Abstract
Borrelia burgdorferi, the bacterial pathogen of Lyme borreliosis, differentially expresses select genes in vivo, likely contributing to microbial persistence and disease. Expression analysis of spirochete genes encoding potential membrane proteins showed that surface-located membrane protein 1 (lmp1) transcripts were expressed at high levels in the infected murine heart, especially during early stages of infection. Mice and humans with diagnosed Lyme borreliosis also developed antibodies against Lmp1. Deletion of lmp1 severely impaired the pathogen's ability to persist in diverse murine tissues including the heart, and to induce disease, which was restored upon chromosomal complementation of the mutant with the lmp1 gene. Lmp1 performs an immune-related rather than a metabolic function, as its deletion did not affect microbial persistence in immunodeficient mice, but significantly decreased spirochete resistance to the borreliacidal effects of anti-B. burgdorferi sera in a complement-independent manner. These data demonstrate the existence of a virulence factor that helps the pathogen evade host-acquired immune defense and establish persistent infection in mammals.
Author Summary
The pathogen of Lyme borreliosis, Borrelia burgdorferi, causes disease in many parts of the world, resulting in multi-system complications in infected humans and animals. The microbe produces certain antigens in response to host environments that potentially allow it to persist and cause disease. Here, we analyzed the expression of B. burgdorferi genes encoding potential membrane proteins in infected hosts and show that one of them, termed Lmp1, is dramatically expressed in infected mice, most prominently in cardiac tissue during early infection. Mice and humans diagnosed with Lyme borreliosis also develop antibodies against Lmp1. Deletion of lmp1 in an infectious isolate of B. burgdorferi impairs the pathogen's ability to persist in murine tissues, especially the heart, and to induce disease, which was reversed when the gene was inserted back into the chromosome of the mutant. Lmp1 performs an immune-related, rather than a metabolic, function as its deletion does not affect microbial persistence in immunodeficient mice, but decreases the spirochete's ability to resist the borreliacidal effects of anti-B. burgdorferi sera. These data identify the existence of a surface-located antigen of B. burgdorferi that helps the pathogen evade host-acquired immune defense and establish persistent infection and disease in mammals.
Introduction
Lyme borreliosis, caused by Borrelia burgdorferi sensu lato, is the most prevalent tick-borne human disease in the United States, Europe and many parts of Asia [1]. Once the pathogen is deposited in the mammalian dermis by feeding Ixodes ticks, it establishes a localized infection at the bite site, then disseminates to distant cutaneous sites and various internal organs, including the spleen, bladder, joints, heart and central nervous system [1]–[3]. While B. burgdorferi persists in several tissue locations in mammals, only a limited set of organs, most frequently the joints and the heart, experience robust host-inflammatory responses resulting in clinical complications, such as Lyme arthritis and carditis. Antibiotic treatment is usually, but not always, successful, and some patients develop a form of antibiotic-resistant arthritis that is thought to be unrelated to persistent infection [4].
The B. burgdorferi transcriptome undergoes dynamic changes during the complex enzootic cycle of the spirochetes [5]–[8]. B. burgdorferi grown in laboratory medium or within host-implanted dialysis membrane chambers readily responds to altered environments, adapting to changes in temperature, pH, nutrients, and host immune responses [6], [9]–[15]. A significant fraction of the B. burgdorferi genome (8.6%), or 150 genes, could be differentially expressed in vitro in response to physiochemical alterations in growth conditions, and a major proportion of these genes (46%) encode proteins with predicted export signals [13]. However, while all B. burgdorferi lipoproteins have outer membrane export signals, some are retained in the periplasm by sequence-specific signals [16]. Studies have identified a few B. burgdorferi genes which are preferentially expressed in specific mammalian and arthropod environments and gene deletion studies [17] have confirmed that some of those differentially-expressed gene products support spirochete infectivity. For example, the B. burgdorferi genes bbk32, dbpA/B and bmpA/B are selectively expressed in mammals and facilitate B. burgdorferi infection of the murine host [8],[18],[19]. In contrast, ospA/B, bb0365 and bb0690 are highly expressed during specific stages of B. burgdorferi persistence in ticks and support the spirochete life cycle in the arthropod [7],[20],[21]. Other genes, such as ospD is dispensable for infectivity [22],[23], regardless of tightly regulated expression in vivo [22]. As gene duplication is a crucial mechanism of evolutionary innovation and the B. burgdorferi genome harbors significant clusters of paralogous genes in addition to large numbers of unique genes with unknown functional annotations [24],[25], many spirochete proteins may have functional redundancy. Thus, despite selective expression and beneficial contribution to the spirochete life cycle, antigens could be functionally redundant and non-essential for infectivity. Therefore, further identification of virulence genes that have significant impact on B. burgdorferi survival in vivo and pathogenesis is important for the development of preventative strategies.
The clinical complications of Lyme borreliosis are primarily triggered by B. burgdorferi-induced host inflammatory responses [26]–[28]. Although spirochetes colonize a wide variety of host tissues, the inflammatory response that results in pathology is observed in a limited set of host organs, most commonly in one or both mouse ankles or human knees and the heart. The diversity of host niches likely influences spirochete gene expression. While microbial antigens that are expressed at higher levels in a time- or tissue-specific manner may assist in B. burgdorferi persistence in local environments, antigens, especially those exposed on the microbial surface, could directly participate in host–pathogen interactions contributing to the genesis of organ-specific pathogenesis. Therefore, we assessed the expression levels of a selected set of B. burgdorferi genes in diverse murine tissues because of their putative membrane localization. We sought to determine if B. burgdorferi gene products that are preferentially expressed at high levels in clinically-relevant host microenvironments directly contribute to microbial virulence. The characterization of microbial ligands that are differentially expressed during the pathogen's life cycle is important for the identification of novel vaccine targets and the prevention of the multi-system disorders caused by B. burgdorferi.
Results
Identification of B. burgdorferi genes that are expressed in higher levels in infected murine tissues
B. burgdorferi persists in diverse tissue environments of the mammalian host. To identify B. burgdorferi genes that are expressed at high levels in vivo, particularly in a tissue-specific manner, we employed a sensitive quantitative RT-PCR (qRT-PCR) approach to compare spirochete transcriptomes in multiple murine tissues and in vitro. A total of 91 spirochete genes were selected for expression analysis, based on their putative association with the spirochete membrane as determined by the database annotation and in silico analysis for extracellular exposure (Table S1). Groups of C3H/HeN mice (5 animals/group) were challenged with B. burgdorferi (105 cells/mouse) and skin, joints, heart and bladder tissue were collected following 1, 2, 3 and 4 weeks of infection. Total RNA was isolated, and corresponding tissues from the indicated time points were combined into four separate pools of skin, joint, heart and bladder samples. qRT-PCR analysis was performed using gene-specific primers as detailed in the Materials and Methods section. Analysis of qRT-PCR data revealed that 44 B. burgdorferi genes (out of 91 assessed) were not transcribed at detectable levels in vivo. The remaining 47 genes displayed variable expression across different tissues, which is presented as fold increase in transcript levels relative to flaB, together with corresponding in vitro expression levels (Figure S1). B. burgdorferi bb0210, annotated as surface-located membrane protein 1 (lmp1), which encodes an exported protein with type I signal peptide with unknown function [25], displayed the most dramatic differential expression in murine tissues, with the highest level of expression found in the heart (Figure S1).
lmp1 is dramatically expressed in the murine heart during early B. burgdorferi infection
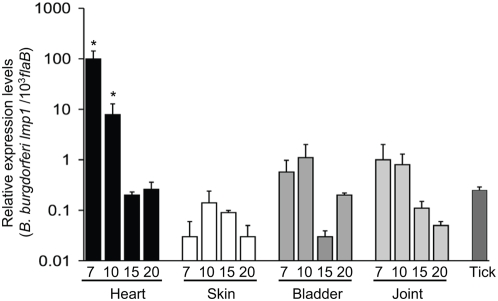
We used the initial qRT-PCR screen as a guide to focus on genes of potential importance, and performed more detailed temporal and spatial expression analyses of lmp1 throughout the B. burgdorferi infection in mice. A similar qRT-PCR experiment (Figure S1), using separate RNA samples collected at weekly intervals, showed that lmp1 was expressed at high levels between weeks 1 and 2, but produced at low levels between weeks 3 and 4 (data not shown). Therefore, a detailed expression analysis of lmp1 focused on the early phases of B. burgdorferi infection in the murine host. To accomplish this, groups of C3H/HeN mice (5 animals/group) were infected with B. burgdorferi, and tissues were isolated at 7, 10, 15, and 20 days. Isolated total RNA was converted to cDNA and subjected to qRT-PCR to measure copies of lmp1 transcripts, relative to flaB expression. The expression of lmp1 was selectively upregulated in the heart at 7 and 10 days post-infection, compared to that in the skin, joints, bladder and infected ticks (Figure 1). Similar to syringe-based infection, the expression of lmp1 was also significantly higher in infected hearts compared to other tissues, when mice were infected via natural tick-borne B. burgdorferi infection. The expression of lmp1 in the murine heart, analyzed at day 7 following B. burgdorferi–infected tick challenge, was significantly higher than corresponding lmp1 expression levels in the infected skin, bladder and joints (Figure S2). Consistent with lmp1 expression data, mice infected with B. burgdorferi and human patients with diagnosed Lyme disease also developed detectable antibody responses to Lmp1 (Figure S3). These results suggest that lmp1 encodes an immunogenic antigen that is expressed in vivo, with dramatic expression in the murine heart in both syringe and tick-transmitted B. burgdorferi infection. Therefore, we further examined the role of Lmp1 in B. burgdorferi virulence in mice.
Figure 1. B. burgdorferi lmp1 is dramatically upregulated during early phases of spirochete infection of the murine heart.
Total RNA was isolated from murine heart, skin, bladder, and joints of B. burgdorferi–infected mice (5 mice/group) at days 7, 10, 15, and 20, following spirochete challenge, as well as from engorged nymphs that fed on 2-week–infected mice, which were then converted to cDNA for measuring lmp1 transcripts using quantitative PCR. The relative expression levels of lmp1 are presented as copies of lmp1 transcript per 1,000 copies of flaB transcripts. Bars represent the mean±SEM from quantitative PCR analyses of three independent infection experiments. The transcript levels of lmp1 in the heart on days 7 and 10 were significantly higher than all time points for the skin, joint, bladder, and tick (*P<0.01–0.001).
lmp1 deletion interferes with the ability of the mutant B. burgdorferi to persist in murine tissues and to induce disease
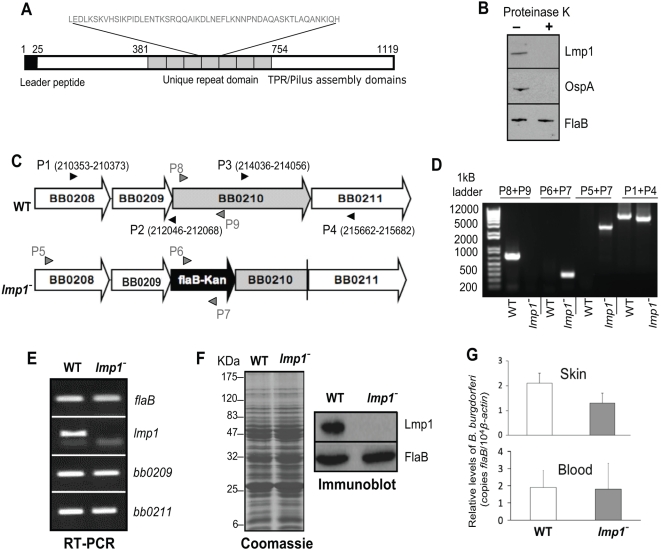
Although the function of B. burgdorferi Lmp1 is unknown, the protein displays putative conserved domains including unique repeat modules housed in the central portion of the protein (Figure 2A). Polyclonal antibodies that specifically recognized native B. burgdorferi Lmp1 were generated (Figure S4) and used in a proteinase K accessibility assay, which indicated that Lmp1 is exposed on the microbial surface (Figure 2B). To further study the role of the Lmp1 in B. burgdorferi infectivity, we created lmp1-deficient B. burgdorferi. An isogenic mutant of lmp1 was created by replacing part of the lmp1 open reading frame with a kanamycin resistance cassette via homologous recombination (Figure 2C). A DNA construct was generated for the intended recombination, sequenced to confirm identity and transformed into B. burgdorferi as detailed in the Materials and Methods section. Transformants were further screened using PCR analysis to ensure that the antibiotic cassette was inserted into the intended chromosomal locus (Figure 2D), and that the plasmid profiles of the wild type and mutant spirochetes were identical (data not shown). RT-PCR analysis showed that while lmp1 mRNA was undetectable in the lmp1 mutant, similar to the parental isolate, the mutant was able to transcribe the surrounding genes, bb0209 and bb0211 (Figure 2E). qRT-PCR analysis further confirmed that the transcript levels of bb0209 and bb0211 in the lmp1 mutant were 95(±9%) and 85 (±8%) of the respective wild type levels (data not shown). The lmp1 mutant spirochetes contained a similar protein profile to that of the wild type (Figure 2F, left panel) and, as expected, the lmp1 mutant did not produce Lmp1 protein (Figure 2F, right panel). We next compared the murine infectivity of lmp1 mutant B. burgdorferi with that of the parental isolates. Groups of 10 C3H/HeN mice were inoculated intradermally with equal numbers of wild type or lmp1 mutant B. burgdorferi (105 spirochetes/mouse). qRT-PCR analysis (Figure 2G) and culture (data not shown) of murine skin biopsy and blood samples collected following one week of infection indicated that both lmp1 mutants and wild type spirochetes were readily detectable in skin and blood. When Ixodes ticks were allowed to feed on mice inoculated with lmp1-deficient B. burgdorferi after two-weeks of infection, the mutants were able to migrate into feeding ticks (data not shown).
Figure 2. Construction and analysis of lmp1 mutant B. burgdorferi.
(A) Schematic diagram representing putative domain features of the Lmp1 protein. Amino acid positions at the beginning of the putative domains are indicated on the top. The leader peptide cleavage site predicted by SignalP 3.0 [58] is located after the 25th amino acid position. The central region of the protein harbors seven unique repeat domains of 54 amino acids (indicated in gray letters), previously identified to house a potential B. burgdorferi adhesin motif [34]. The carboxyl terminal region contains putative conserved domains similar to tetratricopeptide repeat (TPR) or a pilus assembly protein, as identified by NCBI BLASTP conserved domains search program. Lmp1 sequence can be accessed via JCVI B. burgdorferi genome page with the locus tag BB_0210 or via GenBank accession number AAC66595.1 (B) Lmp1 is sensitive to proteinase K–mediated degradation of B. burgdorferi surface proteins. Viable spirochetes were incubated with (+) or without (−) proteinase K for the removal of protease-sensitive surface proteins and processed for immunoblot analysis using Lmp1 antibodies. (B) burgdorferi OspA and FlaB antibodies were utilized as controls for surface-exposed and sub-surface proteins, respectively. (C) Schematic drawings of the wild type isolate (WT) and the lmp1 mutant B. burgdorferi (lmp1−) at the bb0210 (lmp1) locus (gray box arrow). Genes bb0208, bb0209, and bb0211 (white box-arrows) and the kanamycin-resistance cassette driven by the B. burgdorferi flaB promoter (flaB-Kan, black box-arrow) are indicated. Nucleotide positions of primers P1–P4 in the B. burgdorferi genomic database (www.tigr.org) are indicated. Seven nucleotides' overlap between the end of bb0209 and the start of bb0210 was not deleted in the allelic exchange by extending the primer P2 until the end of bb0209. The 5′ and the 3′ arms for homologous recombination flanking upstream and downstream of the lmp1 locus were amplified using primers P1–P2 and P3–P4 (black arrowheads), and ligated to the flaB-Kan cassette as detailed in Materials and Methods. (D) Integration of the mutagenic construct, flaB-Kan, in the intended genomic locus. Primers 5–9 (gray arrowheads, positions indicated in Figure 2C) were used for PCR analysis with isolated DNA from wild type or lmp1 mutant B. burgdorferi as templates. (E) RT-PCR analysis of wild type or lmp1 mutant B. burgdorferi. Total RNA was isolated from cultured spirochete, and transcript levels of lmp1, flaB, bb0209, and bb0211 were detected by RT-PCR analysis. (F) The lysates of wild type and lmp1 mutant B. burgdorferi were separated on a SDS-PAGE gel and either stained with Coomassie blue (left) or transferred onto a nitrocellulose membrane and probed with the antiserum against Lmp1 or FlaB (right). Migration of protein standards is shown to the left in kDa. (G) The lmp1 mutant B. burgdorferi were capable of dissemination from the dermis of infected mice. Skin and blood samples were collected from mice seven days after challenge, and the B. burgdorferi load was analyzed by quantitative PCR measurement of flaB copies and presented as flaB/murine β-actin.
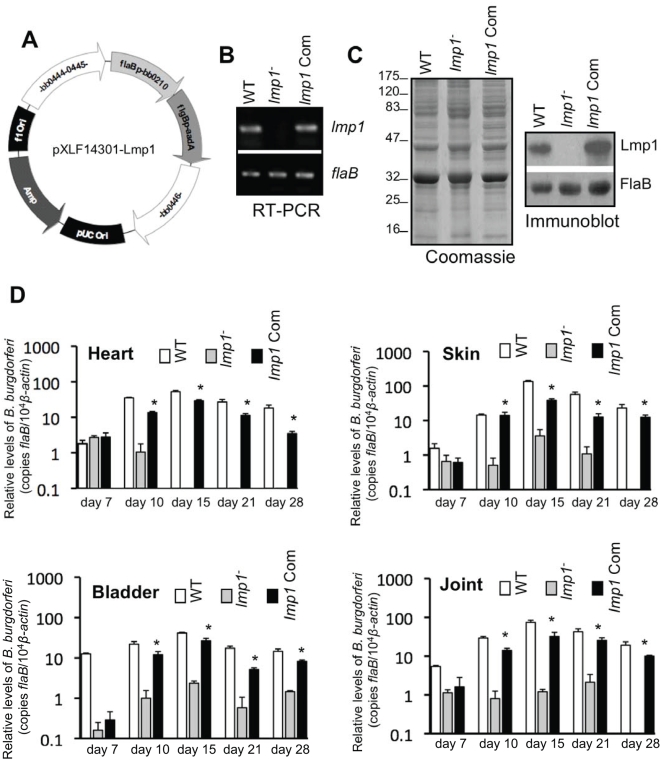
Although these observations indicated that lmp1 mutant B. burgdorferi was infectious in the murine host, the mutant was unable to establish persistent infection in mice and failed to induce disease, as assessed by the development of arthritis and carditis. To rule out the possibility that the observed phenotypic defects of the lmp1 mutant B. burgdorferi to infect the murine host were the result of anomalous effects of genetic manipulation, we sought to complement the lmp1 mutant spirochetes with a wild type copy of the lmp1 gene in cis, and use this isolate in murine infection studies. As lmp1 lacks an obvious upstream promoter, we first fused the open reading frame of lmp1 with the B. burgdorferi flaB promoter. The flaB-lmp1 fusion, along with the streptomycin resistance cassette, aadA [29] was then inserted into pXLF14301 [20] for integration into the B. burgdorferi chromosome (Figure 3A). lmp1 mutants were transformed and selected using antibiotics. PCR analysis confirmed that one of the lmp1-complemented spirochete isolates retained all of the B. burgdorferi plasmids present in the parental isolate (data not shown). RT-PCR and immunoblotting showed that the lmp1-complemented isolate produced both lmp1 mRNA (Figure 3B) and Lmp1 protein (Figure 3C). Lmp1 mRNA and protein production in the complemented and wild type isolates was further assessed by qRT-PCR and densitometric analysis of the immunoblot (Figure 3C), respectively, and normalized against FlaB production, which indicated that both isolates produced comparable levels of Lmp1 (data not shown).
Figure 3. Complementation of lmp1 mutant B. burgdorferi with lmp1 gene restores the ability of spirochetes to persist in mice.
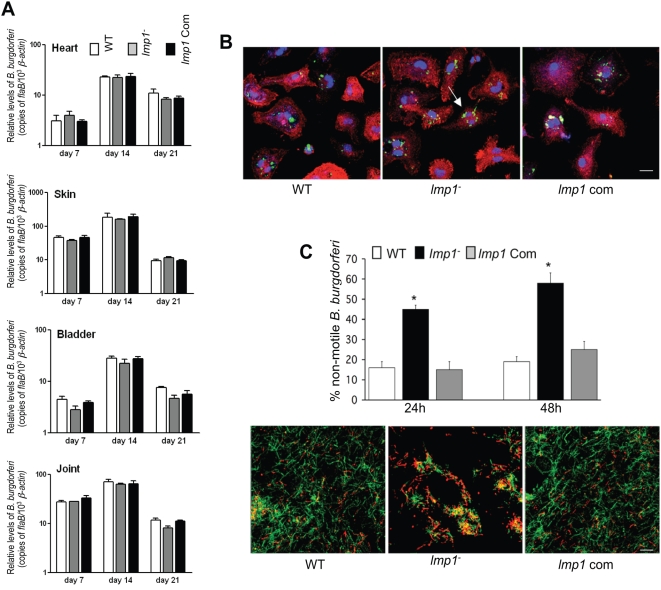
(A) Construction of DNA construct (pXLF14301-Lmp1) for chromosomal integration of the lmp1 gene. The open reading frame of lmp1 gene was fused with B. burgdorferi flaB promoter and cloned into the shuttle vector pKFSS1 that houses the streptomycin resistance gene (aadA) under B. burgdorferi flgB promoter. A DNA fragment encompassing the lmp1 gene with the flaB promoter and aadA cassette was finally excised from pKFSS1 and cloned into BamHI and SmaI sites of pXLF14301, which contains two fragments from B. burgdorferi chromosomal locus (bb0444 and bb0446) that act as the 5′ and 3′ arms needed for homologous recombination and integration of the complemented gene in B. burgdorferi chromosome. (B) RT-PCR analysis of the lmp1 transcripts. Total RNA was isolated from either the wild type (WT), lmp1 mutant (lmp1−), or lmp1-complemented B. burgdorferi (lmp1 Com), converted to cDNA, then subjected to PCR analysis with flaB and lmp1 primers, and analyzed on a 2% agarose gel. (C) Production of the Lmp1 protein by the complemented B. burgdorferi. Lysates of B. burgdorferi were separated on a SDS-PAGE gel, which was either stained with Coomassie blue (left) or transferred to nitrocellulose membrane, and blotted with antiserum against Lmp1 or FlaB (right). (D) The B. burgdorferi burden in mice. Mice were infected with wild type (white bar), lmp1 mutant (gray bar), and lmp1 mutant complemented with lmp1 (black bar) B. burgdorferi, and the spirochete burden was analyzed at days 7, 10, 15, 21, and 28 by measuring copies of the B. burgdorferi flaB gene, which was then normalized to mouse β-actin in each sample. Bars represent the mean±SEM of relative tissue levels of B. burgdorferi from three independent animal infection experiments. With the exception of the day 7 time point, levels of lmp1-complemented isolates were significantly higher than those of lmp1 mutants in all timepoints and murine tissues (* heart, P<0.001; skin, P<0.006; bladder P<0.004, joint P<0.002).
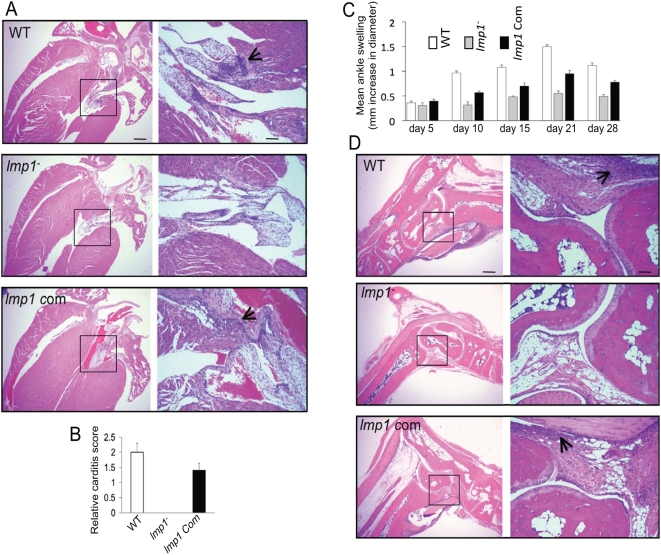
We then compared the ability of the wild type, lmp1 mutant and complemented spirochetes to establish infection and induce disease in the murine host. Groups of 10 C3H/HeN mice were separately inoculated intradermally with wild type, lmp1 mutant and lmp1-complemented B. burgdorferi (105 cells/mouse). The spirochete burdens in heart, skin, bladder and joints were evaluated at day 7, 10, 15, 21 and 28 following B. burgdorferi infection. The results showed that, except for the initial time point (day 7), lmp1 mutants were severely impaired in their ability to colonize all murine tissues and were undetectable in the heart after 10 days of infection (Figure 3D). Similarly, culture analysis of murine heart isolated after 15 days of B. burgdorferi infection showed that the lmp1 mutant spirochetes could not be recovered (data not shown). In contrast, both wild type and lmp1-complemented B. burgdorferi readily persisted in all tested murine tissues throughout infection, with significantly higher burdens than lmp1-deficient spirochetes–heart (P<0.001), skin (P<0.006), bladder (P<0.004) and joints (P<0.002). Both wild type and lmp1-complemented B. burgdorferi caused severe inflammation, but lmp1 mutants induced less severe disease, as reflected by the histopathological signs of carditis (Figure 4A and 4B), development of swelling in the tibiotarsal joints (Figure 4C), and histopathological signs of arthritis (Figure 4D). In most cases, the tissue burdens of lmp1-complemented isolates (Figure 3D) and their ability to induce inflammation (Figure 4B and 4C) were comparable, yet significantly lower (P<0.05), than those of the wild type isolates, possibly due to the constitutive expression of lmp1 from the heterologous flaB promoter which might be detrimental to spirochetes.
Figure 4. Deficiency of B. burgdorferi lmp1 reduces the severity of carditis and arthritis in mice.
(A) Representative histology of hearts isolated from mice infected with wild type B. burgdorferi (WT), lmp1 mutant (lmp1−), or lmp1 mutants complemented with lmp1 gene (lmp1 Com) isolates analyzed two weeks following infection. The left panel indicates lower-resolution (4×, bar = 400 µm) and higher-resolution (20×, bar = 80 µm) images of selected areas from corresponding sections (marked by box) shown in right panels. (B) Quantitative representation of the data shown in Figure 4A. At least ten random sections from each spirochete group were scored for severity of carditis in blinded fashion on a scale of 0–3 as indicated in Materials and Methods. (C) Severity of joint swelling in B. burgdorferi–infected mice. Arthritis was evaluated by assessment of the development of joint swelling of the mice infected with wild type B. burgdorferi (white bar), lmp1 mutant (gray bar), and lmp1 mutants complemented with lmp1 gene (black bar) isolates, measured using a digital caliper on day 5, 10, 15, 21, and 28 following spirochetes challenge. Bars represent the mean±SEM from three independent infection experiments. Differences in the joint swelling between groups of mice infected with lmp1 mutant and those with the lmp1-complemented isolates were significant (P<0.003) at all time points, except for day 5. (D) Representative demonstration of joint histology in mice infected with wild type or genetically manipulated B. burgdorferi isolates. Three weeks following spirochete infection, tibiotarsal joints were analyzed for histopathology. Both lower-resolution (4×, bar = 400 µm, left panel) and corresponding higher-resolution sections (20×, bar = 80 µm, marked by box, right panel) are shown. The arrows indicate the infiltration of inflammatory cells.
lmp1 contributes to B. burgdorferi survival against host-acquired immune defenses
The analysis of spirochete growth in the culture media indicated that the wild type, lmp1 mutants and lmp1-complemented isolates follow a similar growth pattern without significant variation in motility or obvious morphological defects (data not shown). However, lmp1 mutants were impaired to persist in the immunocompetent murine hosts following the first week of infection. We next assessed whether Lmp1 function in vivo is related to the metabolic or immune environment of the host. To accomplish this, we compared the infectivity of wild type and lmp1 mutant B. burgdorferi in the established immunodeficient murine model of Lyme borreliosis using severe combined immunodeficient (SCID) mice [30]. Groups of SCID mice (3 animals/group) were inoculated intradermally with 105 wild type, lmp1 mutant, or lmp1-complemented B. burgdorferi. The spirochete burdens in the heart, skin, bladder, and joints were evaluated at day 7, 14 and 21 following B. burgdorferi infection using quantitative PCR. In parallel, the viability of the spirochete was determined by culture of murine blood and spleen isolated at day 7, 14 and 21. The results showed that the wild type, lmp1 mutant and lmp1-complemented B. burgdorferi could be cultured from murine tissues at all time points (data not shown) and that there was no significant difference in B. burgdorferi burdens in all tested murine tissues throughout the infection (Figure 5A). Consistent with similar burdens of wild type and genetically-manipulated pathogens in SCID mice, one of the innate immune mechanisms, the ability of macrophages to phagocytose invading pathogens, which is important in controlling B. burgdorferi infection, did not differ between wild type and lmp1 mutants (Figure 5B). As neutralizing antibodies that develop in B. burgdorferi infected mammals are primarily responsible for controlling spirochete burden in the host, we explored whether reduced virulence of lmp1mutant spirochetes correlates with the susceptibility of B. burgdorferi to the borreliacidal activity of immune sera. To accomplish this, wild type, lmp1 mutant and lmp1-complemented B. burgdorferi were exposed to antisera collected from B. burgdorferi–infected C3H mice. lmp1 mutants were significantly more susceptible to the bactericidal activities of the anti-B. burgdorferi sera than wild type B. burgdorferi, and were protected by genetic complementation with lmp1 (Figure 5C). The susceptibility of lmp1 mutants to the bactericidal activities of the immune sera did not differ significantly when using active or heat-inactivated serum (data not shown), indicating that lmp1 deletion enhances the borreliacidal effects of antibodies in a complement independent manner. Like parental isolates, the lmp1 mutants were not susceptible to bactericidal activities by the non-immune serum collected from naïve mice (data not shown), suggesting that Lmp1 is not required for serum resistance by spirochetes. Together, these data suggest that Lmp1 contributes to B. burgdorferi defense against host-acquired immune responses by enhancing resistance to the bactericidal antibodies that develop during infection.
Figure 5. Lmp1 contributes to B. burgdorferi defense against host-acquired immune responses.
(A) lmp1 deletion does not influence B. burgdorferi infectivity in severe combined immunodeficient (SCID) mice. Spirochete burdens in murine tissues (heart, joint, skin, and bladder) were analyzed at days 7, 14, and 21 post-infection with wild type (white bar), lmp1 mutant (gray bar), and lmp1 mutant complemented with lmp1 (black bar) B. burgdorferi and presented as copies of the B. burgdorferi flaB gene normalized against mouse β-actin. Bars represent the mean±SEM from two independent infection experiments. Levels of B. burgdorferi isolates are similar in all murine tissues and timepoints (P>0.05). (B) Uptake of B. burgdorferi by primary murine macrophages. Bone marrow-derived macrophages were isolated from C3H mice and incubated with wild type B. burgdorferi (WT), lmp1 mutant (lmp1−), and lmp1 complemented (lmp1 Com) isolates at an MOI of 10. Cells were fixed after 2 hr of spirochete addition and processed for confocal immunofluorescence microscopy. Engulfed spirochetes (arrow), cellular actin, and nuclei were detected using FITC-labeled anti-B. burgdorferi goat IgG, phalloidin-Texas Red, and DAPI, respectively. A representative image of three independent experiments with similar results is presented (bar = 20 µm). (C) The lmp1 mutant is highly susceptible to anti-B. burgdorferi antibody-mediated killing in vitro. The wild type B. burgdorferi (WT), lmp1 mutant (lmp1−), and lmp1 complemented (lmp1 Com) isolates were incubated in the presence of B. burgdorferi–infected mouse serum. Serum used in the assay was pooled from B. burgdorferi–infected mice following 15 days of syringe-based infection (105 cells/mouse). Upper panel represents spirochete viability after 24 and 48 hrs of additional B. burgdorferi–infected serum, as assessed by the increase in the number of non-motile spirochetes using dark-field microscopy. Results are mean±SEM from three representative experiments with similar results. Differences between lmp1 mutant and wild type B. burgdorferi or lmp1-complemented spirochetes were highly significant (*P<0.002). The lower panel shows a representative fluorescence labeling of the live and dead spirochetes after 48 hr of antiserum treatment. Live bacteria were stained green (Syto 9 stain) whereas the dead bacteria stained red (propidium iodide).
Discussion
In nature, B. burgdorferi is maintained through a complex enzootic cycle [1]. Once transmitted to mammals, B. burgdorferi can establish persistent infection in a variety of tissue locations. Limited studies suggest that spirochete genes expressed in higher levels in infected host tissues could be important for B. burgdorferi survival [8]. Since membrane proteins could have a direct contribution to the adaptation of pathogens to host environments, we assessed the expression of 91 B. burgdorferi genes encoding potential membrane proteins covering spirochete infectivity in multiple murine tissues. Our data show that few of the genes analyzed are differentially or highly expressed in the selected tissues. Targeted deletion of one of the spirochete genes that is highly expressed in cardiac tissue, lmp1, while resulting in the initial clearance of pathogen burden in infected hearts, also affected overall virulence of B. burgdorferi in murine infectivity and reduced the outcome of Lyme disease. Our results show that lmp1 mutants persist in SCID mice at similar levels to parental isolates and are susceptible to borreliacidal antibody-mediated killing in vitro, suggesting that Lmp1 contributes to B. burgdorferi defense against host-acquired immune responses. Identification of hitherto unrecognized virulence genes of B. burgdorferi, such as lmp1, that support pathogen infectivity in mammals could shed light on the pathogenesis and prevention of Lyme disease.
Genes that are selectively expressed in vivo could be important for B. burgdorferi persistence in nature, possibly allowing spirochete adaptation to highly heterogeneous metabolic and immune environments. While assessment of pathogen gene expression in vivo is an important prerequisite to understanding microbial pathogenesis, microarray analysis is of limited use for the assessment of the B. burgdorferi transcriptome in vivo, primarily due to the low level of pathogen RNA in infected tissues [31]. Microarray studies were attempted after isolation and amplification of microbial RNA [7],[31], but this requires longer periods of RNA manipulation and risks degradation or clonal alteration during amplification and processing of transcripts. In contrast, optimal quantities of B. burgdorferi RNA can be isolated from spirochetes grown in vitro or in a host-implanted dialysis membrane, and microarray-based studies have been used to assess B. burgdorferi gene expression in these ‘host-like’ conditions that have yielded important information on the role of B. burgdorferi genes in pathogen infectivity [9],[12],[13],[15],[32]. However, the host environment is too complex and dynamic to be duplicated artificially. Therefore, we employed a sensitive qRT-PCR approach for the direct assessment of pathogen gene expression in vivo. This method is reproducible, as two independent sets of animal infection studies identified the same set of 47 B. burgdorferi genes as expressed in vivo, and a majority of them displayed higher expression levels in mice, than in vitro. The variable expression of these genes in multiple tissue locations possibly reflects the adaptive responses of the pathogen to local host environments, enabling immune evasion, adhesion, or nutrient uptake, among other possibilities. Furthermore, regardless of their functional role in pathogen persistence, antigens that are highly produced in certain host sites, such as the joints and heart, could participate in the genesis of inflammatory disease. Our qRT-PCR analysis also identified a set of 44 B. burgdorferi genes that may not be important for mammalian infectivity, as none of these displayed detectable transcription within the first 4 weeks of infection. On the other hand, a set of 26 genes encoding potential lipoproteins displayed detectable expression in vivo and, with the exception of bbo40, expression of many genes (bb0806, bbb09, bbd10, bbj09, bbl39, bbm27, bbn38, bbn39, bbq47, bbs41) agreed with a previous study that evaluated the expression of B. burgdorferi lipoproteins in murine dermis [5]. Although our expression analysis identified selected B. burgdorferi transcripts highly expressed in vivo, rather than proteins, these mRNA are likely the signatures of translated antigens. This speculation is supported by the recent study that identified 103 spirochete immunogens by screening in vitro translated genome-wide proteomic arrays with B. burgdorferi-specific immune sera [33]. Our target list of potential membrane proteins (Table S1) overlaps 10 of these identified immunogens (BB0543, BBB09, BBH06, BBL39, BBM27, BBN38, BBN39, BBO39, BBO40 and BBS41). Each of these immunogens was represented in our identified set of B. burgdorferi genes expressed in murine tissues (Figure S1).
Our study established B. burgdorferi lmp1 as a spirochete gene that is highly expressed in the early stages of mammalian infection, most notably in the murine heart. Lmp1 was first described in an earlier study [34] which highlighted the protein as a potential B. burgdorferi adhesin and indicated that Lmp1-specific antibodies develop in B. burgdorferi–infected mammals. lmp1 does not belong to the paralogous gene families in B. burgdorferi [24] and is thought to encode a relatively large 128-kDa outer membrane protein with putative type I signal peptide. It is not clear if the leader peptide is indeed cleaved or if the hydrophobic N-terminus serves as a membrane anchor; however, the antigen is exposed on the spirochete surface. Lmp1 retains 86–88% amino acid identity across orthologs in the related infectious spirochetes B. afzelii and B. garinii, with the highest sequence conservation in the amino and carboxyl termini. Analysis of the lmp1 locus indicates that the gene overlaps with the immediate upstream gene, bb0209, and shares a short intergenic region with the downstream gene, bb0211, suggesting these three genes are likely part of an operon; however, the lmp1 mutant is able to express both bb0209 and bb0211. Notably, bb0211 encodes for the DNA mismatch repair protein, MutL, and therefore likely bears a house-keeping function in B. burgdorferi biology. Although the functions of bb0209 and lmp1 are unknown, the amino terminus of BB0209 and the carboxyl terminus of Lmp1 both contain multiple tetratricopeptide repeat domains [35], which are shown to mediate protein-protein interaction and the assembly of the pilus and multiprotein complexes [36]. In addition, the central region of Lmp1 harbors a unique cluster of seven repeat motifs, each consisting of 54 amino acids, which is highly conserved amongst Lmp1 orthologs and potentially participates in adherence to host components [34]. Our studies further establish that Lmp1 is a surface-exposed virulence factor of B. burgdorferi, as lmp1 deletion severely interferes with spirochete infectivity and pathogenesis. After its inoculation in the host, B. burgdorferi remains locally in the dermis for a few days and then disseminates to distant organs, likely via the bloodstream, during the first week of infection [37],[38]. Our data indicate that Lmp1 function may not be important during early infection, including spirochete dissemination, since no apparent differences in the burdens of wild type spirochetes and lmp1 mutants were observed in murine blood samples collected at day 3, 5 (data not shown) and 7 (Figure 2G). Instead, lmp1 mutants displayed a severe reduction in numbers in the disseminated organs by day 10, implying that Lmp1 is critical for the persistence of the spirochete in murine tissues. In agreement with the higher level of expression of lmp1 in the cardiac tissue, as compared to other organs, a greater effect of lmp1 deletion was also observed in the heart where the mutants were selectively eliminated after 10 days of infection, suggesting that Lmp1 plays a dominant role in spirochete infection of the heart. B. burgdorferi burdens in mice began to decline after first two weeks of infection [37], which coincides with the development of the acquired immune response, such as neutralizing antibodies that controls spirochete infection [26],[28]. This is consistent with the observation that lmp1 mutants persist in similar levels to the parental isolate in the heart at day 7, despite dramatic wild type expression of lmp1, but begin to decline during the second week. The ability of lmp1 mutants to survive in SCID mice further indicates that the function of the Lmp1 is not related to metabolic requirements of the spirochete survival in vivo, as shown for B. burgdorferi PncA [39] or AdeC [40], but rather is associated with B. burgdorferi survival in host immune environments. B. burgdorferi isolates missing the lp28-1 plasmid [41], which houses VlsE [42],[43], display similar impaired host persistence in immunocompetent mice, as VlsE likely confers protection against host-generated borreliacidal antibodies. Therefore, while lmp1 is variably expressed in diverse tissue environments and carries a predominant role in spirochete persistence in the heart, a basal level of lmp1 expression is noted throughout the murine infection which, based on our in vitro data (Figure 5C), might contribute to the protection of B. burgdorferi against host-acquired immune responses, as was recently proposed for B. burgdorferi OspA in feeding ticks [44].
In summary, we have identified a select set of B. burgdorferi genes encoding potential membrane proteins that are expressed during murine infection. Many of these in vivo-expressed genes are differentially expressed in various host tissues, including joints and heart, and can participate in pathogen persistence and the genesis of disease. Here, we present direct evidence that one microbial gene expressed at higher level in the cardiac tissue, lmp1, encodes an essential virulence factor that plays an important role in immune evasion and dramatically influences spirochete persistence in murine tissues and the genesis of inflammation. Whereas previously identified B. burgdorferi virulence antigens are mostly plasmid-borne, and thus have greater instability and sequence divergence, Lmp1 is chromosomally encoded and is relatively conserved among orthologs in related infectious spirochetes. Further identification of B. burgdorferi virulence determinants that actively support spirochete persistence in vivo could contribute to the development of effective therapeutic strategies against Lyme borreliosis.
Materials and Methods
Bacteria, mice, and ticks
Borrelia burgdorferi infectious isolate A3 [45], a clonal derivative of the B. burgdorferi whole genome sequenced strain B31 M1 [24],[25], was used in this study. Four- to six-week old female C3H/HeN and pathogen-free NCr-SCID mice were purchased from the National Institutes of Health. Mice were inoculated with a single subcutaneous injection of 105 spirochetes per mouse. All animal procedures were performed in compliance with the guidelines and with the approval of the Institutional Animal Care and Use Committee. Ixodes scapularis ticks used in this study belong to a colony that has been reared and maintained in the laboratory.
Quantitative RT-PCR analysis
The identity and oligonucleotide primer sequences for the quantitative RT-PCR analysis of B. burgdorferi genes are indicated in Table S1. The B. burgdorferi target genes [24],[25]were selected based on their predicted localization on the spirochete membrane according to the database annotation (www.tigr.org) and PSORT in silico analysis [46]. Groups of mice (5 animals/group) were infected with B. burgdorferi (105 spirochetes/mouse), and samples of skin, heart, tibiotarsal joint and bladder were collected and frozen in liquid nitrogen at one-week intervals between 1 and 4 weeks of infection. Total RNA was extracted from tissue samples using the TRIzol reagent (Invitrogen). To reduce traces of contaminating DNA, samples were further digested with RNase-free DNaseI (Qiagen), purified using the RNeasy kit (Qiagen) and reverse transcribed to cDNA using the AffinityScript cDNA synthesis kit (Stratagene). The relative levels of B. burgdorferi cDNA in each sample were assessed by quantitative PCR (qPCR), and DNA contamination in each sample was measured using an equal volume of purified RNA as a template. Samples from each time point were pooled by tissue type, and final pools of skin, heart, joints and bladder were used in the qPCR analysis. The primers used for qPCR reaction were designed using OligoPerfect Primer design software (Invitrogen) based on the B. burgdorferi B31 M1 genomic sequence [24],[25]. All PCR primer pairs had a similar annealing temperature (60°C) and spanned 100–300 base pairs of each of the target B. burgdorferi genes. Each primer pair was tested for efficiency and non-specific amplification by melt-curve analysis using B. burgdorferi genomic DNA as a template. In one case of paralogous genes, the same set of primers was assigned for the detection of both genes as indicated in Table S1. To generate reliable in vivo gene expression data and to further ensure specific amplification of B. burgdorferi cDNA in murine tissue samples, the qPCR amplification in each well was followed by melt-curve analysis, and wells showing non-specific amplification were discarded from data analysis. The amplification cycle consisted of initial denaturation at 95°C for 5 min followed by 45 cycles each at 95°C for 10 sec, 60°C for 20 sec and 72°C for 30 sec and final melt curve analysis: 55°C for 30 sec, increase 0.5°C per cycle to 95°C. The amplification was performed in an iQ5 real-time thermal cycler (Bio-Rad) using SYBR Green Supermix (Bio-Rad) as detailed. For expression screening of B. burgdorferi genes, we simultaneously assayed 8 candidate genes in each 96-well PCR plate using duplicate wells of template cDNA (skin, heart, joint and bladder samples) with parallel positive (B. burgdorferi genomic DNA) and negative (no template) controls. Transcript levels of individual genes were assessed in spirochetes grown in vitro in BSK medium (107 cells/ml) and in each of the murine samples, calculated using the 2−ΔΔCt method [47], normalized against flaB transcripts and presented as fold increase in gene expression. Two independent mouse experiments used the same parameters of gene expression analysis to ensure the reproducibility of the assay. For detailed temporal and spatial analysis of lmp1 expression by qRT-PCR analysis, amounts of target transcripts were calculated from standard curves prepared from known quantities of flaB and lmp1 DNA as described [21],[48]. Mice (5 animals/group) were infected via single intradermal injection with B. burgdorferi (105 spirochetes/mouse) or via tick feeding using B. burgdorferi–infected nymphs. Infected murine samples, the heart, skin, bladder and tibiotarsal joints, were removed at different timepoints and frozen in liquid nitrogen. B. burgdorferi–infected ticks were isolated by allowing ticks to feed on 15-day infected mice as described [7]. For quantitative measurement of B. burgdorferi burden in infected tissues, flaB transcripts were measured in infected samples and normalized to mouse or tick β-actin levels.
Generation and phenotypic analysis of genetically manipulated B. burgdorferi isolates
The oligonucleotide primers used for mutagenesis and genetic complementation of B. burgdorferi are indicated in Table S2. The lmp1-deficient B. burgdorferi was created by exchanging a 2068 base pair DNA fragment encompassing the 5′ terminus of the lmp1 gene with a kanamycin-resistance cassette via homologous recombination as described [8]. Briefly, DNA fragments flanking up- and downstream of the lmp1 gene were PCR-amplified using primers P1–P4 and inserted into two multiple-cloning sites flanking the kanAn cassette in plasmid pXLF10601 [49]. This plasmid was sequenced to confirm the identity of the insert and electroporated into wild type B. burgdorferi B31 isolate A3. Transformants were selected for growth in the presence of kanamycin (350 µg/ml). Ten clones were isolated and PCR analysis was used to confirm the intended recombination event using primers P1–P9. The presence of all endogenous plasmids contained in the parental A3 isolate was also assessed in the mutant clones as described [8]. One of the lmp1 mutant clones that retained the same complete set of plasmids as the wild type isolate was used in additional experiments.
Genetic complementation of the lmp1 mutant was achieved by re-insertion of a wild type copy of the lmp1 gene in the B. burgdorferi chromosome [20]. The upstream of the lmp1 open reading frame (ORF) overlaps with the preceding gene by a few nucleotides, lacking an intergenic region with a discernible promoter. We, therefore, fused the lmp1 ORF with the B. burgdorferi flaB promoter [50]. Two B. burgdorferi DNA fragments encompassing the full-length lmp1 gene and the flaB promoter were PCR-amplified, fused and cloned into the BamHI and SalI sites of pKFSS1 housing a streptomycin-resistance cassette (aadA) [29]. A DNA element containing the flaB promoter-lmp1 gene fusion and the aadA cassette was cut with BamHI and SmaI from the recombinant plasmid pKFSS1-lmp1 and inserted into the corresponding restriction sites of the plasmid pXLF14301 [20] that contains the required 5′ and 3′ arms for homologous recombination in the B. burgdorferi chromosomal locus bb0444–0446. The plasmid construct was sequenced to confirm its identity and 25 µg of the plasmid DNA was electroporated into the lmp1 mutant. Four clones were isolated by their ability to grow in the presence of both kanamycin and streptomycin. PCR analysis was used to confirm the intended recombination event, and one of the lmp1-complemented clones that contained the same plasmid profiles as the wild type was chosen for further study. For in vitro growth analysis, an equal number of wild type and genetically-manipulated spirochetes were diluted to a density of 105 cells/ml and grown at 33°C in BSK-H medium until they reached the stationary phase (108 cells/ml). Aliquots of spirochetes were assessed every 12 hours, under a dark-field microscope, for motility, cell clumping and numbers of spirochetes counted using a Petroff-Hausser cell counter.
For phenotypic analysis of lmp1 mutants and lmp1-complemented isolates in vivo, B. burgdorferi were injected into groups of mice (10 animals/group) via needle-inoculation (105 spirochetes/mouse) intradermally on the back. Mice were sacrificed at 7, 10, 15, 21 and 28 days following inoculation. Skin, heart, joints, bladder and blood samples were collected and B. burgdorferi burdens were measured by quantitative PCR analysis as previously described [7],[8]. Development of joint swelling in the infected mice was also evaluated at similar time points as for spirochete burdens. For histological evaluation, heart and joint samples were collected at days 15, 21 and 28 following inoculation. For tick acquisition studies, groups of mice (5 animals/group, 20 ticks/mouse) were fed on by naïve ticks following two weeks of B. burgdorferi infection. The ticks were allowed to feed to repletion and were immediately analyzed for quantitative RT-PCR measurement of B. burgdorferi burden as detailed earlier [7].
Antibodies, ELISA, and immunoblotting
Generation of murine polyclonal antibodies against recombinant Lmp1, ELISA and immunoblotting were performed as described [7],[8]. Recombinant Lmp1 protein was produced in E. coli using the bacterial expression vector pGEX-6P1 (Amersham-Pharmacia Biotech) with specific primers as indicated in Table S2. Expression, purification and enzymatic cleavage of the glutathione transferase (GST) fusion proteins were carried out as detailed [7],[8]. Sixteen serum samples from humans with a clinical history of Lyme disease, collected from the CDC Lyme patient serum panel were used in the ELISA. Five serum samples from normal individuals residing in non-endemic areas for Lyme disease were also collected from CDC and used as negative controls. For immunoblotting, recombinant Lmp1 (0.05 µg/lane) was resolved on a SDS-PAGE gel and probed with 1∶ 1000 dilution of murine or human serum. Murine antiserum was collected from a group of 5 mice, 15 days after infection with B. burgdorferi via syringe inoculation (105 cells/mouse). Murine antiserum generated against recombinant Lmp1 was used in a Proteinase K accessibility assay to determine surface exposure of the Lmp1 as described [48].
Phagocytosis and in vitro killing assay
Bone marrow derived macrophages were isolated from naïve C3H mice as described [51] and cultured for 5 days at 37°C (2×105 cells/well) in L929-conditioned DMEM media. The cells were then washed and resuspended in serum-free DMEM and incubated with B. burgdorferi at a multiplicity of infection (MOI) of 10 at 37°C for 2 hours. Cells were washed with cold PBS to remove unbound B. burgdorferi and fixed in 3.7% paraformaldehyde, and processed for confocal immunofluorescence as described [52]. Spirochetes, cellular actin and nuclei were detected using FITC-labeled anti-B. burgdorferi goat IgG (KPL), phalloidin-Texas Red (Invitrogen), and DAPI (Invitrogen), respectively.
Susceptibility of wild type or genetically manipulated spirochetes to borreliacidal activities in infected mouse sera was performed as described [53]. Briefly, spirochetes were grown in BSK-H medium to a density of 107 spirochetes/ml and exposed to active or heat-inactivated 50% sera isolated from mice infected for 15 days with B. burgdorferi. Samples were incubated at 33°C for 24 to 48 hours and spirochete viability was assessed using dark-field microscopy. Susceptibility of spirochetes to borreliacidal activities in infected mouse sera were also tested in parallel using a combination of two vital stains that specifically label live and dead spirochetes, as described [8]. Briefly, spirochetes were incubated for 48 hours and labeled with the live/dead BacLight Viability kit (Invitrogen) according to the manufacturer's instructions.
Evaluation of arthritis and carditis
B. burgdorferi–infected mice were examined for swelling of the tibiotarsal joints as detailed earlier [8]. Ankle joints from each of the rear legs of each mouse were measured using a precision metric caliper in a blinded fashion. The thickest diameters of the tibiotarsal joints were measured in each mouse prior to B. burgdorferi infection, and the development of ankle swellings was monitored and tabulated on a weekly basis until the sacrifice of the mice. For histological evaluations of arthritis and carditis, at least 5 ankle joints and 5 hearts were collected from each group of mice (5 animal/group) infected with the different isolates. For histology, joints and hearts (cut in half through bisections across the atria and ventricles) were fixed in 10% formalin and processed for Hematoxylin and Eosin staining. Twenty randomly chosen sections from each mouse group were assessed for histopathological comparisons. Signs of arthritis were evaluated as described [8], based on a combined assessment of histological parameters of B. burgdorferi-induced inflammation [54]–[56], such as exudation of fibrin and inflammatory cells into the joints, alteration in the thickness of tendons or ligament sheaths, and hypertrophy and hyperplexia of the synovium. Signs of carditis [54],[57] were evaluated based on the cardiac inflammatory infiltrate, including the transmural infiltration of neutrophils in the blood vessels and infiltration of surrounding connective tissue with macrophages. Carditis was scored on a scale of 0 (no inflammation), 1 (mild inflammation with less than two small foci of infiltration), 2 (moderate inflammation with 2 or more foci of infiltration), or 3 (severe inflammation with focal and diffuse infiltration covering a large area). Both joint and heart tissue sections were blindly examined by two independent researchers.
Statistical analysis
Results are expressed as the mean±standard deviation (SD) or standard error mean (SEM). The significance of the difference between the mean values of the groups was evaluated by two-tailed Student t test.
Supporting Information
Relative expression of selected B. burgdorferi genes during infection of the murine hosts. Total RNA was isolated from B. burgdorferi grown in vitro, and multiple tissues of mice, between 1 and 4 weeks of B. burgdorferi infection, were pooled by tissue type and converted to cDNA for measuring gene-specific transcripts using quantitative PCR. Fold increase in the expression of individual genes in each of the murine samples was calculated based on threshold cycle (Ct) values using the 2−ΔΔCt method [47], normalized against flaB Ct values. Bars represent the mean±SD from four quantitative PCR analyses of two independent infection experiments.
(0.8 MB EPS)
lmp1 is highly expressed in the murine heart during early phases of tick-borne B. burgdorferi infection. Total RNA was isolated from multiple murine tissues following 7 days of challenge with B. burgdorferi–infected nymphal ticks (5 ticks/mouse, 3 animals/group), converted to cDNA, and used for measuring lmp1 transcripts in quantitative PCR assay. The relative expression levels of lmp1 are presented as copies of lmp1 transcript per 1,000 copies of flaB transcripts. Bars represent the mean±SEM from three independent experiments. The transcript levels of lmp1 in the heart were significantly higher than corresponding expression levels in the skin, joint, or bladder (*P<0.02).
(0.4 MB EPS)
Recognition of B. burgdorferi Lmp1 by immune sera. (A) Development of Lmp1-specific antibody response in B. burgdorferi–infected mice and humans. Fifty nanograms of recombinant Lmp1 was probed with normal and B. burgdorferi–infected sera. Arrow indicates the development of Lmp1-specific antibody response in both B. burgdorferi–infected mice and human sera. Infected serum used for immunoblotting was pooled from infected mice following 15 days of syringe-based infection or from a Lyme disease patient as detailed in the text. (B) Reactivity of antibodies in human sera to recombinant Lmp1 as assessed by ELISA. Sera from randomly chosen normal healthy controls (n = 5) and Lyme disease patients (n = 16) were tested for detection of antibodies specific for recombinant Lmp1.
(0.3 MB EPS)
Detection of native B. burgdorferi Lmp1. Murine antibodies generated against Lmp1 specifically recognize native protein in B. burgdorferi. One microgram of B. burgdorferi lysates was probed with normal mouse serum (NMS) or murine anti-serum against Lmp1 (anti-Lmp1). Murine polyclonal antibodies used in the immunoblotting were generated by immunization of mice against recombinant Lmp1 in mice as described in the text. Arrow indicates detection of native B. burgdorferi Lmp1.
(0.3 MB EPS)
Oligonucleotide primers used in the study
(0.2 MB DOC)
Oligonucleotide primers used in the study
(0.07 MB DOC)
Acknowledgments
We are grateful to Patricia Rosa for providing us the B. burgdorferi B31-A3 clone. We sincerely thank Manish Kumar, Ireen Dryburgh-Barry, Deborah Shroder, and Caitlin Hester for their excellent assistance with this study. We are grateful to Martin Schriefer for access to the CDC Lyme serum panel collected from patients with diagnosed Lyme disease and from normal healthy individuals from non-endemic areas.
Footnotes
The authors have declared that no competing interests exist.
This work was supported in part by funding from the American Heart Association, the National Institutes of Health (AR055323), and a Faculty Research Grant.
References
- 1.Steere AC, Coburn J, Glickstein L. The emergence of Lyme disease. J Clin Invest. 2004;113:1093–1101. doi: 10.1172/JCI21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barthold SW, Beck DS, Hansen GM, Terwilliger GA, Moody KD. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162:133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 3.Barthold SW, Persing DH, Armstrong AL, Peeples RA. Kinetics of Borrelia burgdorferi: Dissemination and evolution of disease following intradermal inoculation of mice. Amer J Pathol. 1991;163:263–273. [PMC free article] [PubMed] [Google Scholar]
- 4.Radolf J. Posttreatment chronic Lyme disease–what it is not. J Infect Dis. 2005;192:948–949. doi: 10.1086/432736. [DOI] [PubMed] [Google Scholar]
- 5.Liang FT, Nelson FK, Fikrig E. Molecular adaptation of Borrelia burgdorferi in the murine host. J Exp Med. 2002;196:275–280. doi: 10.1084/jem.20020770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narasimhan S, Santiago F, Koski RA, Brei B, Anderson JF, et al. Examination of the Borrelia burgdorferi transcriptome in Ixodes scapularis during feeding. J Bacteriol. 2002;184:3122–3125. doi: 10.1128/JB.184.11.3122-3125.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pal U, Dai J, Li X, Neelakanta G, Luo P, et al. A Differential Role for BB0365 in the Persistence of Borrelia burgdorferi in Mice and Ticks. J Infect Dis. 2008;197:148–155. doi: 10.1086/523764. [DOI] [PubMed] [Google Scholar]
- 8.Pal U, Wang P, Bao F, Yang X, Samanta S, et al. Borrelia burgdorferi basic membrane proteins A and B participate in the genesis of Lyme arthritis. J Exp Med. 2008;205:133–141. doi: 10.1084/jem.20070962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooks CS, Hefty PS, Jolliff SE, Akins DR. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect Immun. 2003;71:3371–3383. doi: 10.1128/IAI.71.6.3371-3383.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll JA, Garon CF, Schwan TG. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect Immun. 1999;67:3181–3187. doi: 10.1128/iai.67.7.3181-3187.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher MA, Grimm D, Henion AK, Elias AF, Stewart PE, et al. Borrelia burgdorferi sigma54 is required for mammalian infection and vector transmission but not for tick colonization. Proc Natl Acad Sci U S A. 2005;102:5162–5167. doi: 10.1073/pnas.0408536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ojaimi C, Brooks C, Akins D, Casjens S, Rosa P, et al. Borrelia burgdorferi gene expression profiling with membrane-based arrays. Methods Enzymol. 2002;358:165–177. doi: 10.1016/s0076-6879(02)58088-5. [DOI] [PubMed] [Google Scholar]
- 13.Revel AT, Talaat AM, Norgard MV. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc Natl Acad Sci U S A. 2002;99:1562–1567. doi: 10.1073/pnas.032667699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwan TG, Piesman J, Golde WT, Dolan MC, Rosa PA. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci U S A. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tokarz R, Anderton JM, Katona LI, Benach JL. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect Immun. 2004;72:5419–5432. doi: 10.1128/IAI.72.9.5419-5432.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulze RJ, Zückert WR. Borrelia burgdorferi lipoproteins are secreted to the outer surface by default. Mol Microbiol. 2006;59:1473–1484. doi: 10.1111/j.1365-2958.2006.05039.x. [DOI] [PubMed] [Google Scholar]
- 17.Rosa PA, Tilly K, Stewart PE. The burgeoning molecular genetics of the Lyme disease spirochaete. Nat Rev Microbiol. 2005;3:129–143. doi: 10.1038/nrmicro1086. [DOI] [PubMed] [Google Scholar]
- 18.Seshu J, Esteve-Gassent MD, Labandeira-Rey M, Kim JH, Trzeciakowski JP, et al. Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol Microbiol. 2006;59:1591–1601. doi: 10.1111/j.1365-2958.2005.05042.x. [DOI] [PubMed] [Google Scholar]
- 19.Shi Y, Xu Q, McShan K, Liang FT. Both decorin-binding proteins A and B are critical for the overall virulence of Borrelia burgdorferi. Infect Immun. 2008;76:1239–1246. doi: 10.1128/IAI.00897-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Pal U, Ramamoorthi N, Liu X, Desrosiers DC, et al. The Lyme disease agent Borrelia burgdorferi requires BB0690, a Dps homologue, to persist within ticks. Mol Microbiol. 2007;63(3):694–710. doi: 10.1111/j.1365-2958.2006.05550.x. [DOI] [PubMed] [Google Scholar]
- 21.Yang XF, Pal U, Alani SM, Fikrig E, Norgard MV. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J Exp Med. 2004;199:641–648. doi: 10.1084/jem.20031960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Neelakanta G, Liu X, Beck DS, Kantor FS, et al. Role of outer surface protein D in the Borrelia burgdorferi life cycle. Infect Immun. 2007;75:4237–4244. doi: 10.1128/IAI.00632-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart PE, Bestor A, Cullen JN, Rosa PA. A tightly regulated surface protein of Borrelia burgdorferi is not essential to the mouse–tick infectious cycle. Infect Immun. 2008;76:1970–1978. doi: 10.1128/IAI.00714-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casjens S, Palmer N, van Vugt R, Huang WM, Stevenson B, et al. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol. 2000;35:490–516. doi: 10.1046/j.1365-2958.2000.01698.x. [DOI] [PubMed] [Google Scholar]
- 25.Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 26.Connolly SE, Benach JL. The versatile roles of antibodies in Borrelia infections. Nat Rev Microbiol. 2005;3:411–420. doi: 10.1038/nrmicro1149. [DOI] [PubMed] [Google Scholar]
- 27.Nadelman RB, Wormser GP. Lyme borreliosis. Lancet. 1998;352:557–565. doi: 10.1016/S0140-6736(98)01146-5. [DOI] [PubMed] [Google Scholar]
- 28.Steere AC, Glickstein L. Elucidation of Lyme arthritis. Nat Rev Immunol. 2004;4:143–152. doi: 10.1038/nri1267. [DOI] [PubMed] [Google Scholar]
- 29.Frank KL, Bundle SF, Kresge ME, Eggers CE, Samuels DS. aadA Confers Streptomycin Resistance in Borrelia burgdorferi. J Bacteriol. 2003;185:6723–6727. doi: 10.1128/JB.185.22.6723-6727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaible U, Kramer M, Museteanu C, Zimmer H, Mossman H, et al. The severe combine immunodeficient mouse: A laboratory model of Lyme arthritis and carditis. J Exp Med. 1989;170:240–247. doi: 10.1084/jem.170.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narasimhan S, Caimano MJ, Liang FT, Santiago F, Laskowski M, et al. Borrelia burgdorferi transcriptome in the central nervous system of non-human primates. Proc Natl Acad Sci U S A. 2003;100:15953–15958. doi: 10.1073/pnas.2432412100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang FT, Nelson FK, Fikrig E. DNA Microarray Assessment of Putative Borrelia burgdorferi Lipoprotein Genes. Infect Immun. 2002;70:3300–3303. doi: 10.1128/IAI.70.6.3300-3303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbour AG, Jasinskas A, Kayala MA, Davies DH, Steere AC, et al. A genome-wide proteome array reveals a limited set of immunogens in natural infections of humans and white-footed mice with Borrelia burgdorferi. Infect Immun. 2008;76:3374–3389. doi: 10.1128/IAI.00048-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antonara S, Chafel RM, LaFrance M, Coburn J. Borrelia burgdorferi adhesins identified using in vivo phage display. Mol Microbiol. 2007;66:262–276. doi: 10.1111/j.1365-2958.2007.05924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sikorski RS, Boguski MS, Goebl M, Hieter P. A repeating amino acid motif in CDC23 defines a family of proteins and a new relationship among genes required for mitosis and RNA synthesis. Cell. 1990;60:307–317. doi: 10.1016/0092-8674(90)90745-z. [DOI] [PubMed] [Google Scholar]
- 36.Das AK, Cohen PW, Barford D. The structure of the tetratricopeptide repeats of protein phosphatase 5: implications for TPR-mediated protein–protein interactions. Embo J. 1998;17:1192–1199. doi: 10.1093/emboj/17.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hodzic E, Feng S, Freet KJ, Barthold SW. Borrelia burgdorferi population dynamics and prototype gene expression during infection of immunocompetent and immunodeficient mice. Infect Immun. 2003;71:5042–5055. doi: 10.1128/IAI.71.9.5042-5055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hodzic E, Feng S, Freet KJ, Borjesson DL, Barthold SW. Borrelia burgdorferi population kinetics and selected gene expression at the host–vector interface. Infect Immun. 2002;70:3382–3388. doi: 10.1128/IAI.70.7.3382-3388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purser JE, Lawrenz MB, Caimano MJ, Howell JK, Radolf JD, et al. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol Microbiol. 2003;48:753–764. doi: 10.1046/j.1365-2958.2003.03452.x. [DOI] [PubMed] [Google Scholar]
- 40.Jewett MW, Lawrence K, Bestor AC, Tilly K, Grimm D, et al. The critical role of the linear plasmid lp36 in the infectious cycle of Borrelia burgdorferi. Mol Microbiol. 2007;64:1358–1374. doi: 10.1111/j.1365-2958.2007.05746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Labandeira-Rey M, Seshu J, Skare JT. The absence of linear plasmid 25 or 28-1 of Borrelia burgdorferi dramatically alters the kinetics of experimental infection via distinct mechanisms. Infect Immun. 2003;71:4608–4613. doi: 10.1128/IAI.71.8.4608-4613.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lawrenz MB, Wooten RM, Norris SJ. Effects of vlsE complementation on the infectivity of Borrelia burgdorferi lacking the linear plasmid lp28-1. Infect Immun. 2004;72:6577–6585. doi: 10.1128/IAI.72.11.6577-6585.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang JR, Hardham JM, Barbour AG, Norris SJ. Antigenic variation in Lyme disease Borreliae by promiscuous recombination of Vmp-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]
- 44.Battisti JM, Bono JL, Rosa PA, Schrumpf ME, Schwan TG, et al. Outer surface protein A protects Lyme disease spirochetes from acquired host immunity in the tick vector. Infect Immun. 2008;76:5228–5237. doi: 10.1128/IAI.00410-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elias AF, Stewart PE, Grimm D, Caimano MJ, Eggers CH, et al. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect Immun. 2002;70:2139–2150. doi: 10.1128/IAI.70.4.2139-2150.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakai K, Horton P. Computational prediction of subcellular localization. Methods Mol Biol. 2007;390:429–466. doi: 10.1007/978-1-59745-466-7_29. [DOI] [PubMed] [Google Scholar]
- 47.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 48.Coleman AS, Yang X, Kumar M, Zhang X, Promnares K, et al. Borrelia burgdorferi complement regulator-acquiring surface protein 2 does not contribute to complement resistance or host infectivity. PLoS ONE. 2008;3:3010e. doi: 10.1371/journal.pone.0003010. doi:10.1371/journal.pone.0003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, Liu X, Beck DS, Kantor FS, Fikrig E. Borrelia burgdorferi lacking BBK32, a fibronectin-binding protein, retains full pathogenicity. Infect Immun. 2006;74:3305–3313. doi: 10.1128/IAI.02035-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bono JL, Elias AF, Kupko JJ, Stevenson B, Tilly K, et al. Efficient targeted mutagenesis in Borrelia burgdorferi. J Bacteriol. 2000;182:2445–2452. doi: 10.1128/jb.182.9.2445-2452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shin OS, Isberg RR, Akira S, Uematsu S, Behera AK, et al. Distinct roles for MyD88 and Toll-like receptors 2, 5, and 9 in phagocytosis of Borrelia burgdorferi and cytokine induction. Infect Immun. 2008;76:2341–2351. doi: 10.1128/IAI.01600-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pal U, Li X, Wang T, Montgomery RR, Ramamoorthi N, et al. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell. 2004;119:457–468. doi: 10.1016/j.cell.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 53.Pal U, Yang X, Chen M, Bockenstedt LK, Anderson JF, et al. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J Clin Invest. 2004;113:220–230. doi: 10.1172/JCI19894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barthold SW, Hodzic E, Tunev S, Feng S. Antibody-mediated disease remission in the mouse model of lyme borreliosis. Infect Immun. 2006;74:4817–4825. doi: 10.1128/IAI.00469-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bolz DD, Sundsbak RS, Ma Y, Akira S, Kirschning CJ, et al. MyD88 plays a unique role in host defense but not arthritis development in Lyme disease. J Immunol. 2004;173:2003–2010. doi: 10.4049/jimmunol.173.3.2003. [DOI] [PubMed] [Google Scholar]
- 56.Wang X, Ma Y, Weis JH, Zachary JF, Kirschning CJ, et al. Relative contributions of innate and acquired host responses to bacterial control and arthritis development in Lyme disease. Infect Immun. 2005;73:657–660. doi: 10.1128/IAI.73.1.657-660.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bockenstedt LK, Kang I, Chang C, Persing D, Hayday A, et al. CD4+ T helper 1 cells facilitate regression of murine Lyme carditis. Infect Immun. 2001;69:5264–5269. doi: 10.1128/IAI.69.9.5264-5269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Emanuelsson O, Brunak S, von Heijne G, Nielsen H. Locating proteins in the cell using TargetP, SignalP and related tools. Nat Protoc. 2007;2:953–971. doi: 10.1038/nprot.2007.131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relative expression of selected B. burgdorferi genes during infection of the murine hosts. Total RNA was isolated from B. burgdorferi grown in vitro, and multiple tissues of mice, between 1 and 4 weeks of B. burgdorferi infection, were pooled by tissue type and converted to cDNA for measuring gene-specific transcripts using quantitative PCR. Fold increase in the expression of individual genes in each of the murine samples was calculated based on threshold cycle (Ct) values using the 2−ΔΔCt method [47], normalized against flaB Ct values. Bars represent the mean±SD from four quantitative PCR analyses of two independent infection experiments.
(0.8 MB EPS)
lmp1 is highly expressed in the murine heart during early phases of tick-borne B. burgdorferi infection. Total RNA was isolated from multiple murine tissues following 7 days of challenge with B. burgdorferi–infected nymphal ticks (5 ticks/mouse, 3 animals/group), converted to cDNA, and used for measuring lmp1 transcripts in quantitative PCR assay. The relative expression levels of lmp1 are presented as copies of lmp1 transcript per 1,000 copies of flaB transcripts. Bars represent the mean±SEM from three independent experiments. The transcript levels of lmp1 in the heart were significantly higher than corresponding expression levels in the skin, joint, or bladder (*P<0.02).
(0.4 MB EPS)
Recognition of B. burgdorferi Lmp1 by immune sera. (A) Development of Lmp1-specific antibody response in B. burgdorferi–infected mice and humans. Fifty nanograms of recombinant Lmp1 was probed with normal and B. burgdorferi–infected sera. Arrow indicates the development of Lmp1-specific antibody response in both B. burgdorferi–infected mice and human sera. Infected serum used for immunoblotting was pooled from infected mice following 15 days of syringe-based infection or from a Lyme disease patient as detailed in the text. (B) Reactivity of antibodies in human sera to recombinant Lmp1 as assessed by ELISA. Sera from randomly chosen normal healthy controls (n = 5) and Lyme disease patients (n = 16) were tested for detection of antibodies specific for recombinant Lmp1.
(0.3 MB EPS)
Detection of native B. burgdorferi Lmp1. Murine antibodies generated against Lmp1 specifically recognize native protein in B. burgdorferi. One microgram of B. burgdorferi lysates was probed with normal mouse serum (NMS) or murine anti-serum against Lmp1 (anti-Lmp1). Murine polyclonal antibodies used in the immunoblotting were generated by immunization of mice against recombinant Lmp1 in mice as described in the text. Arrow indicates detection of native B. burgdorferi Lmp1.
(0.3 MB EPS)
Oligonucleotide primers used in the study
(0.2 MB DOC)
Oligonucleotide primers used in the study
(0.07 MB DOC)