Abstract
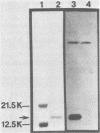
A monoclonal antibody (MCA) to enterotoxigenic Escherichia coli K99 antigen agglutinated K99+ enterotoxigenic E. coli strains B44 (O9:K30;K99;F41:H-) and B41 (O101:K99;F41:H-) grown at 37 degrees C but not at 18 degrees C. The MCA, which was characterized as immunoglobulin G1, reacted specifically with K99 antigen in an enzyme-linked immunosorbent assay and precipitated radiolabeled K99 antigen. A total of 45 colostrum-fed and colostrum-deprived calves were used in three separate trials to determine whether the orally administered K99-specific MCA would prevent diarrhea caused by strain B44. Twenty-eight calves were fed 1 ml of mouse ascitic fluid containing K99-specific MCA at 10 h of age and were orally challenged with strain B44 at 12 to 14 h of age. Control calves either received no placebo or were fed 1 ml of mouse ascitic fluid containing fibronectin-specific MCA at 10 h of age. There was no difference in the incidence of diarrhea between the two groups after challenge. However, the severity of diarrhea, as evaluated by the proportion of calves in each group that developed severe dehydration, the degree of clinical dehydration, the degree of clinical depression, the degree of weight loss, and the duration of diarrhea after challenge was significantly reduced in calves that received the K99-specific MCA. The mortality rate was also significantly lower (P less than 0.001) in the treated (29%) than in the control (82%) group. These results suggest that orally administered K99-specific MCA can prevent severe fatal enteric colibacillosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acres S. D., Forman A. J., Kapitany R. A. Antigen-extinction profile in pregnant cows, using a K99-containing whole-cell bacterin to induce passive protection against enterotoxigenic colibacillosis of calves. Am J Vet Res. 1982 Apr;43(4):569–575. [PubMed] [Google Scholar]
- Acres S. D., Isaacson R. E., Babiuk L. A., Kapitany R. A. Immunization of calves against enterotoxigenic colibacillosis by vaccinating dams with purified K99 antigen and whole cell bacterins. Infect Immun. 1979 Jul;25(1):121–126. doi: 10.1128/iai.25.1.121-126.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chan R., Acres S. D., Costerton J. W. Use of specific antibody to demonstrate glycocalyx, K99 pili, and the spatial relationships of K99+ enterotoxigenic Escherichia coli in the ileum of colostrum-fed calves. Infect Immun. 1982 Sep;37(3):1170–1180. doi: 10.1128/iai.37.3.1170-1180.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaastra W., de Graaf F. K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982 Jun;46(2):129–161. doi: 10.1128/mr.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M. L., Margulies D. H., Scharff M. D. A simple method for polyethylene glycol-promoted hybridization of mouse myeloma cells. Somatic Cell Genet. 1977 Mar;3(2):231–236. doi: 10.1007/BF01551818. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Antibody production by hybridomas. J Immunol Methods. 1980;39(4):285–308. doi: 10.1016/0022-1759(80)90230-6. [DOI] [PubMed] [Google Scholar]
- Guinée P. A., Jansen W. H., Agterberg C. M. Detection of the K99 antigen by means of agglutination and immunoelectrophoresis in Escherichia coli isolates from calves and its correlation with entertoxigenicity. Infect Immun. 1976 May;13(5):1369–1377. doi: 10.1128/iai.13.5.1369-1377.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinée P. A., Veldkamp J., Jansen W. H. Improved minca medium for the detection of K99 antigen in calf enterotoxigenic strains of Escherichia coli. Infect Immun. 1977 Feb;15(2):676–678. doi: 10.1128/iai.15.2.676-678.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadad J. J., Gyles C. L. Scanning and transmission electron microscopic study of the small intestine of colostrum-fed calves infected with selected strains of Escherichia coli. Am J Vet Res. 1982 Jan;43(1):41–49. [PubMed] [Google Scholar]
- Hadad J. J., Gyles C. L. The role of K antigens of enteropathogenic Escherichia coli in colonization of the small intestine of calves. Can J Comp Med. 1982 Jan;46(1):21–26. [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E. K99 surface antigen of Escherichia coli: purification and partial characterization. Infect Immun. 1977 Jan;15(1):272–279. doi: 10.1128/iai.15.1.272-279.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Letchworth G. J., 3rd, Appleton J. A. Passive protection of mice and sheep against bluetongue virus by a neutralizing monoclonal antibody. Infect Immun. 1983 Jan;39(1):208–212. doi: 10.1128/iai.39.1.208-212.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C. Monoclonal antibodies. Sci Am. 1980 Oct;243(4):66–74. doi: 10.1038/scientificamerican1080-66. [DOI] [PubMed] [Google Scholar]
- Moon H. W. Mechanisms in the pathogenesis of diarrhea: a review. J Am Vet Med Assoc. 1978 Feb 15;172(4):443–448. [PubMed] [Google Scholar]
- Morris J. A., Thorns C., Scott A. C., Sojka W. J., Wells G. A. Adhesion in vitro and in vivo associated with an adhesive antigen (F41) produced by a K99 mutant of the reference strain Escherichia coli B41. Infect Immun. 1982 Jun;36(3):1146–1153. doi: 10.1128/iai.36.3.1146-1153.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers L. L. Enteric colibacillosis in calves: immunogenicity and antigenicity of Escherichia coli antigens. Am J Vet Res. 1978 May;39(5):761–765. [PubMed] [Google Scholar]
- Myers L. L. Passive protection of calves against experimentally induced and naturally occurring enteric colibacillosis. Am J Vet Res. 1980 Dec;41(12):1952–1956. [PubMed] [Google Scholar]
- Nagy B. Vaccination of cows with a K99 extract to protect newborn calves against experimental enterotoxic colibacillosis. Infect Immun. 1980 Jan;27(1):21–24. doi: 10.1128/iai.27.1.21-24.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnels P. L., Moon H. W., Schneider R. A. Development of resistance with host age to adhesion of K99+ Escherichia coli to isolated intestinal epithelial cells. Infect Immun. 1980 Apr;28(1):298–300. doi: 10.1128/iai.28.1.298-300.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira P. J., Eldridge A. E. Treponemal haemagglutination test. Br J Vener Dis. 1973 Jun;49(3):242–248. doi: 10.1136/sti.49.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]