Abstract
Objective
To investigate the relationship between physical impairment and brain-computer interface (BCI) performance.
Method
We present a meta-analysis of 29 patients with amyotrophic lateral sclerosis and 6 with other severe neurological diseases in different stages of physical impairment who were trained with a BCI. In most cases voluntary regulation of slow cortical potentials has been used as input signal for BCI control. More recently sensorimotor rhythms and the P300 event-related brain potential were recorded.
Results
A strong correlation has been found between physical impairment and BCI performance, indicating that performance worsens as impairment increases. Seven patients were in the complete locked-in state (CLIS) with no communication possible. After removal of these patients from the analysis, the relationship between physical impairment and BCI performance disappeared. The lack of a relation between physical impairment and BCI performance was confirmed when adding BCI data of patients from other BCI research groups.
Conclusions
Basic communication (yes/no) was not restored in any of the CLIS patients with a BCI. Whether locked-in patients can transfer learned brain control to the CLIS remains an open empirical question.
Significance
Voluntary brain regulation for communication is possible in all stages of paralysis except the CLIS.
Keywords: brain-computer interfaces, brain-computer communication, locked-in state, consciousness, complete locked-in state
Introduction
Different neurological diseases such as amyotrophic lateral sclerosis (ALS), stroke, muscular dystrophy, high spinal cord injury, or chronic polyneurithis (Guillan-Barré syndrome) may lead to severe motor paralysis, albeit their etiology is quite different. In the context of BCI, we refer to patients who are almost completely paralysed, with residual voluntary control over few muscles, such as eye movement, eye blinks, or twitches with the lip, as locked-in state (LIS) patients. Patients may also be in the complete locked-in state (CLIS), e.g., in the end-stage of ALS, in which all motor control is lost (Birbaumer, 2006).
In 1995/96, two patients in LIS were trained to regulate their slow cortical potentials (SCP). SCPs are part of the family of event-related brain potentials (ERPs) imbedded in the cortical electroencephalogram (EEG). After 4–6 weeks of training, both patients achieved significant control over cursor movements by manipulating their SCP amplitude voluntarily (Kuebler et al., 1998). One patient continued training and two more ALS patients with severe paralysis were included. Two patients were able to spell words using SCP-control when provided with a language support program (Kübler et al., 1999). In 1999, the very first verbal message ever communicated with a brain-computer interface, i.e. a message that was written exclusively with regulation of the EEG, was published (Birbaumer et al., 1999). In 2003 a patient described his strategy to regulate SCP and his thoughts about performance variability over sessions, resulting in the longest message ever communicated with a BCI (Neumann et al., 2003). Subsequent reports of other laboratories with severely paralysed patients using a BCI for communication involved also small samples and described similar results (Hochberg et al., 2006; Pfurtscheller et al., 2003; Sellers and Donchin, 2006; Wolpaw and McFarland, 2004).
Over the past 10 years 35 patients with different degrees of physical impairment due to different neurological diseases – but mainly diagnosed with ALS – were trained in BCI use in our laboratory. In ALS, progressive degeneration of the first and second motoneurons leads to severe motor paralysis. Patients die from respiratory failure unless they choose artificial ventilation and feeding. Neuronal degeneration may progress such that patients arrive at the CLIS. Patients were trained with BCIs on the basis of self-regulation of SCP (Birbaumer et al., 1999) and sensorimotor rhythms (SMR) (Kübler et al., 2005) and the P300 evoked potential (Nijboer et al., in press). Patients were confronted with various types of BCI to provide them with a system that works best for the individual patient and to elucidate whether one BCI type would be superior to another.
It has been repeatedly shown that patients with severe motor disability and also patients in the LIS were able to communicate or to control a BCI by means of regulation of SCP or SMR or with the P300-BCI (Birbaumer et al., 1999; Kübler et al., 2005; Neuper et al., 2003; Nijboer et al., in press). Despite these encouraging results, the number of patients using the system without the assistance of experts has not increased. Additionally, we were unable to restore basic communication (yes/no) in patients who were in the complete locked-in state at the beginning of training (Birbaumer, 2006). These patients may have the greatest need for a BCI to restore communication and interaction with the social environment. Thus, two questions arise: first, is there a relationship between physical impairment and BCI performance and if yes, what determines such a relationship and second, what prevents patients in the CLIS from learning BCI-control or any other voluntary control of a psychophysiological variable.
As the state of complete paralysis is hardly reported in the literature we will describe this state in some detail.
Complete locked-in state (CLIS)
The complete locked-in state refers to a state in which no communication and thus, no social interaction is possible. Within the BCI literature this state is seen as a consequence of complete motor paralysis due to a neurological disease such as amyotrophic lateral sclerosis or stroke. Motor paralysis is due to either degeneration of motor neurons or lesions of the pyramidal tracts and cortical motor areas. This held true for six of the seven CLIS patients who were diagnosed with ALS or chronic Guillan-Barré Syndrome (see Table 1 for a list of all patients). Another possibility for entering the CLIS can be due to disorders of consciousness such as in patients diagnosed with vegetative state or coma. These patients are usually not paralysed but unable to initiate motor commands due to extensive brain damage specifically in areas known to be important for wakefulness, attention and awareness such as the posterior brain stem, the thalamus and parietal cortices (Laureys et al., 2004). One of our CLIS patients (PR with hypoxia after heart attack) was probably in such an altered state of consciousness but has never been diagnosed as such. To date, no other patients with disorders of consciousness have been confronted with a BCI.
Table 1.
List of all patients who have been confronted with a BCI since 1995 in the laboratory at the University of Tübingen.
| Patient | diagnosis | age* | sex | duration of participation/year of study entry | Level of impair ment | type of BCI and average CRR** | Level of success*** | CRR published in | ||
|---|---|---|---|---|---|---|---|---|---|---|
| SCP | SMR | P300 | ||||||||
| HPS | ALS spinal | 41 | m | present/1996 | 4 | 87 | 73 | 4 (SCP), 3 (P300)††† | (Kübler et al., 1999) | |
| JB | ALS bulbar | 49 | m | 2 years/1997 | 4 | 86 | 4 (SCP) | (Birbaumer et al., 1999) | ||
| MP | ALS spinal | 37 | m | 2 years/1997 | 3 | 66 | 3 (SCP) | (Kübler et al., 1999) | ||
| MW | brain stem stroke | 26 | f | months/1995 | 4 | X† | 2 (SCP) | (Kübler et al., 1998) | ||
| HE | ALS spinal | 42 | m | present/1998 | 3 | 94 | 4 (SCP) | (Neumann and Birbaumer, 2003) | ||
| EK | ALS spinal | 66 | m | months/1998 | 2 | 57 | 2 (SCP) | (Neumann and Birbaumer, 2003) | ||
| MZ | ALS spinal | 31 | m | months/2000 | 4 | 70 | 3 (SCP) | (Kübler et al., 2001) | ||
| LB | ALS | 63 | m | months/1999 | 5 | 48 | 1 (SCP) | (Neumann and Birbaumer, 2003) | ||
| NB | ALS | 40 | m | months/2000 | 5 | 59 | 2 (SCP) | (Neumann and Birbaumer, 2003) | ||
| KI | cerebral paresis | 33 | m | months/1998 | 3 | 50 | 1 (SCP) | (Kübler, 2000) | ||
| TK | muscular dystrophy | 33 | m | months/1998/2006 | 4 | 43 | 59 | 1 (SCP), 2 (P300)††† | (Kübler, 2000) | |
| RCS | ALS spinal | 56 | m | 1 year/2003 | 4 | 77 | 69 | 3(SMR), 2(P300) | (Kübler et al., 2005) ( Nijboer et al., in press.) | |
| HAC | ALS bulbar | 67 | m | 2 years/2003 | 2 | 78 | 86 | 3(SMR), 4(P300) | (Kübler et al., 2005) ( Nijboer et al., in press) | |
| UBA | ALS spinal | 47 | f | present/2004 | 3 | 81 | 82 | 3(SMR), 4(P300) | (Kübler et al., 2005) ( Nijboer et al., in press) | |
| HM | ALS spinal | 53 | m | 3 years/2002 | 2 | 67 | 76 | 32 | 2(SCP), 3(SMR), 2(P300) | (Kübler et al., 2004; Kübler et al., 2005) ( Nijboer et al., in press) |
| JAK | ALS spinal | 39 | m | 2 years/2005 | 3 | 50 | 2(P300) | ( Nijboer et al., in press) | ||
| LEK | ALS spinal | 49 | f | present/2004 | 3 | 80 | 3(P300) | ( Nijboer et al., in press) | ||
| IR | ALS spinal | 42 | f | present/2003 | 5 | 54 | 43 | 1 (SCP), 1 (SMR)††† | (Kübler et al., 2004) | |
| SM | ALS spinal | 35 | m | months/2002 | 2 | 84 | 3 (SCP) | (Kübler et al., 2004) | ||
| KW | ALS spinal | 47 | f | months/2002 | 2 | 78 | 3 (SCP) | (Kübler et al., 2004) | ||
| GW | ALS bulbar | 59 | f | months/2002 | 2 | 70 | 3 (SCP) | (Kübler et al., 2004) | ||
| GB | ALS spinal | 62 | f | months/2002 | 2 | 79 | 3 (SCP) | (Kübler et al., 2004) | ||
| KR | ALS spinal | 35 | f | present/2002 | 3 | 62 | 87 | 2(SCP), 4(P300) | (Kübler et al., 2004) ( Nijboer et al., in press) | |
| HJZ | ALS | 60 | m | months/2002 | 3 | 74 | 3 (SCP) | (Kübler et al., 2004) | ||
| RB | ALS, spinal | 64 | f | months/2002 | 1 | 68 | 2 (SCP) | (Kübler et al., 2004) | ||
| JF | ALS spinal | 50 | m | months/2002 | 1 | 61 | 2 (SCP) | (Kübler et al., 2004) | ||
| GR | ALS spinal | 37 | m | present/2005 | 4 | 74 | 3 (P300)††† | |||
| PR | heart attack | 55 | m | years‡‡/2002 | 5 | 50 | X†† | X†††† | 1(SCP)†††, 1(SMR), 1(P300)††† | (Hill et al., 2006) |
| AG | chronic GBS‡‡‡ | 42 | f | years‡‡/2000 | 5 | 50 | X†† | X†††† | 1(SCP)†††, 1(SMR), 1(P300)††† | (Hill et al., 2006) |
| WER | ALS spinal | 63 | m | days/2005 | 5 | X†† | X†††† | 1(SMR), 1(P300)††† | (Hill et al., 2006) | |
| G | stroke | 61 | m | one session/2005 | 4 | X†† | 1 (SMR) | (Hill et al., 2006) | ||
| WEW | ALS spinal | 46 | m | months/2004 | 4 | X†††† | 1 (P300) | ( Nijboer et al., in press) | ||
| EM | ALS spinal | 58 | m | weeks/2002 | 5 | 62 | 2 (SCP) | (Hinterberger et al., 2005) | ||
| VWI | ALS spinal | 57 | f | 3 sessions/2007 | 4 | 63 | 2 (P300) ††† | |||
| UB | ALS spinal | m | months/1997 | 2 | X‡ | |||||
age at study entry
average over 3 representative sessions or as reported in publication. If more than one CRR was available (in different publications), the highest published CRR was chosen
the highest level of success was chosen for analysis regardless of type of BCI
above chance level performance, but CRR not reported in publication
chance level performance in first training session and no further SMR-BCI training was conducted; CRR not reported in publication
unpublished
no P300 detectable and thus, no training with the P300-BCI was conducted
data lost due to trainer error
with interruptions
Guillan-Barré Syndrom
From none of our CLIS patients anatomical brain scans were available when BCI training started. Brain scanning in the artificially ventilated patients is usually not possible. Cognitive function in such patients cannot be assessed with the common neuropsychological tests because the patients – by definition – are not able to respond. Cognitive abilities in CLIS patients can only be estimated from passive stimulation paradigms during EEG recording or functional imaging (Owen et al., 2006). We used such passive stimulation and EEG recording to address cognitive processing in our CLIS patients. They were confronted with an oddball paradigm in which rare target stimuli are presented in a stream of frequent standard stimuli; the target stimuli elicit a P300 (Fabiani et al., 1987). The P300 indicates the ability to distinguish between physically different stimuli (e.g., tones of different pitch) (Kotchoubey et al., 2005). They were also confronted with a semantic paradigm known to elicit an N400 which indicates semantic processing (Kotchoubey et al., 2005). Congruent and incongruent word pairs or sentences with unexpected endings or both were presented (for a detailed description of these paradigms see Kotchoubey et al., 2005). All stimuli were presented auditorily and all patients had the N1-P2 complex indicating functionality of primary auditory cortex (Kotchoubey et al., 2005). In the oddball paradigm all but one patients’ response to the target stimuli was significantly different from that to the standard stimuli indicating that they could distinguish the physical quality of auditory stimuli and its differential probability (Kotchoubey et al., 2003a; Kotchoubey et al., 2003b; Kotchoubey et al., 2005). Responses to semantic mismatch were absent in two CLIS patients (see also discussion).
Methods
Patients
Of 35 patients, 29 were diagnosed with ALS. Of the remaining 6 patients one was diagnosed with chronic Guillan-Barré Syndrome, one with muscular dystrophy, one with cerebral paresis, one with diffuse brain damage after hypoxia following a heart attack, and two with stroke (one in the brain stem). BCI training was approved by the Ethical Review Board of the Medical Faculty, University of Tübingen, Germany. For the purpose of evaluating BCI performance in relation to the degree of physical impairment, we subdivided our patient group in five categories: With minor degree of impairment, we refer to patients who had only slightly impaired limb movement and normal speech. Under the category moderate impairment, we summarized those patients with restricted limb movement (wheelchair-bound) and unaffected speech or intact limb movement without speech (such as the bulbar form of ALS which first affects speech and swallowing). Patients who were almost tetraplegic with restricted speech were considered major impaired. Categories four and five were the LIS and the CLIS, respectively (Table 2). All patients who were confronted with a BCI developed for communication purposes were included in the analysis.
Table 2.
Background and disease related data (N = 35)
| Mean age ± SD | sex | diagnosis | Degree of physical impairment | ventilation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| m | f | ALS | other | minor | moderate | major | LIS | CLIS | no | yes | night | |
| 48.4 ± 11.6 | 24 | 11 | 29 | 6 | 2 | 8 | 8 | 10 | 7 | 16 | 16 | 3 |
Varieties of the BCI used in our laboratory
In the following paragraphs, we briefly describe the BCI paradigms used. Both the slow cortical potentials (SCP) BCI and the sensorimotor rhythms (SMR) BCI require the user to learn regulation of the target EEG response by means of online feedback of the EEG. In contrast, in the P300-BCI an evoked brain potential is elicited by external stimuli and learning of voluntary brain regulation is not necessary.
The SCP-BCI
Slow cortical potentials with a negative polarity are recorded as a result of sustained intracortical or thalamocortical input to cortical layers I and II and indicate simultaneous depolarization of large pools of apical dendrites of pyramidal neurons. The depolarization of cortical cell assemblies reduces their excitation threshold, and firing of neurons in regions responsible for motor or cognitive tasks is facilitated (Birbaumer et al., 1990). For SCP recording, the EEG has to be measured with long time constants of several seconds and usually feedback from the central Cz electrode is provided. To learn regulation of the SCP amplitude, patients are presented with two targets, one at the top and one at the bottom of the screen (Figure 1). Continuous feedback is provided in discrete trials via cursor movement on a computer screen. A negative SCP amplitude – compared to a baseline recorded at the beginning of each trial – leads to cursor movement toward the top target, a positive SCP amplitude toward the bottom target of the screen (Kübler et al., 2001) (Figure 1). Patients were instructed to watch the cursor move in relation to their thoughts and to repeat successful strategies. Brain responses in accordance with the task requirement were positively reinforced by a smiling face appearing in the centre of the screen at the end of a trial. Twenty-five patients were confronted with an SCP-BCI and were trained from two up to several hundred sessions over weeks, months or years.
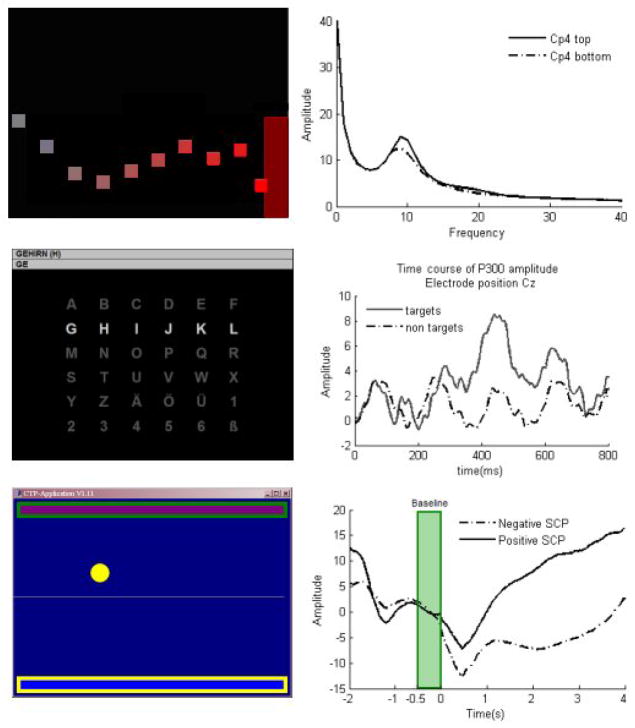
Figure 1.
Three types of BCI (left) and EEG analyses averaged over several trials (right). Top: the SMR-BCI: targets are presented at the top or bottom right margin of the screen. Patients’ task is to move the cursor into the target. Cursor movement is indicated by the squares; during feedback of SMR amplitude, only one square is visible. The cursor moves steadily from left to right, vertical deflections correspond to the SMR amplitude. Top right panel shows amplitude of the EEG as a function of frequency averaged across 230 trials separated by task requirement (top vs. bottom target). Bold line indicates frequency power spectrum when the cursor had to be moved toward the top target; dashed line when the cursor had to be moved toward the bottom target. A difference in amplitude can be clearly seen around the 10 Hz SMR peak. Middle: P300-BCI: a 6x6 letter matrix is presented. Rows and columns flash in random order, indicated by the bright row. In the copy spelling mode (Kübler et al., 2001), the patients’ task is to copy the word presented in the top line (GEHIRN = German for “brain”). In each trial, patients have to count how often the target letter flashes. The target letter is presented in parenthesis at the end of the word. Selected letters are presented in the second line below the word to copy. Middle right panel depicts EEG to target letters (bold line) averaged across 43 trials comprising 430 flashes of the target letter and 2150 flashes of all rows and columns not containing the target letter. Dashed line indicates the course of the EEG to all the non-target rows and columns (for an exact description of letter selection see e.g., Sellers et al., 2006). EEG to target letters is clearly distinguishable from non-target letters. Bottom: SCP-BCI: Targets are presented at the top or bottom of the screen. Patients’ task is to move the cursor (white dot) toward the target with the highlighted frame. The cursor moves steadily from left to right and its vertical deflection corresponds to the SCP amplitude. Bottom right: Time course of the SCP amplitude averaged across 350 trials separated by task requirement. A negative SCP amplitude (dashed line) moves the cursor toward the top, positive SCP amplitude (bold line) toward the bottom target. Before each trial, a baseline is recorded indicated by the gray bar. At time point -2 seconds the task is presented, at 500ms the baseline is recorded and at 0 cursor movement starts. Positive and negative SCP amplitude shifts are clearly distinguishable indicating that the patient learned to manipulate the SCP amplitude.
The SMR-BCI
Sensorimotor rhythms (SMR) refer to localized sinusoidal frequencies in the alpha and lower beta range EEG activity, which can be recorded over primary somatosensory and motor cortical areas (Niedermeyer, 2005). SMR decreases or desynchronizes with movement, preparation for movement or movement imagery and increases or synchronizes in the post-movement period or during motor quiescence (Pfurtscheller and Aranibar, 1979). To learn to modulate the power of SMR, patients receive feedback, e.g., cursor movement on a computer screen in one or two dimensions (Wolpaw and McFarland, 2004). During each trial of one-dimensional control, patients are presented with a target consisting of a red vertical bar that occupies the top or bottom half of the right side of the screen and a cursor on the left side (Figure 1). The patients’ task is to move the cursor in the target so that it hits the target. Low SMR amplitude during motor imagery moves the cursor to the bottom bar, high SMR amplitude (i.e., thinking of nothing in particular) moves the cursor toward the top bar (Kübler et al., 2005). Patients were instructed to use motor movement imagery to control the cursor. Five patients were trained with an SMR-BCI and participated in nine up to 20 sessions.
The P300-BCI
The P300 is a positive deflection in the EEG after a sensory stimulus. It is typically seen when participants are required to attend to a sequence of rare stimuli presented within a stream of frequent standard stimuli, an experimental design referred to as an oddball paradigm. In the classic P300-BCI, first introduced by Farwell and Donchin, participants are presented with a 6x6 matrix where each of the 36 cells contains a letter or a symbol (Farwell and Donchin, 1988). This design becomes an oddball paradigm by first intensifying each row and column for 100 ms in random order and then, by instructing participants to attend to only one (the desired) of the 36 cells. Thus, in one trial of 12 flashes (6 rows and 6 columns), the target cell will flash only twice constituting a rare event, compared to the 10 flashes of all other rows and columns and will therefore elicit a P300 (Sellers and Donchin, 2006) (Figure 1). Eleven patients were trained with a P300-BCI and participated in three up to 60 sessions.
Eight patients were trained with two types and three patients with all three types of BCI. Except for patients LEK and JF (Table 1), who were still able to walk at the beginning of training, all patients were trained at home. BCI training was conducted 1 to 3 times a week.
BCI performance
To evaluate performance of BCI we categorized 4 different levels of success: First, chance level, meaning that cursor control with the SCP- or SMR-BCI or classification of the P300 response was at chance level. For the SCP- and SMR-BCI with two options for selection chance level is 50%; for the P300-BCI with a 6x6 matrix, chance level is 3%. Second, above chance level control indicating that cursor control and classification rates exceeded chance level and reached statistical significance. However, above chance level control of an EEG response does not mean that communication can be achieved. For example, in a BCI with binary choice, a correct response rate (accuracy) of 70% is necessary for communication (Perelmouter and Birbaumer, 2000). Chourlarton and Dale reported for speech recognition software that 70–80% accuracy is necessary to be satisfactory for people to actually use the system (Choularton and Dale, 2004). Thus, we defined performance above 70% correct responses as the third category criterion level control (Kübler et al., 2004). The information that can be transferred with a performance accuracy of 70% depends on the choices the BCI offers to the user. The information transfer rate or bit rate (bits/trial) can be calculated according to the following formula (Wolpaw et al., 2000):
where B is the number of bits per trial, N is the number of possible selections, and P is the probability that a desired selection will occur. Thus, for a two-choice BCI – like the previously described SMR and SCP-BCI – with N=2 and P=0.7 the bit rate is 0.12. For the P300-BCI with a 6x6 matrix with N=36 and P=0.7 the bit rate is 2.75. Finally, all BCIs can be used in a so-called “free-mode”, in which the EEG responses are used for independent communication, internet surfing, or environmental control – depending on the individual person’s desires and needs. We refer to this category as independence. We introduced the second category above chance level control, although this is not sufficient to communicate with a BCI for the following reasons: first, not all patients were trained long enough to achieve the higher levels of success (depending on the purpose of the study), second, some patients quit for personal reasons although they reached a high level of success (and could have become better with more practice), and third because above chance level control performance indicates that at least some learning has occurred. To decide whether performance is above chance the number of trials have to be taken into account. For the P300-BCI the threshold above which the number of selected items was above chance was calculated as follows: the probability to select a correct letter by chance is where N is the number of choices (e.g., 36 in a 6x6 matrix) and the probability to select a wrong letter by chance is q = 1 − p . With n selections (e.g. n = 51 letters to be selected per session ( Nijboer et al., in press)) the probability to select a wrong letter by chance is qn and the probability to select a correct letter by chance given n selections is pn = 1 − qn The number x of letters that is necessary to be above the chance level of α < .05 is determined by .
For the two-choice SMR- and SCP-BCI the observed frequencies (of hits (cursor into the correct target) and misses) was compared to the expected frequencies given chance performance and tested for significance as follows: where fo is the observed and fe the expected frequency and df = 1
Instead of using the correct response rate (CRR) or information transfer rate for analysis of the relation between physical impairment and BCI performance, the varying levels of success were used for two reasons: firstly, because in few patients correct response rates were not available and secondly, because we considered the categories more meaningful with regards to the ability to use a BCI for communication. For example, correct response rates of 47%, 49%, or 52% are different in numbers but are all chance performance provided a BCI with two choices, implying that no control of a BCI is possible. Independence could not at all be represented with correct response rates, because it is a qualitative rather than a quantitative description of BCI performance, but it is clinically highly significant for communication.
The categories were applied identically to all patients. Patients were categorized either according to the percentage of correct responses (correct response rate = CRR) reported in previous publications where the highest value published was chosen or – when data have not yet been published – an average over three representative session was calculated. For few patients CRR was not reported in the publication but performance was labelled as chance level or above chance level (significant) performance; those patients were categorized accordingly (see Table 1).
Results
Performance of patients with different types of BCIs is summarized in Table 3. In Table 4, the level of success is depicted for each category of impairment. Background and performance data of all patients are listed in Table 1. Performance data of one patient were missing due to a trainer error. Although a thorough comparison between the different BCI paradigms (SCP, SMR, P300) is beyond the scope of this paper, we present correct response rates (CRR) for each patient (if available), so that level of success categorization can be traced (Table 1). Mean performance for the SCP-BCI was 66% (N = 23), for the SMR-BCI 71% (N = 5), and for the P300-BCI 66% (N = 11) (Table 3). Because of multiple entries of eleven patients, we refrain from inferential statistics and report descriptive statistics only. To compare levels of success between the 5 categories of impairment, Kruskal-Wallis Rank Test for n independent samples was chosen. In patients who were confronted with two or three types of BCIs and had different levels of performance, the highest level of success was chosen for analysis. Level of success was ranked over the entire patient sample (highest ranks for best performance). To illustrate performance in each category of impairment, the sum of ranks per category was divided by the number of patients in each category leading to mean ranks per category which are depicted in Figure 2. Performance differed significantly as a function of physical impairment (χ2 = 13.4, df = 4, p < .01, Kruskal-Wallis Rank Test for independent samples) with the highest mean ranks in the groups of moderate and major impaired patients (Figure 2). To further elucidate the relationship between physical impairment and BCI performance, we calculated the η2 coefficient (Kirk, 1982) as a measure of the variance in BCI performance explained by the degree of physical impairment: with η2 = .40 (F4/29 = 4.78, p < .01) the relationship was strong. The level of success within each category of impairment (Table 4) shows that in the CLIS group, 5 of 7 patients performed at chance level and only two achieved above chance level control, which was with 59 and 62%, far below criterion level control. If this group was removed from the statistical analysis, ranks did not differ between the remaining groups (χ2 = 3.5, df = 3, p = .32) and η2 was no longer significant (η2 = .12, F3/23 = 1.0, p = .41). We also investigated whether there was a relationship between level of success and dependence on artificial ventilation, but U-Test for comparison of ranks in two independent samples proved not significant (Z = −.87, p = .43; CLIS patients not included).
Table 3.
Performance level achieved, type of BCI used, mean, and range of percentage of correct responses within each BCI type. N = 49 because four patients were confronted with two and seven with three BCI types.
| type of BCI | SCP | SMR | P300 |
|---|---|---|---|
| number of patients | 23† | 5 | 11 |
| mean performance* ± SD | 66.1 ± 14.0 | 71.0 ± 15.8 | 66.0 ± 20.2 |
| range | 43 – 94 | 43 – 81 | 31 – 87 |
| Level of succes | number of patients | ||
| chance level | 5 | 1 | 0 |
| above chance | 8 | - | 5 |
| criterion level | 7 | 4 | 2 |
| independence | 3 | - | 4 |
as percentage of correct responses (correct response rate = CRR)
N=23, because data from one patient was lost due to trainer error and for another no correct response rate was reported in the publication
Table 4.
Best level of success in each category of impairment for each patient, regardless of BCI type (N = 34)
| Level of success | category of impairment | ||||
|---|---|---|---|---|---|
| minor | moderate | major | LIS | CLIS | |
| chance level | - | - | 1 | 2 | 5 |
| above chance level | 2 | 1 | 1 | 3 | 2 |
| criterion level | - | 5 | 3 | 3 | - |
| independence | - | 1 | 3 | 2 | - |
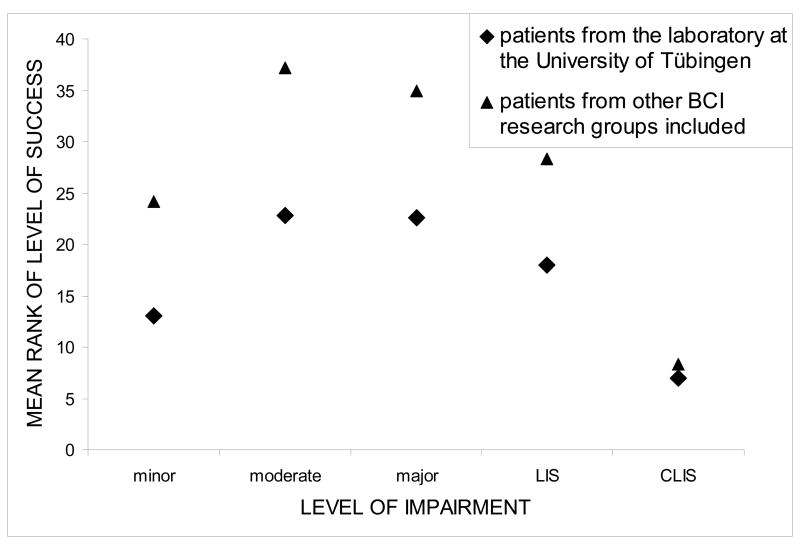
Figure 2.
Mean rank of level of success in each of the five categories of physical impairment. The higher the mean rank the better the performance in the group. Diamonds depict mean ranks of the Tübingen patient group; triangles depict mean ranks when patients from other BCI research groups were included. Other BCI research groups investigated no CLIS and only one LIS patient.
Table 5 summarizes results of BCI training with patients (N = 27) from other BCI research groups. Only one LIS patient (Piccione et al., 2006) took part in those studies; and no CLIS patients participated. If we added these data to the statistical analysis (without CLIS patients), the lack of a relation between physical impairment and BCI performance was confirmed (χ2 = 3.9, df = 3, p = .27; η2 = .07, F3/50 = 1.2, p = .32; Figure 2).
Table 5.
List of patients, BCI type and BCI performance from other BCI research groups. We performed a Medline search with the following keywords: Wolpaw, Pfurtscheller, Neuper, Sellers, Donchin, patients, brain-computer interface, and communication in various combinations. None of these patients were trained with different types of BCI. The search was restricted to non-invasive BCIs which used the EEG as input signal. Only reports of original data (no review papers) were included. Age and sex were not always reported individually and are thus not listed in the Table. It is important to note that here the SMR and P300 BCIs are not always of the same type as described above, classification methods and presentation design varied across BCI research groups and studies. This is why no analysis of BCI performance as a function of BCI type was conducted which included these patients.
| Diagnosis | level of impairment | BCI type | performance (CRR) | level of success | publication |
|---|---|---|---|---|---|
| SCI* (T7) | 2 | SMR | 96/89 | 3 | (McFarland et al., 2005; Wolpaw and McFarland, 2004) |
| SCI (C6) | 2 | SMR | 58/92/>60 - <90** | 3 | (McFarland et al., 2005; Wolpaw and McFarland, 2004; McFarland et al., 2003;) |
| Cerebral Palsy | 2 | SMR | >60 - <90** | (McFarland et al., 2003) | |
| ALS | 1 | SMR | 70–80** > 70** | 3 | (Wolpaw et al., 1997; Miner et al., 1998) |
| ALS | 2 | P300 | 80† | 3 | (Sellers and Donchin, 2006) |
| ALS | 2 | P300 | 73† | 3 | (Sellers and Donchin, 2006) |
| ALS | 3 | P300 | 62† | 2 | (Sellers and Donchin, 2006) |
| SCI (C5) | 2 | SMR | 73 | 3 | (Müller-Putz et al., 2005) |
| SCI (TH8) | 2 | SMR | 95† | 3 | (Krausz et al., 2003) |
| SCI (L1, incomplete) | 2 | SMR | 72† | 3 | (Krausz et al., 2003) |
| SCI (L1) | 2 | SMR | 80† | 3 | (Krausz et al., 2003) |
| SCI (TH12 (incomplete), L1, L4 (complete)) | 2 | SMR | 80† | 3 | (Krausz et al., 2003) |
| Cerebral palsy | 3 | SMR | 70 | 3 | (Neuper et al., 2003) |
| SCI (C5) | 2 | SMR | ≤100/orthosis control*** | 3 | (Pfurtscheller et al., 2000; Pfurtscheller et al., 2003) |
| ALS | 3 | SMR | 83 | 3 | (Müller-Putz et al., 2004) |
| ALS | 3 | P300 | 80† | 3 | (Piccione et al., 2006) |
| Brain stem stroke | 4 | P300 | 63† | 2†† | (Piccione et al., 2006) |
| SCI (C4) | 2 | P300 | 76† | 3 | (Piccione et al., 2006) |
| GBS | 2 | P300 | 67† | 2†† | (Piccione et al., 2006) |
| Multiple sclerosis | 3 | P300 | 58† | 2†† | (Piccione et al., 2006) |
| SCI (C4 or C5 complete) | 2 | SMR | 87† | 3 | (Kauhanen et al., 2006) |
| SCI (C4 or C5 complete) | 2 | SMR | 88† | 3 | (Kauhanen et al., 2006) |
| SCI (C4 or C5 complete) | 2 | SMR | 69† | 2†† | (Kauhanen et al., 2006) |
| Cerebral palsy | 3 | P300 | 100† | 3 | (Hoffmann et al., 2008) |
| Multiple sclerosis | 3 | P300 | 100† | 3 | (Hoffmann et al., 2008) |
| ALS | 3 | P300 | 100† | 3 | (Hoffmann et al., 2008) |
| Traumatic brain and spinal-cord injury, C4 level | 3 | P300 | 100† | 3 | (Hoffmann et al., 2008) |
| Post-anoxic encephalopathy | 3 | P300 | not reported | 1 | (Hoffmann et al., 2008) |
SCI = spinal cord injury;
CRR reported for the total group of BCI users (1 patient among healthy participants) and not corresponding to the individual participant;
FES = functional electric stimulation.
Offline calculation of performance (no online performance reported).
Discussion
In the present study, we analysed the relationship between physical impairment and level of success in BCI use on the basis of a group of 35 patients from our laboratory and an additional group of 23 patients from other BCI research groups. We conclude that there is no continuous decrement in BCI performance with physical decline in this patient sample. Rather, there seems to be a clear-cut separation between the CLIS patients and all other groups.
All but one patient published by other BCI research groups achieved at least significant control over the required brain response. This result is different from ours and might be due to patient selection: for example, in Wolpaw’s group, only individuals who show a sufficient SMR modulation during a screening session are included in further studies (personal communication). Another reason might be the higher amount of spinal cord injured patients whose disease is not accompanied by cortical degeneration. Finally, as almost all individuals have a P300, above chance level performance (above 3% accuracy) is almost always achieved in the P300-BCI.
Due to the different levels of impairment it may be argued that motivation could have influenced BCI performance as it does in all learning and memory functions. It could be expected that the more in need for a BCI the patients were, the higher would be their motivation to achieve control over BCI. Unfortunately, no data are available to confirm or reject this intuitively plausible assumption. To date, only one study with healthy individuals presented data on motivation during BCI training (Nijboer et al., 2008). The authors found that the higher the mastery confidence and lower the fear of incompetence the better participants performed in SMR-BCI training; better mood also led to better performance. Whether these results hold also true for patients and is influenced by level of impairment is currently investigated in our laboratory.
The amount of training sessions and time period of participation varied across patients. It may be argued that performance could be a function of session number. This holds indeed true for early training sessions. We previously showed that learning occurred in the early stages of training and patients remained stable around the performance level, which they achieved in the first 10 to 20 training sessions (Kübler et al., 2004; Neumann and Birbaumer, 2003). We refrained from taking into account the number of training sessions per patient for the statistical analysis, because this would suggest comparable training conditions for all patients. However, this was not the case, because in many patients training was interrupted for various reasons and time periods, e.g., vacation, illness, work load etc. A patient who was confronted with the more recent version of a BCI type could have been better in performance – not because he or she was trained for a longer period of time than another patient, but because the BCI was better adapted to the patients’ needs.
Eleven patients were confronted with two or three BCI types and learning effects across BCI types may have occurred, in addition to proactive interference from one BCI type to the next. However, on the basis of such a small sample size the question whether such carry-over effects occurred cannot be answered.
Patients in CLIS and patients in advanced stages of ALS show slowing of the waking EEG. This slowing may be at least in part be caused by episodes of anoxia due to inadequate artificial respiration. It is often difficult to decide whether the patient is awake or in sleep stage 1 or 2 (Birbaumer, 2006). Thus, one might partially attribute the failure to learn BCI use to artificial ventilation. Besides the CLIS patients, twelve patients were in LIS or in the state of major impairment depended on artificial ventilation, but BCI performance did not differ between ventilated and non-ventilated patients excluding artificial ventilation as a cause of impaired learning.
One might argue that the lack of communication in CLIS patients as a result of ALS or Guillan Barré Syndrome or other neurological diseases were due to deterioration of cognitive function preventing learning and communication. It is difficult to reject this argument empirically because neuropsychological testing for cognitive function is impossible in a completely paralyzed person. The only available information channel about cognitive processing is the brain itself. Consequently, we developed an event-related brain potential test battery with a series of cognitive paradigms, ranging from simple oddball P300 tasks to highly complex semantic mismatch N400 and personalized memory tasks eliciting late cortical positivities (Kotchoubey et al., 2002; Kotchoubey et al., 2005). More than 100 patients in responsive and non-responsive vegetative state and 14 ALS patients at different stages of the disease were tested. The relationships between the complexity of a cognitive task and the presence or absence of a particular component in the EEG were inconsistent (Kotchoubey et al., 2005), meaning a patient may show absent early cortical components such as the N100 but normal P300, or absent P300 to simple tones but intact P600 to highly complex verbal material. With one exception, all CLIS patients of the sample reported here had ERP-responses to one or more of the complex cognitive tasks, indicating at least partially intact cognitive processing in CLIS (Hinterberger et al., 2005; Kotchoubey et al., 2005). These ERP data do not prove or disprove normal information processing in CLIS but suggest some intact “processing modules” in most ALS patients with CLIS despite a reduced general arousal.
During the late sixties and early seventies Miller and colleagues showed that autonomous functions such as heart rate or blood pressure can be brought under voluntary control with operant conditioning (Miller, 1969). After weeks of artificial nutrition and ventilation, curarized rats learned to increase and decrease heart rate, renal blood flow, dilation and constriction of peripheral arteries in an operant conditioning paradigm rewarding the animals (with intracranial brain stimulation) for changes in their physiological parameters. These landmark results challenged the view that the autonomous system is autonomous and independent of the central nervous system. However, in the eighties, Miller and his students failed to replicate these results (Dworkin and Miller, 1986). This failure was attributed to the missing homeostatic effect of the reward: the reward acquires its positive outcome through the homoeostasis-restoring effects, i.e., ingestion of food restores glucostatic balance. In the curarized rat, where all bodily functions are kept artificially constant, the homeostatic function of the reward is no longer present because imbalances of the equilibrium do not occur.
Chronically curarized rats and patients in the CLIS, artificially fed and ventilated, share many similarities. The unsuccessful efforts to restore communication in these patients by means of BCI and the failure of the curarized rat to achieve voluntary control of autonomous parameters may have a common reason (Birbaumer, 2006). Chronically curarized rats and people with longer time periods in CLIS may loose the perception of the contingency between the required physiological behaviour (SMR decrease, changes of the SCP amplitude or heart rate increase) and its consequences (brain stimulation reward in the curarized rat and cursor control or letter selection in the patient). From the viewpoint of learning theory, extinction occurs in the curarized rat because experimental trials with a clear contingency between the physiological response (e.g., heart rate) and the rewards are too rare in a 24 hour day with one or two hours of training. With two hours of training per day the remaining 22 hours without any contingency provide enough time for extinction. Together with the proposed blunting of the reward value due to the lack of homeostatic imbalances, acquisition of voluntary control of the desired physiological response becomes impossible (Birbaumer, 2006; Dworkin and Miller, 1986). We propose that learning BCI-control or any other contingency before onset of CLIS, which can be transferred from LIS to CLIS should prevent extinction in CLIS.
From the failure to control autonomic functions with operant learning in the curarized rat and the studies on contingency perception and voluntary regulation and the intact cognitive event related potentials, we may conclude that passive sensory information processing is intact in CLIS even at the most complex semantic processing levels. It is the complete lack of motor control and feedback which might be responsible for the cessation of voluntary cognitive activity, goal directed thinking and imagery supporting a “motor theory of thinking” already discussed by William James (James, 1890). A single CLIS patient who learns to communicate with a BCI or any other communication method will disprove our hypothesis.
Acknowledgments
We are greatly indebted to our patients, who made this research possible. Particularly HPS allowed us over the past 11 years deep insights in the psychological state of a person in the locked-in state. We thank our colleagues Samuel Faran, Adrian Furdea, Sebastian Halder, Slavica von Hartlieb, Thilo Hinterberger, Miguel Jordan, Ahmed el Karim, Boris Kleber, Boris Kotchoubey, Seung-Soo Lee, Tamara Matuz, Jürgen Mellinger, Ursula Mochty, Emily Mugler, Nicola Neumann, Femke Nijboer, Ute Strehl, Tracy Trevorrow, and Barbara Wilhelm for their contribution. Supported by the Deutsche Forschungsgemeinschaft (SFB 550/TB5) and the National Institutes of Health NIH (NICHD, HD30146) and NIBIB/National Institute of Neurologic Disorders and Stroke (EB00856)). The authors have no conflicts of interest and no financial interest. Both authors declare that they participated in data collection, data analysis, writing of the manuscript and that they have seen and approved the final version. Both authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Birbaumer N. Breaking the silence: Brain-computer interfaces (BCI) for communication and motor control. Psychophysiology. 2006;43:517–32. doi: 10.1111/j.1469-8986.2006.00456.x. [DOI] [PubMed] [Google Scholar]
- 2.Birbaumer N, Elbert T, Canavan AGM, Rockstroh B. Slow potentials of the cerebral cortex and behavior. Physiological Reviews. 1990;70:1–41. doi: 10.1152/physrev.1990.70.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Birbaumer N, Ghanayim N, Hinterberger T, Iversen I, Kotchoubey B, Kübler A, et al. A spelling device for the paralysed. Nature. 1999;398:297–8. doi: 10.1038/18581. [DOI] [PubMed] [Google Scholar]
- 4.Choularton S, Dale R. User responses to speech recognition errors: consistency of behaviour across domains. 10th Australian International Conference on Speech Science & Technology; Macquire University, Sydney. Australia: Australian Speech Science & Technology Association Inc.; 2004. [Google Scholar]
- 5.Dworkin BR, Miller NE. Failure to replicate visceral learning in the acute curarized rat preparation. Behav Neurosci. 1986;100:299–314. doi: 10.1037//0735-7044.100.3.299. [DOI] [PubMed] [Google Scholar]
- 6.Fabiani M, Gratton G, Karis D, Donchin E. Definition, identification, and reliability of measurement of the P300 component of the event-related brain potential. Advances in Psychophysiology. 1987;2:1–78. [Google Scholar]
- 7.Farwell LA, Donchin E. Talking off the top of your head: Toward a mental prosthesis utilizing event-related brain potentials. Electroencephalography and Clinical Neurophysiology. 1988;70:512–523. doi: 10.1016/0013-4694(88)90149-6. [DOI] [PubMed] [Google Scholar]
- 8.Hill NJ, Lal TN, Schroder M, Hinterberger T, Wilhelm B, Nijboer F, et al. Classifying EEG and ECoG signals without subject training for fast BCI implementation: comparison of nonparalyzed and completely paralyzed subjects. IEEE Trans Neural Syst Rehabil Eng. 2006;14:183–6. doi: 10.1109/TNSRE.2006.875548. [DOI] [PubMed] [Google Scholar]
- 9.Hinterberger T, Birbaumer N, Flor H. Assessment of cognitive function and communication ability in a completely locked-in patient. Neurology. 2005;64:1307–8. doi: 10.1212/01.WNL.0000156910.32995.F4. [DOI] [PubMed] [Google Scholar]
- 10.Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–71. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann U, Vesin JM, Ebrahimi T, Diserens K. An efficient P300-based brain-computer interface for disabled subjects. J Neurosci Methods. 2008;167:115–25. doi: 10.1016/j.jneumeth.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 12.James W. The Principles of Psychology. Holt, N.Y.: Reprint: Harvard Univ. Press.Cambridge, Mass; 1983. p. 1890. [Google Scholar]
- 13.Kauhanen L, Nykopp T, Lehtonen J, Jylanki P, Heikkonen J, Rantanen P, et al. EEG and MEG brain-computer interface for tetraplegic patients. IEEE Trans Neural Syst Rehabil Eng. 2006;14:190–3. doi: 10.1109/TNSRE.2006.875546. [DOI] [PubMed] [Google Scholar]
- 14.Kirk RE. Experimental design: Procedures for the behavioral sciences. Belmont, CA: Brooks/Cole; 1982. [Google Scholar]
- 15.Kotchoubey B, Lang S, Bostanov V, Birbaumer N. Is there a mind? Electrophysiology of unconscious patients News Physiol Sci. 2002;17:38–42. doi: 10.1152/physiologyonline.2002.17.1.38. [DOI] [PubMed] [Google Scholar]
- 16.Kotchoubey B, Lang S, Bostanov V, Birbaumer N. Cortical processing in Guillain-Barre syndrome after years of total immobility. J Neurol. 2003a;250:1121–3. doi: 10.1007/s00415-003-0132-2. [DOI] [PubMed] [Google Scholar]
- 17.Kotchoubey B, Lang S, Winter S, Birbaumer N. Cognitive processing in completely paralyzed patients with amyotrophic lateral sclerosis. Eur J Neurol. 2003b;10:551–8. doi: 10.1046/j.1468-1331.2003.00647.x. [DOI] [PubMed] [Google Scholar]
- 18.Kotchoubey B, Lang S, Mezger G, Schmalohr D, Schneck M, Semmler A, et al. Information processing in severe disorders of consciousness: vegetative state and minimally conscious state. Clin Neurophysiol. 2005;116:2441–53. doi: 10.1016/j.clinph.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 19.Krausz G, Scherer R, Korisek G, Pfurtscheller G. Critical decision-speed and information transfer in the "Graz Brain-Computer Interface". Appl Psychophysiol Biofeedback. 2003;28:233–40. doi: 10.1023/a:1024637331493. [DOI] [PubMed] [Google Scholar]
- 20.Kübler A. Brain-computer communication - development of a brain-computer interface for locked-in patients on the basis of the psychophysiological self-regulation training of slow cortical potentials (SCP) Tübingen: Schwäbische Verlagsgesellschaft; 2000. [Google Scholar]
- 21.Kübler A, Neumann N, Wilhelm B, Hinterberger T, Birbaumer N. Predictability of brain-computer communication. Journal of Psychophysiology. 2004;18:121–129. [Google Scholar]
- 22.Kübler A, Neumann N, Kaiser J, Kotchoubey B, Hinterberger T, Birbaumer NP. Brain-computer communication: self-regulation of slow cortical potentials for verbal communication. Arch Phys Med Rehabil. 2001;82:1533–9. doi: 10.1053/apmr.2001.26621. [DOI] [PubMed] [Google Scholar]
- 23.Kübler A, Kotchoubey B, Hinterberger T, Ghanayim N, Perelmouter J, Schauer M, et al. The Thought Translation Device: A neurophysiological approach to communication in total motor paralysis. Exp Brain Res. 1999;124:223–232. doi: 10.1007/s002210050617. [DOI] [PubMed] [Google Scholar]
- 24.Kübler A, Nijboer F, Mellinger J, Vaughan TM, Pawelzik H, Schalk G, et al. Patients with ALS can use sensorimotor rhythms to operate a brain-computer interface. Neurology. 2005;64:1775–7. doi: 10.1212/01.WNL.0000158616.43002.6D. [DOI] [PubMed] [Google Scholar]
- 25.Kuebler A, Kotchoubey B, Salzmann HP, Ghanayim N, Perelmouter J, Homberg V, et al. Self-regulation of slow cortical potentials in completely paralyzed human patients. Neurosci Lett. 1998;252:171–4. doi: 10.1016/s0304-3940(98)00570-9. [DOI] [PubMed] [Google Scholar]
- 26.Laureys S, Owen AM, Schiff ND. Brain function in coma, vegetative state, and related disorders. Lancet Neurol. 2004;3:537–46. doi: 10.1016/S1474-4422(04)00852-X. [DOI] [PubMed] [Google Scholar]
- 27.McFarland DJ, Sarnacki WA, Wolpaw JR. Brain-computer interface (BCI) operation: optimizing information transfer rates. Biol Psychol. 2003;63:237–51. doi: 10.1016/s0301-0511(03)00073-5. [DOI] [PubMed] [Google Scholar]
- 28.McFarland DJ, Sarnacki WA, Vaughan TM, Wolpaw JR. Brain-computer interface (BCI) operation: signal and noise during early training sessions. Clin Neurophysiol. 2005;116:56–62. doi: 10.1016/j.clinph.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Miller NE. Learning of visceral and glandular responses. Science. 1969;163:434–45. doi: 10.1126/science.163.3866.434. [DOI] [PubMed] [Google Scholar]
- 30.Miner LA, McFarland DJ, Wolpaw JR. Answering questions with an electroencephalogram-based brain-computer interface. Arch Phys Med Rehabil. 1998;79:1029–33. doi: 10.1016/s0003-9993(98)90165-4. [DOI] [PubMed] [Google Scholar]
- 31.Müller-Putz G, Scherer R, Neuper C, Lahrmann H, Staiger-Sälzer P, Pfurtscheller G. EEG-basierende Kommunikation: Erfahrungen mit einem Telemonitoringsystem zum Patiententraining. Biomedizinische Technik. Beiträge zur 38. Jahrestagung der Deutschen Gesellschaft für Biomedizinische Technik im VDE - BMT. Vol 49, Ergänzungsband 2, Teil 1. Berlin, 2004: 230–231.
- 32.Müller-Putz GR, Scherer R, Pfurtscheller G, Rupp R. EEG-based neuroprosthesis control: a step towards clinical practice. Neurosci Lett. 2005;382:169–74. doi: 10.1016/j.neulet.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 33.Neumann N, Birbaumer N. Predictors of successful self control during brain-computer communication. J Neurol Neurosurg Psychiatry. 2003;74:1117–21. doi: 10.1136/jnnp.74.8.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neumann N, Kubler A, Kaiser J, Hinterberger T, Birbaumer N. Conscious perception of brain states: mental strategies for brain-computer communication. Neuropsychologia. 2003;41:1028–36. doi: 10.1016/s0028-3932(02)00298-1. [DOI] [PubMed] [Google Scholar]
- 35.Neuper C, Müller GR, Kübler A, Birbaumer N, Pfurtscheller G. Clinical application of an EEG-based brain-computer interface: a case study in a patient with severe motor impairment. Clin Neurophysiol. 2003;114:399–409. doi: 10.1016/s1388-2457(02)00387-5. [DOI] [PubMed] [Google Scholar]
- 36.Niedermeyer E. The normal EEG of the waking adult. In: Niedermeyer E, Lopes da Silva FH, editors. Electroencephalography - Basic Principles, Clinical Applications, and Related Fields. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 167–192. [Google Scholar]
- 37.Nijboer F, Furdea A, Gunst I, Mellinger J, McFarland DJ, Birbaumer N, et al. An auditory brain-computer interface (BCI) J Neurosci Methods. 2008;167:43–50. doi: 10.1016/j.jneumeth.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nijboer F, Sellers E, Mellinger J, Matuz T, Mochty U, Jordan M, et al. A brain-computer interface (BCI) for people with amyotrophic lateral sclerosis (ALS) Clin Neurophys. doi: 10.1016/j.clinph.2008.03.034. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Detecting awareness in the vegetative state. Science. 2006;313:1402. doi: 10.1126/science.1130197. [DOI] [PubMed] [Google Scholar]
- 40.Perelmouter J, Birbaumer N. A binary spelling interface with random errors. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2000;8:227–32. doi: 10.1109/86.847824. [DOI] [PubMed] [Google Scholar]
- 41.Pfurtscheller G, Aranibar A. Evaluation of event-related desynchronization (ERD) preceding and following self-paced movements. Electroencephalography and Clinical Neurophysiology. 1979;46:138–146. doi: 10.1016/0013-4694(79)90063-4. [DOI] [PubMed] [Google Scholar]
- 42.Pfurtscheller G, Guger C, Müller G, Krausz G, Neuper C. Brain oscillations control hand orthosis in a tetraplegic. Neuroscience Letters. 2000;292:211–214. doi: 10.1016/s0304-3940(00)01471-3. [DOI] [PubMed] [Google Scholar]
- 43.Pfurtscheller G, Müller GR, Pfurtscheller J, Gerner HJ, Rupp R. 'Thought' - control of functional electrical stimulation to restore hand grasp in a patient with tetraplegia. Neurosci Lett. 2003;351:33–6. doi: 10.1016/s0304-3940(03)00947-9. [DOI] [PubMed] [Google Scholar]
- 44.Piccione F, Giorgi F, Tonin P, Priftis K, Giove S, Silvoni S, et al. P300-based brain computer interface: reliability and performance in healthy and paralysed participants. Clin Neurophysiol. 2006;117:531–7. doi: 10.1016/j.clinph.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 45.Sellers EW, Donchin E. A P300-based brain-computer interface: initial tests by ALS patients. Clin Neurophysiol. 2006;117:538–48. doi: 10.1016/j.clinph.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 46.Sellers EW, Kübler A, Donchin E. Brain-computer interface research at the University of South Florida cognitive psychophysiology laboratory: The P300 Speller. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2006;14:221–224. doi: 10.1109/TNSRE.2006.875580. [DOI] [PubMed] [Google Scholar]
- 47.Wolpaw JR, McFarland DJ. Control of a two-dimensional movement signal by a noninvasive brain-computer interface in humans. Proc Natl Acad Sci U S A. 2004;101:17849–54. doi: 10.1073/pnas.0403504101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolpaw JR, Flotzinger D, Pfurtscheller G, McFarland DJ. Timing of EEG-based cursor control. J Clin Neurophysiol. 1997;14:529–38. doi: 10.1097/00004691-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 49.Wolpaw JR, Birbaumer N, Heetderks WJ, McFarland DJ, Peckham PH, Schalk G, et al. Brain-computer interface technology: a review of the first international meeting. IEEE Trans Rehabil Eng. 2000;8:164–173. doi: 10.1109/tre.2000.847807. [DOI] [PubMed] [Google Scholar]