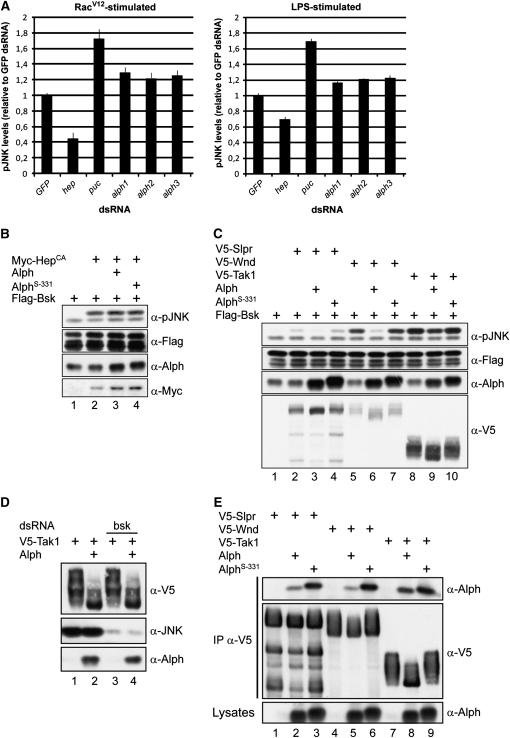
Figure 7.—
Alph negatively regulates the JNK pathway in S2 cells. (A) Means and standard deviations of fluorescence intensities of Rac1V12- or LPS-induced pJNK levels from S2 cells treated with the indicated dsRNAs. JNK pathway activation was monitored by immunofluorescence using an anti-pJNK antibody recognizing dually phosphorylated Bsk in its activation segment. Signals were normalized to GFP dsRNA used as a negative control. The hep and puc dsRNAs were used as positive controls. Three nonoverlapping alph dsRNAs were tested and found to similarly enhance pJNK levels. Three separate experiments were conducted for each dsRNA. Statistical significance was confirmed using a Student's t-test (P < 0.001). (B–E) S2 cells were transfected with the indicated plasmid combinations. Alph proteins overexpressed in S2 cells do not contain an artificial epitope tag. Their levels were measured using anti-Alph polyclonal antibodies, which also detect endogenous Alph. As previously reported (Baril and Therrien 2006) and shown here, the AlphS-331 proteins migrate slightly more slowly than the wild-type variant. A total of 100 ng of the alph-expressing construct was used in B and C, while 500 ng was used in D and E. Lysates were directly probed with the antibodies indicated at the right to monitor protein or Bsk phosphorylation levels. In D, bsk dsRNA was added to the cells 24 hr prior to transfection. In E, the SAPKKKs were independently immunoprecipitated (IP) from NP-40 cell lysates using an anti-V5 (α-V5) antibody. AlphS-331 has a G120E change. Higher SAPKKK-binding activity was also observed for AlphXS-88 and AlphS-355 (not shown), which, respectively, have a D193V and a G173S change (Baril and Therrien 2006).