structures in the back and their role in galloping in some mammals. J Zool (Lond) (Series A) 1985;207:467–482. [Google Scholar]
- Alexander RM, Jayes AS, Maloiy GM, Wathuta E. Allometry of the leg muscles of mammals. J Zool (Lond) Series A. 1981;194:227–267. [Google Scholar]
- Biewener AA, Roberts TJ. Muscle and tendon contributions to force, work, and elastic energy savings: a comparative perspective. Exerc Sport Sci Rev. 2000;28:99–107. [PubMed] [Google Scholar]
- Brown N, Kawcak C, McIlwraith W, Pandy M. Architectural properties of distal forelimb muscles in horses, Equus caballus. J Morphol. 2003;258:106–114. doi: 10.1002/jmor.10113. [DOI] [PubMed] [Google Scholar]
- Burn JF, Portus B, Brockington C. The effect of speed and gradient on hyperextension of the equine carpus. Vet J. 2006;171:169–171. doi: 10.1016/j.tvjl.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Carrier DR, Deban SM, Fischbein T. Locomotor function of forelimb protractor and retractor muscles of dogs: evidence of strut-like behaviour at the shoulder. J Exp Biol. 2007;211:150–162. doi: 10.1242/jeb.010678. [DOI] [PubMed] [Google Scholar]
- Cavagna GA, Heglund NC, Taylor CR. Mechanical work in terrestrial locomotion: two basic mechanisms for minimizing energy expenditure. Am J Physiol. 1977;233:R243–R261. doi: 10.1152/ajpregu.1977.233.5.R243. [DOI] [PubMed] [Google Scholar]
- Goslow GE, Jr, Seeherman HJ, Taylor CR, McCutchin MN, Heglund NC. Electrical activity and relative length changes of dog limb muscles as a function of speed and gait. J Exp Biol. 1981;94:15–42. doi: 10.1242/jeb.94.1.15. [DOI] [PubMed] [Google Scholar]
- Gregersen CS, Silverton NA, Carrier DR. External work and potential for elastic storage at the limb joints of running dogs. J Exp Biol. 1998;201(Pt 23):3197–3210. doi: 10.1242/jeb.201.23.3197. [DOI] [PubMed] [Google Scholar]
- Heglund NC, Cavagna GA, Taylor CR. Energetics and mechanics of terrestrial locomotion. III. Energy changes of the centre of mass as a function of speed and body size in birds and mammals. J Exp Biol. 1982;97:41–56. doi: 10.1242/jeb.97.1.41. [DOI] [PubMed] [Google Scholar]
- Lee DV, Stakebake EF, Walter RM, Carrier DR. Effects of mass distribution on the mechanics of level trotting in dogs. J Exp Biol. 2004;207:1715–1728. doi: 10.1242/jeb.00947. [DOI] [PubMed] [Google Scholar]
- Merkens HW, Schamhardt HC, van Osch GJ, Hartman W. Ground reaction force patterns of Dutch Warmbloods at the canter. Am J Vet Res. 1993;54:670–674. [PubMed] [Google Scholar]
- Payne RC, Hutchinson JR, Robilliard JJ, Smith NC, Wilson AM. Functional specialisation of pelvic limb anatomy in horses (Equus caballus) J Anat. 2005a;206:557–574. doi: 10.1111/j.1469-7580.2005.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne RC, Veenman P, Wilson AM. The role of the extrinsic thoracic limb muscles in equine locomotion. J Anat. 2005b;206:193–204. doi: 10.1111/j.1469-7580.2005.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham K. Veterinary Physiotherapy. London: University of London (The Royal Veterinary College); 2006. Muscle architecture in the forelimb of a racing greyhound. [Google Scholar]
- Shahar R, Milgram J. Morphometric and anatomic study of the forelimb of the dog. J Morphol. 2005;263:107–117. doi: 10.1002/jmor.10295. [DOI] [PubMed] [Google Scholar]
- Smith NC, Wilson AM, Jespers K, Payne RC. Muscle architecture and functional anatomy of the pelvic limb of the ostrich (Struthio camelus) J Anat. 2006;209:765–780. doi: 10.1111/j.1469-7580.2006.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoor CW, van Leeuwen JL. Knee muscle moment arms from MRI and from tendon travel. J Biomech. 1992;25:201–206. doi: 10.1016/0021-9290(92)90276-7. [DOI] [PubMed] [Google Scholar]
- Walter RM, Carrier DR. Ground forces applied by galloping dogs. J Exp Biol. 2007;210:208–216. doi: 10.1242/jeb.02645. [DOI] [PubMed] [Google Scholar]
- Williams SB, Payne RC, Wilson AM. Functional specialisation of the pelvic limb of the hare (Lepus europeus) J Anat. 2007a;210:472–490. doi: 10.1111/j.1469-7580.2007.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SB, Payne RC, Wilson AM. Functional specialisation of the thoracic limb of the hare (Lepus europeus) J Anat. 2007b;210:491–505. doi: 10.1111/j.1469-7580.2007.00703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SB, Wilson AM, Rhodes LJA, Payne RC. Functional anatomy and muscle moment arms of the pelvic limb of an elite sprinting athlete: the racing greyhound (Canis familiaris) J Anat. 2008. [DOI] [PMC free article] [PubMed]
- Wilson AM, McGuigan MP, Su A, van Den Bogert AJ. Horses damp the spring in their step. Nature. 2001;414:895–899. doi: 10.1038/414895a. [DOI] [PubMed] [Google Scholar]
- Wilson AM, Watson JC, Lichtwark GA. A catapult action for rapid limb protraction. Nature. 2003;421:35–36. doi: 10.1038/421035a. [DOI] [PubMed] [Google Scholar]
- Zajac FE, Neptune RR, Kautz SA. Biomechanics and muscle coordination of human walking. Part I: introduction to concepts, power transfer, dynamics and simulations. Gait Posture. 2002;16:215–232. doi: 10.1016/s0966-6362(02)00068-1. [DOI] [PubMed] [Google Scholar]