Abstract
The sensitivity of an organism to hypoxic injury varies widely across species and among cell types. However, a systematic description of the determinants of metazoan hypoxic sensitivity is lacking. Toward this end, we screened a whole-genome RNAi library for genes that promote hypoxic sensitivity in Caenorhabditis elegans. RNAi knockdown of 198 genes conferred an invariant hypoxia-resistant phenotype (Hyp-r). Eighty-six per cent of these hyp genes had strong homologs in other organisms, 73 with human reciprocal orthologs. The hyp genes were distributed among multiple functional categories. Transcription factors, chromatin modifying enzymes, and intracellular signaling proteins were highly represented. RNAi knockdown of about half of the genes produced no apparent deleterious phenotypes. The hyp genes had significant overlap with previously identified life span extending genes. Testing of the RNAi's in a mutant background defective in somatic RNAi machinery showed that most genes function in somatic cells to control hypoxic sensitivity. DNA microarray analysis identified a subset of the hyp genes that may be hypoxia regulated. siRNA knockdown of human orthologs of the hyp genes conferred hypoxia resistance to transformed human cells for 40% of the genes tested, indicating extensive evolutionary conservation of the hypoxic regulatory activities. The results of the screen provide the first systematic picture of the genetic determinants of hypoxic sensitivity. The number and diversity of genes indicates a surprisingly nonredundant genetic network promoting hypoxic sensitivity.
AN extensive understanding of the mechanisms of hypoxic adaptation and death has derived from a variety of experimental models. Both turtles and fish have developed remarkable adaptive mechanisms to survive prolonged and severe hypoxia by metabolic depression and excretion or buffering of anaerobic metabolites (Storey and Storey 2004). Cancer cells have been found to have complex alterations in metabolism, translation and transcription factors, and apoptosis machinery, each of which has been shown to contribute to a general resistance to hypoxic cell death (Soengas et al. 2001; Mackeigan et al. 2005; Brahimi-Horn et al. 2007; Koritzinsky et al. 2007). In animal and cellular models of stroke and myocardial infarction, channels, receptors, and oxygen free radicals are major determinants of neuronal and myocyte hypoxic cell death and have been the focus of therapeutic trials (Kevin et al. 2005; Bano and Nicotera 2007; Green 2008). Unfortunately, multiple human clinical trials with compounds targeting these determinants have not proven efficacious against stroke (O'Collins et al. 2006; Green 2008). The contrast between the remarkable full recovery of hibernating animals subjected to prolonged and severe hypoxia and the profound failure of pharmaceutical neuroprotection trials suggests that we are far from a complete understanding of the determinants of hypoxic survival.
A major omission in the experimental approach to hypoxic injury is a systematic screen for genes that control metazoan hypoxic sensitivity. Large-scale screens are capable of giving a complete or at least a much more complete list of genes and mechanisms that control hypoxic sensitivity. Systematic unbiased screens invariably find surprising genes that had not been found by any other approach. Such screens might be accomplished by two distinct methodologies. A large-scale mutagenesis screen, if done to the point of saturation, could isolate mutations in the entire complement of genes that control hypoxic sensitivity. However, the subsequent identification of these genes would be a prolonged process even in the most genetically tractable models. A second approach is to screen a library of constructs that produce RNAi knockdown of each gene in the genome. While theoretically possible to do in any organism, large-scale RNAi screens for organismal phenotypes have only been accomplished in the nematode Caenorhabditis elegans and in the fruit fly Drosophila melanogaster.
We have previously shown that high-level resistance to hypoxic death of the whole organism is possible in C. elegans by mutation or knockdown of single genes (Scott et al. 2002; Dasgupta et al. 2007). Organismal death is preceded by permanent behavioral deficits, neuronal, and myocyte loss, and necrotic-like cell death (Scott et al. 2002). Mutations in the canonical programmed cell death pathway result in moderate hypoxia resistance of the adult animal, indicating that apoptotic cell death contributes to the demise of the animal after hypoxia (Dasgupta et al. 2007). We made use of the reliable C. elegans death phenotype following hypoxic incubation and an available whole-genome RNAi library to perform a systematic screen for determinants of hypoxic sensitivity in an intact organism.
MATERIALS AND METHODS
Strains:
The wild-type strain used was N2 var Bristol (Brenner 1974). rrf-1(pk1417) and opt-2(lg601) were obtained from the Caenorhabditis Genetics Center funded by the National Institutes of Health National Center for Research Resources. Prior to placing on RNAi bacteria for screening, worms were cultured at 20° on nematode growth medium (NGM) agar plates seeded with OP50 bacteria (Brenner 1974).
Primary screening:
From the Ahringer library (Kamath et al. 2003) (MRC Geneservice), 16,757 RNAi were cultured in 96-well format (van Haaften et al. 2004) and induced with 0.1% β-lactose in S-Basal + 100 μg/ml ampicillin for 24 hr at 22°. Approximately 10 N2 age-synchronous L1 worms in 50 μl S-Basal + 100 μg/ml ampicillin + 0.1% β-lactose + 0.01% Tween-20 were introduced into 100 μl of induced RNAi culture and maintained at 20° with agitation. Seventy-two hours later, worms not reaching adulthood were excluded from the screen. If an entire plate was contaminated or had low food density, the plate was rescreened. For hypoxic testing, plate lids were replaced with an adhesive breathable film and plates were incubated in a hypoxia chamber (Scott et al. 2002) for 22 hr at 26.5° and recovered for 24 hr at 20° with agitation. Oxygen tension was always <0.3%. Wells containing >1 moving worm were scored as hypoxia resistant. Each experiment had a control plate containing hypoxia-sensitive (L4440, empty vector) and hypoxia-resistant [daf-2(RNAi), ced-4(RNAi)] controls.
Secondary screening:
One-generation feeding RNAi (Timmons 2006) was performed on 30–50 age-synchronous adult worms/RNAi plate in triplicate. Visible phenotypes were noted, then hypoxic incubations were performed (Scott et al. 2002). In addition to L4440, a GFP (RNAi)-containing strain L4417 was used as a negative control (Timmons et al. 2001). Plates with ≥30% survival were rescreened. After three rounds of secondary screens, 277 putative positive RNAi clones were obtained. The hypoxia resistance of these 277 was tested in three subsequent quantitative trials. RNAi clones that produced a level of survival that did not overlap with any negative control values were considered highly hypoxia resistant. These 198 clones were statistically different from concurrent controls by a Bonferroni-corrected Fisher's exact test, P < 0.01/277. These 198 clones were also tested in rrf-1(pk1417) in triplicate.
RNAi clone verification and annotation:
During the final test of secondary screening, a portion of RNAi bacterial cultures was reserved for sequencing the plasmid insert of each positive clone with flanking vector primers. Five clones contained wrong targets (chromosome.plate.well): I.16.C4 should be T05F1.8 but is C33F10.12; I.21.A1 should be R06C1.1 but is T11B7.3; II.7.E4 should be K03F6.8 but is C14F11.1; II.33.A11 should be W01G7.2 but is F52B10.1; and IV.24.D2 should be ZK896.8 but is W01A11.3. For incorrect clones, the final validation testing was done on the correct target. All worm and human annotations were from Wormbase and Ensembl. Gene orthologs were determined by reciprocal BLAST with a similarity measure <10−10.
siRNA and hypoxia treatment of cell lines:
All siRNA and scrambled negative control oligos were ordered from QIAGEN (Germantown, MD) and handled according to manufacturer's instruction. T98G and HeLa cell lines were gifts from Steven Barger, University of Arkansas for Medical Sciences and from Philip Stahl, Washington University School of Medicine, respectively, and were maintained in minimal essential medium (MEM) (30-2003, ATCC, Manassas, VA) supplemented with 10% fetal bovine serum (FBS) (35-015-CV, Mediatech, Manassas, VA). The day before siRNA transfections, ∼35,000 cells were plated/well in 24-well plates with MEM + 10% FBS. Each well was transfected with 20–40 nm siRNA mixed with 1 μl lipofectamine 2000 (catalog no. 11668027, Invitrogen, Carlsbad, CA) in triplicate, according to manufacturer's instruction. Twenty-four hours after transfection, siRNA oligos and transfection reagent were removed and cells were maintained in MEM + 10% FBS for another 48 hr then washed twice with MEM and maintained in fresh MEM + 10% FBS. After washes, cells were incubated in hypoxia (<0.3% oxygen, 37°) for 16 hr (HeLa) or 20 hr (T98G). Cells recovered in a humidified incubator (5% CO2, 37°) for 2–3 hr, the time required for full recovery. Cell death and survival were scored for each siRNA. For cell death, lactate dehydrogenase (LDH) was measured for each culture well from an aliquot of media using a kit (G1780, Promega). MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] colorometric assay was used to assay survival (M2128, Sigma-Aldrich) (Mosmann 1983). Six wells of cells transfected with negative control siRNA were maintained in normoxia and lysed as controls for maximal LDH release and total cell number for MTT assays. Percentages of dead and alive in each well were normalized to negative control values and the survival was expressed as a ratio of live/(dead + live). Unpaired t-test (P < 0.05) with Bonferroni correction in every trial (minimum of three independent trials) was used to evaluate statistical significance. For assessment of knockdown efficiency by qRT-PCR, cDNA was synthesized from 2 μg total RNA using RETROscript kit (AM1710, Ambion, Austin, TX). qRT-PCR was performed using Power SYBR Green PCR Master Mix (4367659, Applied Biosystems, Foster City, CA) in a 7500 Fast instrument (Applied Biosystems). ACT-3 expression was used as an endogenous control with ROX passive reference dye. Fold expression changes were calculated using the ΔΔCT method.
cDNA microarrays:
L4-staged worms were subjected to 2, 4.5, and 6 hr of hypoxia (<0.3% oxygen, 26.5°) and to 2, 4.5, and 6 hr of normoxia (21.0% oxygen, 26.5°). Total RNA was isolated immediately using Trizol reagent (Invitrogen). RNA quality was measured using Nanodrop-1000 spectrophotometer (ThermoScientific, Waltham, MA). High quality RNA samples (OD260/280 > 1.9) were used as inputs for microarrays. Eight micrograms of RNA were subjected to reverse transcription and subsequent hybridization using 3DNA Array 350 kit (Genisphere, Hatfield, PA). Cy3- and Cy5-labeled samples were hybridized to C. elegans whole genome oligonucleotide microarrays (Genome Sequencing Center, Washington University). Slides were scanned on a ScanArray 3000 (Perkin Elmer, Waltham, MA) at 10-μm resolution. Three biological replicates (independent RNA isolations), each with a technical replicate (dye swap), were performed for each condition. Raw microarray fluorescent intensity data were processed and analyzed with GeneSpring GX 7.3.1 (Agilent Technologies, Santa Clara, CA). Each time point was subjected to Lowess normalization (per spot and per array) separately. Not all of the 198 genes were present on the microarray and some were represented more than one time. The gene list contained 190 of 198 genes. A flag filter was applied to include all genes that hybridized in three or more trials, which narrowed the gene list to 142. One-way ANOVA (P < 0.05) was performed to find genes that were statistically different between hypoxia and normoxia at each time.
RNAi off-target gene identification:
Using empirically defined levels of sequence identity required for off-target effects (Rual et al. 2007), a computer program (available upon request) was written to parse the intended target gene coding sequence into all possible 25-, 50-, 100-, 200-, and 300-bp overlapping fragments and then BLAST these fragments against the C. elegans genome. The worm database build WS187 was used to run StandAloneBlast; 16 of the genes were not able to be BLASTed because of a bug in StandAloneBlast. All code was written in Perl using ActivePerl 5.8.8 Build 882. Bioperl module 1.5.2_100 was used to assist in running and parsing BLASTs. The requirement for off-target effects with respect to percent sequence identity is defined as 96% for a 25-bp sequence (or 24/25 bp), 92% for a 50-bp sequence (46/50), 88% for a 100-bp sequence (88/100), 83% for a 200-bp sequence (166/200), and 80% for a 300-bp sequence (240/300) (Rual et al. 2007). We defined a potential off-target hit as any BLAST high-scoring pair with the above threshold identity that was not the query sequence. The intended target RNAi fragment was then aligned with the potential off-target sequence to see if the intended target RNAi lay within the same region of homology as the off-target sequence. We found 62 potential off-target RNAi and 45 of these were present in the RNAi library, including 9 that had been tested in our secondary screening. We tested all available off-target RNAi clones for hypoxia resistance (supplemental Table 1).
RESULTS
RNAi screen design:
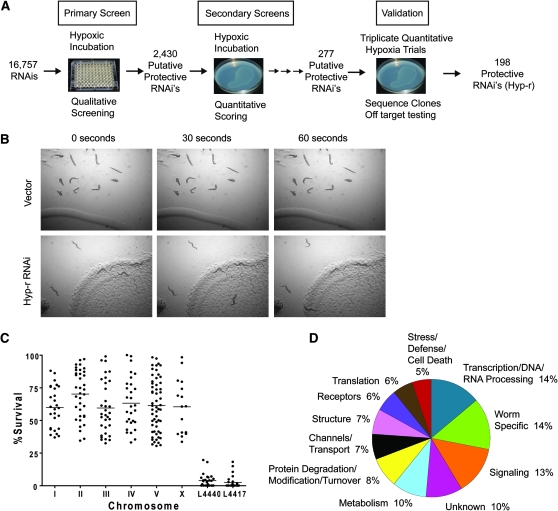
A schematic of the screen design and an example of the hypoxia-resistant (Hyp-r) phenotype are shown in Figure 1, A and B. The design of the RNAi screen had a number of features that deserve comment. First, the screen was designed to identify genes whose reduction-of-function phenotype is hypoxia resistance. Thus, only genes that promote hypoxic sensitivity would be identified. While finding genes that promote survival from hypoxia (knockdown phenotype would be hypoxic hypersensitivity) is equally important, hypoxia resistance was technically easier to score and does not have the concern of a nonspecific sickness that might promote death from any stressor. Second, the primary screen was designed for high throughput and relatively low stringency to narrow efficiently the number of candidates without eliminating moderately hypoxia-resistant animals. Third, the primary screen began RNAi exposure at the first larval stage. This maximized the length of RNAi exposure while allowing embryonic development, where the majority of cell divisions occur, to proceed normally. Thus, genes affecting both early development and hypoxic sensitivity might be identified. Finally, the primary screen was followed by multiple rounds of secondary screening and validation with large numbers of animals that were scored in a quantitative and stringent manner on agar plates. Thus, reduced developmental rates, partial embryonic/larval lethality, and partial sterility could be accommodated and the level and reproducibility of hypoxia resistance could be determined. Finally, in the validation phase, all RNAi clones that were positive in all three secondary screening trials were scored in triplicate assays with simultaneous negative controls and only those clones producing a high level of resistance (no overlap with and >4 SD from the negative controls, P < 0.01/277) were considered further. Additionally, all the Hyp-r clones were sequenced to confirm their identity and the potential for off-target gene effects was determined and found to be possible for only a few genes that had similar functions to their off-target counterparts (supplemental Table 1). Thus, the final hyp gene list is definitive in that all genes on the list promote hypoxic sensitivity in C. elegans. However, while this is the largest set of such genes thus far reported, this list is certainly incomplete for a variety of reasons including the potential for exclusion of essential genes, lack of or failure to grow of RNAi clones for some genes, and poor efficacy of RNAi for some genes.
Figure 1.—
Whole-genome RNAi screen design and results. (A) Overview of feeding RNAi screen design. The primary screen of 16,757 double-stranded RNA clones for those conferring a hypoxia-resistant phenotype (Hyp-r) was performed in liquid culture in 96-well plates. Clones from wells with surviving animals following hypoxia were rescreened in a quantitative assay on agar plates and those that produced significant resistance in three successive trials were validated by an additional three trials, sequence confirmation, and off-target examination. (B) Time lapse image of locomotion after recovery from hypoxia by a Hyp-r RNAi vs. vector control. Worms were fed either vector or a Hyp-r RNAi, incubated in hypoxia for 20 hr then recovered for 24 hr. Note lack of movement or evidence of tracks in the bacterial lawn of vector control animals compared to relatively normal movement and lawn tracks by Hyp-r worms. (C) One hundred ninety-eight RNAi were identified as producing a Hyp-r phenotype. Scatter plot represents the percent survival of each Hyp-r RNAi plotted by chromosome compared to the two negative controls, L4440 vector, and L4417, a dsRNA designed against GFP. The bar represents the median value for that group. (D) Hyp genes organized by functional category.
The hypoxic sensitivity (Hyp) genes:
From the 16,757 clones in the Ahringer C. elegans RNAi library (Kamath et al. 2003), animals on 6.4% of the clones were not scored because of poor bacterial growth or embryonic or larval arrest. The remainder was screened, and 198 RNAi clones were ultimately shown to produce hypoxia resistance (Figure 1C, Table 1, supplemental Table 2, and supplemental Movies 1 and 2). These hypoxic sensitivity (Hyp) genes fell into a variety of functional classes (Figure 1D, Table 1, and supplemental Table 2). A large proportion of the Hyp genes participate in biosynthesis of macromolecules, particularly those classified in the translation and transcription categories. Extensive and varied experiments have shown that downregulation of both translation and transcription enhance recovery from a hypoxic environment (Storey and Storey 2004; Koritzinsky et al. 2007). For translation, all of the Hyp genes encode general factors for either cytoplasmic or mitochondrial protein synthesis. Specific translation initiation and elongation factors have been linked to hypoxia-induced translational arrest and resistance to hypoxic injury (Koritzinsky et al. 2007). Our results show that inhibition of a variety of translation machinery proteins can protect from hypoxic injury. Counterintuitively, components of the proteosomal complex were identified. A priori, inhibition of protein degradation should be deleterious as this process should rid the cell of misfolded and toxic proteins. Yet, in the face of ongoing hypoxic injury, proteosomal activity appears to be maladaptive in C. elegans, and considerable evidence from mammalian models supports this hypothesis (Di Napoli and McLaughlin 2005).
TABLE 1.
Hypoxic sensitivity (Hyp) genes
| Channels/transport
|
Receptor
|
Stress/defense/cell death
|
||||||
|---|---|---|---|---|---|---|---|---|
| Gene ID (name) | % survival | Description/homolog | Gene ID (name) | % survival | Description/homolog | Gene ID (name) | % survival | Description/homolog |
| K04E7.2 (opt-2) | 98.3 | H+/oligopeptide symporter | T07C12.1 (srj-33) | 82.4 | Serpentine receptor, class J | ZK20.3 (rad-23) | 70.7 | Nucleotide excision repair factor NEF2, RAD23 component |
| ZK792.3 (inx-9) | 95.8 | Innexin-type channels | Y59E9AL.1 (thn-6) | 78.0 | 7-transmembrane receptor, Pathogenesis-related, group 5 | K08F4.1 | 61.5 | DNA replication checkpoint protein CHL 12/CTF18 |
| F09E5.5 (sec-6) | 88.6 | Exocyst complex subunit SEC6 | K04F1.2 (srw-96) | 73.8 | Serpentine Receptor, class W | F32D8.3 | 61.2 | Secreted TIL-domain protease inhibitor |
| T10C6.6 | 71.4 | Permease of the major facilitator superfamily | F21A3.1(srh-276) | 73.6 | Serpentine receptor, class H | Y40C5A.3 | 60.7 | Mucin-2 precursor |
| T01H10.8 | 68.0 | Lysosomal trafficking regulator LYST | ZK697.4 (sri-27) | 70.5 | Serpentine receptor, class I | F28D1.5 (thn-2) | 50.9 | Pathogenesis related proteins, group 5, and related proteins |
| Y51A2D.4 (hmit-1.1) | 58.6 | Proton-dependent myoinositol transporter | C06E7.7 (srv-17) | 49.6 | Serpentine receptor, class V | T28F4.5 | 43.1 | Death associated protein 1 |
| R13A5.4 (Igc-12) | 54.0 | Ligand-gated ion channel | K02H11.2 (str-240) | 48.7 | 7-transmembrane receptor | F35E8.8 (gst-38) | 41.4 | Glutathione S-transferase |
| Y71H2AM.15 | 52.4 | SNAP-25-interacting protein | Y102A5C.22 (srx-54) | 40.3 | Serpentine receptor, class X | F17C11.5 (clec-221) | 41.1 | C-type lectin |
| F02E8.6 (ncr-1) | 50.9 | Cholesterol transport (Niemann-Pick C disease protein) | F10G7.7 (sre-39) | 34.4 | Serpentine receptor, class E | C55B6.2 (dnj-7) | 33.6 | dsRNA-activated protein kinase inhibitor P58, DnaJ |
| K10G9.1 | 50.9 | Permease of the major facilitator superfamily | C50h11.14 (srt-5) | 33.3 | 7-transmembrane receptor | F40G9.10 (clec-148) | 32.4 | C-type lectin |
| T01G1.3 | 40.4 | Vesicle coat complex COPII, subunit SEC31 | T05G11.3 (srw-26) | 32.8 | Serpentine receptor, class W | |||
| F02E9.1 | 39.6 | Vacuolar proton-translocating ATPase (V-ATPase) | F59E11.13 (str-84) | 31.6 | 7-transmembrane receptor | |||
| F53H8.1 (apm-3) | 37.6 | Clathrin-associated protein medium chain | ||||||
| F07B10.5 (Igc-36) | 35.1 | GABA receptor | ||||||
| Metabolism
|
Signaling
|
Transcription/DNA/RNA processing
|
||||||
| Gene ID (name) | % survival | Description/homolog | Gene ID (name) | % survival | Description/homolog | Gene ID (name) | % survival | Description/homolog |
| C14B9.8 | 99.0 | Phosphorylase kinase | T01E8.3 (plc-3) | 96.9 | Phospholipase C | F32D1.9 | 98.3 | Polyadenylation factor I complex, subunit FIP 1 |
| T22F3.3 | 97.4 | Glycogen phosphorylase | C29F9.7 (pat-4) | 87.4 | Integrin-linked kinase | T20B12.8 (hmg-4) | 96.0 | Nucleosome-binding factor SPN, POB3 subunit |
| C52A10.2 | 92.1 | Carboxylesterase and related proteins | Y62E10A. 11 | 85.7 | Guanine nucleotide exchange factor | T23G5.1 (rnr-1) | 95.5 | Ribonucleotide reductase |
| C33F10.12 | 86.2 | Mitochondrial phosphate carrier protein | F47F2.1 | 80.4 | cAMP-dependent protein kinase catalytic subunit (PKA) | T27B1.2 | 94.0 | C2H2-type zinc-finger protein |
| F13D12.4 (alh-8) | 85.9 | Methylmalonate semialdehyde dehydrogenase | K08E3.6 (cyk-4) | 80.2 | GTPase-activating protein | T01C3.7 (fib-1) | 93.4 | Fibrillarin and related nucleolar RNA-binding proteins |
| W01A11.2 | 83.3 | Acyl-CoA:diacylglycerol acyltransferase (DGAT) | Y45F10D.7 | 77.6 | WD repeat containing 36 | EEED8.7 (rsp-4) | 92.8 | Splicing factor, SR protein superfamily |
| C04F6.5 (dhs-27) | 79.6 | 17 β-hydroxysteroid dehydrogenase type 3, HSD17B3 | T06E6.2 (cyb-3) | 75.4 | Cyclin B and related kinase-activating proteins | F08F3.9 | 92.4 | Small nuclear RNA activating complex (SNAPc), SNAP43 |
| C32F10.8 | 75.7 | Alanine aminotransferase | K01C8.9 (nst-1) | 73.6 | GTPase | T01G6.4 (nhr-106) | 92.2 | Nuclear hormone receptor |
| C50D2.7 | 68.2 | ADP-dependent glucokinase | F42C5.7 (grl-4) | 67.5 | Hedgehog-like protein | K10C3.6 (nhr-49) | 88.0 | Hepatocyte nuclear factor 4 and similar steroid hormone receptors |
| F09E5.8 | 67.0 | Proline synthetase co-transcribed protein | C50C3.7 | 64.8 | Inositol polyphosphate 5-phosphatase and related proteins | C16A3.4 | 87.0 | C2H2-type zinc-finger protein |
| R04F11.2 | 64.9 | Mitochondrial F1F0-ATP synthase | F13D12.7 (gpb-1) | 60.9 | G-protein β-subunit | T22D1.10 (ruvb-2) | 86.9 | DNA helicase TIP49, TBP-interacting protein |
| F37C12.7 | 55.6 | Acyl-CoA synthetase | K05C4.4 | 59.4 | S-M checkpoint control protein CID1 | F08B4.1 (dic-1) | 82.8 | DEAD box RNA helicase |
| T05H10.6 | 53.5 | Pyruvate dehydrogenase E1, α-subunit | B0213.2 (nlp-27) | 58.2 | Neuropeptide-like | Y111B2A. 18 (rsp-3) | 81.5 | Alternative splicing factor ASF/SF2 (RRM superfamily) |
| K08B12.3 | 50.8 | Sugar phosphatase (HAD superfamily) | B0334.8 (age-1) | 58.2 | Phosphatidylinositol 3-kinase catalytic subunit (p110) | C39F7.2 | 78.3 | Zinc-finger protein |
| T13F2.1 (fat-4) | 48.2 | Δ 6-fatty acid/Δ-8 sphingolipid desaturase | T25B9.4 | 55.6 | Protein tyrosine kinase | Y65B4BR.5 | 77.1 | Transcription factor containing NAC and TS-N domains |
| F22B3.4 | 42.9 | Glucosamine 6-phosphate synthetases | F39H11.3 (cdk-8) | 55.3 | Cyclin C-dependent kinase | R06F6.4 (set-14) | 75.3 | Histone tail methylase containing SET domain |
| F23H11.3 | 36.9 | Succinyl-CoA synthetase, α-subunit | C17H1.7 | 53.5 | PERQ and GYF domain protein | Y55F3AM.12 (dcap-1) | 74.5 | Decapping enzyme complex component DCP1 |
| C14F11.1 | 35.8 | Aspartate aminotransferase | K09F6.3 | 52.8 | Protein-tyrosine phosphatase | C53A5.3 (hda-1) | 72.0 | Histone deacetylase complex, catalytic component RPD3 |
| K04D7.3 (gta-1) | 35.4 | 4-aminobutyrate aminotransferase | PAR2.3 (aak-1) | 52.6 | AMP-activated protein kinase α 2 | C09H6.1 (spr-4) | 68.9 | Suppressor of presenilin defect; zinc finger |
| W01A11.3 (unc-83) | 49.1 | Nuclear envelope protein | Y54E5B.3 (let-49) | 65.2 | Transcriptional coactivator | |||
| Y38E10A.21 (rgs-4) | 41.8 | GTPase-activating protein | T04H1.5 | 61.7 | RNA binding protein, contains G-patch and Zn-finger domains | |||
| F11H8.4 (cyk-1) | 39.9 | RhoA GTPase effector DIA/Diaphanous | F37A4.8 (isw-1) | 61.3 | Chromatin remodeling complex WSTF-ISWI, small subunit | |||
| F56B6.2 (rgs-7) | 39.7 | G protein signaling regulators | M04G12.4 (somi-1) | 54.8 | Suppressor of overexpressed micro RNA | |||
| C04E12.12 | 36.9 | Arrestin-domain protein | T19B10.11 (mxl-1) | 41.4 | Upstream transcription factor 2/L-myc-2 protein | |||
| D1044.8 (nekl-4) | 35.5 | Never in mitosis kinase like | T07C5.5 (nhr-26) | 40.7 | Nuclear hormone receptor | |||
| H24G06.1 | 35.2 | WD40 repeat protein, MAP kinase binding protein | F47D12.4 (hmg-1.2) | 38.3 | HMG box-containing protein | |||
| K04G2.7 | 37.9 | Zinc-finger, RING-type protein | ||||||
| K06B4.6 (nhr-268) | 31.0 | Nuclear hormone receptor | ||||||
| Protein degradation/modification/turnover
|
Structure
|
Translation
|
||||||
| Gene ID (name) | % survival | Description/homolog | Gene ID (name) | % survival | Description/homolog | Gene ID (name) | % survival | Description/homolog |
| K10B2.1 (lin-23) | 90.5 | β-TrCP (transducin repeats containing)/Slimb proteins | F36H1.2 (tag-144) | 100.0 | Integral membrane ankyrin-repeat protein Kidins220/KAP NTPase | C47D12.6 (trs-1) | 96.2 | Threonyl-tRNA synthetase |
| C14C10.5 | 79.8 | Proteosome-activator complex subunit 4 | H02I12.1 | 99.2 | Chitin binding protein | C24G6.8 | 89.4 | Peptidyl tRNA hydrolase |
| AC3.5 | 78.7 | Puromycin-sensitive aminopeptidase | F52B10.1 (nmy-1) | 88.6 | Myosin class II heavy chain | F37C12.13 (exos-9) | 82.7 | Exosomal 3′-5′ exoribonuclease complex, subunit Rrp45 |
| F02E9.7 | 61.5 | Purple (tartrate-resistant) acid phosphatase | K09F6.9 | 85.6 | Trichohyalin homolog | T01H8.1 (rskn-1) | 77.2 | Ribosomal protein S6 kinase |
| ZK1128.6 (ttll-4) | 60.8 | Tubulin-tyrosine ligase-related protein | C27A2.3 (ify-1) | 84.3 | Protein ligand of FZY-1 | Y46H3A.7 | 77.0 | Mitochondrial 39S ribosomal protein homolog |
| T15D6.7 | 60.4 | β-1,3-glucuronyltransferase 1 | T11B7.3 (col-118) | 80.6 | Collagen | ZK218.8 | 66.7 | Translation initiation factor 2C eIF-2C |
| C55B7.5 (uri-1) | 58.5 | Prefoldin RPB5 (RNA polymerase subunit 5) interactor | Y43F8C.16 | 71.2 | Glycoprotein/chitin binding | ZK856.11 | 56.8 | Translation initiation factor related to eIF-1A |
| K03B8.3 (nas-18) | 58.2 | Meprin A metalloprotease | C02F5.8 (tsp-1) | 54.8 | Tetraspanin family integral membrane protein | C07E3.2 (pro-2) | 50.5 | Protein involved in nuclear export of pre-ribosomes |
| Y67D8C.5 (eel-1) | 57.5 | E3 ubiquitin-protein ligase | F42H10.3 | 46.1 | Nebulin repeat protein | Y48A6C.4 | 50.4 | Ortholog of yeast ribosome biogenesis protein |
| F21C3.5 (pfd-6) | 54.8 | Prefoldin subunit 6, KE2 family | F43G9.10 | 42.0 | Microfibrillar-associated protein MFAP1 | Y47H9C.7 | 49.5 | Translation initiation factor 2B, β-subunit (eIF-2Bβ/GCD7) |
| C17A2.4 | 53.2 | Glycosyltransferase | Y38F2AL.1 (nsy-4) | 40.6 | Claudin homolog/tight junctions | T23D8.3 | 38.8 | Low temperature viability 1 homolog/ribosome biogenesis |
| W02A11.4 (uba-2) | 51.9 | E1B/Uba2p - ubiquitin 2 activating enzyme | F18C12.3 | 36.9 | Neurofilament heavy polypeptide | |||
| C08F1.5 (math-4) | 50.0 | Meprin-associated Traf domain containing;BTB/POZ domain | F25B3.1 (ehbp-1) | 36.8 | Ca2+-binding actin-bundling protein, actinin | |||
| Y57A10A.31 | 46.3 | E3 ubiquitin ligase | K01A6.2 (magi-1) | 33.1 | Membrane-associated guanylate kinase, scaffolding protein | |||
| B0281.1 | 45.1 | E3 ubiquitin ligase | ||||||
| T26H5.8 | 39.4 | Glycosyltransferase | ||||||
Gene name is noted for those with assigned C. elegans name; % alive in N2 background, mean of three independent trials; 48 genes without strong homolog/function are not shown.
For transcription, the complement of Hyp genes is larger and more complex, with general and specific transcriptional activators and repressors. We found several genes involved in chromatin remodeling and nucleosomal histone modifications in our screen. Although hypoxia has been shown to induce global transcriptional repression in other systems, recent work suggests that specific histone modifications can occur resulting in selective transcriptional activation and repression, which regulates a cellular response that is not fully understood (Wang et al. 2004; Johnson et al. 2008). Our RNAi screen identified isw-1, a member of the ISW1/NURF chromatin remodeling complex, containing homology to components of the NuA3 complex that activates transcription by acetylating histone H3 (Corona and Tamkun 2004). We also found genes that act on nucleosomal histones to control transcription (set-14, ruvb-2, mxl-1, hda-1, and uba-2). set-14 and ruvb-2 helicases commonly function to activate transcription globally (Poulin et al. 2005). mxl-1 can associate with different partners to either activate or repress transcription. hda-1 generally functions to repress transcription but has recently been shown to activate transcription in specific cases (Kasper et al. 2005). uba-2 has been implicated in sumoylation of hda-1, and sumoylation appears to promote HDAC1 function (Poulin et al. 2005). These findings suggest that transcriptional control via nucleosome and chromatin modification are important events in determining the hypoxic sensitivity of the animal.
Many of the Hyp genes perform fundamental cellular functions that when knocked down could be deleterious to the health of the organism. However, 105 Hyp genes had no other gross phenotypes, such as embryonic/larval lethality, sterility, or developmental delay, when scored by us or as reported by others in large-scale RNAi screens (supplemental Table 2) (Kamath et al. 2003; Simmer et al. 2003; Rual et al. 2004). We attribute this surprising lack of pleiotropy to a number of factors inherent in the design of the screen. First, the screen would have selected against RNAi's producing a highly penetrant embryonic/larval lethality or developmental delay. Second, the animals were exposed to RNAi for only one generation; thus, maternal gene products may have allowed development to adulthood for some genes. Finally, RNAi is often not fully penetrant and may not produce a null phenotype. One particularly interesting pleitropy among the Hyp genes was effects on life span. Our screen identified 11 Hyp genes that have been shown by others to have a long-lived (Age) phenotype (Lee et al. 2003; Hamilton et al. 2005; Hansen et al. 2005; Curran and Ruvkun 2007; Kim and Sun 2007) (supplemental Table 2). We have previously shown that long-lived mutants in the daf-2 gene, which encodes an insulin/IGF-receptor that mediates its effect through repression of the transcription factor DAF-16, are strongly Hyp-r (Scott et al. 2002). Further, aging and hypoxic exposure might reasonably share some common determinants as both can be thought of as a form of stress. Therefore we were not surprised to find overlap between the results of our screen and other RNAi screens for long life span. Three genes that have known genetic interactions with the DAF-2 pathway were particularly interesting. age-1 is a PI-3 kinase subunit in the DAF-2 insulin signaling pathway, which validated our screening approach. Loss of age-1 makes worms long lived and resistant to hypoxia (Friedman and Johnson 1988; Scott et al. 2002). We also identified lin-23 in our screen. Paradoxically, lin-23(RNAi) in both daf-2(lf) and N2 backgrounds shortens life span by affecting the localization of DAF-16/FOXO transcription factor (Ghazi et al. 2007). DAF-16 mislocalization should increase hypoxic sensitivity; thus, lin-23 must act through a DAF-16-independent mechanism distinct from that controlling life span to increase hypoxic sensitivity. Another interesting Hyp and Age gene is opt-2, an oligopeptide transporter. opt-2(lf) enhances daf-2(rf)-dependent elongation of life span and is suppressed by daf-16; however, increased stress resistance in opt-2(lf) is not suppressable by daf-16 (Nehrke 2003; McElwee et al. 2004; Meissner et al. 2004). We have confirmed that an opt-2 deletion allele (Meissner et al. 2004) is strongly hypoxia resistant [opt-2(lg601), 99.1 ± 0.5% alive after 20-hr hypoxia]. It will be of interest to determine whether the hypoxia-resistance phenotype of opt-2(rf) requires DAF-16.
Hyp genes function in both somatic and germ cells to control hypoxic sensitivity:
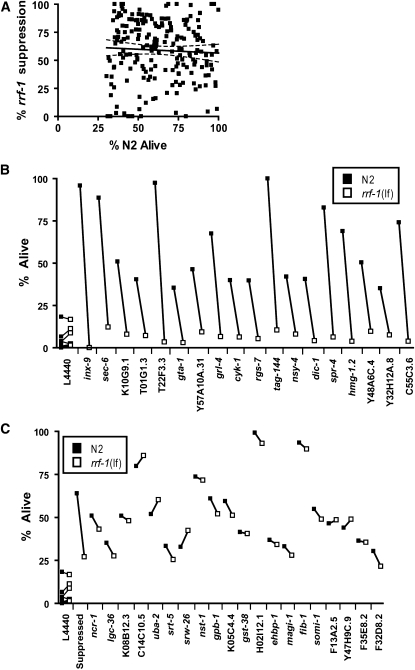
Given that the hyp genes were defined in a screen for survival of a multicellular organism, we considered the possibility that one cell type might be particularly important for organismal hypoxic sensitivity. Specifically, the question of the relative contribution of knockdown of Hyp genes in germline cells vs. somatic cells was relevant. Germ cells are rapidly dividing and pluripotent while somatic cells are terminally differentiated and turn over slowly if at all. Indeed, in a mutagenesis screen for hypoxia resistance, we isolated several sterile mutants, and we found that at least one preexisting sterile mutant, glp-1(bn18), is hypoxia resistant (data not shown). Additionally, ablation of germ cells increases C. elegans life span (Hsin and Kenyon 1999; Arantes-Oliveira et al. 2002), and germline stem cells regulate cell nonautonomously organismal fat metabolism (Wang et al. 2008). Thus, we considered the possibility that germline cells might be particularly sensitive to hypoxia and that knockdown of expression of Hyp genes in the germline might contribute disproportionately or even exclusively to the Hyp-r phenotype. To test this hypothesis, we utilized a loss-of-function mutation in rrf-1, a gene required for RNAi knockdown of expression in somatic but not germ cells (Sijen et al. 2001). If the Hyp-r phenotype for a particular gene was due exclusively to knockdown in somatic cells, the hypoxia resistance would be completely suppressed in a rrf-1(lf) background, whereas if the locus of action was exclusively germline, the hypoxia resistance would be unchanged in the rrf-1 mutant. We tested all 198 genes in the rrf-1 background and found that the RNAi Hyp-r phenotype for the majority of genes was strongly suppressed by the rrf-1 mutant (Figure 2A and supplemental Table 2). There was no correlation between the strength of the Hyp-r phenotype and rrf-1(lf) suppression. Examination of a microarray data set that documented genes with enriched expression in the C. elegans germline (Reinke et al. 2000) revealed that most of the Hyp genes (88/150 Hyp genes in the Reinke data set) were not germline enriched (supplemental Table 2). These data argue that both somatic cells and germ cells contribute to organismal hypoxic sensitivity. However, there were several examples of either full suppression by rrf-1(lf) (Figure 2B) or no suppression (Figure 2C). Thus, knockdown of some genes acting primarily in either somatic cells or germ cells can protect the entire organism from hypoxic death.
Figure 2.—
Somatic and germ cell contribution to organismal hypoxic sensitivity. (A) A correlation plot of survival in a wild-type background with level of suppression by rrf-1(pk1417lf). The r2 of the linear regression line was 0.003, P = 0.5. (B) Hyp genes whose RNAi Hyp-r phenotype was fully suppressed by rrf-1(lf). The connected points represent the means of three trials of the RNAi in either an N2 or rrf-1(lf) background. (C) Hyp genes whose RNAi Hyp-r phenotype was not significantly suppressed by rrf-1(pk1417), P > 0.05/198, Fisher's exact test. The trials with the empty vector L4440 showing no effect in the absence of a Hyp-r RNAi and the mean suppression of all the significantly suppressed Hyp genes are shown for comparison.
Hyp gene expression in normoxia and hypoxia:
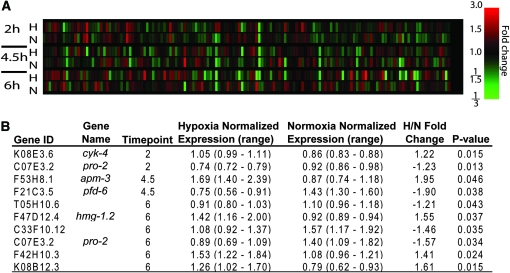
We wondered if Hyp genes were transcriptionally regulated by hypoxia. We expected two different types of transcriptional responses. Decreases in expression of the Hyp genes in response to hypoxia would suggest a proadaptive response to repress the Hyp gene. Upregulation of expression of Hyp genes, on the other hand, would be maladaptive. To examine the response to hypoxia of all hyp genes, we utilized whole-genome oligonucleotide microarrays (http://genome.wustl.edu/genome/celegans/microarray/ma_gen_info.cgi). Significant but relatively short hypoxic exposures were chosen to minimize cell death in sensitive cell types and thereby reflect changes in cellular expression rather than alterations in cell type abundance. Expression from 142 of 190 hyp genes found on the microarray were reliably detected in both normoxia and hypoxia (Figure 3A). Any genes not expressed in normoxia and hypoxia were excluded from analysis. The failure to detect these genes by microarray is presumably due to low expression levels in general, expression only in a particular larval stage or in a small subset of cells, lack of representation on the microarray of the dominant splice variant, or poor reverse transcription or hybridization. Nine hyp genes were found to be significantly up- or downregulated by hypoxia at one or more time points (Figure 3B). Two genes were particularly interesting in this regard. pro-2 was downregulated by hypoxia. pro-2 is involved in nuclear export of preribosomes and has downregulated expression in both 2 and 6 hr of hypoxia. A decrease in nuclear ribosome export would be one means to arrest translation and reduce the level of aggregrated polysomes that have been proposed to contribute to hypoxic cytotoxicity (Degracia and Hu 2007). hmg-1.2 was found to be upregulated by hypoxia. hmg-1.2 encodes a highly conserved HMG1/2-like DNA binding protein that may function as a general transcription factor (Sessa and Bianchi 2007). Human HMGB1 expression is activated early in ischemia-reperfusion injury of the heart and appears to promote myocardial injury (Andrassy et al. 2008). Upregulation of hmg-1.2 by hypoxia suggests that it may perform a similar role in worms as a maladaptive death-promoting gene early in hypoxic exposure.
Figure 3.—
Hypoxia regulation of Hyp genes. (A) An ordered heat map was created for 142 genes that had above threshold signals in more than half of the hybridizations. Each column corresponds to a gene's normalized expression over a time course of 2, 4.5, and 6 hr of incubation in hypoxia (H) or normoxia (N). RNA was isolated immediately at the end of the incubation. (B) One-way ANOVA identified 10 genes whose expression is statistically different (P < 0.05) between hypoxia and normoxia at one or more time points (2, 4.5, and 6 hr). The mean normalized expression was determined from six independent hybridizations of three biological replicates.
Hypoxia resistance by knockdown of human orthologs of Hyp genes:
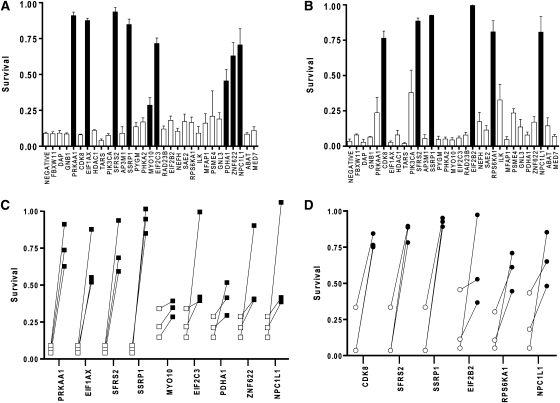
Finally, we asked whether orthologs of the Hyp genes could function similarly in mammalian cells to promote hypoxic sensitivity. We were motivated to perform these experiments for two reasons. First and most obvious, we wanted to determine which if any of genes functioned similarly in cells from other organisms besides C. elegans. Second, we wanted to determine the effect of these genes or their orthologs, not only on the hypoxic sensitivity of the organism but also on cells in isolation, thereby defining directly their effects on hypoxic cell death. Of the 73 human reciprocal orthologs (supplemental Table 2), we selected 30 to test for their role in hypoxic cell death by siRNA knockdown. The human genes were chosen only by strength of orthology and by distribution among the functional classes. Two distinct cell types were used: HeLa cells, a human cervical epithelial cancer permanent cell line (Masters 2002) and T98G cells, a human glioblastoma-derived cell line (Stein 1979). The cell types were chosen for their diverse tissue origin and well-established competence for transfection. Of the 30 human orthologs tested, siRNA knockdown of 9 of them significantly protected HeLa cells from hypoxic death (Figure 4, A and C); 6 siRNAs protected T98G cells (Figure 4, B and D). Three siRNAs protected both cell types. Thus, taken together 12/30 or 40% of the siRNAs were protective in either of these cell types. Given the impressive number of hypoxia-resistant siRNAs, we were concerned despite appropriate controls that the siRNAs themselves might be nonspecifically inducing hypoxia resistance. Thus, we randomly chose an additional 10 siRNAs in the same library to test (supplemental Table 3). Only 1 of the 10, programmed cell death 11 (PDCD11), conferred hypoxia resistance (data not shown). Thus, the orthologs of the C. elegans Hyp genes appear to be markedly enriched for genes that promote hypoxic sensitivity in at least these two cell types.
Figure 4.—
Hypoxic protection by knockdown of orthologs of worm Hyp genes. siRNAs were used to knockdown human orthologs of Hyp genes in HeLa (A and C) and T98G (B and D) cells. Cells were treated with hypoxia after siRNA transfection. Cell death and survival were determined by LDH and MTT assays, respectively. Survival was measured by MTT values normalized to MTT + LDH values for a given experiment. A and B are the results from one trial for all human orthologs tested (bars represent mean ± SEM of triplicate cultures). Solid bars represent the siRNAs providing significant protection in all trials (P < 0.05 with Bonferroni correction, unpaired, two-sided t-test). Results from all trials of the siRNAs producing significant protection of HeLa and T98G are summarized in C and D, respectively. Each line connects survival values for negative control siRNA (□ or ○) with the given siRNA (▪ or •) for each independent trial.
DISCUSSION
Previous forward mutagenesis screens, candidate screens, existing mutant screens, and multigenerational selections have identified genes that regulate hypoxic sensitivity in C. elegans (Jiang et al. 2001; Padilla et al. 2002; Scott et al. 2002; Nystul et al. 2003; Shen et al. 2005; Mendenhall et al. 2006; Samokhvalov et al. 2008; Anderson et al. 2009;) and/or Drosophila (Ma et al. 2001; Chen et al. 2002; Zhou et al. 2007; Zhou et al. 2008). In Drosophila, most of these hypoxic sensitivity genes function to reduce hypoxic sensitivity and would not have been expected to be found in our screen. However, the hypoxia-resistance phenotype of a P-element insertion in Drosophila sec6 and phenotypic reversion by precise P-element excision showed that sec6 promotes hypoxic organismal death in Drosophila (Zhou et al. 2007) as does its C. elegans ortholog, sec-6 (Table 1, supplemental Table 2—Channels/transport). Thus, the role of SEC-6 in promoting hypoxic sensitivity appears to be evolutionarily conserved.
In C. elegans, we have shown (Scott et al. 2002) and others have confirmed (Mendenhall et al. 2006) that daf-2(rf) alleles are hypoxia resistant. We have also shown that mutants in age-1, which functions downstream of daf-2 to promote hypoxic sensitivity, are also hypoxia resistant (Scott et al. 2002). age-1 was identified in our RNAi screen but daf-2 was not. The daf-2 RNAi construct in the Ahringer library does not provide good knockdown or hypoxia resistance when we tested it directly (data not shown); thus, it is not surprising that it was not found in our screen. However, a different daf-2(RNAi) construct (Dillin et al. 2002), kindly provided by Andrew Dillin and Cynthia Kenyon, confers a strong hypoxia-resistant phenotype and served as a hypoxia-resistant positive control for our screen. The daf-2 false negative result serves to illustrate one of the many reasons that our screen would not have identified all C. elegans genes, whose reduction-of-function phenotype is hypoxia resistance. The Hyp-r phenotype of daf-2(rf) has been shown to be partially suppressed by RNAi targeting of gpd-2/3, which encode glyceraldehyde-3-phosphate dehydrogenases (GAPDHs) (Mendenhall et al. 2006). GAPDH catalyzes a requisite step in glycolysis. However, Mendenhall et al. (2006) found that RNAi knockdown of other glycolytic enzymes did not suppress the Hyp-r phenotype of daf-2(rf). Thus, the mechanism whereby GAPDH regulates hypoxic sensitivity may be through a nonglycolytic function. Our screen showed that knockdown of some glycolytic enzymes produces hypoxia resistance, suggesting that reducing glycolytic activity is beneficial in the setting of acute hypoxic injury. We have also recently reported the results of a mutagenesis screen where a reduction-of-function mutation in an aminoacyl-tRNA synthetase was isolated that conferred very high level hypoxia resistance (Anderson et al. 2009). RNAi targeting of other aminoacyl-tRNA synthetases showed that knockdown of most of these enzymes results in hypoxia resistance and that the primary mechanism of resistance was translational suppression (Anderson et al. 2009). Only one aminoacyl-tRNA synthetase was found in the RNAi screen, but several other translational machinery genes were found. It will be interesting to determine if the translation factors implicated in the screen are inherently more important in regulating hypoxia than others.
Only ∼5% of the Hyp genes were found to be hypoxia regulated in our microarray assays. With a similar hypoxic exposure, a previous microarray experiment in C. elegans found that ∼110 of 17,817 C. elegans genes were hypoxia regulated (Shen et al. 2005). Thus, the Hyp genes may be enriched for hypoxia-regulated genes. Of the hypoxia-regulated genes reported by Shen et al. (2005), 3, C24B9.9(dod-3), F02E8.6(npc-1), and F45D11.16, were found in our RNAi screen. None of these genes reached statistical significance in our microarray experiments. Nevertheless, both dod-3 and ncr-1 are quite interesting. dod-3 was also found to be regulated by the daf-2/daf-16 pathway (Murphy et al. 2003), and the human ortholog of ncr-1 was shown to regulate the hypoxic sensitivity of both HeLa and T98G cells (Figure 4 and supplemental Table 3). Thus, these two genes are particularly attractive candidates for future study.
The results of our screen pose an enigma: If 198 genes all contribute proportionally to the hypoxic sensitivity of the organism, how does knockdown of any one gene produce strong hypoxia resistance? Simple additivity of the genes' functions would require that the phenotype of any one gene would be immeasurable. Yet, this is clearly not what we found. One explanation is that the network of hyp genes is highly interdependent and synergistic with loss of activity of any one gene markedly reducing the effect of all the other genes on hypoxic sensitivity. However, examination of the functional categories in which the genes function indicates that the hyp genes act in diverse pathways that do not directly intersect. Indeed, examination of potential network interactions among the human orthologs of the hyp genes revealed relatively small networks that did not include genes from all functional categories (data not shown; Ingenuity Systems, Redwood City, CA) (Calvano et al. 2005). An alternative view of the hyp genes more consistent with their molecular identities is that multiple independent parallel pathways control hypoxic sensitivity and that hypoxic sensitivity is not the arithmetic sum of its component determinants. Rather, hypoxic survival does not behave as a continuous variable but is somewhat binary with inhibition of any one of multiple pathways capable of flipping the switch in favor of survival.
Acknowledgments
We thank Elaine Mardis and the Washington University Genome Sequencing Center for their help with the microarray experiments and for rearraying the 384-well RNAi library plates into 96-well plates. We thank Raphael Kopan in the Department of Developmental Biology at Washington University School of Medicine for his encouragement to perform the mammalian cell line experiments. We thank Swathi Arur in Tim Schedl's lab for mining the Reinke database for determination of germline enrichment. C. elegans strains used in this work were kindly provided by the Caenorhabditis Genetics Center funded by the National Institutes of Health National Center for Research Resources. Financial support was provided by the McKnight Endowment for Neurosciences, the American Heart Association, and the National Institute of Neurological Disorders and Stroke.
References
- Anderson, L. L., X. Mao, B. A. Scott and C. M. Crowder, 2009. Survival from hypoxia by inactivation of aminoacyl-tRNA-synthetases. Science (in press). [DOI] [PMC free article] [PubMed]
- Andrassy, M., H. C. Volz, J. C. Igwe, B. Funke, S. N. Eichberger et al., 2008. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation 117 3216–3226. [DOI] [PubMed] [Google Scholar]
- Arantes-Oliveira, N., J. Apfeld, A. Dillin and C. Kenyon, 2002. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science 295 502–505. [DOI] [PubMed] [Google Scholar]
- Bano, D., and P. Nicotera, 2007. Ca2+ signals and neuronal death in brain ischemia. Stroke 38 674–676. [DOI] [PubMed] [Google Scholar]
- Brahimi-Horn, M. C., J. Chiche and J. Pouyssegur, 2007. Hypoxia and cancer. J. Mol. Med. 85 1301–1307. [DOI] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvano, S. E., W. Xiao, D. R. Richards, R. M. Felciano, H. V. Baker et al., 2005. A network-based analysis of systemic inflammation in humans. Nature 437 1032–1037. [DOI] [PubMed] [Google Scholar]
- Chen, Q., E. Ma, K. L. Behar, T. Xu and G. G. Haddad, 2002. Role of trehalose phosphate synthase in anoxia tolerance and development in Drosophila melanogaster. J. Biol. Chem. 277 3274–3279. [DOI] [PubMed] [Google Scholar]
- Corona, D. F., and J. W. Tamkun, 2004. Multiple roles for ISWI in transcription, chromosome organization and DNA replication. Biochim. Biophys. Acta 1677 113–119. [DOI] [PubMed] [Google Scholar]
- Curran, S. P., and G. Ruvkun, 2007. Lifespan regulation by evolutionarily conserved genes essential for viability. PLoS Genet. 3 e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta, N., A. M. Patel, B. A. Scott and C. M. Crowder, 2007. Hypoxic preconditioning requires the apoptosis protein CED-4 in C. elegans. Curr. Biol. 17 1954–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGracia, D. J., and B. R. Hu, 2007. Irreversible translation arrest in the reperfused brain. J. Cereb. Blood Flow Metab. 27 875–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Napoli, M., and B. McLaughlin, 2005. The ubiquitin-proteasome system as a drug target in cerebrovascular disease: therapeutic potential of proteasome inhibitors. Curr. Opin. Investig. Drugs 6 686–699. [PMC free article] [PubMed] [Google Scholar]
- Dillin, A., D. K. Crawford and C. Kenyon, 2002. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science 298 830–834. [DOI] [PubMed] [Google Scholar]
- Friedman, D. B., and T. E. Johnson, 1988. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics 118 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazi, A., S. Henis-Korenblit and C. Kenyon, 2007. Regulation of Caenorhabditis elegans lifespan by a proteasomal E3 ligase complex. Proc. Natl. Acad. Sci. USA 104 5947–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, A. R., 2008. Pharmacological approaches to acute ischaemic stroke: reperfusion certainly, neuroprotection possibly. Br. J. Pharmacol. 153(Suppl 1): S325–S338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, B., Y. Dong, M. Shindo, W. Liu, I. Odell et al., 2005. A systematic RNAi screen for longevity genes in C. elegans. Genes Dev. 19 1544–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, M., A. L. Hsu, A. Dillin and C. Kenyon, 2005. New genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS Genet. 1 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsin, H., and C. Kenyon, 1999. Signals from the reproductive system regulate the lifespan of C. elegans. Nature 399 362–366. [DOI] [PubMed] [Google Scholar]
- Jiang, H., R. Guo and J. A. Powell-Coffman, 2001. The Caenorhabditis elegans hif-1 gene encodes a bHLH-PAS protein that is required for adaptation to hypoxia. Proc. Natl. Acad. Sci. USA 98 7916–7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, A. B., N. Denko and M. C. Barton, 2008. Hypoxia induces a novel signature of chromatin modifications and global repression of transcription. Mutat. Res. 640 174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath, R. S., A. G. Fraser, Y. Dong, G. Poulin, R. Durbin et al., 2003. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421 231–237. [DOI] [PubMed] [Google Scholar]
- Kasper, L. H., F. Boussouar, K. Boyd, W. Xu, M. Biesen et al., 2005. Two transactivation mechanisms cooperate for the bulk of HIF-1-responsive gene expression. EMBO J. 24 3846–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevin, L. G., E. Novalija and D. F. Stowe, 2005. Reactive oxygen species as mediators of cardiac injury and protection: the relevance to anesthesia practice. Anesth. Analg. 101 1275–1287. [DOI] [PubMed] [Google Scholar]
- Kim, Y., and H. Sun, 2007. Functional genomic approach to identify novel genes involved in the regulation of oxidative stress resistance and animal lifespan. Aging Cell 6 489–503. [DOI] [PubMed] [Google Scholar]
- Koritzinsky, M., B. G. Wouters, B. Helmut Sies and Bernhard, 2007. Hypoxia and regulation of messenger RNA translation. Methods Enzymol. 435 247–273. [DOI] [PubMed] [Google Scholar]
- Lee, S. S., R. Y. Lee, A. G. Fraser, R. S. Kamath, J. Ahringer et al., 2003. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat. Genet. 33 40–48. [DOI] [PubMed] [Google Scholar]
- Ma, E., X. Q. Gu, X. Wu, T. Xu and G. G. Haddad, 2001. Mutation in pre-mRNA adenosine deaminase markedly attenuates neuronal tolerance to O2 deprivation in Drosophila melanogaster. J. Clin. Invest. 107 685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKeigan, J. P., L. O. Murphy and J. Blenis, 2005. Sensitized RNAi screen of human kinases and phosphatases identifies new regulators of apoptosis and chemoresistance. Nat. Cell Biol. 7 591–600. [DOI] [PubMed] [Google Scholar]
- Masters, J. R., 2002. HeLa cells 50 years on: the good, the bad and the ugly. Nat. Rev. Cancer 2 315–319. [DOI] [PubMed] [Google Scholar]
- McElwee, J. J., E. Schuster, E. Blanc, J. H. Thomas and D. Gems, 2004. Shared transcriptional signature in Caenorhabditis elegans dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. J. Biol. Chem. 279 44533–44543. [DOI] [PubMed] [Google Scholar]
- Meissner, B., M. Boll, H. Daniel and R. Baumeister, 2004. Deletion of the intestinal peptide transporter affects insulin and TOR signaling in Caenorhabditis elegans. J. Biol. Chem. 279 36739–36745. [DOI] [PubMed] [Google Scholar]
- Mendenhall, A. R., B. LaRue and P. A. Padilla, 2006. Glyceraldehyde-3-phosphate dehydrogenase mediates anoxia response and survival in Caenorhabditis elegans. Genetics 174 1173–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann, T., 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65 55–63. [DOI] [PubMed] [Google Scholar]
- Murphy, C. T., S. A. McCarroll, C. I. Bargmann, A. Fraser, R. S. Kamath et al., 2003. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424 277–283. [DOI] [PubMed] [Google Scholar]
- Nehrke, K., 2003. A reduction in intestinal cell pHi due to loss of the Caenorhabditis elegans Na+/H+ exchanger NHX-2 increases life span. J. Biol. Chem. 278 44657–44666. [DOI] [PubMed] [Google Scholar]
- Nystul, T. G., J. P. Goldmark, P. A. Padilla and M. B. Roth, 2003. Suspended animation in C. elegans requires the spindle checkpoint. Science 302 1038–1041. [DOI] [PubMed] [Google Scholar]
- O'Collins, V. E., M. R. Macleod, G. A. Donnan, L. L. Horky, B. H. van der Worp et al., 2006. 1,026 experimental treatments in acute stroke. Ann. Neurol. 59 467–477. [DOI] [PubMed] [Google Scholar]
- Padilla, P. A., T. G. Nystul, R. A. Zager, A. C. Johnson and M. B. Roth, 2002. Dephosphorylation of cell cycle-regulated proteins correlates with anoxia-induced suspended animation in Caenorhabditis elegans. Mol. Biol. Cell 13 1473–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin, G., Y. Dong, A. G. Fraser, N. A. Hopper and J. Ahringer, 2005. Chromatin regulation and sumoylation in the inhibition of Ras-induced vulval development in Caenorhabditis elegans. EMBO J. 24 2613–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke, V., H. E. Smith, J. Nance, J. Wang, C. Van Doren et al., 2000. A global profile of germline gene expression in C. elegans. Mol. Cell 6 605–616. [DOI] [PubMed] [Google Scholar]
- Rual, J.-F., J. Ceron, J. Koreth, T. Hao, A.-S. Nicot et al., 2004. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res. 14 2162–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rual, J. F., N. Klitgord and G. Achaz, 2007. Novel insights into RNAi off-target effects using C. elegans paralogs. BMC Genomics 8 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samokhvalov, V., B. A. Scott and C. M. Crowder, 2008. Autophagy protects against hypoxic injury in C. elegans. Autophagy 4 1034–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, B. A., M. S. Avidan and C. M. Crowder, 2002. Regulation of hypoxic death in C. elegans by the insulin/IGF receptor homolog DAF-2. Science 296 2388–2391. [DOI] [PubMed] [Google Scholar]
- Sessa, L., and M. E. Bianchi, 2007. The evolution of high mobility group box (HMGB) chromatin proteins in multicellular animals. Gene 387 133–140. [DOI] [PubMed] [Google Scholar]
- Shen, C., D. Nettleton, M. Jiang, S. K. Kim and J. A. Powell-Coffman, 2005. Roles of the HIF-1 hypoxia-inducible factor during hypoxia response in Caenorhabditis elegans. J. Biol. Chem. 280 20580–20588. [DOI] [PubMed] [Google Scholar]
- Sijen, T., J. Fleenor, F. Simmer, K. L. Thijssen, S. Parrish et al., 2001. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell 107 465–476. [DOI] [PubMed] [Google Scholar]
- Simmer, F., C. Moorman, A. M. van der Linden, E. Kuijk, P. V. van den Berghe et al., 2003. Genome-wide RNAi of C. elegans using the hypersensitive rrf-3 strain reveals novel gene functions. PLoS Biol. 1 E12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soengas, M. S., P. Capodieci, D. Polsky, J. Mora, M. Esteller et al., 2001. Inactivation of the apoptosis effector Apaf-1 in malignant melanoma. Nature 409 207–211. [DOI] [PubMed] [Google Scholar]
- Stein, G. H., 1979. T98G: an anchorage-independent human tumor cell line that exhibits stationary phase G1 arrest in vitro. J. Cell. Physiol. 99 43–54. [DOI] [PubMed] [Google Scholar]
- Storey, K. B., and J. M. Storey, 2004. Metabolic rate depression in animals: transcriptional and translational controls. Biol. Rev. Camb. Philos. Soc. 79 207–233. [DOI] [PubMed] [Google Scholar]
- Timmons, L., 2006. Delivery methods for RNA interference in C. elegans. Methods Mol. Biol. 351 119–125. [DOI] [PubMed] [Google Scholar]
- Timmons, L., D. L. Court and A. Fire, 2001. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263 103–112. [DOI] [PubMed] [Google Scholar]
- van Haaften, G., N. L. Vastenhouw, E. A. Nollen, R. H. Plasterk and M. Tijsterman, 2004. Gene interactions in the DNA damage-response pathway identified by genome-wide RNA-interference analysis of synthetic lethality. Proc. Natl. Acad. Sci. USA 101 12992–12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, F., R. Zhang, T. V. Beischlag, C. Muchardt, M. Yaniv et al., 2004. Roles of Brahma and Brahma/SWI2-related gene 1 in hypoxic induction of the erythropoietin gene. J. Biol. Chem. 279 46733–46741. [DOI] [PubMed] [Google Scholar]
- Wang, M. C., E. J. O'Rourke and G. Ruvkun, 2008. Fat metabolism links germline stem cells and longevity in C. elegans. Science 322 957–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, D., J. Xue, J. Chen, P. Morcillo, J. D. Lambert et al., 2007. Experimental selection for Drosophila survival in extremely low O2 environment. PLoS ONE 2 e490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, D., J. Xue, J. C. Lai, N. J. Schork, K. P. White et al., 2008. Mechanisms underlying hypoxia tolerance in Drosophila melanogaster: hairy as a metabolic switch. PLoS Genet. 4 e1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]