Summary
Head and neck squamous cell carcinoma of the upper aerodigestive tract is well known for its frequently late presentation and diagnosis at an advanced stage. In addition, it is well recognized that it may arise in multiple sites, either synchronously or metachronously. Thus it should be imperative to endoscopically screen the upper aerodigestive tract of patients at risk for head and neck squamous cell carcinoma with a new diagnostic tool, especially due to the fact that early lesions are very difficult to detect even by multiple passes with a standard endoscopy, if they are ≤ 1 cm in diameter. Lugol chromoendoscopy, which is mainly used in the oesophagus, is not suitable for the head and neck region due to severe mucosal irritation. Herein, narrow-band imaging is described, a diagnostic tool already proved as a useful screening method in other endoscopic fields, and its application in the early detection of head and neck squamous cell carcinoma is reviewed, as reported by previous studies in the otolaryngologic literature. Narrow-band imaging relies on the principle of depth of penetration of light, with the narrow-band blue light having a short wavelength (415 nm) penetrating into the mucosa and highlighting the superficial vasculature. Furthermore, the blue filter is designed to correspond to the peak absorption spectrum of haemoglobin to enhance the image of capillary vessels on surface mucosa. Thus, superficial mucosal lesions that would be missed by regular white light endoscopy, are identified, in view of their neoangiogenetic pattern of vasculature, using the blue light of the narrow-band imaging. Narrow-band imaging has been used extensively in the lower aerodigestive system, yet there are only 2 reports of applications in the region of the head and neck, specifically the oropharynx and the hypopharynx. However, these are not the only sites that can benefit from narrow-band imaging. Herewith, the uses and importance are highlighted of narrow-band imaging as a future diagnostic tool in otolaryngology, in the pre-, intra- and post-operative settings.
Keywords: Upper aero-digestive tract, Malignant tumours, Diagnosis, Endoscopy, Narrow-band imaging
Riassunto
Il carcinoma squamocellulare della testa e del collo a livello delle vie aereo-digestive superiori è ben noto per la sua frequente presentazione tardiva e diagnosi in fase avanzata. In aggiunta, è ampiamente risaputo che queste neoplasie possono originare in aree differenti, sia con modalità sincrona che metacrona. Dovrebbe pertanto essere possibile valutare endoscopicamente le vie aereo-digestive superiori dei pazienti a rischio per carcinoma squamocellulare della testa e del collo con un nuovo strumento diagnostico, soprattutto considerando che le lesioni in fase iniziale sono molto difficili da identificare anche dopo molteplici passaggi con un normale endoscopio, soprattutto se di dimensioni pari ad 1 cm o meno. La cromoendoscopia Lugol, che è principalmente utilizzata a livello esofageo, non è applicabile a livello testa e collo per la grave irritazione mucosale che ne consegue. Descriviamo pertanto la narrow-band imaging, uno strumento diagnostico che è già stato dimostrato essere un utile metodo di valutazione in altri campi dell’endoscopia, e passiamo in rassegna la sua applicazione nella diagnosi precoce dei carcinomi squamocellulari della testa e del collo come descritto in lavori precedentemente comparsi nella letteratura otorinolaringoiatrica. La narrow-band imaging si basa sul principio della profondità di penetrazione della luce, con la luce blu a banda ristretta dotata di una breve lunghezza d’onda (415 nm) in grado di penetrare nella mucosa in modo da evidenziarne la vascolarizzazione superficiale. Inoltre, il filtro blu corrisponde al picco di assorbimento dello spettro dell’emoglobina in modo da far risaltare l’immagine dei vasi capillari a livello della superficie mucosale. In tal modo, lesioni mucosali superficiali che sfuggirebbero ad una normale valutazione endoscopica con luce bianca, vengono identificate utilizzando la luce narrow-band imaging grazie alle loro caratteristiche di vascolarizzazione neoangiogenetica. La narrow-band imaging è stata ampiamente impiegata a livello delle vie aereo-digestive inferiori, mentre esistono finora soltanto 2 studi sul suo impiego in ambito testa e collo, in particolar modo a livello di orofaringe ed ipofaringe. In ogni caso, queste non sono le uniche sedi che possono trarre beneficio dall’impiego della narrow-band imaging. Nostro scopo è pertanto quello di sottolineare gli impieghi e l’importanza della narrow-band imaging come futuro mezzo diagnostico in ambito otorinolaringoiatrico, sia in fase pre-, che intra- e post-operatoria.
Introduction
Head and neck squamous cell carcinoma (HNSCC) of the upper aerodigestive tract (UADT) is well known for its frequently late presentation and diagnosis at an advanced stage 1. This is mainly due to the complex head and neck anatomy and the presence of relatively silent areas. In addition, it is well recognized that HNSCCs may arise in multiple sites, either synchronously or metachronously 2. This worsens the prognosis and makes it more difficult to treat the patient without risking detrimental effects on speech and swallowing. Thus it should be imperative to endoscopically screen the UADT of patients at risk for HNSCC (i.e., those to be treated for an already recognized primary tumour and those previously managed and undergoing follow-up), in order to detect either synchronous or second malignancies at an early stage. Moreover, endoscopic screening of cancer-free patients, at high risk due to excessive alcohol consumption and smoking, would be of great value to detect any HNSCC as early as possible.
The best case scenario for early detection of SCC of the UADT would be at the stage of squamous dysplasia or even carcinoma in situ (CIS). These early lesions are very difficult to detect even with multiple passes with the endoscope, if they are ≤ 1 cm in diameter 2. A well established technique, already in use for the oesophagus, is Lugol chromoendoscopy, where squamous dysplasia and CIS appear as Lugol voiding lesions (LVL) 3 4. These precancerous lesions can be easily resected via endoscopic mucosal resection (EMR), which is a minimally invasive procedure with short hospital stay. However, this technique cannot be applied in the head and neck region due to the severe mucosal irritation that may lead to severe dysphagia and even aspiration. Watanabe et al. 3 even reported that they needed to put their patients, for whom they used Lugol chromoendoscopy on the UADT, under general anaesthesia, which defeats the purpose of a screening method. It would be of great value if a new technique could be used to somehow enhance the endoscopic view of the UADT to reveal these small mucosal lesions that would otherwise escape diagnosis even by the experienced endoscopist. In the same vein, this new technique should be non-invasive, and preferably not relying on the experience of the endoscopist, in order to be as objective as possible. Achieving these goals would set a milestone in the screening and early detection of HNSCC.
We herein describe narrow-band imaging (NBI) (Olympus Medical Systems Corporation, Tokyo, Japan), a diagnostic tool already proven as a useful screening method in other endoscopic fields, and demonstrate its usefulness in the early detection of HNSCC, as reported by previous studies in the otolaryngologic literature 2.
Technology
The perception of the endoscopist of abnormal conditions of the UADT with regard to the morphology of pre-malignant and malignant lesions is changing 5. These lesions often present with non-elevated morphology, with slight discolouration, as the superficial mucosal vascular structures are constantly changing during the process of tumour neoangiogenesis. Digital reconstruction of images, captured by the videoendoscope, makes image processing possible 6. The haemoglobin index (i.e., haemoglobin content in the mucosa) can be estimated by adjusting the colour of reflected light 7. The technique of NBI combines the potentials of both technologies (magnification of videoendoscopy and image processing) 8, based on the principle of modifying the spectral characteristics of the illuminating light by narrowing the bandwidth of the optical filter in the light source 9.
From a technical point of view, the NBI system contains the same components of a conventional red, green, and blue (RGB) sequential videoendoscope system. In NBI mode, an optical filter allows a narrow-band light to pass at a short wave length of 400-430 nm (centered at 415 nm), that only penetrates the mucosa superficially enhancing the mucosal vasculature. Another narrow-band light of longer wavelength 525-555 nm (centered at 540 nm) penetrates deeper into the tissues revealing the submucosal vessels 9.
Basically, NBI relies on the principle of depth of light penetration. In contrast to red light, blue light has less penetration and less scattering thus enhancing image resolution 10. Furthermore, the blue filter is designed to correspond to the peak absorption spectrum of haemoglobin to enhance the image of capillary vessels on surface mucosa. The reflection is increased by a monochromatic charge coupled device (CCD), and an image processor creates a composite pseudocolour image, which is displayed on a high definition video screen 9, enabling NBI to enhance mucosal contrast without the use of dyes. Thus, superficial mucosal lesions that would previously have been missed by regular white light during endoscopy would be identified by the blue light of NBI, based on the increased vascularity and neoangiogenesis of the tumour.
NBI is not cumbersome and is easy to set up and operate with just the switch of a button on the videoendoscope, videocamera or monitor console between conventional white light and filtered narrow-band light. Moreover, the diagnostic value of this optical technology is enhanced by combining it with magnifying endoscopy (NBI-ME) that allows the endoscopist to zoom on the mucosa, simply switching a dedicated button.
The best image definition, both for conventional rigid endoscopy and NBI, is achieved by the use of a HDTV (High Definition Television) camera incorporated on the endoscope, thus no degradation occurs in the quality of images during magnification. HDTV offers up to 1080 lines of resolution, allowing a signal definition 4.26 times superior to that of regular standard definition television (SDTV). The result is a very sharp, detailed and bright picture that looks almost three dimensional 11. Unfortunately, for technical reasons, at the time of preparing this report, the HDTV camera can only be incorporated on the rigid endoscopes, with the flexible type only taking advantage of a standard definition camera.
Non-ENT NBI applications
There are many reports in the literature on the use of NBI in various anatomic regions of the aerodigestive tract, namely the tracheobronchial tree, oesophagus, stomach, duodenal ampullary, and colorectal regions. Hamamoto et al. 12 evaluated the usefulness of combining NBI-ME in the diagnosis of Barrett’s oesophagus (BE). Images and videos, both by conventional ME and by NBI-ME, were obtained in a group of patients by an experienced endoscopist, and the differences were evaluated by another. They found that visualization of the oesophago-gastric junction, net-like blood vessels, and columnar-lined oesophagus were all visualized far better by NBI-ME.
The value of NBI in the detection of high grade dysplasia and early cancer (HGD/EC) in BE was evaluated by Kara et al. 13 in a prospective randomized crossover study. Patients with BE underwent high resolution endoscopy (HRE) followed by indigo carmine chromoendoscopy (ICC), or HRE followed by NBI in a randomized sequence. They found that a good quality HRE, in experienced hands, is capable of detecting most lesions with HGD/EC in patients with BE, and that ICC and NBI, in combination with HRE, are of limited additional value for primary detection of lesions. They found similar results between ICC and NBI in the detection of HGD/EC in patients with BE, concluding that these techniques are most suitable for targeted detailed inspection of suspected areas. Moreover, Kara et al. 14 introduced a classification system of mucosal and vascular patterns in BE with NBI. They found that NBI was useful in revealing mucosal morphology characteristics of non-dysplastic BE and high-grade intraepithelial neoplasia without the need of staining, concluding that such a technique had a relatively high diagnostic value for these lesions when used for targeted detailed examination of areas of interest.
Sharma et al. 15 in a prospective blinded study on patients with known or suspected BE, found that NBI was a diagnostic tool with a high degree of accuracy for the detection of metaplastic and dysplastic tissue within the BE segment, permitting what they called “virtual in vivo histology” and potentially eliminating the need for random biopsies.
In the stomach, Uedo et al. 9 investigated the use of NBI-ME for the detection of intestinal metaplasia in the gastric mucosa by the appearance of what they called a light blue crest (LBC). LBC was defined as a fine blue-white line on the crests of the epithelial surface or gyri. They devised a grading system for LBCs and found that their appearance correlated with histological evidence of intestinal metaplasia with a sensitivity of 89%, a specificity of 93%, a positive predictive value of 91%, a negative predictive value of 92%, and an accuracy of 91%. They concluded that detection of LBC with NBI is a highly accurate sign of the presence of histological intestinal metaplasia.
In another study, Uchiyama et al. 16 investigated the reliability of NBI-ME to diagnose and differentiate between benign and malignant duodenal ampullary tumours. In their study, they investigated patients whose ampullas were noted, with conventional endoscopy, to be significantly enlarged or protruding. The correlation between NBI-ME images and histo-pathological findings were investigated. Once again, they found that the NBI system was able to predict the histological characteristics of ampullary lesions and concluded that there is a great potential for foci of adenocarcinoma to be accurately diagnosed by NBI, which escaped detection with forceps biopsy.
Machida et al. 17 studied the use of NBI for evaluating colo-rectal lesions. They estimated the accuracy of differentiation between neoplastic and non-neoplastic lesions using the NBI system in comparison with conventional colonoscopy, and with chromoendoscopy. They found that in the examination of colonic lesions the NBI system provides additional imaging features to those obtained both with conventional endoscopy and chromoendoscopy. For distinguishing neoplasms from non-neoplastic lesions, NBI was equivalent to chromoendoscopy. A further investigation was reported by Su et al. 18 comparing conventional colonoscopy, chromoendoscopy, and NBI in the differential diagnosis of neoplastic and non-neoplastic colonic polyps. They also found NBI to be far more specific, sensitive, and accurate than conventional colonoscopy, and equal to chromoendoscopy. However, NBI is a far less demanding endoscopic technique when compared to chromoendoscopy.
On the other hand, in the lower airway, Shibuya et al. 19 investigated the use of high magnification bronchovideoscopy combined with NBI for detailed examination of angiogenic squamous dysplasia (ASD) in the bronchial tree of heavy smokers at high risk for lung cancer. The study was carried out on sputum cytology specimens suspicious or positive for malignancy. They found that the microvessels, vascular networks of various grades, and dotted vessels in ASD tissues were clearly visible on NBI. Diameters of the dotted vessels identified by NBI images were in keeping with the diameters of ASD capillary blood vessels diagnosed by pathological examination, confirming the importance of NBI in detecting ASD and its discrimination from other pre-invasive bronchial lesions.
ENT NBI applications
When it comes to the field of otolaryngology and head and neck surgery, the literature on the use of NBI is very sparse. Indeed, we found only two studies in the literature where NBI was used in the assessment of UADT SCC.
Muto et al. 1 were the first to use a prototype NBI system for screening the oropharynx and hypopharynx for SCC in patients with oesophageal cancer. The clinical examination was performed during routine endoscopic screening or surveillance procedures, and carried out by a single endoscopist. At first, a non-magnifying observation with NBI was performed to identify abnormal mucosal areas. If identified as well demarcated brownish lesions, photographs of the non-magnified view were taken. Subsequently, they observed the lesion and surrounding normal mucosa under magnification. If scattered brownish dots were observed, within the lesion, under the magnifying NBI, they diagnosed the lesion as malignant, according to the criteria reported for oesophageal lesions 20. Magnified NBI clearly revealed scattered brown dots within all malignant lesions. These dots were histologically confirmed to be dilated capillaries on immunohistochemical staining with antihuman monoclonal CD-31 antibody. This microvascular proliferation (MVP) pattern was observed in all lesions by NBI, whereas it was rarely detected by conventional observation, even with magnification. Over a period of 17 months, 34 consecutive superficial lesions were found in 18 patients. Multifocal carcinoma was found in 5 (28%) of them. All lesions exhibited a MVP pattern on magnified NBI as well demarcated brownish areas. Overall, 13 patients with a combined total of 29 lesions underwent EMR under general anaesthesia. Of these, the piriform sinus was the most frequent primary site (66%, or 19 out of 29 lesions). The median tumour diameter was 20 mm (range, 1.3-40 mm). Twenty-one (72%) lesions were histologically confirmed to be carcinoma in situ, and the remaining showed evidence of microinvasion (0.05-1 mm) beneath the epithelium. Vascular invasion was observed in only one lesion. After a median follow-up period of 8 months (range 1-16 months), there were no cases of local recurrence. The Authors concluded that the NBI technique could significantly improve the efficacy of screening for, and surveillance of, lesions of the head and neck region, especially those at oropharyngeal and hypopharyngeal sites, during routine endoscopic examination.
Watanabe et al. 2 further investigated the efficacy of the NBI system in endoscopic screening of the oropharynx and hypopharynx in patients with oesophageal cancer. The study was carried out on 217 consecutive patients by the same otolaryngologist. Among these patients, 6 superficial lesions were detected with the NBI system, one in the oropharynx and 5 in the hypopharynx. Of these lesions, only 4 could hardly be recognized by conventional endoscopy because of their small diameter (5 mm or less). The other two lesions had a diameter of 1 cm or more and could be recognized by both conventional endoscopy and NBI. However, the Authors stated that NBI was more beneficial in recognizing the superficial lesions than the conventional endoscopic view. On NBI observation, all lesions were also recognized as well demarcated brownish areas, exhibiting scattered brown dots within these areas on close view. EMR was performed for all the 6 patients and the histological examination revealed CIS. Even though detecting 6 lesions of the UADT in 217 patients with oesophageal cancer may seem a small number, Watanabe et al. concluded that, over 10 years, they had previously performed 9940 conventional endoscopic screenings for 1118 patients with oesophageal cancer. Among those, 142 early HNSCCs were detected in 127 patients, resulting in an average of 70 endoscopic screenings needed to detect one lesion with their conventional endoscopy screening programme. If the same ratio were to be applied in their more recent study, 217 conventional endoscopic examinations should reveal about 3 superficial lesions only. Thus they felt that the NBI system might improve the sensitivity by about two-fold over the conventional method. Furthermore, they emphasized the major differences between the NBI system they used and that being used for the digestive tract, reported in previous papers 21. The diameter of the endoscope is much smaller, only 3.2 mm in diameter, allowing a thorough examination of the UADT to be performed transnasally, and the time needed for the examination about 3-5 minutes, much shorter than the time required in the digestive tract examination setting. These differences allow the use of NBI as an outpatient procedure with the patient in a seated position, and totally avoiding the endoscopy laboratory.
NBI future trends in ENT
Even though the experience of the Department of Otorhinolaryngology – Head and Neck Surgery of Brescia, in the use of NBI, is still in its infancy, the easy application of this technical device and the optimistic impressions already developed in the otolaryngologic as well as in other endoscopic disciplines, prompted us to prospectively recruit patients for clinical trials to precisely evaluate its diagnostic accuracy. As already demonstrated, the NBI system plays an important role in the screening and diagnosis of superficial lesions in various anatomic sites of the aerodigestive tract. The paucity of studies in the otolaryngologic literature should stimulate future multi-institutional investigations for the early detection of HNSCCs in high-risk patients (including those enrolled in a surveillance protocol after treatment of a previous head and neck cancer). The oropharynx and hypopharynx are not the only sites that can benefit from this kind of technology, but even the oral cavity (Figs. 1, 2, 3), nasal fossa, nasopharynx, and larynx should be accordingly investigated with NBI. Future refinements in the HDTV system and flexible videoendoscopic instrumentation will allow a more accurate evaluation of the UADT in head and neck cancer patients even in the pre-operative setting. Moreover, another interesting field of research is represented by the intra-operative examination of tumour boundaries by rigid endoscopes coupled to the already existing HDTV monitors with the NBI system in order to assess the validity of such a tool in ensuring complete tumour resection and assessing the surgical margins. Far from being the panacea of early HNSCC detection, we foresee NBI as a useful tool in the future pre-, intra-, and post-operative endoscopic work-up of neoplastic lesions of the UADT.
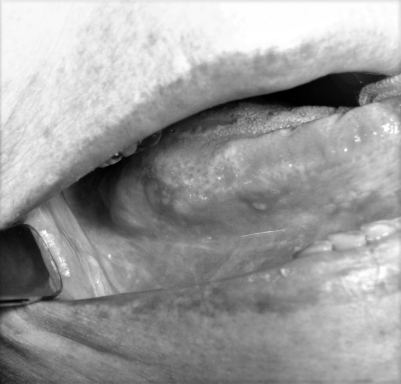
Fig. 1.
A 65-year-old female with long-standing lichen planus of the oral cavity. During a routine follow-up evaluation, multiple erythro- and leukoplasic lesions were detected at the level of the floor of mouth and lateral margin of mobile tongue.
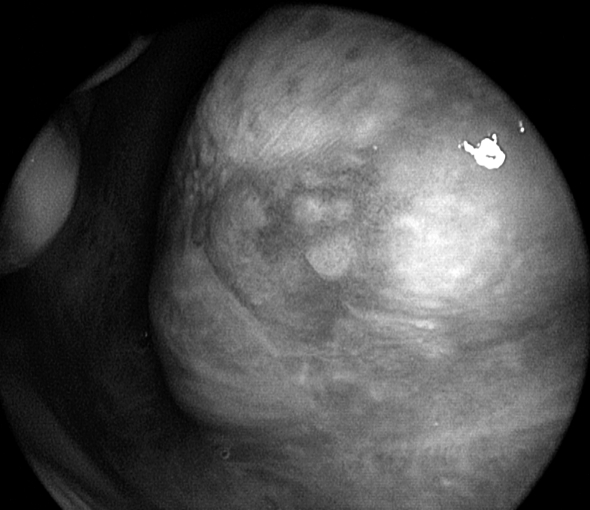
Fig. 2.
Same patient as in Figure 1. Close endoscopic view with 0° telescope coupled to the HDTV camera of the most posterior leuko-erythroplasic tongue lesion.
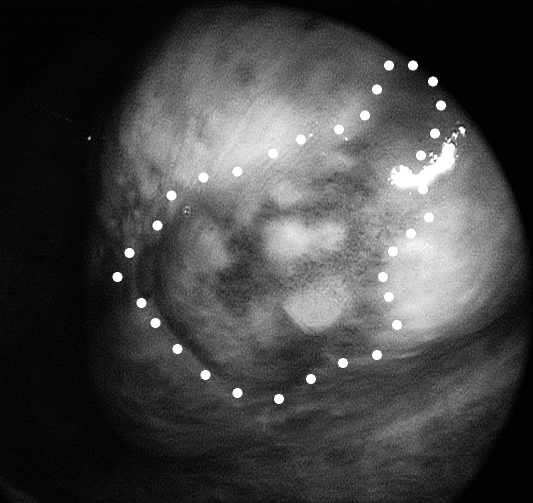
Fig. 3.
Same patient as in Figures 1 and 2. Close endoscopic view with NBI obtained by 0° telescope coupled to the HDTV camera of the previously observed tongue lesion. Peripheral margins of the abnormal neoangiogenetic vascularization are clearly visible and located well beyond the limits of the lesion as identified by white light endoscopy. Excisional biopsy under local anaesthesia, following the NBI detected borders, was demonstrated to be carcinoma in situ with free margins.
References
- 1.Muto M, Nakane M, Katada C, Sano Y, Ohtsu A, Esumi H, et al. Squamous cell carcinoma in situ at oropharyngeal and hypopharyngeal mucosal sites. Cancer 2004;101:1375-81. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe A, Tsujie H, Taniguchi M, Hosokawa M, Fujita M, Sasaki S. Laryngoscopic detection of pharyngeal carcinoma in situ with narrowband imaging. Laryngoscope 2006;116:650-4. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe A, Hosokawa M, Taniguchi M, Sasaki S. Periodic pharyngolaryngoscopy detects early head and neck cancer and improves survival in esophageal cancer. Ann Thorac Surg 2003;76:1699-705. [DOI] [PubMed] [Google Scholar]
- 4.Muto M, Hironaka S, Nakane M, Boku N, Ohtsu A, Yoshida S. Association of multiple Lugol-voiding lesions with synchronous and metachronous oesophageal squamous cell carcinoma in patients with head and neck cancer. Gastrointest Endosc 2002;56:517-21. [DOI] [PubMed] [Google Scholar]
- 5.Lambert R, Jeannerod M, Rey JF. Eyes wide shut. Endoscopy 2004;36:723-5. [DOI] [PubMed] [Google Scholar]
- 6.Tajiri H, Matsuda K, Fujisaki J. What can we see with the endoscope? Present status and future perspectives. Dig Endosc 2002;14:131-7. [Google Scholar]
- 7.Kuznetsov K, Lambert R, Rey J-F. Narrow-band imaging: potential and limitations. Endoscopy 2006;38:76-81. [DOI] [PubMed] [Google Scholar]
- 8.Gono K, Obi T, Yamaguchi M, Ohyama N, Machida H, Sano Y, et al. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt 2004;9:568-77. [DOI] [PubMed] [Google Scholar]
- 9.Uedo N, Ishihara R, Iishi H, Yamamoto S, Yamamoto S, Yamada T, et al. A new method of diagnosing gastric intestinal metaplasia: narrow-band imaging with magnifying endoscopy. Endoscopy 2006;38:819-24. [DOI] [PubMed] [Google Scholar]
- 10.Kara MA, Bergman JJGHM. Autofluorescence imaging and narrow-band imaging for the detection of early neoplasia in patients with Barrett’s esophagus. Endoscopy 2006;38:627-31. [DOI] [PubMed] [Google Scholar]
- 11.Szold A. Seeing is believing. Surg Endosc 2005;19:730-3. [DOI] [PubMed] [Google Scholar]
- 12.Hamamoto Y, Endo T, Nosho K, Arimura Y, Sato M, Imai K. Usefulness of narrow-band imaging endoscopy for diagnosis of Barrett’s esophagus. J Gastroenterol 2004;39:14-20. [DOI] [PubMed] [Google Scholar]
- 13.Kara MA, Peters FP, Rsomolen WD, Krishnadath KK, ten Kate FJW, Fockens P, et al. High-resolution endoscopy plus chromoendoscopy or narrow-band imaging in Barrett’s esophagus: a prospective randomized crossover study. Endoscopy 2005;37:926-36. [DOI] [PubMed] [Google Scholar]
- 14.Kara MA, Ennahachi M, Fockens P, ten Kate FJW, Bergman JJGHM. Detection and classification of the mucosal and vascular patterns (mucosal morphology) in Barrett’s esophagus by using narrow band imaging. Gastrointest Endosc 2006;64:155-66. [DOI] [PubMed] [Google Scholar]
- 15.Sharma P, Bansal A, Mathur S, Wani S, Cherian R, McGregor D, et al. The utility of a novel narrow band imaging endoscopy system in patients with Barrett’s esophagus. Gastrointest Endosc 2006;64:167-75. [DOI] [PubMed] [Google Scholar]
- 16.Uchiyama Y, Imazu H, KaKutani H, Hino S, Sumiyama K, Kuramochi A, et al. New approach to diagnosing ampullary tumors by magnifying endoscopy combined with a narrow-band imaging system. J Gastroenterol 2006;41:483-90. [DOI] [PubMed] [Google Scholar]
- 17.Machida H, Sano Y, Hamamoto Y, Muto M, Kozu T, Tajiri H, et al. Narrow-band imaging in the diagnosis of colorectal mucosal lesions: a pilot study. Endoscopy 2004;36:1094-8. [DOI] [PubMed] [Google Scholar]
- 18.Su MY, Hsu CM, Ho YP, Chen PC, Lin CJ, Chiu CT. Comparative study of conventional colonoscopy, chromoendoscopy, and narrow-band imaging systems in differential diagnosis of neoplastic and nonneoplastic colonic polyps. Am J Gastroenterol 2006;101:2711-6. [DOI] [PubMed] [Google Scholar]
- 19.Shibuya K, Hoshino H, Chiyo M, Iyoda A, Yoshida S, Sekine Y, et al. High magnification bronchovideoscopy combined with narrow-band imaging could detect capillary loops of angiogenic squamous dysplasia in heavy smokers at high risk of lung cancer. Thorax 2003;58:989-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumagai Y, Inoue H, Nagai K, Kawano T, Iwai T. Magnifying endoscopy, stereoscopic microscopy, and the microvascular architecture of superficial oesophageal carcinoma. Endoscopy 2002;34:369-75. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida T, Inoue H, Usui S, Satodate H, Fukami N, Kudo SE. Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesion. Gastrointest Endosc 2004;59:288-95. [DOI] [PubMed] [Google Scholar]