Abstract
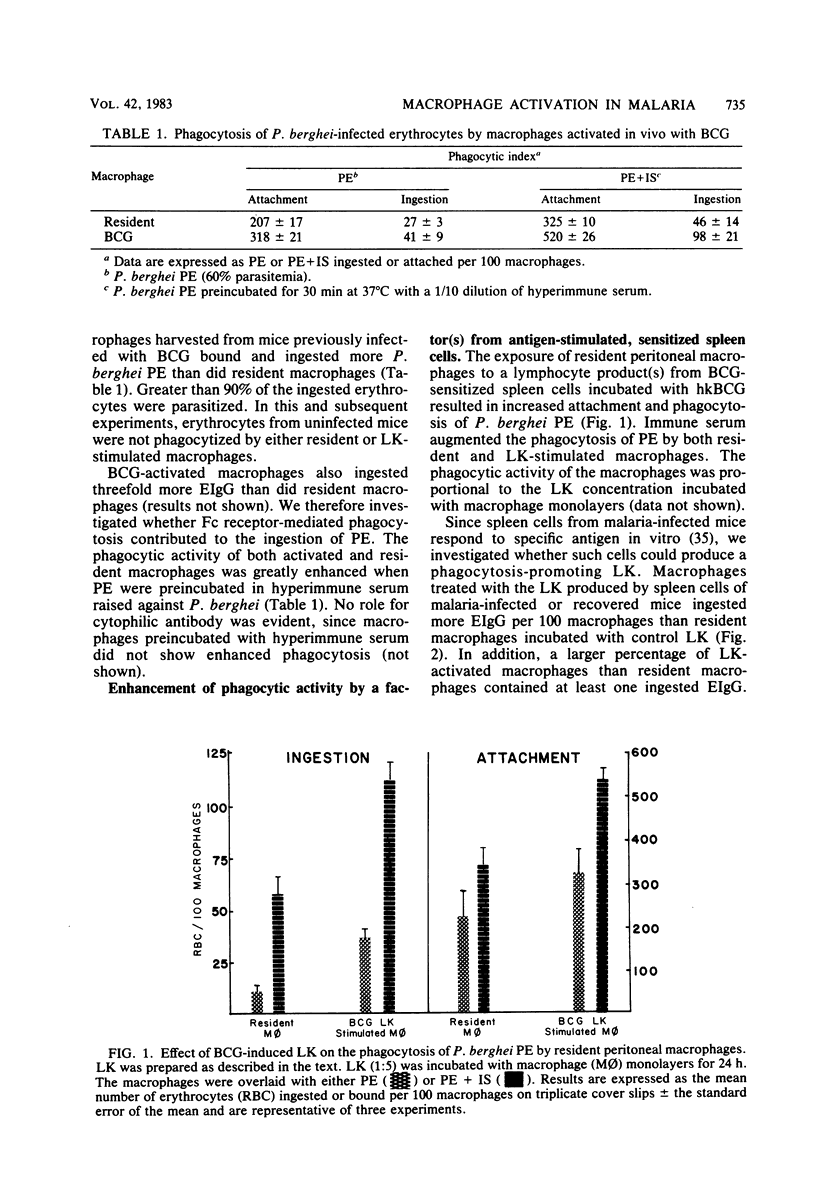
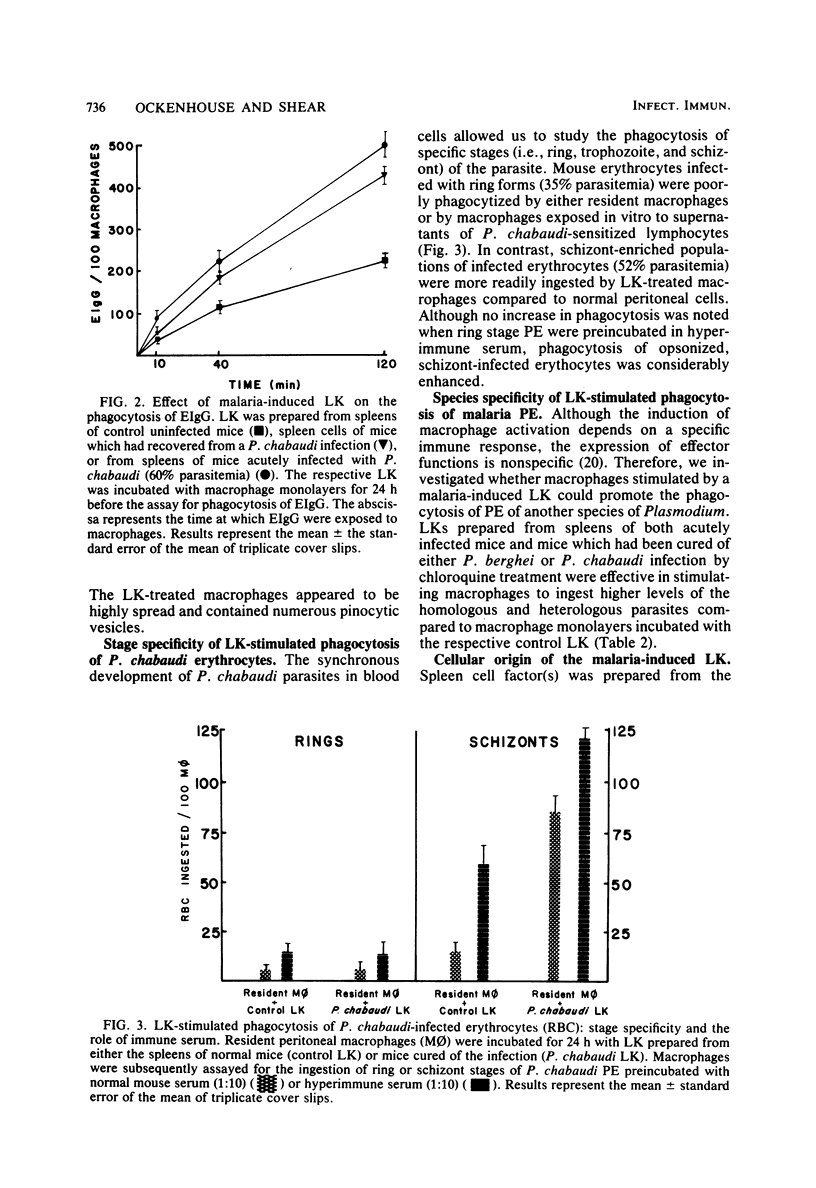
Culture supernatants from antigen-pulsed spleen cells of mice infected previously with either BCG or Plasmodium chabaudi were used to study macrophage activation as judged by phagocytosis of immunoglobulin G-sensitized erythrocytes and Plasmodium berghei- and P. chabaudi-infected erythrocytes. Resident peritoneal macrophages were incubated in vitro with spleen cell factor and then assayed for ingestion of immunoglobulin G-sensitized or parasitized erythrocytes. Macrophages activated with BCG-induced lymphokine bound and ingested two- to threefold more P. berghei parasitized erythrocytes than macrophages incubated with control spleen cell factor. Similarly, Plasmodium-stimulated spleen cells from mice infected with malaria produced a lymphokine(s) capable of activating macrophages for enhanced Fc receptor-mediated phagocytosis. The stimulation of phagocytosis by the lymphokine is nonspecific in nature, since phagocytosis of parasitized erythrocytes from one species of murine malaria is enhanced by the lymphokine prepared from a heterologous species. Nylon wool-nonadherent, malaria-sensitized spleen cells elaborated a lymphokine which stimulates macrophages for enhanced phagocytosis, whereas anti-0-treated spleen cells failed to produce the phagocytosis-promoting lymphokine. Consequently, this lymphokine appears to be elaborated by sensitized T lymphocytes. Interestingly, enhanced phagocytosis of opsonized trophozoites and schizonts, but not ring stage parasites of P. chabaudi, was displayed by macrophages activated with the lymphokine(s) prepared from P. chabaudi-recovered mice. Preincubation of the malaria parasitized erythrocytes with hyperimmune serum raised against the parasites greatly facilitated both binding and ingestion by the stimulated macrophages.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianco C., Griffin F. M., Jr, Silverstein S. C. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975 Jun 1;141(6):1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. N., McLaren D. J., Hills L. A., Jarra W. The binding of antibodies from Plasmodium berghei-infected rats to isoantigenic and parasite-specific antigenic sites on the surfaces of infected reticulocytes. Parasite Immunol. 1982 Jan;4(1):21–31. doi: 10.1111/j.1365-3024.1982.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Celada A., Cruchaud A., Perrin L. H. Opsonic activity of human immune serum on in vitro phagocytosis of Plasmodium falciparum infected red blood cells by monocytes. Clin Exp Immunol. 1982 Mar;47(3):635–644. [PMC free article] [PubMed] [Google Scholar]
- Chow J. S., Kreier J. P. Plasmodium berghei: adherence and phagocytosis by rat macrophages in vitro. Exp Parasitol. 1972 Feb;31(1):13–18. doi: 10.1016/0014-4894(72)90042-2. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Allison A. C., Cox F. E. Protection of mice against Babesia and Plasmodium with BCG. Nature. 1976 Jan 29;259(5541):309–311. doi: 10.1038/259309a0. [DOI] [PubMed] [Google Scholar]
- Cohen S., Butcher G. A. Properties of protective malarial antibody. Nature. 1970 Feb 21;225(5234):732–734. doi: 10.1038/225732a0. [DOI] [PubMed] [Google Scholar]
- Facer C. A., Bray R. S., Brown J. Direct Coombs antiglobulin reactions in Gambian children with Plasmodium falciparum malaria. I. Incidence and class specificity. Clin Exp Immunol. 1979 Jan;35(1):119–127. [PMC free article] [PubMed] [Google Scholar]
- Hommel M., David P. H., Oligino L. D. Surface alterations of erythrocytes in Plasmodium falciparum malaria. Antigenic variation, antigenic diversity, and the role of the spleen. J Exp Med. 1983 Apr 1;157(4):1137–1148. doi: 10.1084/jem.157.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayawardena A. N., Janeway C. A., Jr, Kemp J. D. Experimental malaria in the CBA/N mouse. J Immunol. 1979 Dec;123(6):2532–2539. [PubMed] [Google Scholar]
- Jayawardena A. N., Murphy D. B., Janeway C. A., Gershon R. K. T cell-mediated immunity in malaria. I. The Ly phenotype of T cells mediating resistance to Plasmodium yoelii. J Immunol. 1982 Jul;129(1):377–381. [PubMed] [Google Scholar]
- Jayawardena A. N., Targett G. A., Carter R. L., Leuchars E., Davies A. J. The immunological response of CBA mice to P. yoelii. I. General characteristics, the effects of T-cell deprivation and reconstitution with thymus grafts. Immunology. 1977 Jun;32(6):849–859. [PMC free article] [PubMed] [Google Scholar]
- Jayawardena A. N., Targett G. A., Leuchars E., Carter R. L., Doenhoff M. J., Davies A. J. T-cell activation in murine malaria. Nature. 1975 Nov 13;258(5531):149–151. doi: 10.1038/258149a0. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Khusmith S., Druilhe P., Gentilini M. Enhanced Plasmodium falciparum merozoite phagocytosis by monocytes from immune individuals. Infect Immun. 1982 Mar;35(3):874–879. doi: 10.1128/iai.35.3.874-879.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig H. J., Nussenzweig V., Nussenzweig R. S. Erythrocyte membrane-associated immunoglobulins during malaria infection of mice. J Immunol. 1977 Jul;119(1):210–216. [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas M. G., Nussenzweig R. S., Nussenzweig V., Shear H. L. Complement-mediated defect in clearance and sequestration of sensitized, autologous erythrocytes in rodent malaria. J Clin Invest. 1981 Jan;67(1):183–192. doi: 10.1172/JCI110011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn T. C., Wyler D. J. Intravascular clearance of parasitized erythrocytes in rodent malaria. J Clin Invest. 1979 Jun;63(6):1187–1194. doi: 10.1172/JCI109413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. W., Weidanz W. P. Splenomegaly, enhanced phagocytosis, and anemia are thymus-dependent responses to malaria. Infect Immun. 1978 Jun;20(3):728–731. doi: 10.1128/iai.20.3.728-731.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. W., Weidanz W. P. T-cell immunity to malaria in the B-cell deficient mouse. Am J Trop Med Hyg. 1979 Jan;28(1):1–3. doi: 10.4269/ajtmh.1979.28.1. [DOI] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheagren J. N., Tobie J. E., Fox L. M., Wolff S. M. Reticuloendothelial system phagocytic function in naturally acquired human malaria. J Lab Clin Med. 1970 Mar;75(3):481–487. [PubMed] [Google Scholar]
- Shear H. L., Nussenzweig R. S., Bianco C. Immune phagocytosis in murine malaria. J Exp Med. 1979 Jun 1;149(6):1288–1298. doi: 10.1084/jem.149.6.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. P., Hunter K. W., Oldfield E. C., Strickland G. T. Murine malaria: blood clearance and organ sequestration of Plasmodium yoelii-infected erythrocytes. Infect Immun. 1982 Oct;38(1):162–167. doi: 10.1128/iai.38.1.162-167.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbaum F. I., Evans C. B., Tigelaar R. E. An in vitro assay for T cell immunity to malaria in mice. J Immunol. 1976 May;116(5):1280–1283. [PubMed] [Google Scholar]
- Wyler D. J., Gallin J. I. Spleen-derived mononuclear cell chemotactic factor in malaria infections: a possible mechanism for splenic macrophage accumulation. J Immunol. 1977 Feb;118(2):478–484. [PubMed] [Google Scholar]
- Wyler D. J., Quinn T. C., Chen L. T. Relationship of alterations in splenic clearance function and microcirculation to host defense in acute rodent malaria. J Clin Invest. 1981 May;67(5):1400–1404. doi: 10.1172/JCI110168. [DOI] [PMC free article] [PubMed] [Google Scholar]



