Abstract
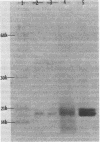
Bacteria which attach to different mucous membranes should have differing specificities of adherence in vitro. Human Escherichia coli isolates from blood and urine (pathogens) and from stool and throat (commensals) were characterized as to the patterns of hemagglutination (HA), as well as the structure and function of their pili. Bacterial HA was done in microtiter plates and on slides after bacterial growth in broth or agar. Human erythrocytes were agglutinated by 95% of the pathogens and 65 to 70% of the commensals grown in broth or agar. Mannose-resistant HA was characteristically caused by pathogens, and commensals characteristically caused mannose-sensitive HA of guinea pig cells. Strains often had both mannose-resistant and mannose-sensitive reactions, or even a mannose-paradoxical reaction. Pathogens more often caused HA, but titers were lower than those for commensals. Slide HA was less sensitive than the microtiter method. All isolates were piliated. Commensals also had more pili than pathogens when grown in broth (117.8 versus 38.3 pili per bacterium), but pathogens had more pili after growth on agar (32.1 versus 8.1 pili per bacterium). Isolates causing high-titer HA had large numbers of pili (greater than 85 pili per bacterium), but some well-piliated strains were non-hemagglutinating. Pili were purified from seven E. coli strains from different sites of isolation and with different erythrocyte-binding specificity. Pili usually migrated as a single band on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. However, more than one type of pilus could be copurified from some strains since there were two or more bands after separation in octyl-glucoside and two different amino terminal sequences. Protein sequencing was done on five different pili: four resembled type 1 pili and one was a P fimbria. The type 1-like pili (strains 2239 and 9353) had an initial variable sequence of 1 to 5 residues, followed by a common region of 21 residues. The P fimbria (strain 7714) had different erythrocyte-binding specificity but was still 27% homologous with 2239 and 9353. E. coli strains from different body sites have characteristic attachments to erythrocytes. Pili derived from these different sources may also have different binding specificity, but they are similar in primary structure.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Dodd D. C., Eisenstein B. I. Antigenic quantitation of type 1 fimbriae on the surface of Escherichia coli cells by an enzyme-linked immunosorbent inhibition assay. Infect Immun. 1982 Nov;38(2):764–773. doi: 10.1128/iai.38.2.764-773.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. P., Clegg S., Wilson M. I. The fimbrial and non-fimbrial haemagglutinins of Escherichia coli. J Med Microbiol. 1979 May;12(2):213–227. doi: 10.1099/00222615-12-2-213. [DOI] [PubMed] [Google Scholar]
- Eshdat Y., Silverblatt F. J., Sharon N. Dissociation and reassembly of Escherichia coli type 1 pili. J Bacteriol. 1981 Oct;148(1):308–314. doi: 10.1128/jb.148.1.308-314.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Silver R. P., Evans D. J., Jr, Chase D. G., Gorbach S. L. Plasmid-controlled colonization factor associated with virulence in Esherichia coli enterotoxigenic for humans. Infect Immun. 1975 Sep;12(3):656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Höhne C., Noble M. A., Haldane E. V., Lior H., Young L. S. Hemolysin and K antigens in relation to serotype and hemagglutination type of Escherichia coli isolated from extraintestinal infections. J Clin Microbiol. 1981 Jan;13(1):171–178. doi: 10.1128/jcm.13.1.171-178.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Young L. S., Pitt J. Hemagglutination typing of Escherichia coli: definition of seven hemagglutination types. J Clin Microbiol. 1980 Aug;12(2):235–242. doi: 10.1128/jcm.12.2.235-242.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Direct microsequence analysis of polypeptides using an improved sequenator, a nonprotein carrier (polybrene), and high pressure liquid chromatography. Biochemistry. 1978 May 30;17(11):2124–2133. doi: 10.1021/bi00604a016. [DOI] [PubMed] [Google Scholar]
- Isaacson R. E., Fusco P. C., Brinton C. C., Moon H. W. In vitro adhesion of Escherichia coli to porcine small intestinal epithelial cells: pili as adhesive factors. Infect Immun. 1978 Aug;21(2):392–397. doi: 10.1128/iai.21.2.392-397.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E., Richter P. Escherichia coli 987P pilus: purification and partial characterization. J Bacteriol. 1981 May;146(2):784–789. doi: 10.1128/jb.146.2.784-789.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P., Orskov I., Orskov F. F7 and type 1-like fimbriae from three Escherichia coli strains isolated from urinary tract infections: protein chemical and immunological aspects. Infect Immun. 1982 May;36(2):462–468. doi: 10.1128/iai.36.2.462-468.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P., Orskov I., Orskov F. Isolation and characterization of F12 adhesive fimbrial antigen from uropathogenic Escherichia coli strains. Infect Immun. 1983 Apr;40(1):91–96. doi: 10.1128/iai.40.1.91-96.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P. Primary structure of the CFA1 fimbrial protein from human enterotoxigenic Escherichia coli strains. Eur J Biochem. 1982 May 17;124(2):339–348. doi: 10.1111/j.1432-1033.1982.tb06597.x. [DOI] [PubMed] [Google Scholar]
- Klemm P. The complete amino-acid sequence of the K88 antigen, a fimbrial protein from Escherichia coli. Eur J Biochem. 1981 Jul;117(3):617–627. doi: 10.1111/j.1432-1033.1981.tb06382.x. [DOI] [PubMed] [Google Scholar]
- Korhonen T. K., Väisänen V., Kallio P., Nurmiaho-Lassila E. L., Ranta H., Siitonen A., Elo J., Svenson S. B., Svanborg-Edén C. The role of pili in the adhesion of Escherichia coli to human urinary tract epithelial cells. Scand J Infect Dis Suppl. 1982;33:26–31. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Black R. E., Brinton C. C., Jr, Clements M. L., Fusco P., Hughes T. P., O'Donnell S., Robins-Browne R., Wood S., Young C. R. Reactogenicity, immunogenicity and efficacy studies of Escherichia coli type 1 somatic pili parenteral vaccine in man. Scand J Infect Dis Suppl. 1982;33:83–95. [PubMed] [Google Scholar]
- McMichael J. C., Ou J. T. Structure of common pili from Escherichia coli. J Bacteriol. 1979 Jun;138(3):969–975. doi: 10.1128/jb.138.3.969-975.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R. L., Isaacson R. E., Moon H. W., Brinton C. C., To C. C. Immunization of suckling pigs against enterotoxigenic Escherichia coli-induced diarrheal disease by vaccinating dams with purified 987 or K99 pili: protection correlates with pilus homology of vaccine and challenge. Infect Immun. 1978 Dec;22(3):771–777. doi: 10.1128/iai.22.3.771-777.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Ferencz A., Orskov F. Tamm-Horsfall protein or uromucoid is the normal urinary slime that traps type 1 fimbriated Escherichia coli. Lancet. 1980 Apr 19;1(8173):887–887. doi: 10.1016/s0140-6736(80)91396-3. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F. Serology of Escherichia coli fimbriae. Prog Allergy. 1983;33:80–105. [PubMed] [Google Scholar]
- Orskov I., Orskov F., Smith H. W., Sojka W. J. The establishment of K99, a thermolabile, transmissible escherichia coli K antigen, previously called "Kco", possessed by calf and lamb enteropathogenic strains. Acta Pathol Microbiol Scand B. 1975 Feb;83(1):31–36. doi: 10.1111/j.1699-0463.1975.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Rutter J. M., Jones G. W. Protection against enteric disease caused by Escherichia coli--a model for vaccination with a virulence determinant? Nature. 1973 Apr 20;242(5399):531–532. doi: 10.1038/242531a0. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Blake M., Gotschlich E. C. Intra-strain heterogeneity of gonococcal pili is related to opacity colony variance. J Exp Med. 1980 Mar 1;151(3):716–725. doi: 10.1084/jem.151.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Hemagglutination by purified type I Escherichia coli pili. J Exp Med. 1977 Nov 1;146(5):1169–1181. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Type I Escherichia coli pili: characterization of binding to monkey kidney cells. J Exp Med. 1977 Nov 1;146(5):1182–1194. doi: 10.1084/jem.146.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E. Hemagglutination by Neisseria meningitidis. Can J Microbiol. 1981 Jun;27(6):586–593. doi: 10.1139/m81-089. [DOI] [PubMed] [Google Scholar]
- Sellwood R., Gibbons R. A., Jones G. W., Rutter J. M. Adhesion of enteropathogenic Escherichia coli to pig intestinal brush borders: the existence of two pig phenotypes. J Med Microbiol. 1975 Aug;8(3):405–411. doi: 10.1099/00222615-8-3-405. [DOI] [PubMed] [Google Scholar]
- Smithies O., Gibson D., Fanning E. M., Goodfliesh R. M., Gilman J. G., Ballantyne D. L. Quantitative procedures for use with the Edman-Begg sequenator. Partial sequences of two unusual immunoglobulin light chains, Rzf and Sac. Biochemistry. 1971 Dec 21;10(26):4912–4921. doi: 10.1021/bi00802a013. [DOI] [PubMed] [Google Scholar]
- Svanborg Edén C., Gotschlich E. C., Korhonen T. K., Leffler H., Schoolnik G. Aspects on structure and function of pili on uropathogenic Escherichia coli. Prog Allergy. 1983;33:189–202. doi: 10.1159/000318330. [DOI] [PubMed] [Google Scholar]
- Watts T. H., Scraba D. G., Paranchych W. Formation of 9-nm filaments from pilin monomers obtained by octyl-glucoside dissociation of Pseudomonas aeruginosa pili. J Bacteriol. 1982 Sep;151(3):1508–1513. doi: 10.1128/jb.151.3.1508-1513.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]