Abstract
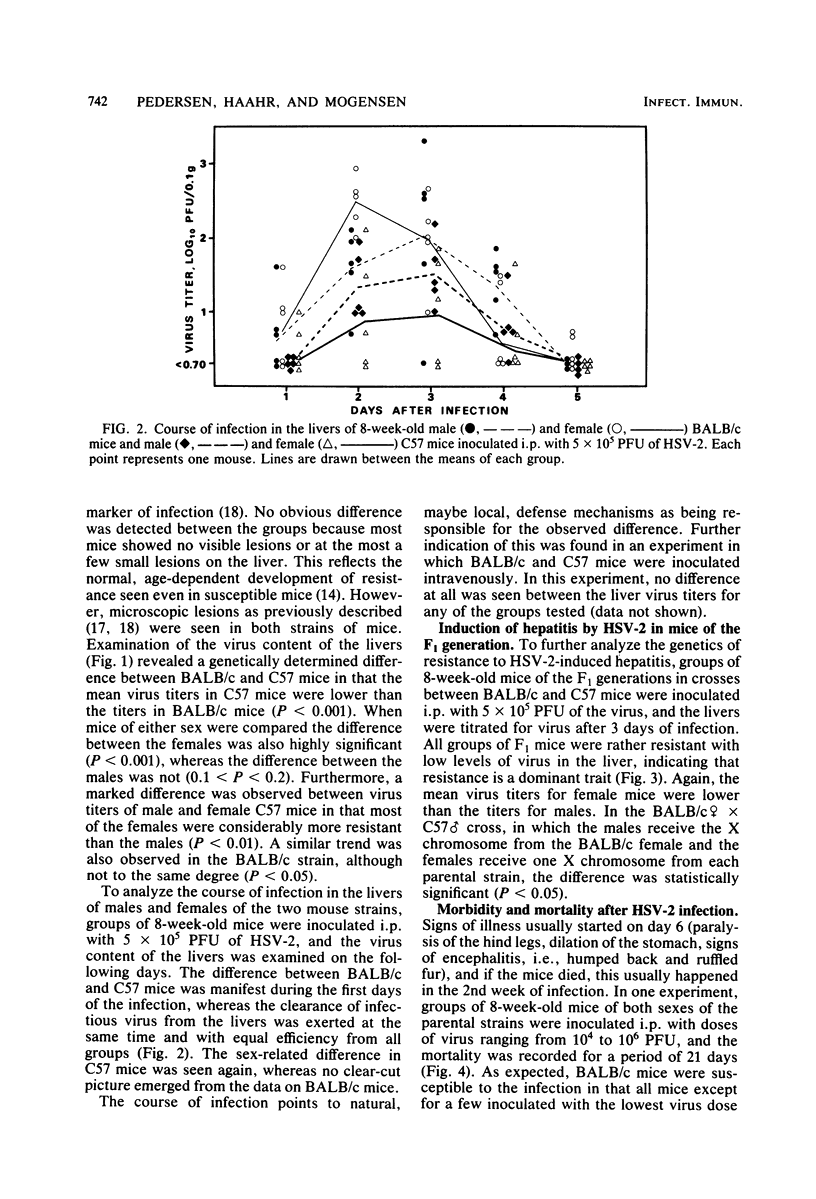
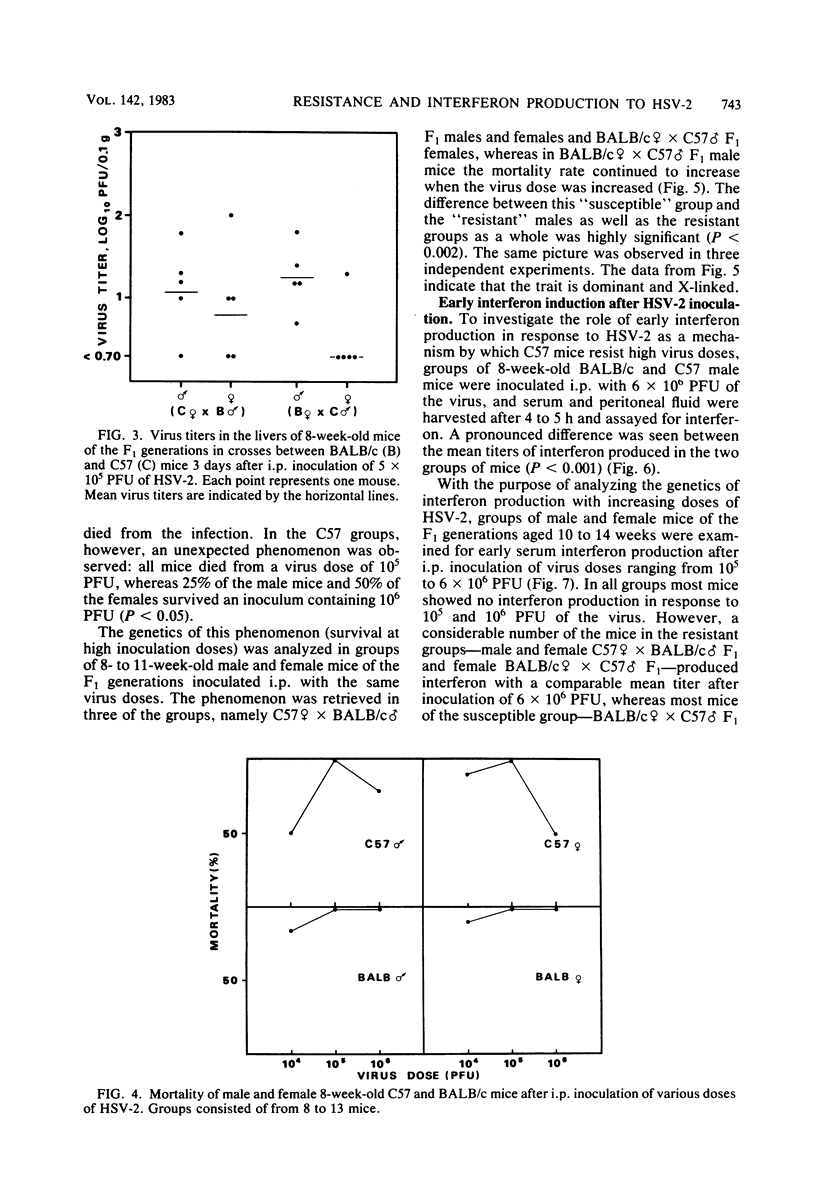
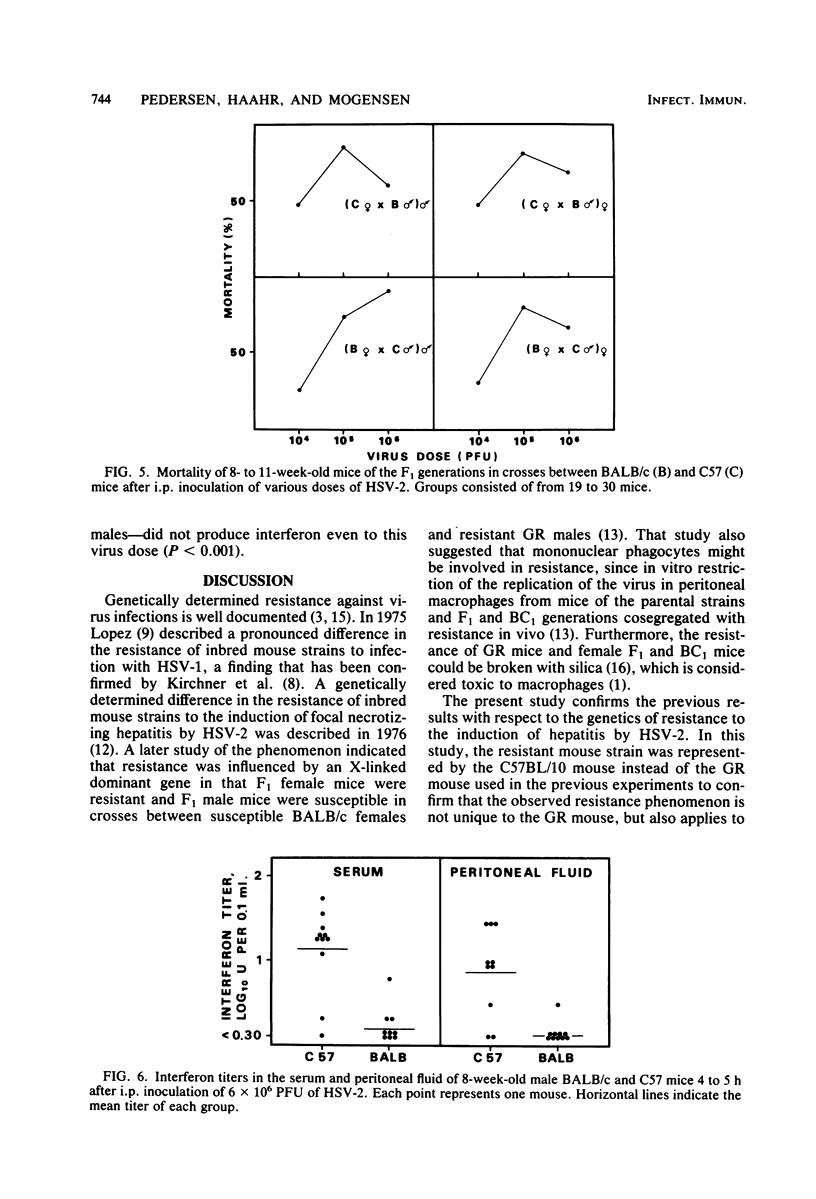
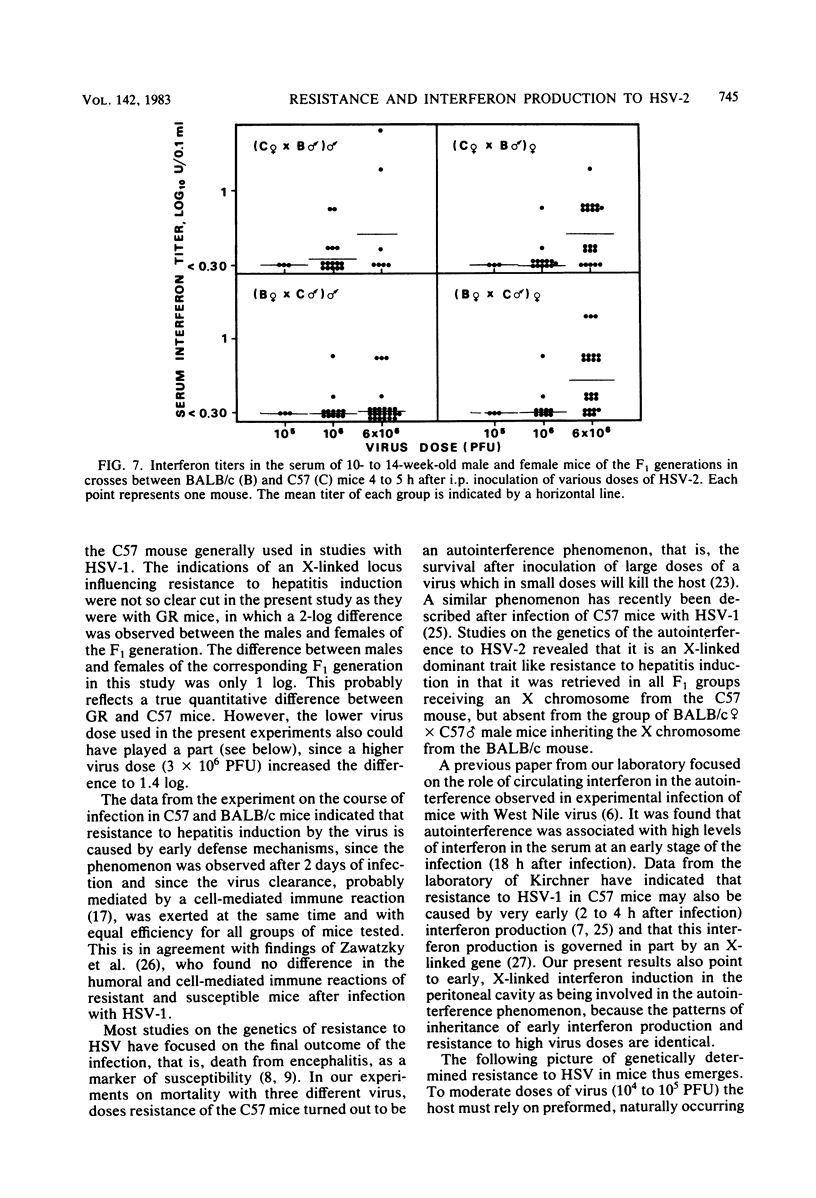
Mice inoculated intraperitoneally with herpes simplex virus type 2 develop focal necrotizing hepatitis and eventually die from ascending myelitis and encephalitis. The genetics of resistance to the infection were analyzed in crosses between resistant C57BL/10 mice and susceptible BALB/c mice. It was shown that the resistance of C57BL/10 mice to hepatitis induction was influenced by an X-linked dominant gene as previously shown for the GR mouse strain. The course of infection in the liver pointed to early, natural defense mechanisms as being responsible for the difference between the mouse strains, whereas the clearance of virus from the liver, probably mediated by specific immunity, was exerted at the same time and with equal efficiency for all groups of mice. In mortality experiments, resistance was shown to be an autointerference phenomenon in that a considerable number of C57BL/10 mice survived an intraperitoneal injection of 10(6) PFU, whereas all mice were killed by 10(5) PFU. This resistance of C57BL/10 mice to high doses of HSV-2 was retrieved in all groups of F1 mice in crosses between C57BL/10 and BALB/c mice except the (BALB/c female X C57 male) male group, in which the mice receive the X chromosome from the susceptible BALB/c female. Thus, the autointerference phenomenon also seems to be influenced by loci on the X chromosome. A similar pattern of inheritance was observed when early interferon induction (4 to 5 h after infection) in response to HSV-2 was measured. The possible relevance of this early interferon response in conjunction with other potential natural defense mechanisms is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armerding D., Rossiter H. Induction of natural killer cells by herpes-simplex virus type 2 in resistant and sensitive inbred mouse strains. Immunobiology. 1981;158(4):369–379. doi: 10.1016/S0171-2985(81)80007-1. [DOI] [PubMed] [Google Scholar]
- Bang F. B. Genetics of resistance of animals to viruses: I. Introduction and studies in mice. Adv Virus Res. 1978;23:269–348. doi: 10.1016/S0065-3527(08)60102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser I., Tovey M. G., Maury C., Bandu M. T. Role of interferon in the pathogenesis of virus diseases in mice as demonstrated by the use of anti-interferon serum. II. Studies with herpes simplex, Moloney sarcoma, vesicular stomatitis, Newcastle disease, and influenza viruses. J Exp Med. 1976 Nov 2;144(5):1316–1323. doi: 10.1084/jem.144.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haahr S. The possible role of circulating interferon on autointerference in mice infected intraperitoneally with West Nile virus. Acta Pathol Microbiol Scand. 1969;77(3):425–432. doi: 10.1111/j.1699-0463.1969.tb04249.x. [DOI] [PubMed] [Google Scholar]
- Kirschner H., Kochen M., Hirt H. M., Munk K. Immunological studies of hsv-infection of resistant and susceptible inbred strains of mice. Z Immunitatsforsch Immunobiol. 1978 Mar;154(2):147–154. [PubMed] [Google Scholar]
- Lopez C. Genetics of natural resistance to herpesvirus infections in mice. Nature. 1975 Nov 13;258(5531):152–153. doi: 10.1038/258152a0. [DOI] [PubMed] [Google Scholar]
- MIMS C. A. ASPECTS OF THE PATHOGENESIS OF VIRUS DISEASES. Bacteriol Rev. 1964 Mar;28:30–71. doi: 10.1128/br.28.1.30-71.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan T. C. Host defenses against viral disease. N Engl J Med. 1974 Feb 7;290(6):323–329. doi: 10.1056/NEJM197402072900608. [DOI] [PubMed] [Google Scholar]
- Mogensen S. C., Andersen H. K. Recovery of mice from herpes simplex virus type 2 hepatitis: adoptive transfer of recovery with immune spleen cells. Infect Immun. 1981 Sep;33(3):743–749. doi: 10.1128/iai.33.3.743-749.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C. Biological conditions influencing the focal necrotic hepatitis test for differentiation between herpes simplex virus types 1 and 2. Acta Pathol Microbiol Scand B. 1976 Jun;84(3):154–158. [PubMed] [Google Scholar]
- Mogensen S. C. Genetics of macrophage-controlled resistance to hepatitis induced by herpes simplex virus type 2 in mice. Infect Immun. 1977 Aug;17(2):268–273. doi: 10.1128/iai.17.2.268-273.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C. Macrophages and age-dependent resistance to hepatitis induced by herpes simplex virus type 2 im mice. Infect Immun. 1978 Jan;19(1):46–50. doi: 10.1128/iai.19.1.46-50.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C. Role of macrophages in natural resistance to virus infections. Microbiol Rev. 1979 Mar;43(1):1–26. doi: 10.1128/mr.43.1.1-26.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C., Teisner B., Andersen H. K. Focal necrotic hepatitis in mice as a biological marker for differentiation of Herpesvirus hominis type 1 and type 2. J Gen Virol. 1974 Oct;25(1):151–155. doi: 10.1099/0022-1317-25-1-151. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Roizman B. Infection with herpes-simplex viruses 1 and 2. 1. N Engl J Med. 1973 Sep 27;289(13):667–674. doi: 10.1056/NEJM197309272891305. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Roizman B. Infection with herpes-simplex viruses 1 and 2. 3. N Engl J Med. 1973 Oct 11;289(15):781–789. doi: 10.1056/NEJM197310112891505. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Roizman B. Infection with herpes-simplex viruses 1 and 2. II. N Engl J Med. 1973 Oct 4;289(14):719–725. doi: 10.1056/NEJM197310042891404. [DOI] [PubMed] [Google Scholar]
- Welsh R. M. Natural cell-mediated immunity during viral infections. Curr Top Microbiol Immunol. 1981;92:83–106. doi: 10.1007/978-3-642-68069-4_6. [DOI] [PubMed] [Google Scholar]
- Zawatzky R., Gresser I., DeMaeyer E., Kirchner H. The role of interferon in the resistance of C57BL/6 mice to various doses of herpes simplex virus type 1. J Infect Dis. 1982 Sep;146(3):405–410. doi: 10.1093/infdis/146.3.405. [DOI] [PubMed] [Google Scholar]
- Zawatzky R., Hilfenhaus J., Marcucci F., Kirchner H. Experimental infection of inbred mice with herpes simplex virus type 1. I. Investigation of humoral and cellular immunity and of interferon induction. J Gen Virol. 1981 Mar;53(Pt 1):31–38. doi: 10.1099/0022-1317-53-1-31. [DOI] [PubMed] [Google Scholar]
- Zawatzky R., Kirchner H., DeMaeyer-Guignard J., DeMaeyer E. An X-linked locus influences the amount of circulating interferon induced in the mouse by herpes simplex virus type 1. J Gen Virol. 1982 Dec;63(2):325–332. doi: 10.1099/0022-1317-63-2-325. [DOI] [PubMed] [Google Scholar]



