Abstract
The inhibitor of growth (ING) family of type II tumor suppressors are encoded by five genes in mammals and by three genes in Caenorhabditis elegans. All ING proteins contain a highly conserved plant homeodomain (PHD) zinc finger. ING proteins are activated by stresses, including ionizing radiation, leading to the activation of p53. ING proteins in mammals and yeast have recently been shown to read the histone code in a methylation-sensitive manner to regulate gene expression. Here we identify and characterize ing-3, the C. elegans gene with the highest sequence identity to the human ING3 gene. ING-3 colocalizes with chromatin in embryos, the germline, and somatic cells. The ing-3 gene is part of an operon but is also transcribed from its own promoter. Both ing-3(RNAi) and ing-3 mutant strains demonstrate that the gene likely functions in concert with the C. elegans p53 homolog, cep-1, to induce germ-cell apoptosis in response to ionizing radiation. Somatically, the ing-3 mutant has a weak kinker uncoordinated (kinker Unc) phenotype, indicating a possible neuronal function.
THE founding member of the inhibitor of growth (ING) family was first identified as a growth inhibitor and type II tumor suppressor expressed in normal, but not in cancerous, cells (Garkavtsev et al. 1996). ING proteins have since been implicated in diverse biological processes, including angiogenesis, apoptosis, DNA repair, cell cycle regulation, oncogenesis, regulation of gene expression, and stress signaling (reviewed in Russell et al. 2006). ING proteins bind a wide range of cellular proteins, including proliferating cell nuclear antigen (Scott et al. 2001) as well as histone acetyltransferase (HAT) and histone deacetylase (HDAC) complexes (reviewed in Russell et al. 2006). In particular, ING proteins interact genetically and physically with the p53 tumor suppressor during stress responses and apoptosis (Garkavtsev et al. 1998; Nourani et al. 2003).
More than 60 ING genes have been identified in a broad range of species on the basis of sequence similarity (He et al. 2005), and all contain a highly conserved plant homeodomain (PHD), a Cys4-His-Cys3 form of zinc finger (Bienz 2006) associated with chromatin remodeling. The PHD region of ING proteins interacts directly with histone H3, and a subset of ING proteins does this in a methylation-sensitive manner (Martin et al. 2006; Pena et al. 2006; Shi et al. 2006). These properties, combined with observations that the ING proteins serve as receptors and transducers of stress-activated phosphoinositides (Gozani et al. 2003; Kaadige and Ayer 2006), suggest that ING proteins link key pathways involved with regulating chromatin structure, histone methylation, and histone acetylation.
The five mammalian ING genes (Nagashima et al. 2001, 2003; Feng et al. 2002) fall into three subgroups (He et al. 2005). Although the mammalian ING family subgroups (ING1–5) share structural (He et al. 2005) and functional features (Doyon et al. 2006) and appear to play roles in interpreting the epigenetic histone code (Martin et al. 2006; Palacios et al. 2006; Pena et al. 2006; Shi et al. 2006), little is known about their function in intact organisms. In Saccharomyces cerevisiae, deletion of any of the three ing homologous genes, yng1, yng2, and yng3, results in pleiotropic phenotypes (Loewith et al. 2000). For example, most mutants and mutant combinations germinate and grow, but yng2 knockout cells grow slowly and are enlarged and multibudded, with buds frequently devoid of DNA. Triple yng mutants are viable but show severe growth inhibition and more exaggerated morphological and multibudded phenotypes. Mutants in each gene are hypersensitive to heat shock. Deletion of yng2 alone results in sensitivity to UV irradiation but not to γ-irradiation or treatment with alkylating agents (Loewith et al. 2000). The yng stress-associated functions may be related to the observation that mammalian ING1 and ING2 both induce hsp70 heat-shock protein (Feng et al. 2006).
As with humans, mice harbor five ING genes. The only reported knockout, ING1, is viable, but animals have an increased incidence of B-cell lymphomas and sensitivity to γ-radiation (Kichina et al. 2006; Coles et al. 2007), consistent with ING1 being a type II tumor suppressor. However, although previous cell culture results clearly indicate that ING proteins interact both physically and genetically with p53 (Garkavtsev et al. 1998; Shinoura et al. 1999; Shiseki et al. 2003; reviewed in Soliman and Riabowol 2007), neither ING1 deletion mice nor their derived cell lines demonstrated that ING1 function depends upon p53 or that p53 function is perturbed in ING1 knockouts (Coles et al. 2007).
To explore the developmental role(s) of ING proteins, and particularly a relationship to p53, we studied the function of Caenorhabditis elegans ing-3, the ING gene most similar in worms and humans. We found that ING-3 is widely expressed and localizes to chromatin. Unlike yeast (Loewith et al. 2000), no changes were seen in response to UV damage after ing-3(RNAi). However, similar to what is seen in mice, reduction or loss of C. elegans ING-3 alters the response to γ-radiation, as indicated by decreased germline apoptosis, which in turn leads to increased embryonic lethality. Unlike the mouse ING1 mutant, we find that ing-3 does operate in concert the C. elegans p53 homolog cep-1 to mediate apoptosis. The phenotypes that we report are the first to demonstrate a role for an ING protein in the germline or embryo.
MATERIALS AND METHODS
Strains:
Animals were cultured at 20° as described (Brenner 1974). Wild type was Bristol N2, and mutant strains include cep-1(gk138), rrf-3(pk1426), and mcd-1(tm2169) (http://www.wormbase.org; WS170 data freeze). ing-3(tm2530) and ing-3(ttTi5439) were generously provided by the Japanese National Bioresource Project for the Nematode (Gengyo-Ando and Mitani 2000) and the Mos transposon insertion from the NEMAGENETAG Consortium (Granger et al. 2004), respectively. ing-3 mutants were outcrossed three times.
Identification of homologs:
Homologs of the ing-3 gene were identified in Caenorhabditis briggsae, Drosophila, Xenopus, mice, and humans with the BLAST program at the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/BLAST). The multiple sequence alignments of ING3 were created with the CLUSTAL W program at the European Bioinformatics Institute (http://www.ebi.ac.uk/clustalw/).
Nomarski differential interference contrast microscopy:
Worms were observed on 3% agarose pads and anesthetized with 10 mm levamisole (Shaham 2006). Worms, both by Nomarski and immunofluorescence, were visualized using an Axioscope microscope (Zeiss) and images were processed using AxioVision (Zeiss) and Photoshop 7.0 (Adobe) software.
Antibody production and Western blots:
Mouse polyclonal antibodies against C. elegans ING-3 were generated at the Southern Alberta Cancer Research Institute Antibody Services against the keyhole limpet hemocyanin-conjugated peptide CEMEADNSGVTEMIE [amino acid residues 108–122, a highly conserved hydrophilic motif within the lamin interacting domain (LID)]. Gravid adults were used in Western blots, which were performed as described (Feng et al. 2006) using a 2000-fold dilution of anti-ING-3 antiserum incubated overnight at 4°.
Immunostaining:
Adult hermaphrodite gonads were fixed in 100% methanol as described (Hansen et al. 2004). Dissected gonads were incubated overnight at 4° with mouse anti-ING-3 antiserum, diluted 1:100. Goat anti-mouse IgG-Texas Red (Sigma) was used at 1:100 dilution as the secondary antibody. DAPI (4′,6-diamidino-2-phenylindole) was added at 0.5 μg/ml to stain DNA.
Embryos were fixed and permeablized by freeze-cracking as described (Miller and Shakes 1995). Staining was at the same antibody concentrations as for the gonads, but incubations with primary and secondary antibodies were for 45 min and 1 hr, respectively.
Plasmid construction:
To determine the structure of ing-3 transcripts, a cDNA pool of mixed-stage worms was subjected to PCR amplification. A pair of specific primers (F1 and R1, listed below) flanking the start and stop codons amplified the coding region. A poly(T) primer and nested gene-specific primers (F2 and F3) amplified the 3′-end while the splice leaders SL1 and SL2 and the nested gene-specific primers (R2 and R3) amplified the 5′-end. PCR products were gel purified and then sequenced by the University of Calgary DNA lab.
The genomic sequences of the operon promoter (600 bp) and the ing-3 internal promoter (880 bp) were obtained by PCR and subcloned into the promoterless GFP-LacZ reporter vector pPD96.04 (gift from Andrew Fire, Stanford University) to create the transcriptional ing-3 promoter-gfp-LacZ fusions.
The following primers were used: F1, 5′-CATTGCATGCCTCTTCCTCGATGATTTTTT-3′; R1, 5′-CAGAAGCTTTTAGACTTCTCCCTCTTCGG-3′; SL1, 5′-GGTTTAATTACCCAAGTTTGAG-3′; SL2, 5′-GGTTTTAACCCAGTTACTCAAG-3′; R2, 5′-ATTTGCGTTGAGCTAGCACG-3′; R3, 5′-CGGTCACTCCTGAATTGTCG-3′; F2, 5′-AGAAGAGCTATGGAGATATG-3′; and F3, 5′-ATCGGAGG ATGAGGAGGATG-3′.
Transgenesis:
Transgenic strains were created by injecting 50 ng/ml of the promoter-gfp-LacZ plasmid DNA with 50 ng/ml of pRF4 [rol-6(su1006dm)] into the gonad of young adult hermaphrodites (Mello et al. 1991). The presence of the constructs was confirmed in Roller offspring by PCR. All six transgenic lines made for each promoter plasmid showed the same GFP and LacZ expression patterns. For β-Gal staining, worms in M9 buffer were dried on slides for 10 min, fixed, and permeablized with acetone for 30 min and then incubated overnight with X-Gal (Zdinak et al. 1997).
RNA interference:
RNA interference (RNAi) for ing-3 was performed using standard bacterial feeding and injection methods (Timmons et al. 2001). A 463-bp fragment of ing-3 (see Figure 1C) was cloned into the RNAi feeding vector L4440, transformed into Escherichia coli HT115, and induced with isopropyl-β-d-thiogalactopyranoside. Wild-type worms fed on E. coli HT115 containing the vector L4440 were used as controls. The following primers were used: ING3F (5′-ACAGTCTCCATTCCAAGTGCTCT-3′) and ING3R (5′-AACTCGGTCCTTCCTCATCATCA-3′).
Figure 1.—
ing-3 protein and gene structure. (A) Diagram of the predicted ING-3 protein, indicating the PHD, leucine zipper-like domain (LZL), the NLS, and the LID. The corresponding regions and amino acid similarities with human ING-3 are shown. (B) The ing-3 gene is the second gene in a predicted operon that includes mitochondrial dehydrogenase (Y51H1A.3) and histone deacetylase hda-6 and mcd-1. (C) The six exons of the ing-3 gene are indicated by yellow boxes while green boxes represent the 5′ and 3′ untranslated regions. The relative exon positions on cosmid Y51H1A are indicated. The position of the alleles ttTi5439 and tm2530 are shown in red, and the blue box indicates the region used to raise the antisera. The region used to generate dsRNA for RNAi is marked with red arrows.
For injection, double-strand RNA (dsRNA) was generated by in vitro transcription with a MEGAscript purification kit (Ambion) and dsRNA gfp was used as a control. Sense and antisense RNAs were transcribed separately and mixed together in equimolar amounts. dsRNA was injected at 1 μg/μl into the gonads of young adult hermaphrodites, worms were transferred to new plates 12 hr later, and phenotypes were scored in the next 24-hr brood. ing-3 feeding and injection gave similar results.
Ionizing radiation-induced embryonic lethality:
One-day post-L4 gravid adults treated with RNAi were exposed to different doses of γ-radiation using a 137Cs source. Following irradiation, worms were immediately transferred to new plates, and eggs laid during the first 0–8 hr (irradiated at diakinesis through embryogenesis) or 8–22 hr (irradiated at pachytene; Takanami et al. 2000) were scored for hatching 24 hr after removal of the parents.
Ionizing radiation-induced germ-cell death:
L4-stage larvae were treated with 120 Gy of γ-radiation. The number of apoptotic germ cells per gonad arm was counted 24 hr later using Nomarski optics (Gumienny et al. 1999; Gartner et al. 2000) or acridine orange (AO; Gartner et al. 2004; Lettre et al. 2004). Worms were incubated with 500 μl of 100 mm AO on plates for 1 hr, transferred to new plates in the dark, and 1 hr later were mounted on slides and observed by fluorescence microscopy.
Statistical analyses:
The mean number of apoptotic germ cells per gonad and mean embryonic death rate ± standard deviation (SD) are presented. Hypotheses were tested with the two-sided Student's t-test.
RESULTS
C. elegans has three ING genes:
To identify genes with the highest similarity to human ING genes, multiple searches were conducted using the NCBI BLAST program. Three genes, T06A10.4, Y51H1A.4, and C11G6.3, encode ING-related proteins, corresponding to human ING1–2, ING3, and ING4–5 subgroups, respectively. As shown in supplemental Table 1, ING3 and Y51H1A.4 had the most significant reciprocal scores in BLAST searches (the human-to-worm score was 1.9 × 10−33). However, in phylograms drawn by different methods (He et al. 2005), there is not a consistent correspondence between these C. elegans genes and the three vertebrate ING subgroups. Using parsimony analysis, Y51H1A.4 and C11G6.3 group with ING3, with Y51H1A.4 being closer, while neighbor-joining analysis suggests that the three worm genes form an outgroup from the vertebrate genes. We chose to focus on the most similar of the worm genes, Y51H1A.4, which we designated ing-3 because this gene and human ING3 have the highest reciprocal BLAST scores.
Comparison of the primary amino acid sequence of the ING3 proteins from different organisms showed that several regions appeared well conserved (Figure 1A and supplemental Figure 1), including the PHD domain, the leucine zipper-like domain (LZL), the nuclear localization sequence (NLS, although it is in different locations in human and worm proteins), and the lamin interacting domain (LID, Soliman and Riabowol 2007; Han et al. 2008).
The ing-3 gene is expressed in a broad range of tissues:
The C. elegans ing-3 gene is the second gene in a predicted operon that includes mitochondrial dehydrogenase (Y51H1A.3), histone deacetylase hda-6, and the mediator of the cell death gene mcd-1 (Figure 1B). As confirmed by RT–PCR, ing-3 is encoded by six exons (Figure 1C). In C. elegans, most mRNAs are trans-spliced to one of two 22-nucleotide spliced leaders, SL1 or SL2 (Blumenthal and Steward 1997). SL2 is spliced onto the downstream genes in operons, whereas SL1 is used by the first gene in operons and for most non-operon genes. Some downstream genes in operons are also transcribed from both the operon promoter and an internal promoter, in which case the transcripts can be spliced to either SL1 or SL2 (Blumenthal and Steward 1997). Our RT–PCR analysis indicates that ing-3 transcripts are spliced to both SL1and SL2 (data not shown).
To determine promoter activity of the regions immediately upstream of the operon and upstream of ing-3 itself, 600 bp of the 5′ genomic sequence of the first gene in the operon (the “operon promoter”) and the 880 bp between ing-3 and the immediate upstream gene (the “internal promoter”) were separately inserted into a GFP-LacZ reporter and used to generate transgenic lines (Figure 2A). Expression of the operon promoter∷gfp-LacZ was first detected during embryogenesis at gastrulation and persisted through adulthood (data not shown). As shown in Figure 2, B–E, GFP and LacZ expression included pharynx, vulva, spermatheca, neurons, and epidermal cells, but not the intestine. In contrast, the expression of the internal promoter∷gfp-LacZ reporter was first detected at the comma stage, which is later in development than when the operon promoter∷gfp-LacZ is first detected. Unlike the operon promoter, the internal promoter drives expression in the anterior and posterior intestine cells (Figure 2, F and G), where it persists through adulthood.
Figure 2.—
Reporters driven by the operon or internal promoter give different expression patterns. (A) The 5′ genomic sequence (600 bp) of the first gene of this operon (Y51H1A.3) was used as the “operon promoter,” while the 880 bp between ing-3 and Y51H1A.3 was used as the “internal promoter.” (B) GFP and (C) LacZ indicate that the operon promoter was widely expressed in L1 larva, especially in the pharynx. (D) The operon promoter showed GFP expression in the adult vulva, spermatheca (asterisks), neurons, and epidermal cells, among others. (E) Differential interference contrast image corresponding to D. The internal promoter expressed GFP (F) and LacZ (G) in the L1 intestine, the limits of which are indicated by asterisks. The signal between the asterisks in F represents gut auto-fluorescence. Note higher expression in the anterior intestinal cells.
ING-3 immunolocalization:
Since C. elegans transgenes are frequently silenced in the germline (Kelly and Fire 1998), we produced polyclonal antibodies against a unique and highly conserved hydrophilic region within the LID region of ING-3 (Figure 1A; supplemental Figure 1). Western blots were used to test the specificity of the ING-3 antibody . As shown in Figure 3A, the antibody recognizes only a single band in ing-3(+) worms. ing-3(tm2530) has a partial deletion in the fourth intron and the fifth exon (Figure 1C) and would potentially encode a truncated product of 28 kDa. In ing-3(ttTi5439) worms, the gene is interrupted by a Mos-1 insertion in the fourth exon and would result in a predicted product of 17 kDa. Our mouse polyclonal antibodies recognize a motif encoded before both mutations and so potentially detect these mutant proteins. However, Western blots showed that the wild-type 45-kDa band was missing in both strains and no smaller bands appeared (Figure 3A), suggesting that mutant proteins (or mRNA) are degraded. Thus, these alleles likely represent strong losses of function if not nulls.
Figure 3.—
Immunolocalization of ING-3. (A) Mouse polyclonal anti-ING-3 recognized endogenous ING-3 as a single 45-kDa band on Western blots of wild-type gravid hermaphrodites. This band was absent in the two ing-3 mutants but was present in cep-1 lysates. Actin was used as the loading control. (B) ING-3 colocalized with chromatin in the newly fertilized embryo (top left) but is absent from ing-3(tm2530) (top right). ING-3 was also found on chromatin at mitosis in developing embryos (arrows, bottom row). (C) Immunostaining of dissected wild-type adult gonads (left-most column) shows that ING-3 overlapped DAPI. Lower levels were present in the mitotic region (I) compared to the transition zone (II). High ING-3 persisted into the early pachytene region (III), but then decreased as cells proceeded through meiosis. Low levels, mainly on chromosomes, were seen at diplotene (IV). Higher magnification of the early pachytene section of the gonad (columns 2–4) showed extensive overlap between ING-3 and DAPI. The nuclear signal disappeared when antisera was preincubated with the immunizing peptide (peptide block) or in ing-3(tm2530). Thus the low level of ING-3 staining between cells likely represents background. The right-most column shows the staining pattern for ING-3 in the anterior nuclei of a dissected gut.
We next examined ING-3 localization patterns, particularly in adult germlines and embryos. Figure 3B shows that ING-3 was present in the newly fertilized embryo, where it colocalized with DNA. Similar localization of ING-3 and DAPI continued throughout the embryonic cell cycle, including during mitosis (Figure 3B). This suggests that ING-3 is tightly bound to chromatin at all stages of the cell cycle and so differs from mammalian ING1, which is found throughout the cell during mitosis (Han et al. 2008). ING-3 was also present in the gonad (Figure 3C). Staining was weak in the mitotic proliferating germ cells (region I) but was stronger in the transition zone (region II) and the following early pachytene stages (region III). It decreased by late pachytene and diplotene (region IV). As shown in the higher magnification of gonadal cells, nuclear ING-3 staining, although sometimes uneven, overlapped extensively with DNA. Nuclear staining was absent when antisera were preincubated with the immunizing peptide as well as in the deletion allele ing-3(tm2530), demonstrating antibody specificity. However, nonspecific background staining persisted between cells (this is an unlikely cross-reaction with the other worm ING proteins since they share at most 3/5 consecutive identical amino acids with the immunizing peptide). ING-3 was also expressed in somatic cells where it again overlapped with DAPI, and strong staining was seen for some cells in the anterior gut (Figure 3C), consistent with results obtained with internal promoter–reporter constructs (Figure 2, F and G).
Depletion of ing-3 inhibits ionizing radiation-induced germ-cell apoptosis:
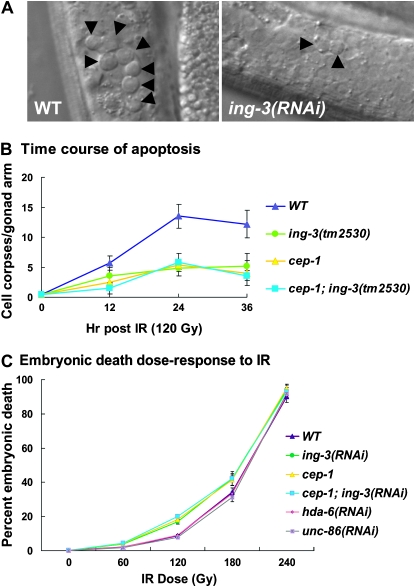
Since we were particularly interested in the functional relationship between ing-3 and cep-1/p53, we investigated the role of ing-3 in germline apoptosis, for which cep-1 has a well-documented phenotype (Derry et al. 2001; Schumacher et al. 2001; Hofmann et al. 2002). In mammals, ING proteins also play roles in apoptosis (Helbing et al. 1997; Nagashima et al. 2003; Wang and Li 2006). We found strong suppression of programmed cell death following ionizing radiation (IR) from γ-rays upon ing-3 depletion. Twenty-four hours after exposure to 120 Gy of IR, wild type showed a large number of pachytene germ cells undergoing programmed cell death, but many fewer dying cells were present in irradiated ing-3(RNAi) animals (Figure 4A). Although the basal level of germline apoptosis was unchanged from wild type in unirradiated ing-3(RNAi) animals, the average number of germ-cell corpses was half that of wild type following radiation (Table 1; P < 0.001). The Mos insertion allele ing-3(ttTi5439) was similar to RNAi, while the internal deletion allele ing-3(tm2530) had a slightly stronger phenotype (the phenotypes are due to ing-3 rather than to unknown linked mutations since they are present in two independent alleles). RNAi alone results in a strong loss of function and RNAi treatment did not alter the phenotype of tm2530, indicating that tm2530 is null. Decreased levels of IR-induced apoptosis when ing-3 was depleted could represent a delay rather than a block to cell death. However, the time course in Figure 4B indicates that this was not the case.
Figure 4.—
Germ-cell apoptosis and embryonic death following IR. (A) L4 stage larvae were exposed to 120 Gy of IR and the number of apoptotic germ cells (arrowheads) was scored 24 hr later. Animals treated with ing-3(RNAi) showed a significantly reduced number of apoptotic germ cells. (B) Time course of accumulation of cell corpses following 120 Gy of IR. Similar to wild type and cep-1, apoptosis plateaus by 24 hr in ing-3 and ing-3; cep-1, indicating that ing-3 is blocking rather than delaying cell death. (C) Dose-response curve of wild type and mutants vs. embryonic inviability of the 8- to 22-hr brood following the indicated levels of IR. Error bars represent 1 standard deviation in B and C.
TABLE 1.
Effects of ing-3 on germ-cell apoptosis 24 hr after exposure to 120 Gy of IR
| Genotype | Germ-cell corpses/gonad arm (±SD) | N (gonad arms) |
|---|---|---|
| Wild type (no radiation) | 3.0 (±1.4) | 40 |
| Wild type | 13.5 (±2.0) | 40 |
| ing-3(RNAi) (no radiation) | 2.8 (±1.3) | 40 |
| ing-3(RNAi) | 6.3 (±1.5) | 40 |
| ing-3(ttTi5439) | 6.7 (±1.8) | 45 |
| ing-3(tm2530) | 4.8 (±1.2) | 40 |
| ing-3(tm2530; RNAi) | 4.9 (±1.4) | 30 |
| cep-1(null) | 5.4 (±0.8) | 30 |
| ing-3(RNAi); cep-1(null) | 5.9 (±1.2) | 40 |
| ing-3(tm2530); cep-1(null) | 5.8 (±1.5) | 30 |
DNA-damaged germ cells that survive due to loss of ing-3 should give rise to inviable zygotes. The 0- to 8-hr brood after IR corresponds to post-pachytene fertilized embryos or germ cells at the time of exposure. These cells are not sensitive to DNA-damage-induced apoptosis (Gartner et al. 2000; Takanami et al. 2000), and therefore apoptosis cannot prevent cells damaged during this time from giving rise to inviable zygotes. Hence, as expected, no change was seen in the viability of wild-type vs. ing-3(RNAi) embryos laid 0–8 hr after IR exposure (data not shown). In contrast, ing-3(RNAi) nearly doubled the level of embryonic inviability in the 8- to 22-hr brood compared to wild type (Table 2; P < 0.001; all data reported below are for this time window). In contrast to IR, we did not find differences in the sensitivity of wild type and ing-3(RNAi) to UV (data not shown). As we found for the prevention of germline apoptosis, the Mos insertion allele ttTi5439 was similar to RNAi in increasing embryonic death, while the internal deletion allele tm2530 showed a stronger, likely null phenotype, since its phenotype was not altered by RNAi (Table 2). We generated an IR dose-response curve for embryonic lethality and found that ing-3(RNAi) differed from wild type at both 120 and 180 Gy, with the maximum fold difference (twofold) occurring at 120 Gy (Figure 4C). The RNAi effects were specific as feeding of control unc-86 dsRNA did not affect embryonic viability (Table 2, Figure 4C).
TABLE 2.
Unhatched embryos among the brood laid 8–22 hr following exposure of adult hermaphrodites to 120 Gy of IR
| Genotype | % embryonic death (±SD) | N |
|---|---|---|
| Wild type (no radiation) | 0.4 (±0.3) | 1753 |
| Wild type | 9.4 (±0.6) | 3078 |
| ing-3(RNAi) (no radiation) | 0.2 (±0.1) | 1524 |
| ing-3(RNAi) | 16.8 (±1.3) | 4266 |
| ing-3(ttTi5439) | 18.9 (±2.1) | 3340 |
| ing-3(tm2530) | 22.7 (±2.5) | 3431 |
| ing-3(tm2530; RNAi) | 23.3 (±4.3) | 3125 |
| unc-86(RNAi) | 7.9 (±0.8) | 2283 |
| hda-6(RNAi) | 9.6 (±0.7) | 2372 |
| mcd-1(tm2169) | 10.2 (±0.9) | 2275 |
| cep-1 (no radiation) | 0.5 (±0.4) | 1602 |
| cep-1 | 18.2 (±1.9) | 3789 |
| ing-3(RNAi); cep-1 | 20.0 (±1.7) | 3672 |
| ing-3(tm2530); cep-1 | 21.8 (±2.9) | 2120 |
ing-3 is in the same operon as hda-6 histone deacetylase (Figure 1B). This is intriguing because mammalian and yeast ING proteins are found in chromatin acetylation and deacetylation complexes (Loewith et al. 2000; Vieyra et al. 2002b; Doyon et al. 2006), and functionally related genes are sometimes found in the same C. elegans operons (Clark et al. 1994; Huang et al. 1994; Treinin et al. 1998). However, Figure 4C and Table 2 indicate that hda-6(RNAi) did not influence embryonic viability following IR (with the caveat that the RNAi is sometimes not effective). Similarly, another member of the operon, mcd-1, whose gene product promotes somatic apoptosis (Reddien et al. 2007), had no effect in our assay (Table 2; the allele used is predicted to truncate the protein in the second exon, prior to the zinc finger).
ing-3 acts in the same pathway as cep-1/p53:
To determine if ing-3 acts in concert with p53, we examined genetic interactions between ing-3 and cep-1/p53. Compared to wild type, a cep-1 deletion allele decreased the number of IR-induced germ-cell corpses and decreased embryonic viability (Tables 1 and 2) to a similar extent as the loss of ing-3 did [unlike previous reports (Schumacher et al. 2001; Quevedo et al. 2007), we found that the irradiated cep-1 levels were slightly above unirradiated controls]. The dose response of embryonic inviability to IR was also similar in ing-3(RNAi) and cep-1 (Figure 4C). If the two mutations act in independent, parallel pathways (and at least one mutation is a null), then the double mutant would show additive effects. However, if the genes act in the same or in convergent pathways, as reported for mammalian ING1 and p53 in some studies (Garkavtsev et al. 1998) but not in others (Coles et al. 2007), then the double mutant should resemble the individual single mutants. As shown in Table 1, the behavior of ing-3(RNAi); cep-1 or ing-3(tm2830); cep-1 indicates that the genes act together since the amount of apoptosis in the double mutants did not decrease below that in the single mutants and remained substantially higher than the basal level of apoptosis in unirradiated wild type (P < 0.001). Likewise, the level of embryonic lethality in ing-3(RNAi); cep-1 or ing-3(tm2830); cep-1 was similar to cep-1 alone (Table 2, Figure 4C). In addition, loss of cep-1 did not affect the level of ING-3 expression (Figure 3A), consistent with reports in other species that expression of ING proteins is independent of p53 (Cheung et al. 2000; Coles et al. 2007). We observed no changes to the intracellular localization of ING-3 following IR (data not shown).
Since loss of ing-3 function results in a hyposensitive DNA-damage-induced apoptosis phenotype, we tested if overexpression of ING-3 sensitized germ cells to DNA-damage-induced apoptosis using a heat-inducible construct. No changes in the rate of germ-cell apoptosis after IR were noted (data not shown). However, we did not detect a stronger immunofluorescence signal in the germline, likely due to poor germline transgene expression (Kelly and Fire 1998).
ing-3 results in a kinker Unc phenotype:
Applying RNAi to ing-3 in wild type or to the RNAi-sensitive strain rrf-3 (Simmer et al. 2002) resulted in no overt morphological phenotypes. The brood size of ing-3(tm2530) was similar to that of wild type (201 ± 62 vs. 220 ± 29 for N2, n = 8 and 7 hermaphrodites, respectively). However, both the Mos insertion and the ing-3 mutants showed a weak, but fully penetrant, kinker Unc phenotype. This indicates possible neuronal function, which was likely not seen with dsRNA exposure because neurons are refractory to RNAi (Simmer et al. 2002).
DISCUSSION
C. elegans ing-3 and IR-induced apoptosis:
Mammalian ING proteins have well-documented roles in apoptosis (Helbing et al. 1997; Garkavtsev et al. 1998; Shinoura et al. 1999; Scott et al. 2001; Shimada et al. 2002; Vieyra et al. 2002a; Wagner and Helbing 2005; Zhu et al. 2005, 2006; Feng et al. 2006; Wang and Li 2006; Coles et al. 2007). Specifically, overexpression of human ING3, a putative homolog of the C. elegans ing-3 gene studied here, promotes apoptosis (Nagashima et al. 2003; Wang and Li 2006), and low levels of nuclear ING3 are associated with decreased survival of melanoma patients (Wang et al. 2007). In C. elegans we found that IR-induced germ-cell apoptosis was inhibited in both ing-3(RNAi) knockdown and ing-3 knockouts (Figure 4, Table 1). As a result, many DNA-damaged germ cells survived and went on to form inviable zygotes (Figure 4, Table 2). Western blotting showed that RNAi reduced ING-3 levels by 50–80% (data not shown) whereas in the knockouts ING-3 was completely depleted. However, the phenotypes of the internal deletion mutant ing-3(tm2530) were only slightly stronger than those of the RNAi, suggesting that C. elegans is sensitive to relatively small changes in ING-3 levels or that the residual ING-3 after RNAi was in tissues not relevant to the phenotype.
Unlike γ-radiation, ing-3(RNAi) did not elevate the embryonic death rate following non-ionizing DNA-damaging treatments, including UV radiation, paraquat treatment, and heat shock (data not shown). These results suggest that, in contrast to mammalian ING proteins, C. elegans ING-3 may transduce signals generated by ionizing irradiation but not by other stresses. These functions may be carried out by the other two worm ING-related genes, and a division of labor might be expected since mammalian ING genes appear to function in a variety of histone acetylation complexes with potentially overlapping function (Feng et al. 2002; Doyon et al. 2006).
It is possible that the decreased number of programmed cell deaths in ing-3 following IR resulted from the mutants simply having fewer germ cells. A number of lines of evidence argue against this possibility. First, the brood size of ing-3(tm2180) is similar to that of wild type. Second, if there were fewer cells that could die, then there would not have been a corresponding increase in the percentage of dead embryos following IR as we observed (Figure 4). Third, the response to IR and UV differed for ing-3, with only the former showing increased embryo lethality in the mutants; alterations in germ-cell populations would affect UV and IR similarly. Fourth, if the decrease in apoptosis following IR were due to a decrease in germ-cell numbers, then the effects of ing-3 and cep-1 would have been additive because they were mechanistically different, which is contrary to what we found.
The roles of ing-3 and cep-1 in promoting stress-induced apoptosis:
While dispensable for physiological germ-cell death and developmental programmed cell death (Salinas et al. 2006), cep-1, the C. elegans p53 homolog, is required for DNA-damage-induced apoptosis (Derry et al. 2001; Schumacher et al. 2001). In mammalian cells, ING proteins can promote p53-dependent apoptosis, but a recent report indicates that p53 and ING1 operate independently regarding sensitivity to apoptosis (Coles et al. 2007). In contrast to ING1 function in mammals, our data show that the IR-induced phenotypes of ing-3, cep-1, and ing-3; cep-1 are very similar (Tables 1 and 2, Figure 4). This indicates that the two genes function in the same apoptotic pathway; otherwise additive effects would have been seen. Consistent with this idea, ING-3 localizes in the pachytene nuclei (Figure 3) as CEP-1 does (Schumacher et al. 2005). The simplest model is that the two genes act together to induce transcription of genes required for apoptosis. However, although CEP-1 induces transcription of the proapoptotic gene egl-1 (Hofmann et al. 2002; Schumacher et al. 2005; Quevedo et al. 2007), preliminary evidence indicates that ing-3(tm2830) does not reduce the level of the egl-1 transcript (S. Shah, unpublished results). Thus, ing-3 may influence only a subset of cep-1 targets, it may act downstream of CEP-1-mediated transcription, or it could function in a parallel, but nonredundant, pathway.
The function of CEP-1 and ING-3 also differs in that CEP-1 is required for both UV and IR-induced germ-cell apoptosis (Derry et al. 2001; Schumacher et al. 2001, 2005; Stergiou et al. 2007) while we found no role for ING-3 in the UV response. In addition to ing-3 and cep-1, genes such as abl-1, lin-35, dpl-1, efl-2, clk-2, hus-1, mrt-2, ced-3, ced-4, and ced-9 are also involved in DNA-damage-induced C. elegans germ-cell apoptosis (Deng et al. 2004; Schertel and Conradt 2007). How ing-3 operates within pathways represented by these genes is unknown, although mammalian ING3 has been reported to function with the apoptotic caspase 8 in melanoma cancer cells (Wang and Li 2006).
Although we examined ing-3 phenotypes only in the germline and embryo, ING-3, like CEP-1, is widely expressed. In addition, ing-3 appears to be expressed differently when transcribed as part of an operon or from its own internal promoter (Figure 2). This may allow differential expression in different tissues, which in turn might reflect different functions. Both ing-3(tm2530) and ing-3(ttTi5439) mutants possess a weak kinker Unc phenotype. The kinker phenotype usually indicates a defect in neurons (Geng et al. 2003), a cell type in which ING-3 is expressed (Figure 2). It will be interesting to determine if ING-3 neural function is evolutionarily conserved in vertebrates (Wagner and Helbing 2005).
It is intriguing that ing-3 resides in an operon with two other genes that could potentially influence similar processes. hda-6 encodes a histone deacetylase while mcd-1 encodes a C2H2 zinc-finger mediator of somatic cell death (Reddien et al. 2007). Although we did not detect genetic interactions between these genes and ing-3 during germline apoptosis, they may interact with ing-3 in other processes.
ING-3 and chromatin regulation:
In both mammals and yeast, ING proteins interact with histone acetyltransferase or deacetylase complexes (reviewed in Russell et al. 2006). We found that ING-3 overlapped the DAPI pattern throughout the cell cycle, suggesting that ING-3 might function in chromatin regulation as suggested by reports showing a direct interaction between the PHD regions of mammalian ING proteins and histone H3 (Martin et al. 2006; Pena et al. 2006; Shi et al. 2006). The staining of ING-3 was not evenly distributed on chromatin, suggesting that ING-3 might accumulate in specific regions. If so, ING-3 might regulate chromatin structure with associated HAT and HDAC complexes and influence gene silencing or transcription in C. elegans as previously reported in cell culture systems.
Acknowledgments
We thank members of the Riabowol and Mains labs and members of the Calgary C. elegans group, in particular J. Gaudet, J. D. McGhee, and D. Hansen, as well as the anonymous reviewers, for discussion and help with methods. We thank S. Mitani and L. Segalat for generously providing ing-3 mutants and the Caenorhabditis Genetics Center (funded by the National Institutes of Health, Center for Research Resources) for providing strains. We thank the Southern Alberta Cancer Research Institute Hybridoma Facility for generating antibodies and Susan Lees-Miller for providing the ionizing irradiation source. This work was supported by grants from the Canadian Institutes of Health Research (CIHR) and the Alberta Cancer Board to K.R. and grants from the CIHR and the Alberta Heritage Foundation for Medical Research to P.E.M.
References
- Bienz, M., 2006. The PHD finger, a nuclear protein-interaction domain. Trends Biochem. Sci. 31 35–40. [DOI] [PubMed] [Google Scholar]
- Blumenthal, T., and K. Steward, 1997. RNA processing and gene structure, pp. 117–146 in C. elegans II, edited by D. L. Riddle, T. Blumenthal, B. J. Meyer and J. R. Priess. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung, K. J., Jr, J. A. Bush, W. Jia, and G. Li, 2000. Expression of the novel tumour suppressor p33(ING1) is independent of p53. Br. J. Cancer 83 1468–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, S. G., X. Lu and H. R. Horvitz, 1994. The Caenorhabditis elegans locus lin-15, a negative regulator of a tyrosine kinase signaling pathway, encodes two different proteins. Genetics 137 987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles, A. H., H. Liang, Z. Zhu, C. G. Marfella, J. Kang et al., 2007. Deletion of p37Ing1 in mice reveals a p53-independent role for Ing1 in the suppression of cell proliferation, apoptosis, and tumorigenesis. Cancer Res. 67 2054–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, X., E. R. Hofmann, A. Villanueva, O. Hobert, P. Capodieci et al., 2004. Caenorhabditis elegans ABL-1 antagonizes p53-mediated germline apoptosis after ionizing irradiation. Nat. Genet. 36 906–912. [DOI] [PubMed] [Google Scholar]
- Derry, W. B., A. P. Putzke and J. H. Rothman, 2001. Caenorhabditis elegans p53: role in apoptosis, meiosis, and stress resistance. Science 294 591–595. [DOI] [PubMed] [Google Scholar]
- Doyon, Y., C. Cayrou, M. Ullah, A. J. Landry, V. Cote et al., 2006. ING tumor suppressor proteins are critical regulators of chromatin acetylation required for genome expression and perpetuation. Mol. Cell 21 51–64. [DOI] [PubMed] [Google Scholar]
- Feng, X., Y. Hara and K. Riabowol, 2002. Different HATS of the ING1 gene family. Trends Cell Biol. 12 532–538. [DOI] [PubMed] [Google Scholar]
- Feng, X., S. Bonni and K. Riabowol, 2006. HSP70 induction by ING proteins sensitizes cells to tumor necrosis factor alpha receptor-mediated apoptosis. Mol. Cell. Biol. 26 9244–9255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garkavtsev, I., A. Kazarov, A. Gudkov and K. Riabowol, 1996. Suppression of the novel growth inhibitor p33ING1 promotes neoplastic transformation. Nat. Genet. 14 415–420. [DOI] [PubMed] [Google Scholar]
- Garkavtsev, I., I. A. Grigorian, V. S. Ossovskaya, M. V. Chernov, P. M. Chumakov et al., 1998. The candidate tumour suppressor p33ING1 cooperates with p53 in cell growth control. Nature 391 295–298. [DOI] [PubMed] [Google Scholar]
- Gartner, A., S. Milstein, S. Ahmed, J. Hodgkin and M. O. Hengartner, 2000. A conserved checkpoint pathway mediates DNA damage-induced apoptosis and cell cycle arrest in C. elegans. Mol. Cell 5 435–443. [DOI] [PubMed] [Google Scholar]
- Gartner, A., A. J. MacQueen and A. M. Villeneuve, 2004. Methods for analyzing checkpoint responses in Caenorhabditis elegans. Methods Mol. Biol. 280 257–274. [DOI] [PubMed] [Google Scholar]
- Geng, W., P. Cosman, J. H. Baek, C. C. Berry and W. R. Schafer, 2003. Quantitative classification and natural clustering of Caenorhabditis elegans behavioral phenotypes. Genetics 165 1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengyo-Ando, K., and S. Mitani, 2000. Characterization of mutations induced by ethyl methanesulfonate, UV, and trimethylpsoralen in the nematode Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 269 64–69. [DOI] [PubMed] [Google Scholar]
- Gozani, O., P. Karuman, D. R. Jones, D. Ivanov, J. Cha et al., 2003. The PHD finger of the chromatin-associated protein ING2 functions as a nuclear phosphoinositide receptor. Cell 114 99–111. [DOI] [PubMed] [Google Scholar]
- Granger, L., E. Martin and L. Segalat, 2004. Mos as a tool for genome-wide insertional mutagenesis in Caenorhabditis elegans: results of a pilot study. Nucleic Acids Res. 32: e117. [DOI] [PMC free article] [PubMed]
- Gumienny, T. L., E. Lambie, E. Hartwieg, H. R. Horvitz and M. O. Hengartner, 1999. Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development 126 1011–1022. [DOI] [PubMed] [Google Scholar]
- Han, X., X. Feng, J. B. Rattner, H. Smith, P. Bose et al., 2008. Tethering by lamin A stabilizes and targets the ING1 tumour suppressor. Nat. Cell Biol. 10 1333–1340. [DOI] [PubMed] [Google Scholar]
- Hansen, D., L. Wilson-Berry, T. Dang and T. Schedl, 2004. Control of the proliferation versus meiotic development decision in the C. elegans germline through regulation of GLD-1 protein accumulation. Development 131 93–104. [DOI] [PubMed] [Google Scholar]
- He, G. H., C. C. Helbing, M. J. Wagner, C. W. Sensen and K. Riabowol, 2005. Phylogenetic analysis of the ING family of PHD finger proteins. Mol. Biol. Evol. 22 104–116. [DOI] [PubMed] [Google Scholar]
- Helbing, C. C., C. Veillette, K. Riabowol, R. N. Johnston and I. Garkavtsev, 1997. A novel candidate tumor suppressor, ING1, is involved in the regulation of apoptosis. Cancer Res. 57 1255–1258. [PubMed] [Google Scholar]
- Hofmann, E. R., S. Milstein, S. J. Boulton, M. Ye, J. J. Hofmann et al., 2002. Caenorhabditis elegans HUS-1 is a DNA damage checkpoint protein required for genome stability and EGL-1-mediated apoptosis. Curr. Biol. 12 1908–1918. [DOI] [PubMed] [Google Scholar]
- Huang, L. S., P. Tzou and P. W. Sternberg, 1994. The lin-15 locus encodes two negative regulators of Caenorhabditis elegans vulval development. Mol. Biol. Cell 5 395–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaadige, M. R., and D. E. Ayer, 2006. The polybasic region that follows the plant homeodomain zinc finger 1 of Pf1 is necessary and sufficient for specific phosphoinositide binding. J. Biol. Chem. 281 28831–28836. [DOI] [PubMed] [Google Scholar]
- Kelly, W. G., and A. Fire, 1998. Chromatin silencing and the maintenance of a functional germline in Caenorhabditis elegans. Development 125 2451–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kichina, J. V., M. Zeremski, L. Aris, K. V. Gurova, E. Walker et al., 2006. Targeted disruption of the mouse ing1 locus results in reduced body size, hypersensitivity to radiation and elevated incidence of lymphomas. Oncogene 25 857–866. [DOI] [PubMed] [Google Scholar]
- Lettre, G., E. A. Kritikou, M. Jaeggi, A. Calixto, A. G. Fraser et al., 2004. Genome-wide RNAi identifies p53-dependent and -independent regulators of germ cell apoptosis in C. elegans. Cell Death Differ. 11 1198–1203. [DOI] [PubMed] [Google Scholar]
- Loewith, R., M. Meijer, S. P. Lees-Miller, K. Riabowol and D. Young, 2000. Three yeast proteins related to the human candidate tumor suppressor p33(ING1) are associated with histone acetyltransferase activities. Mol. Cell. Biol. 20 3807–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, D. G., K. Baetz, X. Shi, K. L. Walter, V. E. Macdonald et al., 2006. The Yng1p plant homeodomain finger is a methyl-histone binding module that recognizes lysine 4-methylated histone H3. Mol. Cell. Biol. 26 7871–7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello, C. C., J. M. Kramer, D. Stinchcomb and V. Ambros, 1991. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, D. M., and D. C. Shakes, 1995. Immunofluorescence microscopy, pp. 365–394 in Caenorhabditis elegans: Modern Biological Analysis of an Organism, edited by H. F. Epstein and D. C. Shakes. Academic Press, San Diego.
- Nagashima, M., M. Shiseki, K. Miura, K. Hagiwara, S. P. Linke et al., 2001. DNA damage-inducible gene p33ING2 negatively regulates cell proliferation through acetylation of p53. Proc. Natl. Acad. Sci. USA 98 9671–9676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima, M., M. Shiseki, R. M. Pedeux, S. Okamura, M. Kitahama-Shiseki et al., 2003. A novel PHD-finger motif protein, p47ING3, modulates p53-mediated transcription, cell cycle control, and apoptosis. Oncogene 22 343–350. [DOI] [PubMed] [Google Scholar]
- Nourani, A., L. Howe, M. G. Pray-Grant, J. L. Workman, P. A. Grant et al., 2003. Opposite role of yeast ING family members in p53-dependent transcriptional activation. J. Biol. Chem. 278 19171–19175. [DOI] [PubMed] [Google Scholar]
- Palacios, A., P. Garcia, D. Padro, E. Lopez-Hernandez, I. Martin et al., 2006. Solution structure and NMR characterization of the binding to methylated histone tails of the plant homeodomain finger of the tumour suppressor ING4. FEBS Lett. 580 6903–6908. [DOI] [PubMed] [Google Scholar]
- Pena, P. V., F. Davrazou, X. Shi, K. L. Walter, V. V. Verkhusha et al., 2006. Molecular mechanism of histone H3K4me3 recognition by plant homeodomain of ING2. Nature 442 100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo, C., D. R. Kaplan and W. B. Derry, 2007. AKT-1 regulates DNA-damage-induced germline apoptosis in C. elegans. Curr. Biol. 17 286–292. [DOI] [PubMed] [Google Scholar]
- Reddien, P. W., E. C. Andersen, M. C. Huang and H. R. Horvitz, 2007. DPL-1 DP, LIN-35 Rb and EFL-1 E2F act with the MCD-1 zinc-finger protein to promote programmed cell death in Caenorhabditis elegans. Genetics 175 1719–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, M., P. Berardi, W. Gong and K. Riabowol, 2006. Grow-ING, Age-ING and Die-ING: ING proteins link cancer, senescence and apoptosis. Exp. Cell Res. 312 951–961. [DOI] [PubMed] [Google Scholar]
- Salinas, L. S., E. Maldonado and R. E. Navarro, 2006. Stress-induced germ cell apoptosis by a p53 independent pathway in Caenorhabditis elegans. Cell Death Differ. 13 2129–2139. [DOI] [PubMed] [Google Scholar]
- Schertel, C., and B. Conradt, 2007. C. elegans orthologs of components of the RB tumor suppressor complex have distinct pro-apoptotic functions. Development 134 3691–3701. [DOI] [PubMed] [Google Scholar]
- Schumacher, B., K. Hofmann, S. Boulton and A. Gartner, 2001. The C. elegans homolog of the p53 tumor suppressor is required for DNA damage-induced apoptosis. Curr. Biol. 11 1722–1727. [DOI] [PubMed] [Google Scholar]
- Schumacher, B., C. Schertel, N. Wittenburg, S. Tuck, S. Mitani et al., 2005. C. elegans ced-13 can promote apoptosis and is induced in response to DNA damage. Cell Death Differ. 12 153–161. [DOI] [PubMed] [Google Scholar]
- Scott, M., P. Bonnefin, D. Vieyra, F. M. Boisvert, D. Young et al., 2001. UV-induced binding of ING1 to PCNA regulates the induction of apoptosis. J. Cell Sci. 114 3455–3462. [DOI] [PubMed] [Google Scholar]
- Shaham, S. (Editor), 2006. WormBook: Methods in Cell Biology, edited by The C. elegans Research Community. WormBook, http://www.wormbook.org.
- Shi, X., T. Hong, K. L. Walter, M. Ewalt, E. Michishita et al., 2006. ING2 PHD domain links histone H3 lysine 4 methylation to active gene repression. Nature 442 96–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada, H., T. L. Liu, T. Ochiai, T. Shimizu, Y. Haupt et al., 2002. Facilitation of adenoviral wild-type p53-induced apoptotic cell death by overexpression of p33(ING1) in T.Tn human esophageal carcinoma cells. Oncogene 21 1208–1216. [DOI] [PubMed] [Google Scholar]
- Shinoura, N., Y. Muramatsu, M. Nishimura, Y. Yoshida, A. Saito et al., 1999. Adenovirus-mediated transfer of p33ING1 with p53 drastically augments apoptosis in gliomas. Cancer Res. 59 5521–5528. [PubMed] [Google Scholar]
- Shiseki, M., M. Nagashima, R. M. Pedeux, M. Kitahama-Shiseki, K. Miura et al., 2003. p29ING4 and p28ING5 bind to p53 and p300, and enhance p53 activity. Cancer Res. 63 2373–2378. [PubMed] [Google Scholar]
- Simmer, F., M. Tijsterman, S. Parrish, S. P. Koushika, M. L. Nonet et al., 2002. Loss of the putative RNA-directed RNA polymerase RRF-3 makes C. elegans hypersensitive to RNAi. Curr. Biol. 12 1317–1319. [DOI] [PubMed] [Google Scholar]
- Soliman, M. A., and K. Riabowol, 2007. After a decade of study-ING, a PHD for a versatile family of proteins. Trends Biochem. Sci. 32 509–519. [DOI] [PubMed] [Google Scholar]
- Stergiou, L., K. Doukoumetzidis, A. Sendoel and M. O. Hengartner, 2007. The nucleotide excision repair pathway is required for UV-C-induced apoptosis in Caenorhabditis elegans. Cell Death Differ. 14 1129–1138. [DOI] [PubMed] [Google Scholar]
- Takanami, T., A. Mori, H. Takahashi and A. Higashitani, 2000. Hyper-resistance of meiotic cells to radiation due to a strong expression of a single recA-like gene in Caenorhabditis elegans. Nucleic Acids Res. 28 4232–4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons, L., D. L. Court and A. Fire, 2001. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263 103–112. [DOI] [PubMed] [Google Scholar]
- Treinin, M., B. Gillo, L. Liebman and M. Chalfie, 1998. Two functionally dependent acetylcholine subunits are encoded in a single Caenorhabditis elegans operon. Proc. Natl. Acad. Sci. USA 95 15492–15495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieyra, D., T. Toyama, Y. Hara, D. Boland, R. Johnston et al., 2002. a ING1 isoforms differentially affect apoptosis in a cell age-dependent manner. Cancer Res. 62 4445–4452. [PubMed] [Google Scholar]
- Vieyra, D., R. Loewith, M. Scott, P. Bonnefin, F. M. Boisvert et al., 2002. b Human ING1 proteins differentially regulate histone acetylation. J. Biol. Chem. 277 29832–29839. [DOI] [PubMed] [Google Scholar]
- Wagner, M. J., and C. C. Helbing, 2005. Multiple variants of the ING1 and ING2 tumor suppressors are differentially expressed and thyroid hormone-responsive in Xenopus laevis. Gen. Comp. Endocrinol. 144 38–50. [DOI] [PubMed] [Google Scholar]
- Wang, Y., and G. Li, 2006. ING3 promotes UV-induced apoptosis via Fas/caspase-8 pathway in melanoma cells. J. Biol. Chem. 281 11887–11893. [DOI] [PubMed] [Google Scholar]
- Wang, Y., D. L. Dai, M. Martinka and G. Li, 2007. Prognostic significance of nuclear ING3 expression in human cutaneous melanoma. Clin. Cancer Res. 13 4111–4116. [DOI] [PubMed] [Google Scholar]
- Zdinak, L. A., I. B. Greenberg, N. J. Szewczyk, S. J. Barmada, M. Cardamone-Rayner et al., 1997. Transgene-coded chimeric proteins as reporters of intracellular proteolysis: starvation-induced catabolism of a LacZ fusion protein in muscle cells of Caenorhabditis elegans. J. Cell Biochem. 67 143–153. [PubMed] [Google Scholar]
- Zhu, J. J., F. B. Li, X. F. Zhu and W. M. Liao, 2006. The p33ING1b tumor suppressor cooperates with p53 to induce apoptosis in response to etoposide in human osteosarcoma cells. Life Sci. 78 1469–1477. [DOI] [PubMed] [Google Scholar]
- Zhu, Z., J. Lin, J. H. Qu, M. A. Feitelson, C. R. Ni et al., 2005. Inhibitory effect of tumor suppressor p33(ING1b) and its synergy with p53 gene in hepatocellular carcinoma. World J. Gastroenterol. 11 1903–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]