Abstract
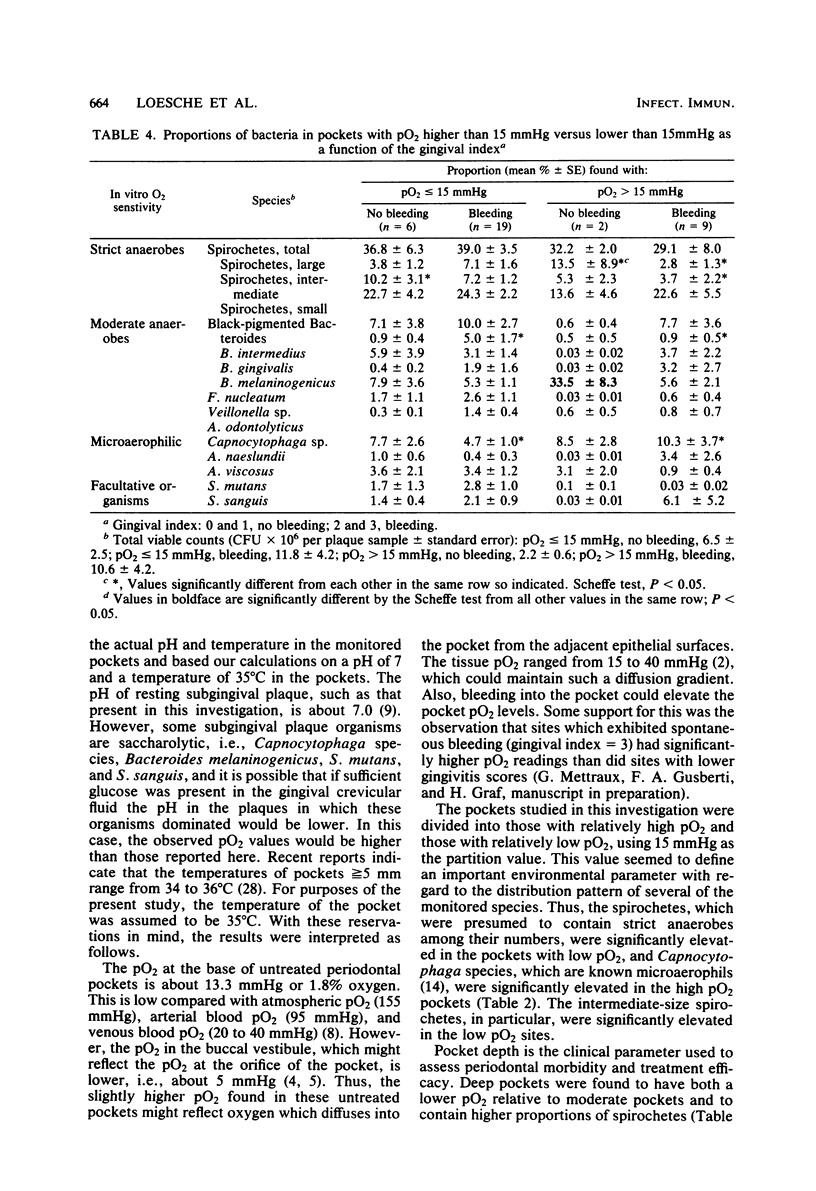
The predominance of anaerobic bacteria in subgingival plaque samples suggests that the pocket environment is anaerobic. In the present investigation, a small oxygen tension (pO2) electrode was inserted into the base of the pocket and the pO2 was recorded. In addition, the plaque in these pockets was examined culturally and microscopically. The oxygen tension at the bottom of 36 pockets (5 to 10 mm in depth) ranged from 5 to 27 mmHg (1 mmHg congruent to 133.3 Pa) with a mean value of 13.3 mmHg. Moderate pockets (5 and 6 mm) exhibited a mean pO2 of 15.7 mmHg, which was significantly higher than the 12.0 mmHg found in the deeper pockets. The deep pockets had higher percentages of spirochetes and Bacteroides intermedius, whereas the moderate pockets had elevated proportions of Actinomyces naeslundii and Streptococcus mutans. The sites with oxygen tensions equal to or less than 15 mmHg had significantly higher percentages of spirochetes, whereas the microaerophilic Capnocytophaga species were found in pockets with a pO2 greater than 15 mmHg. The presence of bleeding in the pocket was associated with higher proportions of B. intermedius, Capnocytophaga sp., and A. naeslundii. These pO2 readings of periodontal pockets indicated that there is a spectrum of pO2 values which seem to define, in a general way, the microbiological composition of the pocket.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arank A., Syed S. A., Kenney E. B., Freter R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl Microbiol. 1969 Apr;17(4):568–576. doi: 10.1128/am.17.4.568-576.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard P., Fehlmann W., Mindt W. An electrochemical sensor for continuous intravascular oxygen monitoring. Biotelem Patient Monit. 1979;6(1-2):16–31. [PubMed] [Google Scholar]
- Eskow R. N., Loesche W. J. Oxygen tensions in the human oral cavity. Arch Oral Biol. 1971 Sep;16(9):1127–1128. doi: 10.1016/0003-9969(71)90218-4. [DOI] [PubMed] [Google Scholar]
- Kenney E. B., Ash M. M., Jr Oxidation reduction potential of developing plaque, periodontal pockets and gingival sulci. J Periodontol. 1969 Nov;40(11):630–633. doi: 10.1902/jop.1969.40.11.630. [DOI] [PubMed] [Google Scholar]
- Kleinberg I., Hall G. PH and depth of gingival crevices in different areas of the mouths of fasting humans. J Periodontal Res. 1969;4(2):109–117. doi: 10.1111/j.1600-0765.1969.tb01955.x. [DOI] [PubMed] [Google Scholar]
- Knowles J. W., Burgett F. G., Nissle R. R., Shick R. A., Morrison E. C., Ramfjord S. P. Results of periodontal treatment related to pocket depth and attachment level. Eight years. J Periodontol. 1979 May;50(5):225–233. doi: 10.1902/jop.1979.50.5.225. [DOI] [PubMed] [Google Scholar]
- Kornman K. S., Loesche W. J. Effects of estradiol and progesterone on Bacteroides melaninogenicus and Bacteroides gingivalis. Infect Immun. 1982 Jan;35(1):256–263. doi: 10.1128/iai.35.1.256-263.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornman K. S., Loesche W. J. New medium for isolation of Actinomyces viscosus and Actinomyces naeslundii from dental plaque. J Clin Microbiol. 1978 Jun;7(6):514–518. doi: 10.1128/jcm.7.6.514-518.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornman K. S., Loesche W. J. The subgingival microbial flora during pregnancy. J Periodontal Res. 1980 Mar;15(2):111–122. doi: 10.1111/j.1600-0765.1980.tb00265.x. [DOI] [PubMed] [Google Scholar]
- LOE H., SILNESS J. PERIODONTAL DISEASE IN PREGNANCY. I. PREVALENCE AND SEVERITY. Acta Odontol Scand. 1963 Dec;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- Leadbetter E. R., Holt S. C., Socransky S. S. Capnocytophaga: new genus of gram-negative gliding bacteria. I. General characteristics, taxonomic considerations and significance. Arch Microbiol. 1979 Jul;122(1):9–16. doi: 10.1007/BF00408040. [DOI] [PubMed] [Google Scholar]
- Listgarten M. A., Helldén L. Relative distribution of bacteria at clinically healthy and periodontally diseased sites in humans. J Clin Periodontol. 1978 May;5(2):115–132. doi: 10.1111/j.1600-051x.1978.tb01913.x. [DOI] [PubMed] [Google Scholar]
- Listgarten M. A. Structure of the microbial flora associated with periodontal health and disease in man. A light and electron microscopic study. J Periodontol. 1976 Jan;47(1):1–18. doi: 10.1902/jop.1976.47.1.1. [DOI] [PubMed] [Google Scholar]
- Loesche W. J. Oxygen sensitivity of various anaerobic bacteria. Appl Microbiol. 1969 Nov;18(5):723–727. doi: 10.1128/am.18.5.723-727.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J., Straffon L. H. Longitudinal investigation of the role of Streptococcus mutans in human fissure decay. Infect Immun. 1979 Nov;26(2):498–507. doi: 10.1128/iai.26.2.498-507.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A., Laughon B. E., Stoll J. The bacteriology of acute necrotizing ulcerative gingivitis. J Periodontol. 1982 Apr;53(4):223–230. doi: 10.1902/jop.1982.53.4.223. [DOI] [PubMed] [Google Scholar]
- MACDONALD J. B., GIBBONS R. J., SOCRANSKY S. S. Bacterial mechanisms in periodontal disease. Ann N Y Acad Sci. 1960 Mar 29;85:467–478. doi: 10.1111/j.1749-6632.1960.tb49975.x. [DOI] [PubMed] [Google Scholar]
- Mukherjee S. The temperature of the periodontal pockets. J Clin Periodontol. 1981 Feb;8(1):17–20. doi: 10.1111/j.1600-051x.1981.tb02020.x. [DOI] [PubMed] [Google Scholar]
- Newman M. G., Socransky S. S. Predominant cultivable microbiota in periodontosis. J Periodontal Res. 1977 Mar;12(2):120–128. doi: 10.1111/j.1600-0765.1977.tb00114.x. [DOI] [PubMed] [Google Scholar]
- ROGOSA M. THE GENUS VEILLONELLA. I. GENERAL CULTURAL, ECOLOGICAL, AND BIOCHEMICAL CONSIDERATIONS. J Bacteriol. 1964 Jan;87:162–170. doi: 10.1128/jb.87.1.162-170.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. Subgingival microflora and periodontal disease. J Clin Periodontol. 1979 Oct;6(5):351–382. doi: 10.1111/j.1600-051x.1979.tb01935.x. [DOI] [PubMed] [Google Scholar]
- Socransky S. S., Hubersak C. Replacement of ascitic fluid or rabbit serum requirement of Treponema dentium by alpha-globulin. J Bacteriol. 1967 Nov;94(5):1795–1796. doi: 10.1128/jb.94.5.1795-1796.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky S. S. Microbiology of periodontal disease -- present status and future considerations. J Periodontol. 1977 Sep;48(9):497–504. doi: 10.1902/jop.1977.48.9.497. [DOI] [PubMed] [Google Scholar]
- Syed S. A., Loesche W. J., Pape H. L., Jr, grenier E. Predominant cultivable flora isolated from human root surface caries plaque. Infect Immun. 1975 Apr;11(4):727–731. doi: 10.1128/iai.11.4.727-731.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed S. A., Loesche W. J. Survival of human dental plaque flora in various transport media. Appl Microbiol. 1972 Oct;24(4):638–644. doi: 10.1128/am.24.4.638-644.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed S. A., Svanberg M., Svanberg G. The predominant cultivable dental plaque flora of beagle dogs with gingivitis. J Periodontal Res. 1980 Mar;15(2):123–136. doi: 10.1111/j.1600-0765.1980.tb00266.x. [DOI] [PubMed] [Google Scholar]



