Abstract
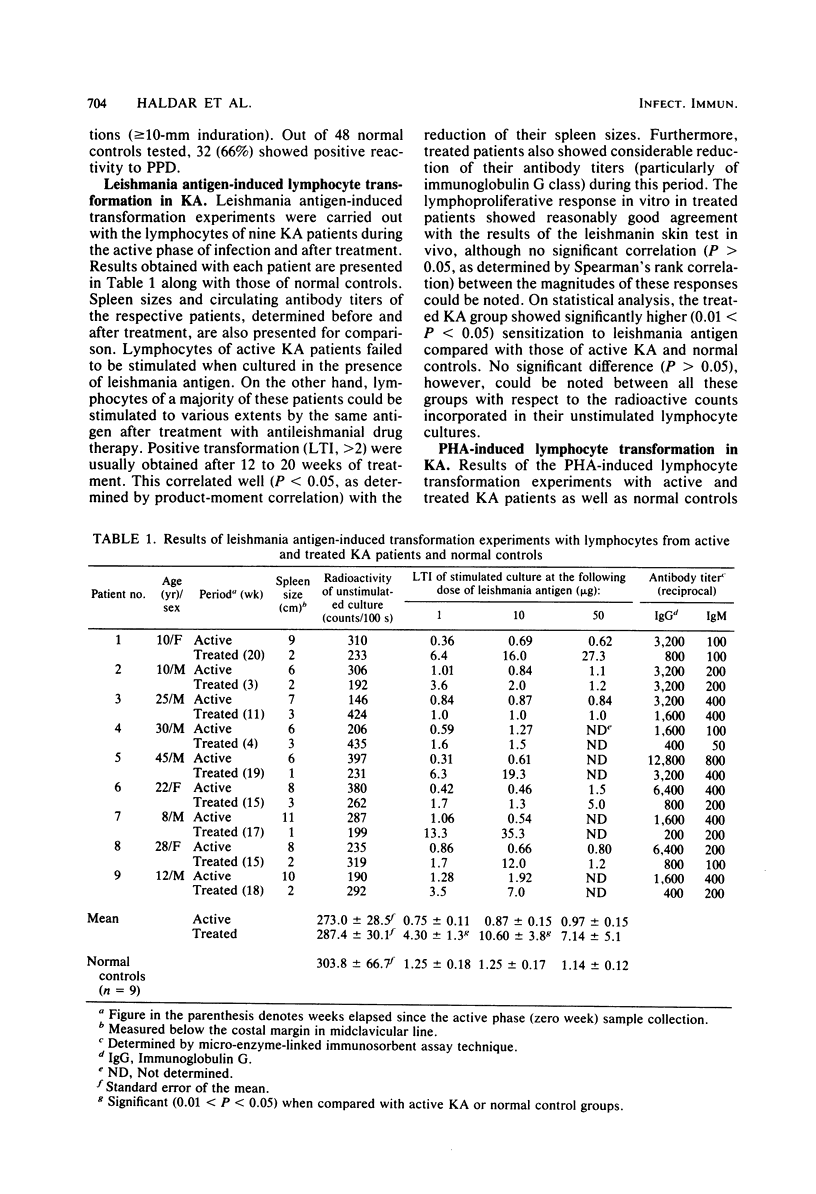
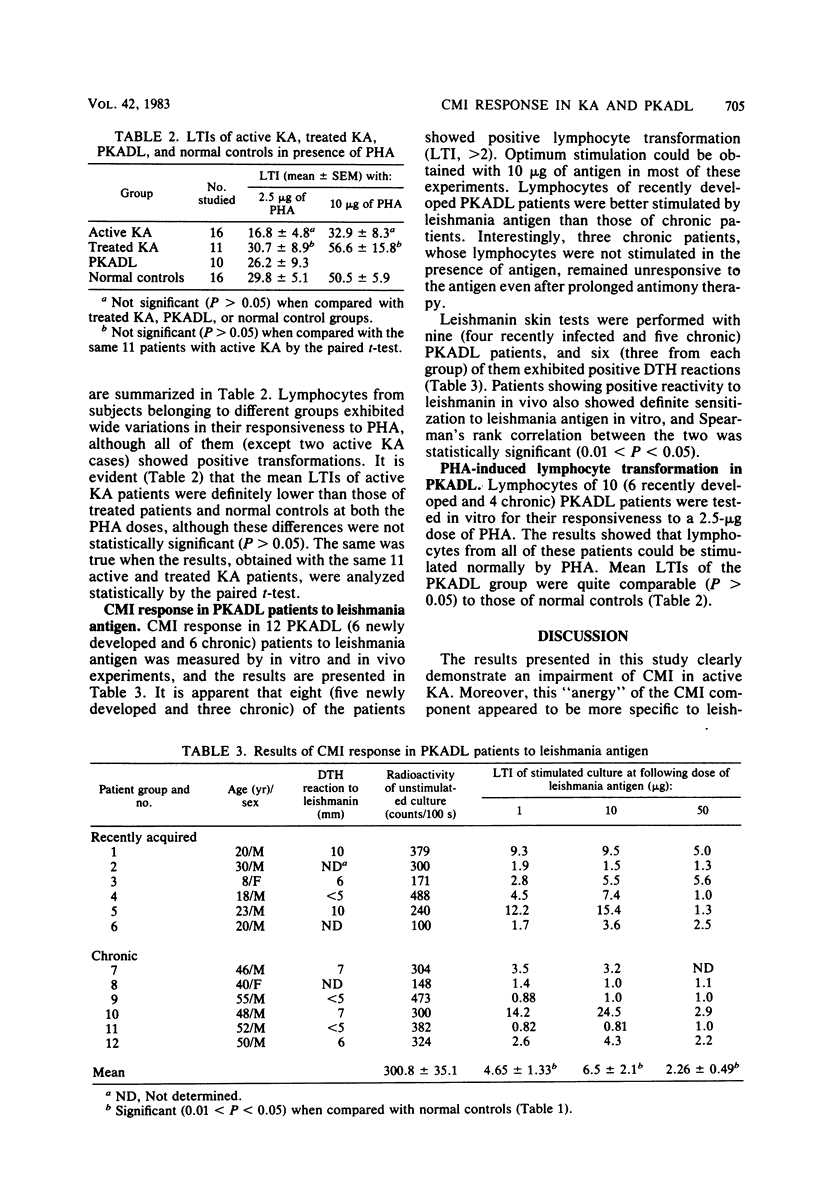
Cell-mediated immune (CMI) response in 16 Indian kala-azar (KA) and 12 post-kala-azar dermal leishmaniasis (PKADL) patients was studied in detail by in vitro lymphocyte transformation experiments and by in vivo skin testing. Peripheral blood lymphocytes of active KA patients failed to be stimulated by leishmania antigen. On the other hand, lymphocytes from a majority of the active KA patients could be stimulated by phytohemagglutinin. Active KA patients also failed to show delayed type hypersensitivity reaction to leishmanin, although 72% of them showed delayed type hypersensitivity to a purified protein derivative of tuberculin. Longitudinal studies indicated that antigen-specific CMI response usually appeared in treated KA patients after 12 to 20 weeks of antileishmanial drug therapy, although individual variations were noted. CMI response in PKADL patients was variable as about two-thirds of them showed positive sensitization to leishmania antigen in either in vivo or in vitro tests. Usually, patients with newly acquired PKADL exhibited better CMI response than those with chronic PKADL. However, lymphocytes from all of these patients could be stimulated normally by phytohemagglutinin. Results presented in this study show an impairment of CMI response in active KA which appears to be more specific to leishmania than generalized in nature. Moreover, restoration of specific T-cell responsiveness was aided by antileishmanial drug therapy which resulted in the reduction of antigenic load by parasite destruction and a concomitant decrease in circulating antibody levels, particularly that of the immunoglobulin G class. We suggest that the protection afforded by specific CMI response against Leishmania donovani infection may not be absolute and probably depends on other host-related factors leading to parasite destruction and patient recovery.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER S. LEISHMANIA. Adv Parasitol. 1964;2:35–96. doi: 10.1016/s0065-308x(08)60586-2. [DOI] [PubMed] [Google Scholar]
- Aikat B. K., Pathania A. G., Sehgal S., Bhattacharya P. K., Dutta U., Pasricha N., Singh S., Parmar R. S., Sahaya S., Prasad L. S. Immunological response in Indian kala-azar. Indian J Med Res. 1979 Oct;70:583–591. [PubMed] [Google Scholar]
- Broeckaert-van Orshoven A., Michielsen P., Vandepitte J. Fatal leishmaniasis in renal-transplant patient. Lancet. 1979 Oct 6;2(8145):740–741. doi: 10.1016/s0140-6736(79)90664-0. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cahill K. M. Field techniques in the diagnosis of kala-azar. Trans R Soc Trop Med Hyg. 1970;64(1):107–110. doi: 10.1016/0035-9203(70)90201-4. [DOI] [PubMed] [Google Scholar]
- Carvalho E. M., Teixeira R. S., Johnson W. D., Jr Cell-mediated immunity in American visceral leishmaniasis: reversible immunosuppression during acute infection. Infect Immun. 1981 Aug;33(2):498–500. doi: 10.1128/iai.33.2.498-500.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose A. C., Haldar J. P., Pal S. C., Mishra B. P., Mishra K. K. Phytohaemagglutinin-induced lymphocyte transformation test in Indian kala-azar. Trans R Soc Trop Med Hyg. 1979;73(6):725–726. doi: 10.1016/0035-9203(79)90032-4. [DOI] [PubMed] [Google Scholar]
- Ghose A. C., Haldar J. P., Pal S. C., Mishra B. P., Mishra K. K. Serological investigations on Indian kala-azar. Clin Exp Immunol. 1980 May;40(2):318–326. [PMC free article] [PubMed] [Google Scholar]
- Haldar J. P., Saha K. C., Ghose A. C. Serological profiles in Indian post kala-azar dermal leishmaniasis. Trans R Soc Trop Med Hyg. 1981;75(4):514–517. doi: 10.1016/0035-9203(81)90188-7. [DOI] [PubMed] [Google Scholar]
- Hepner Levy L., Mendes E. Impaired cell-mediated immunity in patients with kala-azar. Allergol Immunopathol (Madr) 1981 Mar-Apr;9(2):109–112. [PubMed] [Google Scholar]
- Ho M., Koech D. K., Iha D. W., Bryceson A. D. Immunosuppression in Kenyan visceral leishmaniasis. Clin Exp Immunol. 1983 Feb;51(2):207–214. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANSON-BAHR P. E. Immunity in kala-azar. Trans R Soc Trop Med Hyg. 1961 Nov;55:550–555. doi: 10.1016/0035-9203(61)90078-5. [DOI] [PubMed] [Google Scholar]
- Pampiglione S., Manson-Bahr P. E., La Placa M., Borgatti M. A., Musumeci S. Studies in Mediterranean leishmaniasis. 3. The leishmanin skin test in kala-azar. Trans R Soc Trop Med Hyg. 1975;69(1):60–68. doi: 10.1016/0035-9203(75)90012-7. [DOI] [PubMed] [Google Scholar]
- Ray R., Ghose A. C. Cultivation of Leishmania donovani in vitro in a high yielding liquid culture medium. Indian J Med Res. 1980 Feb;71:203–206. [PubMed] [Google Scholar]
- Rezai H. R., Ardehali S. M., Amirhakimi G., Kharazmi A. Immunological features of kala-azar. Am J Trop Med Hyg. 1978 Nov;27(6):1079–1083. doi: 10.4269/ajtmh.1978.27.1079. [DOI] [PubMed] [Google Scholar]
- Sercarz E. E., Yowell R. L., Turkin D., Miller A., Araneo B. A., Adorini L. Different functional specificity repertoires for suppressor and helper T cells. Immunol Rev. 1978;39:108–136. doi: 10.1111/j.1600-065x.1978.tb00398.x. [DOI] [PubMed] [Google Scholar]
- Southgate B. A., Manson-Bahr P. E. Studies in the epidemiology of East African leishmaniasis. 4. The significance of the positive leishmanin test. J Trop Med Hyg. 1967 Feb;70(2):29–33. [PubMed] [Google Scholar]
- Turk J. L., Bryceson A. D. Immunological phenomena in leprosy and related diseases. Adv Immunol. 1971;13:209–266. doi: 10.1016/s0065-2776(08)60185-6. [DOI] [PubMed] [Google Scholar]
- Veress B., Omer A., Satir A. A., El Hassan A. M. Morphology of the spleen and lymph nodes in fatal visceral leishmaniasis. Immunology. 1977 Nov;33(5):605–610. [PMC free article] [PubMed] [Google Scholar]
- Wyler D. J. Circulating factor from a kala-azar patient suppresses in vitro antileishmanial T cell proliferation. Trans R Soc Trop Med Hyg. 1982;76(3):304–306. doi: 10.1016/0035-9203(82)90174-2. [DOI] [PubMed] [Google Scholar]
- Wyler D. J., Weinbaum F. I., Herrod H. R. Characterization of in vitro proliferative responses of human lymphocytes to leishmanial antigens. J Infect Dis. 1979 Aug;140(2):215–221. doi: 10.1093/infdis/140.2.215. [DOI] [PubMed] [Google Scholar]


