Abstract
Objectives
Laparoscopic partial nephrectomy requires advanced training in minimally invasive techniques in order to accomplish precise tumor resection and renal reconstruction while minimizing warm ischemia times. Complex renal tumors may preclude a minimally invasive approach to nephron sparing surgery in some patients. We describe our technique, illustrated with video, of robotic partial nephrectomy for challenging renal tumors, including hilar, endophytic, and multiple tumors.
Methods
Robotic assistance was used to resect 14 tumors in eight patients (mean age 50.3 years, range 30–68 years). Three patients had hereditary kidney cancer. All patients had complex tumor features on preoperative imaging, including hilar tumors (five patients), endophytic tumors (four patients), and/or multiple tumors (three patients).
Results
Robotic partial nephrectomy procedures were performed successfully without complications. Hilar clamping was utilized with a mean warm ischemia time of 31 minutes (range 24–45 minutes). Mean blood loss was 230 ml (range 100–450 ml). Histopathology confirmed clear cell renal cell carcinoma (n=3), hybrid oncocytic tumor (n=2), chromophobe renal cell carcinoma (n=2), and oncocytoma (n=1). All patients had negative surgical margins. Mean index tumor size was 3.6 cm (range 2.6–6.4 cm). Mean hospital stay was 2.6 days. At three months follow-up, no patients experienced perioperative complications or a statistically significant change in serum creatinine or estimated glomerular filtration rate and there was no evidence of tumor recurrence.
Conclusions
Robotic partial nephrectomy is a safe and feasible approach for select patients with complex renal tumors, including hilar, endophytic, and multiple tumors. Robotic assistance may facilitate tumor resection and renal reconstruction for challenging cases, offering a minimally invasive surgical option for select patients with complex tumors who might otherwise require open surgery.
Keywords: kidney cancer, laparoscopy, partial nephrectomy, robotics, technique
INTRODUCTION
Minimally invasive nephron sparing surgery has become increasingly popular as expertise in laparoscopy has increased and has demonstrated excellent long-term renal functional and oncological outcomes1, 2. Laparoscopic partial nephrectomy is a technically challenging procedure, requiring advanced laparoscopic skills to accomplish tumor resection, hemostasis, and renal reconstruction with sufficient speed of intracorporeal suturing to minimize warm ischemia times. Complex renal tumors, such as hilar, endophytic, and multiple tumors, present additional challenges to a nephron-sparing approach using conventional laparoscopy.
The da Vinci® surgical system may facilitate performing these complex renal tumors using the minimally invasive surgical approach. Potential advantages include 3-dimensional stereoscopic vision, articulating instruments, and scaled-down movements reducing tremor. The articulating instruments and increased freedom of movement may also allow the surgeon to replicate well established open surgical maneuvers more readily. Robotic assistance is well established in radical prostate surgery and it has also become more common for renal procedures, including pyeloplasty3, radical nephrectomy4, and donor nephrectomy5. Preliminary reports have demonstrated the safety and feasibility of robotic partial nephrectomy6–9.
We describe our technique of robotic partial nephrectomy in the setting of complex renal tumors, including hilar, endophytic, and multiple tumors. We include a detailed outline of the procedure with corresponding video segments illustrating how robotic assistance can facilitate the challenges of tumor excision and renal reconstruction for complex renal masses.
METHODS
INTRODUCTION AND IMAGING (Video Clip #1)
At the National Cancer Institute, 28 patients underwent partial nephrectomy between March 2007 and July 2007. Robotic partial nephrectomy was offered to eight consecutive patients with complex renal tumors, defined as hilar (tumor abutting hilar vessels), endophytic (complete), or multiple tumors. Other surgical approaches to partial nephrectomy performed during that time included open (n = 16), laparoscopic intraperitoneal (n=2), and laparoscopic retroperitoneal (n = 2). Selection criteria for a robotic approach was based on complex tumor features and patient preference rather than by randomization. Within our cohort of robotic patients, no patients had a solitary kidney or prior renal surgery. Both the console surgeon and assistant were fellowship trained with experience in laparoscopic and robotic surgery.
Patient demographic data for those who underwent robotic partial nephrectomy are summarized in Table 1. Mean patients age was 50.3 yr (range: 30–61 yr) and all patients had normal preoperative creatinine levels and were without major medical comorbidities. We present a brief clinical description of four patients mentioned in our accompanying video or figures.
Table 1.
Patient demographic data
| Patient total (n) | 8 |
| Number of tumors resected | 14 |
| Mean age, yr (range) | 50.3 (30–61) |
| Sex (n) | |
| Male | 2 |
| Female | 6 |
| Mean preoperative serum creatinine in mg/dl (range) | 0.9 (0.7–1.2) |
| Mean preoperative estimated GFR | 84.9 (67–122) |
| Side of involvement (n) | |
| Right | 3 |
| Left | 5 |
| GFR=glomerular filtration rate (ml/min/1.73m2) | |
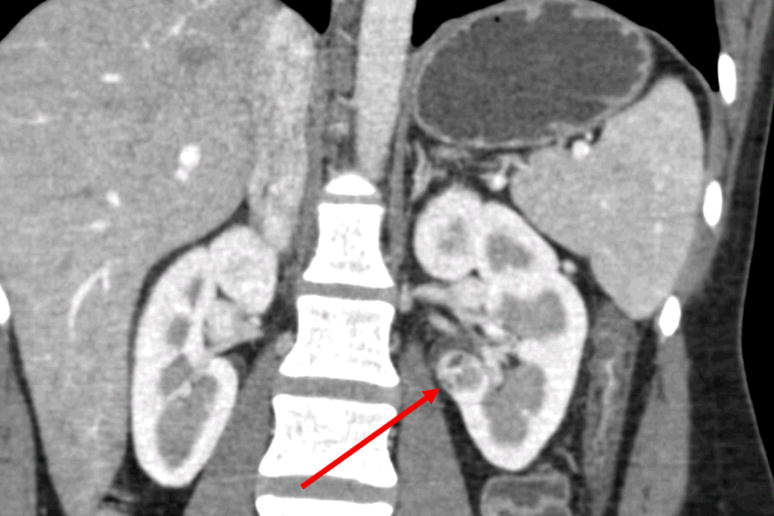
A 30 year old female presented with an incidental left renal hilar mass. A CT scan demonstrated a 2.6 cm solid, enhancing mass located near the renal hilum adjacent to the posterior renal pelvis and major branches of the renal hilar vessels (Figure 1). A percutaneous renal biopsy had been performed at another facility, demonstrating clear cell renal cell carcinoma, Fuhrman grade 2.
Fig 1.
Hilar tumor. CT demonstrates a 2.6 cm solid tumor located near the left renal vessels and abutting the renal pelvis.
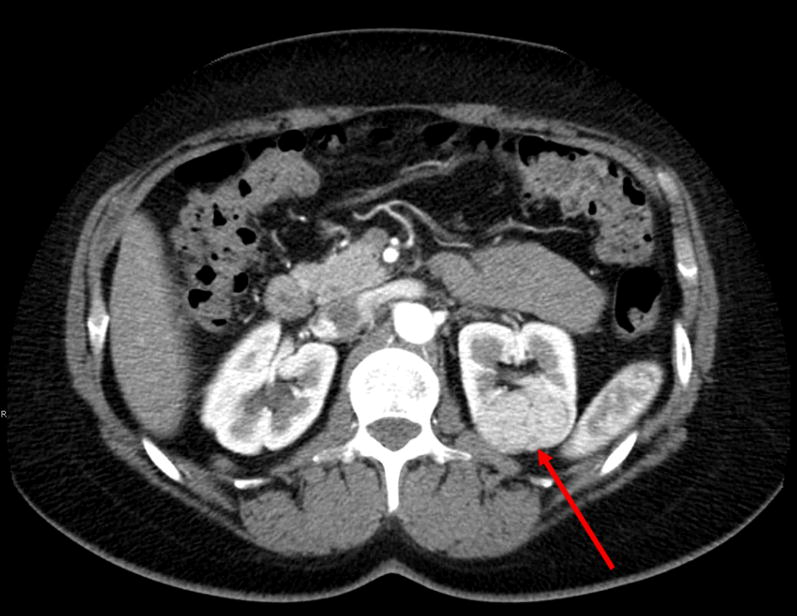
A 60 year old female with Birt-Hogg Dube syndrome presented with a 2.6 cm solid, enhancing renal mass in the left upper pole that was completely endophytic (Figure 2) as well as smaller masses in the midpole measuring 1.1 cm and 0.8 cm. The accompanying video demonstrates resection of the endophytic and multiple tumors.
Fig 2.
Endophytic tumor. CT demonstrates a left 2.6 cm solid upper pole renal mass.
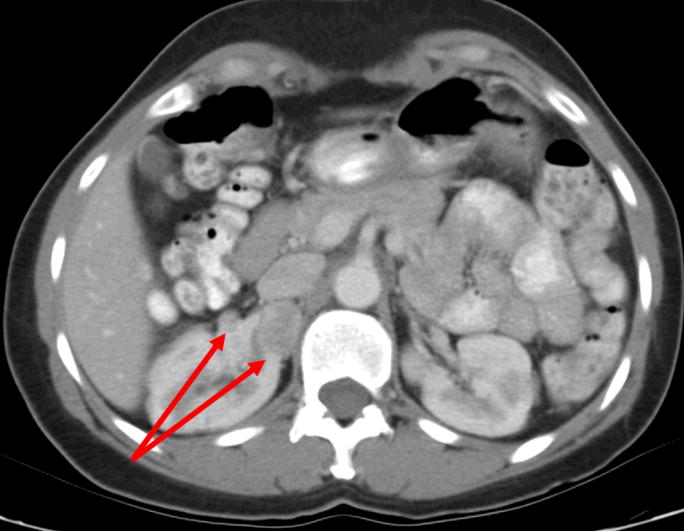
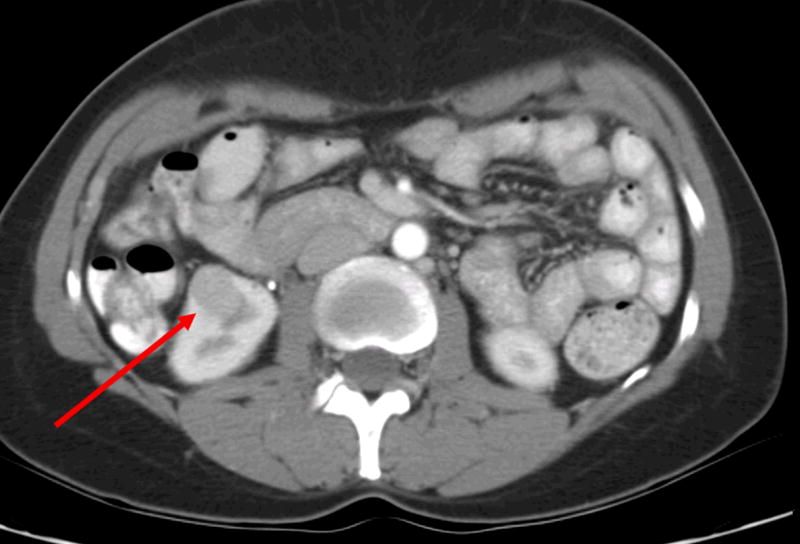
A 47 year old female was incidentally diagnosed with bilateral renal masses. A CT scan demonstrated four tumors in the right kidney with three tumors in the upper pole (1.2–2.9 cm) and a 2.1 cm mass in the right lower pole (Figure 3).
Fig 3.
a. Multiple renal tumors. CT demonstrates two tumors in the mid/upper pole of the right kidney.
b. Multiple renal tumors in the same patient. CT scan also demonstrates a right lower pole renal mass.
SURGICAL TECHNIQUE
We describe our surgical technique of robotic partial nephrectomy for complex renal tumors, illustrated with video. This technique emulates both open and laparoscopic techniques of nephron-sparing surgery.
Positioning, Ports, and Docking: (Video Clip #2)
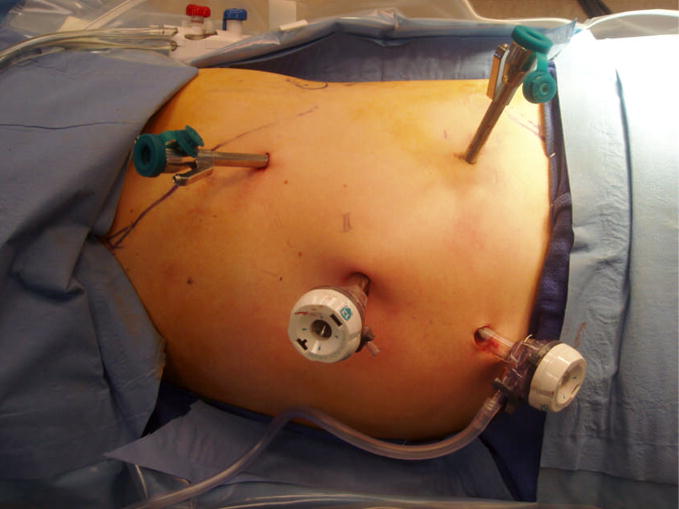
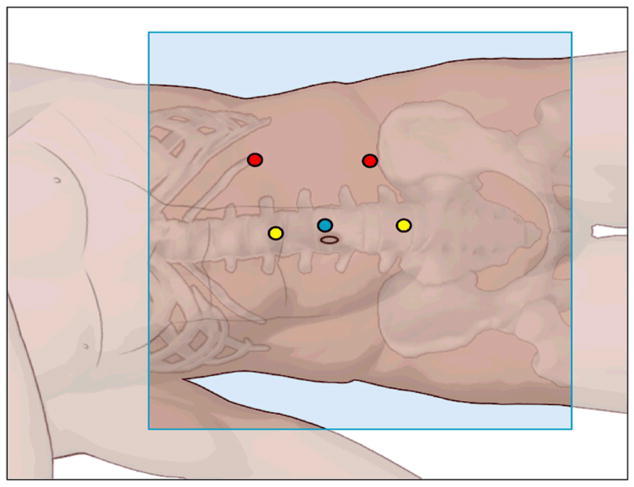
Prior to positioning the patient, cystoscopy was performed and a ureteral catheter was placed to instill methylene blue for identification of the collecting system. The patient is positioned in either modified flank or full flank position and a pneumoperitoneum of 15 mm Hg is established. Ports are placed as demonstrated in Figures 4a,b. A 12mm periumbilical port is placed for the camera. Two robotic instrument ports are placed approximately 8 cm from the camera in a wide “V” configuration centered on the renal tumor. These ports may be shifted laterally and/or superiorly for patients with a large body habitus or upper pole tumor location. A 12 mm assistant port is placed inferior to the camera port. An optional 5mm assistant port may be placed above the camera port if needed. For docking, the robot is brought in posteriorly at approximately a 20 degree angle toward the head of the patient.
Fig 4.
a. Port site placement for robotic partial nephrectomy. Periumbilical camera port for hilar tumor. Ports may be shifted laterally and superiorly for upper pole tumors.
b. Schematic of port site placement demonstrating a periumbilical camera port (blue), robotic instrument ports (red), and assistant ports (yellow).
Bowel Mobilization: (Video Clip #3)
A zero degree lens is used initially, but a 30 degree downward lens may also be utilized as needed. Robotic instruments used include a Bipolar Maryland forceps, monopolar cautery scissors, and needle drivers. The peritoneum is incised sharply along the Line of Toldt and the bowel is mobilized medially using sharp and blunt dissection, developing the plane between the anterior Gerota’s fascia and the posterior mesocolon. The bedside assistant maintains medial counter-traction. A full flank position further aids with bowel mobilization. Dissection is continued along the upper pole of the kidney to mobilize the spleen or liver. We utilize robotic assistance from the beginning of bowel mobilization. However, a “hybrid technique”, initially utilizing conventional laparoscopy to reflect the bowel, may also be used if needed.
Anatomical Landmarks and Hilar Dissection: (Video Clip #4)
Continued medial reflection of the bowel allows for exposure of the gonadal vessels and the ureter. These structures are retracted anteriorly, exposing the underlying psoas muscle. Care is taken not to strip the fascia from the psoas muscle. Dissection then proceeds towards the renal hilum. The Maryland bipolar forceps are used to place the kidney on stretch and the renal hilar vessels are dissected to allow access for clamp placement. Lateral renal attachments are left in place to aid in countertraction. Venous branches can be ligated as needed for exposure.
Ultrasound and Tumor Exposure: (Video Clip #5)
A laparoscopic ultrasound probe is used to map the location and size of renal tumors and to confirm resection margins and depth. Gerota’s fascia is opened and the fat is cleaned off the renal capsule to expose the tumor(s). If the fat overlying the tumor must be removed to optimize view of adjacent critical structures to preserve them, this fat can be sent separately to pathology for analysis for possible pT3a disease. The use of color Doppler may be used to identify adjacent vessels. The margin of resection is scored circumferentially using monopolar cautery.
Hilar Clamping, Tumor Excision and Renal Reconstruction
A. Tumor excision under warm ischemia for endophytic/hilar tumors (Video Clip #6)
For tumors that are endophytic or adjacent to the renal hilum, resection is done under warm ischemia. The assistant clamps the renal hilar vessel(s) using laparoscopic bulldog clamp(s) through the primary 12mm assistant port. We generally clamp the renal hilar vessels using separate bulldog clamps for the renal artery and renal vein. No patients in our study had multiple renal vessels. Mannitol (12.5 gm) may be administered intravenously prior to clamping. The tumor is resected along the previously scored margin using cold resection with the robotic monopolar scissors. The Maryland bipolar forceps are used to manipulate the tumor for exposure and to aid in dissection. The assistant uses suction to expose and maintain visualization of the resection plane of the tumor. After excision, the tumor can be placed beside the kidney or on top of the liver for later retrieval.
Hemostasis is achieved using a combination of cautery, hemostatic agents, and suturing. A pre-placed ureteral catheter may be used to inject methylene blue to identify entry into the collecting system. The robotic instruments are exchanged for robotic needle drivers. A 3-0 Vicryl suture on an SH needle is used to achieve hemostasis and repair any previously identified entry into the collecting system (an RB-1 needle may also be used). Sutures may be secured with either absorbable suture clips or by tying knots. Renal parenchymal defects are approximated over surgicell bolsters using 2-0 Vicryl sutures on an SH needle (a 0-Vicry suture on a CT-1 needle may also be used). A hemostatic agent, such as Floseal®, is applied. Preplacing surgicell bolsters and sutures in the abdomen may reduce warm ischemia time during earlier experience. The kidney is placed back on stretch using the robotic needle driver and the hilar clamp is removed by the assistant. Hemostasis is confirmed. A pre-placed lap pad may be used to apply pressure to the resection site.
The specimen is placed in a retrieval bag and removed through the primary assistant 12 mm port, enlarging the port site if needed. Gerota’s fascia is approximated over the defect using a running 3-0 Vicryl suture on an SH needle. A drain is placed in the perinephric space.
B. Non-ischemic technique for hereditary multiple or exophytic tumors (Video Clip #7)
For hereditary multiple renal tumors or small exophytic tumors, excision and reconstruction may be performed without hilar clamping. Similar to the technique described for excision under warm ischemia, tumors are removed using cold resection with the robotic monopolar scissors and Maryland bipolar instrument. A preplaced lap pad is used to tamponade the defect prior to achieving hemostasis and performing reconstruction using the technique described above.
RESULTS
Eight patients underwent successful robotic partial nephrectomy for complex renal tumors with a total of 14 tumors resected. Table 2 contains a summary of overall results while Table 3 shows results for individual patients. Mean warm ischemia time was 31 minutes (range 24–45 minutes). Mean estimated blood loss was 230 ml (range 100–450 ml). No patient required blood transfusions and there were no intraoperative complications. Histopathology confirmed clear cell renal cell carcinoma (n=3), hybrid oncocytic tumor (n=2), and chromophobe renal cell carcinoma (n=2) and oncocytoma (n=1). Mean overall tumor size was 2.4 cm (range 0.8–6.4 cm). The mean tumor size of the largest tumor in each patient, or index tumor, was 3.6 cm (range 2.6–6.4 cm). All patients had negative surgical margins. At the 3-month follow-up, no patients experienced a significant change in serum creatinine level or estimated glomerular filtration rate compared to preoperative levels (p>0.05), and no evidence of tumor recurrence was found.
Table 2.
Summary of results after robotic partial nephrectomy for complex renal tumors
| Mean warm ischemia time, min (range) | 31 (24–45) |
| Mean operative time, min(range) | 192 (165–214) |
| Mean blood loss, ml (range) | 230 (100–450) |
| Mean hospital stay, d (range) | 2.6 (2.0–3.0) |
| Mean increase in serum creatinine at discharge, mg/dl | +0.03 (−0.2–0.2) |
| Mean decrease in eGFR, ml/min/1.73m2 (range) | −5.6 (−;3.4–15) |
| Complex Tumor Features (n) | |
| Hilar | 5 |
| Endophytic | 4 |
| Multiple | 3 |
| eGFR=estimated glomerular filtration rate. | |
Table 3.
Results for individual patients undergoing robotic partial nephrectomy
| No. | No. of tumors | Tumor Location | Tumor size, cm | Operative time, min | Warm ischemia time, min | Mean blood loss, ml | Pathology |
|---|---|---|---|---|---|---|---|
| 1 | 1 | Hilar | 2.6 | 165 | 29 | 100 | Clear cell RCC, Fuhrman 2 |
| 2 | 3 | Endophytic, UP | 2.6 | 214 | 24* | 100 | Hybrid oncocytic tumor** |
| Midpole (2) | 1.1, 0.8 | -- | |||||
| 3 | 4 | LP (1) | 2.1 | 204 | 45*** | 450 | Chromophobe RCC** |
| UP (3) | 2.9, 1.2, 1.3 | ||||||
| 4 | 1 | Hilar | 6.4 | 185 | 33 | 300 | Hybrid oncocytic tumor |
| 5 | 1 | Hilar, endophytic | 2.9 | 200 | 39 | 150 | Clear cell RCC, Fuhrman 2 |
| 6 | 1 | Hilar | 4.0 | 180 | 24 | 200 | Clear cell RCC, Fuhrman 2 |
| 7 | 2 | Endophytic | 3.0 | 191 | 26 | 340 | Oncocytoma |
| UP | 0.8 | Angiomyolipoma | |||||
| 8 | 1 | Hilar/UP,endo | 4.5 | 195 | 31 | 200 | Chromophobe RCC |
Key: UP=upper pole, LP=lower pole, RCC=renal cell carcinoma, endo=endophytic
Tumors resected off clamp.
Same histologic subtype for all renal tumors resected.
Total warm ischemia time for resection of all tumors.
DISCUSSION
Laparoscopic radical nephrectomy was first described by Clayman et al.10 As expertise in laparoscopy has increased, minimally invasive nephron sparing surgery has become increasingly popular and has demonstrated excellent long-term renal functional and oncological outcomes1, 2. Our experience demonstrates that robotic partial nephrectomy is feasible in select patients with challenging renal tumors, such as multiple, endophytic, or hilar tumors. We feel that robotic assistance has allowed us to improve our minimally invasive approach to laparoscopic partial nephrectomy in these challenging cases by facilitating crucial steps, including tumor resection and renal reconstruction. Complex situations, such as multiple tumors, tumors in a location where angles of resection and suturing would be difficult, or tumors near vital hilar structures, can add to the technical challenge of a laparoscopic approach. Laparoscopic nephron-sparing surgery requires advanced skills in laparoscopy to accomplish tasks of tumor resection and renal reconstruction using intracorporeal suturing in a time-sensitive manner to minimize warm ischemia times.
Robotic technology addresses some of these technical limitations. Potential advantages of robotic assistance for partial nephrectomy include having a magnified, 3-dimensional view, which can help to assess and maintain the proper plane of tumor resection as well as aid in the identification of small open vessels for hemostasis or small openings in the collecting system for closure by intracorporeal suturing. The articulating robotic instruments and computer elimination of tremor facilitate precise, yet quick, tumor resection and renal reconstruction reducing the technical challenge significantly when approaching complex renal tumors, particularly in the setting of difficult surgical angles or adjacent hilar structures. Although we did not resect with wide margins in these particular cases based on the complexity of the clinical situation, the visualization and precision of the robotic system helped us to maintain an accurate plane of tumor resection.
Other reports have also demonstrated the safety and feasibility of robotic partial nephrectomy (Table 4)6–9. Our reports compare favorably to other reports of robotic partial nephrectomy, despite the challenging cases in our series. Caruso et al. compared robotic partial nephrectomy and laparoscopic partial nephrectomy in a small, nonrandomized study consisting of 10 patients in each group. They did not see a significant difference between the two groups in regards to blood loss, hospital stay, ischemia times, transfusion rates, operating times, or complication rates. However, they did mention the possibility of robotic partial nephrectomy providing a more tangible benefit for complex lesions requiring extensive reconstruction6. Our study supports this view that robotic assistance can facilitate tumor resection and renal reconstruction, offering a potential advantage in select patients with challenging renal tumors, such as multiple, endophytic, or hilar tumors.
Table 4.
Comparison of contemporary series of robotic partial nephrectomy
| Series | No. of patients | No. of tumors | Mean tumor size, cm | Operative time, min | Warm ischemia time, min | Mean hospital stay, d | Mean blood loss, ml |
|---|---|---|---|---|---|---|---|
| Gettman et al. | 13 | 13 | 3.5 | 215 | 22 | 4.3 | 170 |
| Phillips et al. | 12 | 12 | 1.4 | 265 | 26 | 2.7 | 240 |
| Kaul et al. | 10 | 10 | 2.3 | 155 | 21 | 1.5 | 92 |
| Present study | 8 | 14 | 3.6 | 192 | 31 | 2.6 | 230 |
Our institution previously described a surgical technique for concurrent laparoscopic management of multiple renal tumors11. We have since refined our surgical technique so as to be able to accomplish these surgeries with robotic assistance. To our knowledge, this is the first report of robotic partial nephrectomy in the setting of multiple renal tumors and hereditary kidney cancer.
Hilar tumors present a significant technical challenge for laparoscopic as well as open surgeons. Some of these patients undergo laparoscopic radical nephrectomy or open partial nephrectomy. Laparoscopic partial nephrectomy for hilar tumors has been described12. However, this is an advanced procedure done on select patients by a surgeon with considerable laparoscopic experience. This approach would not be possible for the majority of urologists. Comparing our hilar tumors with this laparoscopic partial nephrectomy series, our mean warm ischemia times was shorter (31min vs. 36 min) despite a larger mean tumor size (4.1 cm vs. 3.7 cm). Several studies suggest than 30 min is not an absolute limit for warm ischemia during partial nephrectomy13, 14. Although expertise is also required for complex renal tumors using robotic assistance, the visualization and precision provided facilitates tumor resection and renal reconstruction, helping the surgeon to replicate open surgical techniques.
Evidence suggests that robotic assistance may have a faster learning curve than conventional laparoscopy when comparing laparoscopic and robotic prostatectomy15. It remains to be determined if this is the case for robotic partial nephrectomy. In our study, both the console surgeon and assistant had advanced fellowship training in robotic and laparoscopic techniques for kidney and prostate cancer. Ideally, the console surgeon and other members of the robotic team should have some experience in robotic or laparoscopic surgery (or both) before attempting robotic partial nephrectomies, particularly for complex renal tumors.
We did not experience any episodes of major bleeding or other complications in our series that would necessitate open conversion. However, if significant bleeding were to be encountered requiring open conversion, the da Vinci system can be quickly undocked by removing the robotic instruments clutching the robotic arms, and removing the robotic arm and trocar as a unit. In the rare instance of a malfunction of the robot, the robotic trocars can be used to continue the case by conventional laparoscopy.
The medial camera port placement used in our study offers a global perspective of anatomic structures and a view that simulates that of conventional laparoscopy. A technique of lateral camera port placement with medial instrument placement, as described by Kaul et al8, may reduce arm collisions, provide more space for the assistant, and facilitate use of the fourth arm. Which technique to use is largely based on surgeon preference.
Limitations of our study include its small sample size. However, we feel that the video footage in our select group of patients demonstrates the potential utility of robotic assistance when approaching complicated tumors. Potential disadvantages of robotic partial nephrectomy include the cost, lack of haptic feedback, and the need for an experienced bedside assistant, particularly for important steps such as exposure, suctioning, hilar clamping, and instrument exchange. As surgical techniques improve and robotic systems with fourth arm capabilities become routinely used, surgeons may be able to gain more independence during these important steps.
Cost remains a potential limitation to the wide-spread use of robotic partial nephrectomy. We recognize that robotic assistance may not be practical for all patients, particularly those with small, exophytic tumors that could easily be removed with conventional laparoscopy. It was beyond the scope of this study to perform a comparative cost analysis of robotic partial nephrectomy versus laparoscopic versus open partial nephrectomy and it remains to be determined if robotic assistance for partial nephrectomy is the best use of our health resources. However, if robotic assistance facilitates a minimally invasive and nephron-sparing approach in select patients with complex tumors, then the benefit to society may justify its cost for this particular group of patients. A cost analysis comparing robotic assistance with conventional laparoscopy is necessary in future studies.
Our study was not designed to compare robotic assistance with other approaches to partial nephrectomy, but rather to describe our surgical technique and outcomes with robotic partial nephrectomy for a select group of patients with complex renal tumors. We do not claim superiority of robotic partial nephrectomy over conventional laparoscopy and we are not advocating a robotic approach for all partial nephrectomy cases. Our study merely suggests that robotic assistance may facilitate a minimally invasive approach to partial nephrectomy in select patients with complex tumors. We feel that open partial nephrectomy remains the standard that should be emulated in minimally invasive approaches to nephron-sparing surgery. A comparative analysis of open versus laparoscopic versus robotic partial nephrectomy, ideally in the form of a randomized clinical trial, would be useful as a follow-up study.
CONCLUSIONS
Robotic partial nephrectomy is a safe and feasible approach for select patients with complex renal tumors including hilar, endophytic, and multiple renal tumors. The features of the robotic system can facilitate the technical challenges of a minimally invasive approach to partial nephrectomy for these difficult cases, but the advantages must be weighed against its costs. For complex renal cancer cases, robotic assistance may provide patients the benefit of minimally invasive surgery without the need for total nephrectomy or open surgery.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. We would like to thank Francine Thomas, Clinical Imaging Processing Services, National Cancer Institute, National Institutes of Health for assisting with the 3-dimensional CT scan reconstructions.
Footnotes
Conflicts of Interest: none
Reference List
- 1.Allaf ME, Bhayani SB, Rogers C, Varkarakis I, Link RE, Inagaki T, Jarrett TW, Kavoussi LR. Laparoscopic partial nephrectomy: evaluation of long-term oncological outcome. J Urol. 2004;172:871–873. doi: 10.1097/01.ju.0000134292.36152.fa. [DOI] [PubMed] [Google Scholar]
- 2.Lane BR, Gill IS. 5-Year outcomes of laparoscopic partial nephrectomy. J Urol. 2007;177:70–74. doi: 10.1016/j.juro.2006.08.093. [DOI] [PubMed] [Google Scholar]
- 3.Patel V. Robotic-assisted laparoscopic dismembered pyeloplasty. Urol. 2005;66:45–49. doi: 10.1016/j.urology.2005.01.053. [DOI] [PubMed] [Google Scholar]
- 4.Klingler DW, Hemstreet GP, Balaji KC. Feasibility of robotic radical nephrectomy--initial results of single-institution pilot study. Urol. 2005;65:1086–1089. doi: 10.1016/j.urology.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 5.Horgan S, Benedetti E, Moser F. Robotically assisted donor nephrectomy for kidney transplantation. Am J Surg. 2004;188:45S–51S. doi: 10.1016/j.amjsurg.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 6.Caruso RP, Phillips CK, Kau E, Taneja SS, Stifelman MD. Robot assisted laparoscopic partial nephrectomy: initial experience. J Urol. 2006;176:36–39. doi: 10.1016/S0022-5347(06)00499-X. [DOI] [PubMed] [Google Scholar]
- 7.Gettman MT, Blute ML, Chow GK, Neururer R, Bartsch G, Peschel R. Robotic-assisted laparoscopic partial nephrectomy: technique and initial clinical experience with DaVinci robotic system. Urol. 2004;64:914–918. doi: 10.1016/j.urology.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 8.Kaul S, Laungani R, Sarle R, Stricker H, Peabody J, Littleton R, Menon M. da Vinci-assisted robotic partial nephrectomy: technique and results at a mean of 15 months of follow-up. Eur Urol. 2007;51:186–191. doi: 10.1016/j.eururo.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Phillips CK, Taneja SS, Stifelman MD. Robot-assisted laparoscopic partial nephrectomy: the NYU technique. J Endourol. 2005;19:441–445. doi: 10.1089/end.2005.19.441. [DOI] [PubMed] [Google Scholar]
- 10.Clayman RV, Kavoussi LR, Soper NJ, Dierks SM, Meretyk S, Darcy MD, Roemer FD, Pingleton ED, Thomson PG, Long SR. Laparoscopic nephrectomy: initial case report. J Urol. 1991;146:278–282. doi: 10.1016/s0022-5347(17)37770-4. [DOI] [PubMed] [Google Scholar]
- 11.Vira MN, Kansall NS, Lee SJ. Laparoscopic partial nephrectomy for multiuple renal tumors. American Urological Association National Meeting; 2006. [Google Scholar]
- 12.GILL IS, COLOMBO JR, FRANK IGOR, MOINZADEH ALIR, KAOUK JIHA, DESAI MIHI. LAPAROSCOPIC PARTIAL NEPHRECTOMY FOR HILAR TUMORS. Journal of Urology, The. 2005;174:850–854. doi: 10.1097/01.ju.0000169493.05498.c3. [DOI] [PubMed] [Google Scholar]
- 13.Bhayani SB, Rha KH, Pinto PA, Ong AM, Allaf ME, Trock BJ, Jarrett TW, Kavoussi LR. Laparoscopic partial nephrectomy: effect of warm ischemia on serum creatinine. J Urol. 2004;172:1264–1266. doi: 10.1097/01.ju.0000138187.56050.20. [DOI] [PubMed] [Google Scholar]
- 14.Desai MM, Gill IS, Ramani AP, Spaliviero M, Rybicki L, Kaouk JH. The impact of warm ischaemia on renal function after laparoscopic partial nephrectomy. BJU Int. 2005;95:377–383. doi: 10.1111/j.1464-410X.2005.05304.x. [DOI] [PubMed] [Google Scholar]
- 15.Rozet F, Harmon J, Cathelineau X, Barret E, Vallancien G. Robot-assisted versus pure laparoscopic radical prostatectomy. World J Urol. 2006;24:171–179. doi: 10.1007/s00345-006-0065-3. [DOI] [PubMed] [Google Scholar]