Abstract
It is becoming increasingly evident that cancer stem cells play a vital role in development and progression of cancers and relapse following chemotherapy. The present study examines the presence of cancer stem-like cells (CSC) in adenomatous polyps and in normal appearing colonic mucosa in humans with aging. The number of polyps was found to increase linearly with advancing age (r2=0.92, p<0.02). Immunohistochemical analysis revealed co-localization of CSC markers CD44 and CD166 in colonic polyps. Real-time RT-PCR analysis of normal appearing mucosa from subjects with adenomatous polyps showed an age-related rise in CSC as evidenced by the increased expression of CD44, CD166 and ESA. A similar phenomenon was also observed for EGFR. In addition, the expression each CSC marker was found to be about 2-fold higher in subjects with 3-4 polyps than those with 1-2 polyps. In conclusion, our results show that colon cancer stem-like cells are present in the premaliganant adenomatous polyps as well in normal appearing colonic muocsa. Moreover, our observation of the age-related rise in CSC in macroscopically normal colonic mucosa suggests a predisposition of the organ to developing colorectal cancer.
Keywords: Cancer stem Cells, Colon cancer, Aging, EGFR
Introduction
It is being increasingly recognized that epithelial cancers including colorectal cancer are diseases driven by subpopulation of self renewing cancer stem cells (1). Cancer stem cells represent a unique population of cells within the tumor based on their ability to self-renew as well as generate progenitor cells which proliferate and give rise to the rest of the tumor cells. In this sense cancer stem cells resemble their normal adult stem cells counterparts which are present in various tissues including the colonic epithelium. Although the origin of the cancer stem cells is not fully known, it is widely believed that they arise from the normal stem cells or progenitors upon mutation(s) (1). Currently, cancer stem cells are identified by specific surface epitopes. Cells expressing these surface epitopes have the ability to form tumors at a much diluted concentration in SCID mice and histologically resemble the primary tumor from which they were derived (2,3). Recent investigation suggests colorectal cancer stem cells express surface markers CD44, CD166 and ESA (epithelial-specific antigen, also known as EpCAM) (3).
Aging is associated with increased incidence of various cancers including colorectal cancer. In fact, the occurrence of both nonmalignant and malignant colorectal neoplasm increases with advancing age (4). Although the reasons for this increase is not fully understood, results from animal experimental models show that aging is associated with increased proliferation and attenuated apoptosis in both gastric and colonic mucosa, leading to increased susceptibility to cancer (5). This has in part been attributed to increased expression and activation of epidermal growth factor receptor (EGFR) and its family members in the gastrointestinal (GI) mucosa (4). Activation of various growth factor receptors particularly EGFR in the GI mucosa may contribute to cancer stem cell growth and/or survival which would lead to greater chance of malignant transformation. Whether cancer stem-like cells (CSC) are present in normal appearing mucosa in subjects with premalignant and/or malignant lesion are unknown. We hypothesize that CSC are present in the premalignant polyps as well as in normal appearing colonic mucosa in subjects with adenomas and that aging may affect CSC population. The current investigation was undertaken to test this hypothesis by examining the expression of CSC markers in colonic polyps and in normal appearing colonic mucosa.
Materials and Methods
Tissue procurement
Colonic polyps and normal appearing colonic mucosa (≤10 cm anal verge) were from the patients who were enrolled in our recently completed folic acid chemoprevention trial (6). In this chemoprevention trial, patient with adenomatous polyps who underwent polypectomy were randomized to receive placebo or daily 5 mg of folic acid per day for 3 years (6). Biopsies of normal appearing colonic mucosa distant from the site of the polyp were also obtained (6). However, the present study utilized the colon biopsies obtained at baseline polypectomy of 16 subjects, prior to randomization.
Immunohistological analysis
For immunohistochemical staining, an immunoperoxidase method was used with a streptavidin biotinylated horseradish peroxidase complex (Dako, Carpinteria, California, USA). Sections of formalin fixed paraffin embedded tissue blocks were deparaffinized and subsequently incubated in methanol containing 0.3% hydrogen peroxide at room temperature for 20 minutes to quench endogenous peroxidase activity and then treated with pepsin for 10 minutes at room temperature. Sections were incubated with protein block serum free (Dako) at room temperature for 10 minutes to block non-specific staining and then incubated for two hours at room temperature with rabbit polyclonal anti-EGFR antibodies. After washing, sections were incubated with a biotin conjugated secondary antibody at room temperature for 30 minutes and finally with peroxidase conjugated streptavidin at room temperature for 30 minutes. Peroxidase activity was detected with the enzyme substrate 3-amino-9-ethyl carbazole. For negative controls, sections were treated in the same way except that they were incubated with Tris buffered saline instead of the primary antibody. The slides were examined and scored by a trained pathologist. The percentage of cells with immunostaining was recorded and intensity of staining was separately graded as follows: 0=no staining, 1=faint staining, 2=substantial staining, 3=very strong staining. The combined score was generated by multiplying % positive with intensity of staining.
For determination of localization of CD44 and CD166, Sections of formalin fixed paraffin embedded tissue blocks were deparaffinized and were rehydrated in 0.1 M phosphate-buffered saline (PBS), pH 7.4, treated for 15 min with blocking serum (Vector Laboratories, Burlingame, California, USA), and subsequently incubated overnight in a refrigerated humidity chamber with antibodies to either CD44 (Cell signaling, Beverly, MA) or CD166 (Cell Signaling, Beverly, MA). The slides were washed three times in PBS, and then treated for 1 hr with appropriate secondary antibodies labeled with FITC (for CD44) or TRITC (for CD166). Secondary antibodies were obtained from Sigma Chemical Co., St. Louis, Missouri, USA. In order to evaluate co-localization, additional specimens incubated with CD44 labeled with secondary antibodies were washed three times in PBS, then incubated for 2 hr with anti-CD166 antibody, followed by three washes with PBS and treatment with TRITC conjugated secondary antibodies. Negative control specimens were incubated overnight in blocking serum, and then treated for 1 hr with FITC- or TRITC-conjugated secondary antibodies, followed by three washes with PBS. All slides were cover-slipped and examined with a Zeiss LSM 510 confocal microscope utilizing a 40£ water-immersion objective.
Isolation of RNA
Total RNA was extracted from normal appearing rectal mucosal biopsies using RNA-STAT solution (Tel Test, Friendswood, TX) according to the manufacturer’s instruction. The total RNA was treated with DNase I to remove contaminating genomic DNA, subsequently purified using RNAeasy Mini Kit (Qiagen, Valencia, CA).
Reverse transcription-polymerase chain reaction (RT-PCR)
Two-step RT-PCR was performed by using the GeneAmp Gold RNA PCR Kit (Applied Biosystems, Foster City, CA). Briefly, 1 μg of purified RNA was reverse transcribed in the presence of 2.5 mM MgCl2, 1X RT-PCR Buffer, 1 mM dNTPs, 10 mM dithiothreitol, 10 units RNase inhibitor, 1.25 μM random hexamers and 15 units Multiscribe Reverse Transcriptase in a final reaction volume of 20 μl. The mixture was incubated at 25°C for 10min for hybridization, subsequently at 42°C for 15 minutes in a Gene Amp PCR system 9600 (Pepkin-Elmer), and then by cooling to 4°C. The RT reactions were subjected to polymerase chain reaction (PCR) amplification. Five microliter of cDNA products were amplified with 2.5 units of Ampli Taq Gold Polymerase (Applied Biosystems), 1X RT-PCR Buffer, 1.75 mM MgCl2, 0.8 mM dNTPs, 0.15 μM of following primers: D44 forward:5’-AAGGTGGAGCAAACACAACC-3’ and reverse:5’AACTGCAATGCAAACTGCAAG-3’; CD166 forward: 5’-TAGCAGGAATGCAACTGTGG-3’ and reverse: 5’-CGCAGACATAGTTTCCAGCA-3’ and ESA forward: 5’-CTGGCCGTAAACTGCTTTGT-3’ and reverse 5’-TCCCAAGTTTTGAGCCATTC-3’. Beta-actin forward (5’-CCCAGCACAATGAAGATCAA-3’) and reverse (5’-ACATCTGCTGGAAGGTGGAC-3’) primers (107 bp product) were used as internal controls. Reactions were carried out in the Gene Amp PCR system 9600, first hold of 10 minutes at 95°C for activated AmpliTaq Gold DNA Polymerase, and were followed by 20 seconds at 94°C, 60 seconds at 62°C for 40 cycles for amplification target gene.
Statistical analysis
The statistical analyses were all performed using the Statistical Package for Social Sciences (SPSS, version 8.0; 1997, Chicago, IL). All t-tests were two sided. A contingency table was computed via Chi-Square analysis.
Results
Aging and number of adenomas
Baseline colonoscopy revealed a positive linear relationship between advancing age and the number of polyps per patient (Fig. 1A). This relationship was highly significant (r2=0.92; P<0.02). (Fig 1A). In addition, the proportion of advanced adenomas (polyps >1cm) was higher subjects over 70 years of age than those under 70 years (p<0.02). Analysis of the data revealed that 36% of the subjects younger than 70 years had advanced adenomas compared to 61% of the subjects older than 70 years (Fig 1B).
Figure 1.
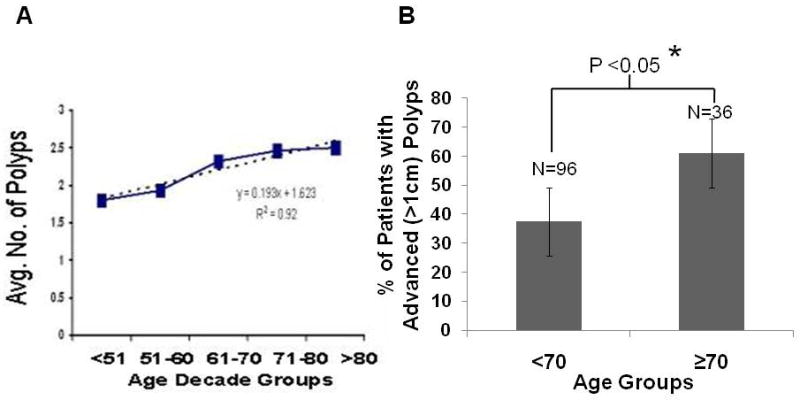
Number of polyps increased linearly with aging (A), and incidence of advanced polyps (>1cm) is higher in subjects over 70 years than those under 70 years of age (B).
CSC in adenomatous polyps
Although expression of CSC is well characterized in cancer, its presence and putative location are poorly studied in precancerous lesions. We examined the presence of CSC in adenomatous polyps using confocal immunofluoresence microscopy with anti-CD44 and anti-CD166 antibodies. CD44 and CD166 positive staining was observed in colonic polyps (Fig. 2). More importantly, they were found to be co-localized in membranes of CSC that were present in the crypts (Fig.2).
Figure 2.
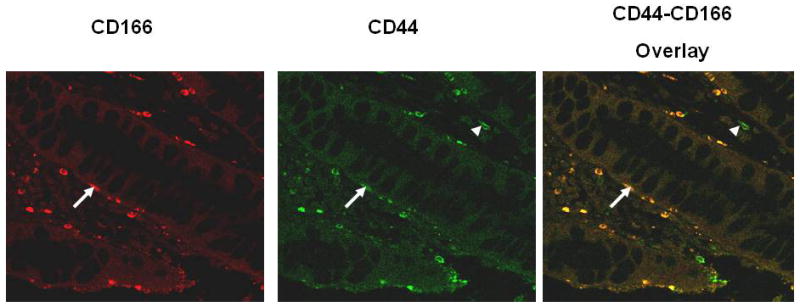
Immunohistochemical (Confocal immunofluoresence microscopy) analysis of CSC markers CD44 and CD166 in adenomatous polyps. Arrows show co-localization of CD44 and CD166, whereas the arrow-heads illustrate CD44 staining in infiltrating hematopoietic cells.
Aging and EGFR as well as CSC expression in normal colonic mucosa
The age-related increase in adenomas was accompanied by a parallel rise in EGFR expression in the normal appearing rectal mucosa (distant from the site of adenoma) (Figs. 3A and B). There was a greater than 5-fold increase in EGFR immunoreactivity between subjects younger than 50 years and older than 70 years. To determine whether CSC would be present in the normal appearing colonic mucosa from patients with adenomatous polyps, we examined the expression of colorectal CSC markers CD44, CD166 and ESA in macroscopically normal rectal mucosa in 16 patients from different age groups whose tissue samples had sufficient amount of good quality RNA available for RT-PCR analysis. We found a higher expression of CD44, CD166, ESA (as determined by real time RT-PCR) in subjects older than 55 compared to those between 40-55 years of age (Fig. 4A). In addition, we found a positive relationship in expression of CSC markers with number of polyps such that 2-4 polyps showed 1.6-2 fold higher levels of CSC markers compared to 1-2 polyps (Fig. 4B). This age-related as well as number of polyps related increase in CSC markers in normal appearing mucosa was associated with increase in EGFR expression.
Figure 3.
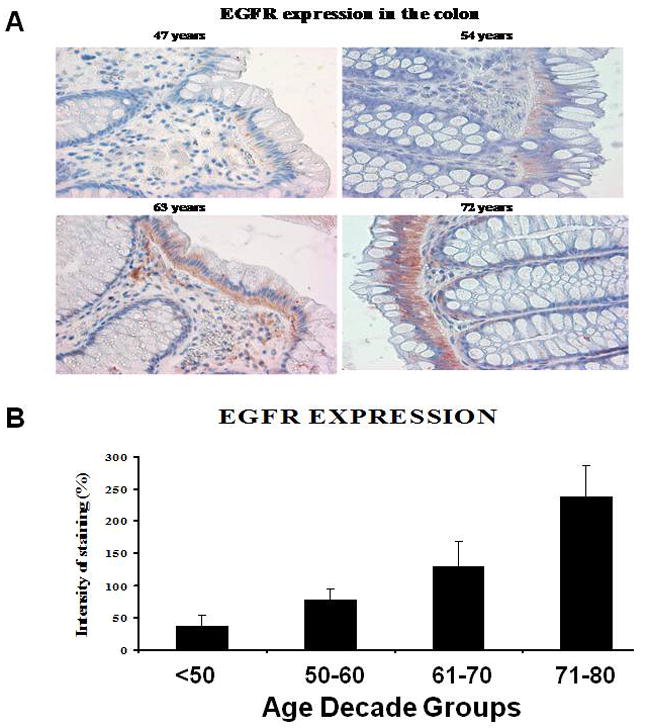
Age related increase in EGFR immunoreactivity in normal appearing colonic mucosa from subjects with adenomatous polyps.
Figure 4.
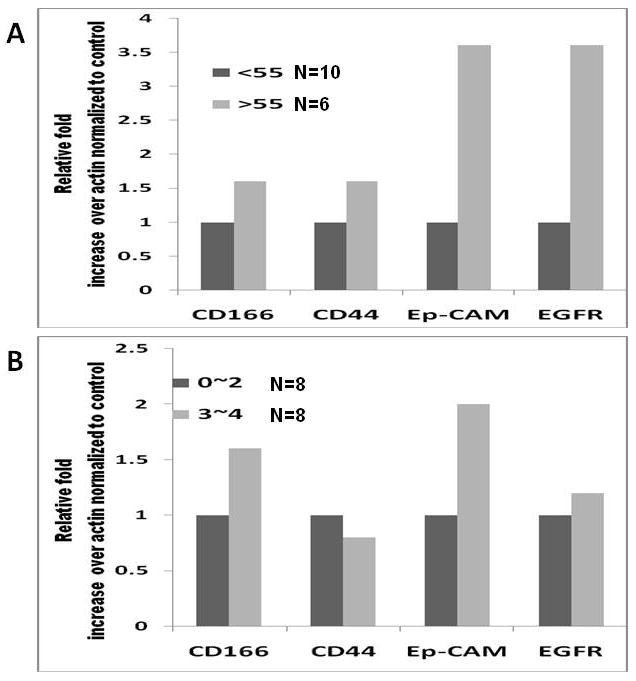
Increase in cancer stem-like cells expression (as measured by RT-PCR for CD44, CD166 and ESA) with A) aging and B) number of polyps in normal appearing colonic mucosa (≤10cm from anal verge).
Discussion
Incidence of colorectal cancer increases with aging (7). But currently there are no reliable biomarkers to detect biochemical changes preceding the development of neoplastic lesions in the gastrointestinal tract. In the present study we demonstrate a linear increase in number of adenomas with aging accompanied by a parallel rise in EGFR expression in the normal appearing colonic mucosa (distant from the site of adenoma). The latter suggests a role for EGFR in the development of colonic neoplasia, an event that has been observed in almost all epithelial cancers (8,9). In fact 40-50% colon tumors show increased expression of EGFR (9).
Since it is becoming increasingly evident that CSC play a critical role in the initiation as well as resistance and relapse following chemotherapy, we examined the presence of stem-like cell expression in premalignant polyps as well as normal colonic mucosa. Our current observation that CSC markers such as CD44 and CD166 are co-localized in adenomatous glands, supports the notion that stem cells may play a critical role in development and progression of colorectal malignancy. Furthermore, that the expression of CD44, CD166 and ESA, is higher in normal appearing mucosa of subjects over 55 years than those below 55 years with adenomas suggests that with aging increases number of stem-like cells that may lead to a greater predisposition colonic mucosa to subsequent transformation. Further support for this comes of the observation there is a positive relationship between expression of cancer stem-like cells in normal mucosa and the number of polyps. Moreover, the fact that age related increase in CSC is associated with a parallel rise in EGFR expression, indicates a relationship between the two events. This raises the possibility that EGFR could potentially be a marker of colon CSC that may play a role in the development and progression of colorectal cancer.
In conclusion, this is the first of a kind study that demonstrates the presence of CSC in macroscopically normal appearing colonic mucosa in patients with adenomatous polyps. More importantly, our data show that CSC in the normal mucosa increases with aging accompanied by a parallel rise in EGFR, which may partly be responsible for the age-related rise in colorectal cancer.
Acknowledgments
This work was supported by grants (APNM) from the NIH/NIA (AG014343) and the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jordan CT, Guzman ML, Noble M, et al. Cancer stem cells. N Engl J Med. 2006;355:1253–61. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien CA, Pollett A, Gallinger S, et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–10. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 3.Dalerba P, Dylla SJ, Park IK, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Nat Acad Sci (U S A) 2007;104:10158–63. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao ZQ, Moragoda L, Jaszewski R, Hatfield JA, Fligiel SE, Majumdar APN. Aging is associated with increased proliferation and decreased apoptosis in the colonic mucosa. Mech Ageing Dev. 2001;122:1849–64. doi: 10.1016/s0047-6374(01)00323-2. [DOI] [PubMed] [Google Scholar]
- 5.Majumdar APN, Du J, Yu Y, et al. Cell cycle and apoptosis regulatory protein-1: a novel regulator of apoptosis in the colonic mucosa during aging. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1215–22. doi: 10.1152/ajpgi.00324.2007. [DOI] [PubMed] [Google Scholar]
- 6.Jaszewski R, Misra S, Tob M, et al. Folic acid supplementation inhibits recurrence of colorectal adenomas: A randomized chemoprevention trial. World J Gastroenterol. 2008;14:4492–98. doi: 10.3748/wjg.14.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.http://seer.cancer.gov/faststats/selections.php?run&output=2&data=1&statistic=3&year=200802&race=1&sex=1&age=1&series=cancer&cancer=20
- 8.Patel BB, Majumdar APN. HER family of receptors as treatment targets. In: Lowy AM, Leach SD, Phillip PA, editors. Pancreatic cancer, M.D. Anderson Solid Tumor Oncology Series. 1. Springer; 2008. pp. 609–34. [Google Scholar]
- 9.Nautiyal J, Rishi AK, Majumdar APN. Emerging therapies in gastrointestinal cancers. World J Gastroenterol. 2006;12:7440–50. doi: 10.3748/wjg.v12.i46.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raguz S, Yagüe E. Resistance to chemotherapy: new treatments and novel insights into an old problem. Br J Cancer. 2008;99:387–91. doi: 10.1038/sj.bjc.6604510. [DOI] [PMC free article] [PubMed] [Google Scholar]


