Abstract
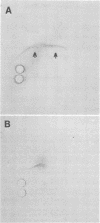
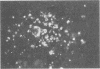
Fresh normal chicken serum (NCS) which lacked virus-neutralizing antibody to fowlpox virus (FPV) was found to inhibit the appearance of the cytopathic effect of the virus, virus growth, and plaque formation in chicken embryo cells. Immunofluorescent examination revealed the deposition of the third component of complement (C3) on FPV-infected chicken embryo cells incubated with fresh NCS. The inhibitory activity of fresh NCS on viral cytopathic effect was independent of the Ca2+ ion and was abrogated by treatment of fresh NCS with inulin or zymosan. Similarly, deposition of C3 on FPV-infected cells occurred independently of the Ca2+ ion and was inhibited by treatment of fresh NCS with inulin or zymosan but was not inhibited by absorption with FPV-infected cells. These results suggest that antibody-independent activation of complement by FPV-infected cells via the alternative pathway caused the inhibition of the virus growth as well as the C3 deposition. Involvement of complement activation as nonspecific host response to virus infection was also suggested by the demonstration of the C3 deposition in the skin lesions of FPV-infected chickens.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Frank M. M. The complement system in host defense and inflammation. Rev Infect Dis. 1979 May-Jun;1(3):483–501. doi: 10.1093/clinids/1.3.483. [DOI] [PubMed] [Google Scholar]
- Hirsch R. L. The complement system: its importance in the host response to viral infection. Microbiol Rev. 1982 Mar;46(1):71–85. doi: 10.1128/mr.46.1.71-85.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai C., Yoshikawa Y., Yamanouchi K., Okada H. Isolation and identification of the third component of complement of Japanese quails. J Immunol. 1983 Jun;130(6):2814–2820. [PubMed] [Google Scholar]
- Kierszenbaum F., Ivanyi J., Budzko D. B. Mechanisms of natural resistance to trypanosomal infection. Role of complement in avian resistance to Trypanosoma cruzi infection. Immunology. 1976 Jan;30(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Morita C. Role of humoral and cell-mediated immunity on the recovery of chickens from fowlpox virus infection. J Immunol. 1973 Nov;111(5):1495–1501. [PubMed] [Google Scholar]
- Norley S. G., Wardley R. C. Complement-mediated lysis of African swine fever virus-infected cells. Immunology. 1982 May;46(1):75–82. [PMC free article] [PubMed] [Google Scholar]
- Pathak P. N., Rao G. V., Tompkins W. A. In vitro cellular immunity to unrelated pathogens in chickens infected with fowlpox virus. Infect Immun. 1974 Jul;10(1):34–41. doi: 10.1128/iai.10.1.34-41.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Albrecht P., Hicks J. T., Meyer B. C., Ennis F. A. Sensitive neutralization test for virus antibody. 1. Mumps antibody. Arch Virol. 1978;58(4):301–311. doi: 10.1007/BF01317822. [DOI] [PubMed] [Google Scholar]
- Sissons J. G., Oldstone M. B. Antibody-mediated destruction of virus-infected cells. Adv Immunol. 1980;29:209–260. doi: 10.1016/S0065-2776(08)60045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sissons J. G., Oldstone M. B., Schreiber R. D. Antibody-independent activation of the alternative complement pathway by measles virus-infected cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):559–562. doi: 10.1073/pnas.77.1.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy D. N., Hanson L. E., Myers W. L. Detection of fowlpox virus antigen in tissue culture by fluorescent antibody technique. Avian Dis. 1970 Nov;14(4):810–812. [PubMed] [Google Scholar]
- Ueda Y., Tagaya I., Amano H., Ito M. Studies on the early antigens induced by vaccinia virus. Virology. 1972 Sep;49(3):794–800. doi: 10.1016/0042-6822(72)90535-1. [DOI] [PubMed] [Google Scholar]
- Yoshikawa Y., Yamanouchi K., Hishiyama M., Kobune K. Lysis of RSV-transformed Japanese quail cells by a factor from normal quail serum. Int J Cancer. 1978 May 15;21(5):658–666. doi: 10.1002/ijc.2910210518. [DOI] [PubMed] [Google Scholar]