Abstract
Type I interferon is important in anti-viral responses and in coordinating the innate immune response. Here we explore the use of interferon-β to adjuvant the response to a rabies virus (RV) vaccine vector expressing both HIV-1 Gag and IFN-β. Viral load and immune responses of immunized mice were analyzed over time. Our results indicate that the RV expressing IFN-β (IFN(+)) is highly attenuated when compared to control RV and demonstrate that the expression of IFN-β reduces viral replication approximately 100-fold. Despite the decrease in replication, those mice immunized with the IFN(+) RV had a significantly greater number of activated CD8+ T cells. The increased activation of CD8+ T cells was dependent on IFN-β signaling, as we saw no difference following infection of IFNAR−/− mice. Although mice immunized with IFN(+) have a greater primary immune response than controls, immunized mice that were challenged with vaccinia expressing Gag had no significant difference in the number or functionality of CD8+ T cells. The increased CD8+ T cell activation in the presence of IFN-β, even with greatly reduced viral replication, indicates the beneficial effect of IFN-β for the host.
Keywords: Vaccine, Adjuvant, Interferon-beta, Rabies
INTRODUCTION
Traditional vaccine approaches, such as live-attenuated viruses, have been very successful in providing immunity to some of mankind’s largest microbial threats. One such success story was the use of an attenuated vaccinia virus to immunize people against smallpox infection (Parrino and Graham, 2006). However, similar approaches cannot be used or have failed to control other infectious diseases, such as HIV-1. Thus, vaccine development requires novel approaches and potentially the use of a molecular adjuvant to increase immune response (Letvin, 2006).
Live attenuated vaccine vectors have the greatest potential for generating broad-scope immunity and their usefulness has been shown in different settings. We have generated a vaccine vector based on attenuated Rabies virus (RV) expressing HIV-1 or SIV antigens that has proven to be highly immunogenic in mice and has protected non-human primates from a AIDS-like disease (McGettigan et al., 2001; McKenna et al., 2007).
RV is an enveloped non-segmented negative strand RNA virus of the rhabdoviridae family. Although wild type RV almost always causes a fatal CNS disease in mammalian species (Dietzschold, Faber, and Schnell, 2003), in its attenuated form, RV has proved to be an excellent vaccine vector (Faber et al., 2005; McGettigan et al., 2003b; McKenna et al., 2007; Siler et al., 2002). Furthermore, attenuated RV has been shown to induce both a strong cellular and humoral immune response to foreign genes (McGettigan et al., 2003a; McKenna et al., 2007; McKenna et al., 2003). RV has a relatively simple genome organization encoding five structural proteins: a nucleoprotein (N), a phosphoprotein (P), a matrix protein (M), a glycoprotein (G), and an RNA-dependent RNA polymerase (L). RV-N encapsidates the viral RNA and together with RV-P and polymerase L composes the ribonucleoprotein (RNP) complex. RV-M bridges the RNP complex with the transmembrane domain of RV-G (Mebatsion, Weiland, and Conzelmann, 1999). Both RV-M and RV-G facilitate virion budding from an infected cell (Mebatsion, Konig, and Conzelmann, 1996; Mebatsion, Weiland, and Conzelmann, 1999).
The RV lifecycle is thought to be sensitive to several antiviral proteins that are induced by type I interferon (IFN) and thus, RV has developed at least two mechanisms by which it can antagonize type I IFN induction and signaling. In addition to serving as the non-catalytic cofactor to polymerase L, RV-P also inhibits the phosphorylation of IRF-3 at serine-386 by interfering with TBK-1 (Brzozka, Finke, and Conzelmann, 2005). Furthermore, the C-terminal domain of RV-P can bind to the coil-coil domain of Stat1 and efficiently prevent the nuclear accumulation of Stat1 (Brzozka, Finke, and Conzelmann, 2006; Vidy, Chelbi-Alix, and Blondel, 2005; Vidy et al., 2007). Thus, RV-P interferes with both the induction and signaling of type I interferon.
Type I interferon, although originally recognized for its anti-viral properties, has recently gained recognition for its role in coordinating the innate and adaptive immune responses. Type I IFN expression may bias immune responses toward Th1 responses (Wenner et al., 1996), and, in addition, IFN-α/β plays an important role in the clonal expansion of antigen-specific CD8+ T cells. Adoptive transfer of interferon receptor knockout (IFNAR−/−) CD8+ T cells into wild type hosts highlighted the necessity of IFN-α/β signaling in T cells to induce survival of LCMV-specific CD8+ T cells during the proliferative phase (Kolumam et al., 2005).
Type I IFN not only affects T cell responses, but, has also been shown to augment the immune response by activating antigen presenting cells (APC). Dendritic cell (DC) maturation following infection with LCMV (Montoya et al., 2005) and Herpes simplex virus type-1 (Pollara et al., 2004) has been shown to be type I IFN dependent. Additionally, subsequent to transfection with two recombinant adenovirus (Ad) vectors, it was seen that DCs up-regulated the expression of phenotypic activation markers. The increase was independent of viral replication and toll like receptor (TLR) signaling. However, there was only a marginal increase of co-stimulatory molecules on the surface of BM-DC derived from IFNAR−/− mice, suggesting that the maturation of Ad-transduced cells was dependent on type I IFN signaling (Hensley et al., 2005). Furthermore, following maturation in the presence of type I interferon and GM-CSF, monocyte-derived DCs more effectively stimulate an antigen-specific CD8+ T cell response than DCs matured with GM-CSF and IL-4 (Santodonato et al., 2003).
It is apparent that IFN-α/β plays an important role in directing the adaptive immune response. This function also suggests that it may be a valuable adjuvant in vaccine development. However, much of the work supporting the immune-modifying functions of IFN-β have been done in vitro; therefore, the potential use of IFN as an adjuvant in vaccine development needs to be further investigated. In an attempt to induce IFN-α/β expression in a vaccine for respiratory syncytial virus (RSV), Martinez-Sobrido et al. inserted the RSV-F protein into a Newcastle disease virus (NDV) vaccine vector. Although the recombinant virus increased IFN-α/β levels, as predicted, and protected mice against RSV challenge the mechanism of protection was unclear, because similar protection was seen in IFNAR−/− and wild type mice (Martinez-Sobrido et al., 2006). When combining multiple viral proteins, it may be impossible to distinguish the direct effects of the interferon from the immunological response to the additional protein. Furthermore, different viruses have several distinct features in addition to their ability to induce IFN, which make results difficult to analyze. Here we use a system in which IFN-β is expressed by the RV vaccine vector in order to compare the immune responses induced in the presence or absence of increased levels of IFN-β.
The use of IFN-β to promote the induction of a stronger CD8+ T cell response might be beneficial for certain vaccine approaches. Although little evidence is available to link the beneficial outcome of a RV infection to increased CTL responses against RV as a vaccine vector, increased CTL responses to the foreign antigen may be required. In order to further investigate the potential immune-enhancing effects of IFN-β, we constructed two recombinant Rabies viruses, one expressing both HIV-1 Gag and IFN-β and the other expressing HIV-1 Gag and IFN-β minus the ATG start codon. Our data support the hypothesis that IFN-β works at the interface of the innate and adaptive immune response by sustaining the pool of activated antigen-specific CD8+ lymphocytes. Thus, in addition to controlling viral replication (a well-studied effect of type I IFN), we provide evidence that IFN-β also directs the cells of the adaptive immune system to adequately respond to a pathogen. Elucidating the immune-modifying effects of type I IFN is important in determining whether the addition of this cytokine can enhance the cellular response and increase the potency of RV based vaccine vectors.
RESULTS
Construction and characterization of the recombinant RV
To investigate the effect of type I interferon on the adaptive immune response, we used a previously well-characterized RV vaccine vector encoding HIV-1 Gag (Figure 1A, denoted BNSP-Gag) (McGettigan et al., 2001). For our purposes, the gene encoding mouse IFN-β was introduced between the RV G and L genes resulting in virus that expressed both HIV-1 Gag and IFN-β (Figure 1A, denoted IFN(+)). Of note, it is well established that the expression of an additional gene can change the growth characteristics of RV (McGettigan et al., 2006). Thus, in order to have the proper control, we also cloned the mouse IFN-β gene without the ATG start codon between the RV G and L genes (Figure 1A, denoted IFN(−)). Recombinant viruses were recovered by standard methods as previously described (Tan et al., 2007).
FIGURE 1. Construction of recombinant RV and expression of Gag and IFN-β.
(A) We cloned mouse IFN-β (IFN(+)) or mouse IFN-β lacking the ATG start codon (IFN(−)) into the vaccine RV strain BNSP-Gag, which expresses HIV-1 Gag. Recovered viruses were analyzed for expression of the IFN-β by ELISA (B) and the functionality of the IFN-β was determined by a VSV protection assay (C). For this approach, BSR cells were infected, and at 48 hpi the supernatant was UV-inactivated. NA cells were pretreated with UV inactivated supernatant at a dilution of 1:10 or 1:1000 for 24 hours and then infected with VSV-GFP for 5 hours. GFP expression, an indication of VSV replication, is seen by green fluorescence. (D) Recovered viruses were also analyzed for expression of HIV-1 p55 by immunofluorescent staining. Cells were infected with recombinant RV and stained for RV-N or HIV-1 p55.
We then evaluated expression of IFN-β by the two recombinant RVs IFN(+) and IFN(−). Production of IFN-β was quantified in viral supernatant via ELISA 48 h post infection (Figure 1B). We detected only background levels of IFN-β in the supernatants of BSR cells infected with IFN(−), presumably due to the IFN antagonistic activity of RV-P(Brzozka, Finke, and Conzelmann, 2005; Brzozka, Finke, and Conzelmann, 2006; Vidy, Chelbi-Alix, and Blondel, 2005; Vidy et al., 2007). On the other hand, BSR cells infected with the IFN(+) virus produced approximately 3.5 μg/ml IFN-β, which is a 1000-fold increase over background. Next, we aimed to assess the biological functionality of the IFN-β expressed by the recombinant RV. Briefly, we infected BSR cells with IFN(+), IFN(−) or BNSP for 48 hours. The supernatant from each sample was then collected and UV-inactivated. Serial dilutions of the supernatants were then used to pre-treat mouse NA cells for 24 h prior to infecting the NA cells with recombinant VSV expressing GFP (Stojdl et al., 2003). Five hours after infection with VSV-GFP, NA cells were analyzed for expression of GFP, a marker for VSV replication in our system. Type I IFN is known to efficiently inhibit VSV replication in neurons (Trottier, Palian, and Reiss, 2005); thus, if functional IFN-β is present in the supernatant, we should not see GFP expression. As shown in Figure 1C, only supernatants from IFN(+) infected cells efficiently inhibited VSV replication, indicating the presence of functional IFN-β in that sample.
The expression of HIV-1 p55 was determined by immunostaining infected BSR cells. Recombinant RV BNSP IFN(−) and IFN(+) were analyzed with antibodies directed against RV-N or HIV-1 p24. The results indicate that all viruses infect the cells (Figure 1D, anti-RV-N) and both IFN(−) and IFN(+) RV express HIV-1 Gag (Figure 1D, anti-HIV-1 p24).
Characterization of recombinant viruses growth kinetics
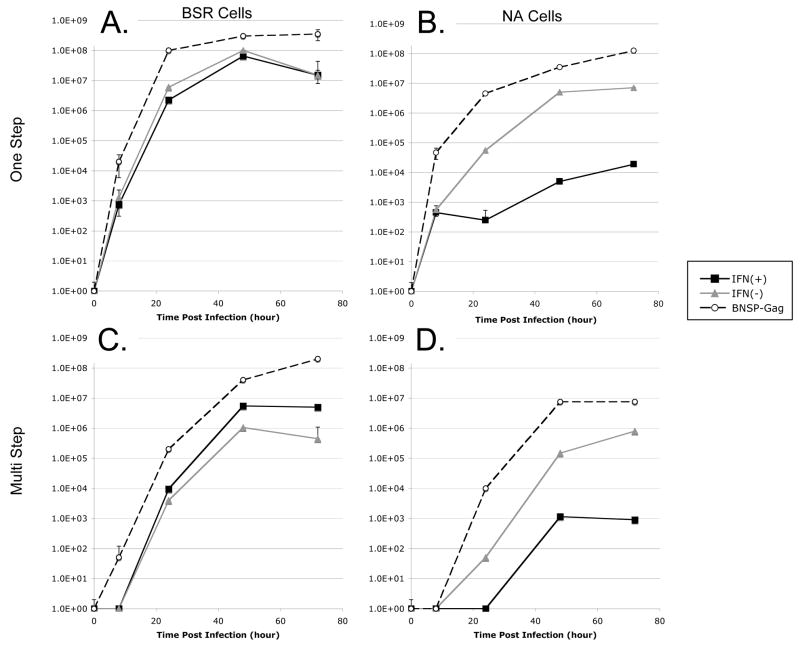
To determine whether the expression of both IFN-β and HIV-1 Gag in the RV genome altered the growth kinetics of RV we analyzed viral growth by multi-step and one-step growth curves. It is well documented that IFN-β is a potent anti-viral; therefore, we analyzed growth kinetics on both type I IFN responsive and non-responsive cells. It was determined that both NA and BSR cells can produce type I IFN following Sendai virus infection. Of the two, however, only NA cells are responsive to mouse IFN-β and, thus, able to inhibit VSV replication (data not shown). Cells were infected with an MOI of 0.1 (multi-step) or 10 (one-step). Aliquots of the supernatants were collected at various time points, and the titers were determined. The multi-step and one-step growth curves derived from infected BSR cells illustrated that IFN(+) and IFN (−) viruses grew with similar kinetics and to relatively equal titers (Figure 2A, C), indicating that the expression of IFN-β did not affect viral growth by a type I IFN independent mechanism. Of note, the titer for both IFN(+) and IFN(−) viruses was about 10-fold less than the titer of the parent virus strain, BNSP-Gag, at all time points presumably due to the insertion of the IFN-β gene between RV G and RV L. However, when IFN-β sensitive cells were used, there was a significant difference in the growth kinetics of IFN(+) and IFN(−) RV. For both one-step and multi-step growth curves there is a delay in viral growth and approximately a 2.5 to 3 log decrease in the final titer of the IFN(+) virus (Figure 2B, D). Thus, IFN-β expressed by RV exhibits antiviral properties when grown on IFN sensitive cells.
FIGURE 2. Growth kinetics of recombinant RV.
(A, B) For the one-step growth curve BSR cells (A) or NA cells (B) were infected at a high MOI of 10 with recombinant RV to determine the effect of IFN-β expression on viral production. (C, D) To generate a multistep growth curve, BSR cells (C) or NA cells (D) were infected with recombinant RV at a low MOI of 0.01; this illustrates the effect of IFN-β expression on viral replication and spread.
Immunogenicity of recombinant RV in Balb/c mice following mucosal immunization
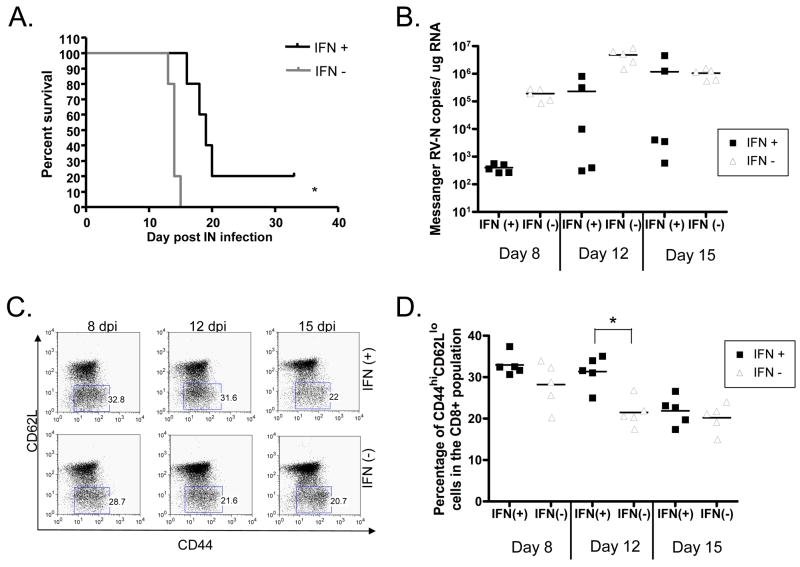
In order to characterize how the increased expression of IFN-β following a RV infection would impact the immunogenicity and pathogenicity of RV vaccine vectors, BALB/c mice were immunized intranasally with 1.5×105 ffu of IFN(+) or IFN(−). We infected the mice intranasally in order to see the effect on pathogenicity, because it has been shown that the first generation vaccine vector, BNSP-Gag, is apathogenic after peripheral inoculation while still lethal after intranasal or intracranial inoculation (McGettigan et al., 2003b). We saw that the survival of mice infected with the IFN(+) virus was significantly greater than the survival of those mice infected with the recombinant RV which did not express IFN-β (Figure 3A). As expected, this suggested that expression of IFN-β results in increased survival of IFN(+) immunized mice.
FIGURE 3. Pathogenicity and immunogenicity of recombinant RV in the primary response to immunization.
Mice were immunized intranasally with 1.5×105 ffu of sucrose purified RV. (A) Survivorship of the mice was monitored by a Kaplan-Meier test for significance. We saw a significant increase in the survival of mice immunized intranasally with IFN(+) as compared to those immunized with IFN(−). (B) Viral messenger RNA recovered from the brains of infected mice was quantified using real time PCR at 8, 12, and 15 days after infection. Significantly more virus was detected in the brains of mice infected with IFN(−) virus as compared to mice infected with the IFN(+) virus. (C and D) Despite lower amounts of virus, mice infected with IFN(+) virus had a significantly greater percentage of activated (CD44hi CD62Llo) CD8+ T cells at 12 dpi. One representative mouse from each group is shown (C) and also the average of 5 mice per group for each time point (D). Horizontal lines indicate the average value and (*) indicate a p value of < 0.002.
We next sought to determine whether the increased survival was due to the antiviral activity or immune-modifying activity of IFN-β. Again, mice were immunized intranasally with IFN(+) and IFN(−), and then the brains and the spleens of infected animals were taken at various times post infection. Viral RNA in the brain was quantified using qPCR specific for RV-N as previously described (Tan et al., 2007). As shown in Figure 3B, there was an average 1000-fold decrease in viral messenger RNA on day 8 and a 100-fold decrease in mRNA on day 12 when mice were infected with the IFN(+) virus as compared to mice infected with the control IFN(−) virus. Of note, the viral genomic RNA on day 8, 12, and 15 was about 10-fold lower than the messenger RNA for each mouse (data not shown). Thus, in vivo IFN-β expression had a similar attenuating effect on viral growth as was seen in tissue culture and efficiently decreased the viral load during the first 12 days of infection.
The splenocytes of the mice from above were analyzed to evaluate the immune-modifying effect of IFN-β on the RV vaccine vectors. Due to the low precursor frequency of antigen-specific cells in a wild type mouse, we looked at the percentage of activated cells (CD44hiCD62Llo) in the CD8+ lymphocyte population. This shows the immune response to the entire vaccine vector and not just to the HIV-1 gag protein. Surprisingly, we saw that, despite a large decrease in viral load in IFN(+) infected animals at 8 dpi, there were similar numbers of activated CD8+ T cells recovered from IFN(+) and IFN(−) infected animals. Furthermore, at 12 dpi, there was a significant increase in the percentage of activated CD8+ splenocytes in the IFN(+) infected animals despite the 100-fold decrease in viral antigen (Figure 3C, D). Thus, although mice infected with the IFN(+) virus probably had a lower amount of viral antigen at 8 and 12 dpi due to the anti-viral effects of IFN-β, these mice had similar (day 8) or significantly greater (day 12) CD8+ T cell responses. In order to determine whether this effect is specific to the route of immunization we also looked for T cell activation in the inguinal lymph nodes and spleens of mice immunized intramuscularly. Consistent with what was seen after intranasal immunization, 8 dpi there was no significant difference between the activated T cells found in the IFN(+) and IFN(−) immunized mice (data not shown). Thus we conclude that during the primary immune response, IFN-β is acting as an adjuvant for the cellular immunity.
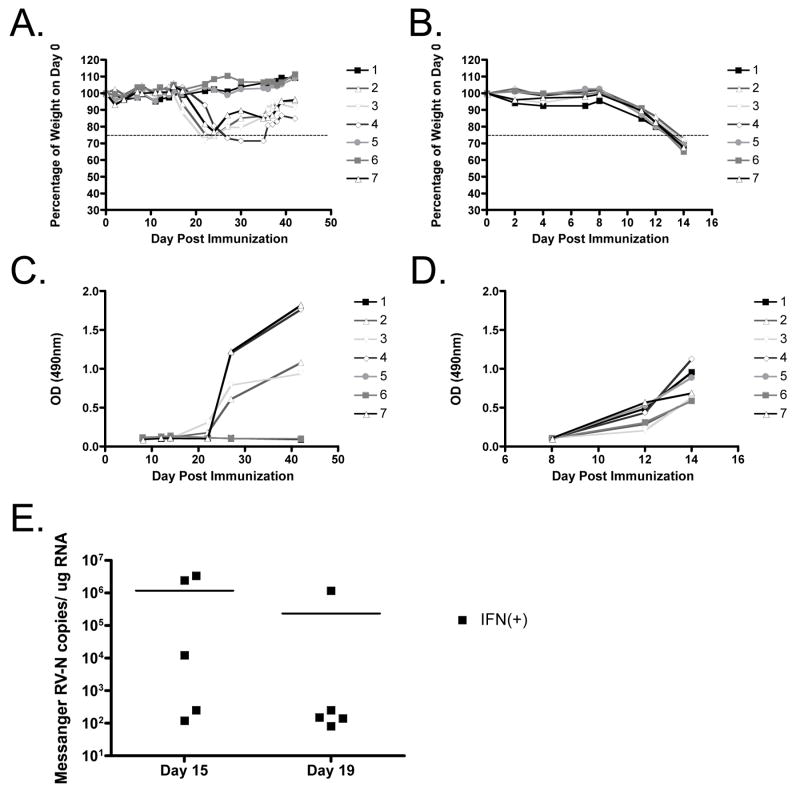
Type I IFN has also been shown to affect antibody development following influenza infection (Heer et al., 2007). To determine the impact that IFN-β expression has on the humoral immune response in our vaccine strategy, mice were bled at various times post infection, and sera was analyzed for RV-G antibodies. It was seen that total IgG levels increased when the mice began to lose weight (Figure 4A-D), and IFN(−) immunized mice had appreciable RV-G IgG levels in their sera at earlier timepoints than IFN(+) immunized mice. Moreover, three out of seven IFN(+) mice never developed RV-induced pathology but also never produced anti-RV antibodies, indicating that the innate immune response via IFN-β can clear RV infection. We concluded from this experiment that humoral responses are determined in large part by viral replication.
FIGURE 4. Vector specific IgG antibody formation.
Mice were immunized intranasally with 1.5×105 ffu of sucrose purified IFN(+) RV (A, C, E) or IFN(−) RV (B, D). The weight fluctuations were monitored over time (A, B) and blood was collected from mice at various times post immunization. (C, D) An ELISA was done to determine the total IgG specific for RV-G on a given day. RV-G specific IgG formation is delayed following immunization with IFN(+). (E) Viral messenger RNA recovered from the brains of IFN(+) infected mice was quantified using real time PCR at 15 and 19 days after infection to determine if the infection persisted. Horizontal lines indicate the average value.
To determine whether the delayed kinetics of antibody production in mice vaccinated with IFN(+) was due to a persistent infection of IFN(+) RV, we quantified the viral messenger RNA at day 15 (when mice first exhibited signs of disease) and at 19 dpi (when mice were at their lowest body weight). We saw that at day 19 the level of viral RNA message was decreasing and most of the mice have around 102 copies of viral mRNA/μg RNA (Figure 4E). Of note, the one mouse that had a high level of viral messenger RNA 19 dpi had the lowest amount of virus at 15dpi, thus it is possible that the kinetics of viral replication in that mouse was highly delayed. Therefore, it seems unlikely that the IFN(+) virus is persisting long term in the mice, but rather the kinetics of viral replication may be delayed by the addition of IFN-β.
Immunogenicity of recombinant RV in IFNAR−/− mice following mucosal immunization
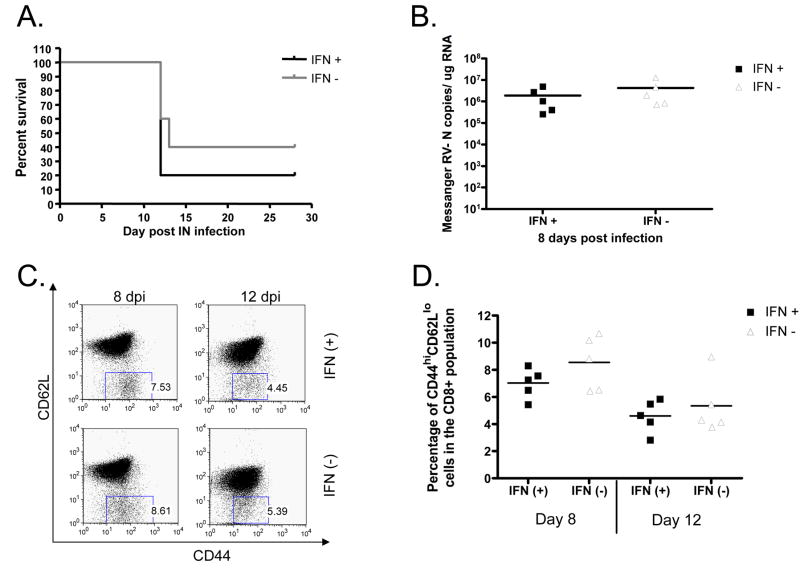
In order to establish that the differences seen in BALB/c mice were dependent on the expression of IFN-β, we repeated the evaluation of viral load and immunogenicity in interferon receptor knockout (IFNAR −/−) mice. Type I IFN signals through a heterodimeric receptor to initiate the JAK/STAT signaling pathway (Randall and Goodbourn, 2008). One of the receptor subunits is knocked out in the IFNAR−/− mice rendering them unable to initiate the IFN-β signaling cascade (Muller et al., 1994). Following immunization, the IFNAR−/− mice receiving either the IFN(+)or the IFN(−) immunization did not show significant differences in survival (Figure 5A). Likewise, 8 dpi there was no significant difference in the amount of viral messenger RNA isolated from the brains of mice that had been immunized with IFN(+) or IFN(−) vaccine vectors (Figure 5B). In addition, the percentage of CD44hiCD62Llo cells in the CD8+ population was equal in mice immunized with IFN(+) or IFN(−) (Figure 5C, D). Taken together, these data suggest that the differences we observed in wild type BALB/c mice immunized with IFN(+) and IFN(−) viruses were due to IFN-α/β signaling.
FIGURE 5. Pathogenicity and immunogenicity of recombinant RV vectors in IFNAR−/− mice.
Mice were immunized intranasally with 1.5×105 ffu of sucrose purified RV. (A) Survivorship of the mice was monitored by a Kaplan-Meier test for significance. We saw no significant difference in the survival of mice immunized with IFN(+) or IFN(−). (B) Viral messenger RNA recovered from the brains of infected mice was quantified using real time PCR 8 days after infection. There was no significant difference in the viral load found in mice immunized with IFN(+) or IFN(−). (C and D) There was no difference in the activation (CD44hi CD62Llo) of CD8+ T cells 8 or 12 dpi. One representative mouse from each group is shown (C) and also the average of 5 mice per group for each time point (D). Horizontal lines indicate the average value.
Increased levels of type I IFN do not increase the activation level of antigen presenting cells, but may promote increased survival of CD8+ T cells following antigen encounter
In order to more fully understand the role of IFN-β in the primary immune response following viral infection, we sought to determine how the expression of IFN-β acts to increase the level of CD8+ T cell activation. Several groups have seen that IFN-β acts on antigen presenting cells, such as macrophages and dendritic cells, to induce the upregulation of co-stimulatory molecules that, in turn, may generate a greater T cell response. Alternately, the IFN-β may act directly on the CD8+ T cell to contribute to their activation.
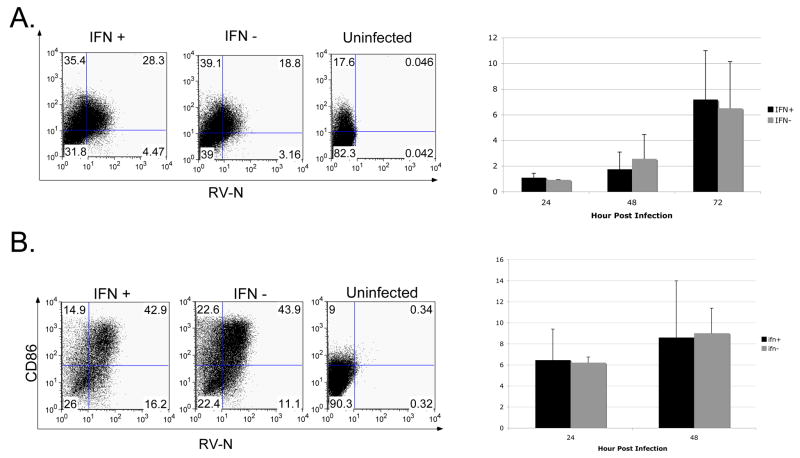
To test the first hypothesis, we infected a macrophage cell line (Raw264.7) or a dendritic (JAWSII) cell line with IFN(+) or IFN(−) viruses. At various hours post infection, we analyzed the activation of the APC by determining the expression of CD80 or CD86. Following infection of macrophage cells with either IFN(+) or IFN(−), there was a dramatic upregulation of CD80 by 48 hpi (Figure 6A). However, there was no significant difference in the fold increase over uninfected cells in CD80 expression when comparing IFN(+) and IFN(−) infected cells at any time point. Similarly, we saw that following infection of JAWSII cells with either IFN(+) or IFN(−) RV equally induced the upregulation of CD86 expression (Figure 6B). In order to more clearly distinguish the effect of IFN-β from the RV infection a supplementary experiment was performed in which the supernatant from IFN(+) or IFN(−) infected JAWSII cells was used to activate uninfected JAWSII cells. After 24 or 48h, there was no significant difference in the activation state, as determined by expression of CD80 and CD86, of JAWSII cells treated with UV-inactivated supernatant from IFN(+) or IFN(−) infected cells compared to cells treated with uninfected supernatant. Thus, it seems that incorporating IFN-β into the RV construct does not significantly increase DC activation. Therefore, it seems unlikely that the difference we observed in CD8+ T cell activation was a result of increased co-stimulation by APCs.
FIGURE 6. The effect of IFN-β on antigen presenting cell activation.
(A) Macrophage cells (Raw264.7) increased expression of CD80 over time following infection at an MOI of 10 with IFN(+) and IFN(−). The left panel shows one representative experiment at 72 hpi. The right panel shows the average fold increase in MFI over the uninfected sample (representative of 3 independent experiments). (B) The expression of CD86 on dendritic cell line JAWSII over time following infection at an MOI of 10 with IFN(+) or IFN(−) RV. The left panel shows one representative experiment at 48 hpi. The right panel shows the average fold increase in MFI over the uninfected sample (2 independent experiments).
IFN-β does not appear to increase maturation of APCs; therefore, we next investigated the impact that IFN-β has on the antigen-specific CD8+ T cell population in our vaccine strategy. To determine the direct effect of IFN-β on T cells, we developed a transfer model in which all of the T cells in the mouse were either wild type (responsive to IFN) or IFNAR−/− (non-responsive to IFN). Briefly, we isolated CD3+ T cells from BALB/c or IFNAR−/− mice and then transferred 3×106 cells intraperitoneally into Nude mice (Figure 7A). Nude mice have a very small number of mature α/β-T cells with limited functionality in the periphery (Kindred, 1979; Kishihara et al., 1987; Yoshikai, Reis, and Mak, 1986).
FIGURE 7. The direct effect of IFN-β on CD8+ T cells.
(A) The experimental design is shown here. Briefly, CD3+ T cells from BALB/c or IFNAR−/− mice were transferred into Nude mice, and at one day post-transfer, mice were immunized intramuscularly with IFN(+). At 12dpi, the percentage of activated (CD44hi CD62Llo) CD8+ T cells (B) and the total cell number of CD4+ and CD8+ cells recovered from the spleen (C) was determined by flow cytometry. Horizontal lines indicate the average value.
In this transfer model, the only T cells able to respond to infection are those we transferred to the mice one day prior to infection with the IFN(+) virus. In analyzing the splenocytes, we saw no difference in the percentage of activated CD8+ T cells in the spleens of BALB/c or IFNAR−/− recipient mice 12 dpi (Figure 7B). However, we did notice that the total number of CD8+ cells recovered from the BALB/c recipient mice tended to be greater than the number recovered from the IFNAR−/− mice (Figure 7C). This trend approached significance when comparing CD8+ T cell number (p= 0.0661), however was not significant when comparing CD4+ T cell number (p=0.3326). We conclude that IFN-β signaling may be important to sustain the population of antigen-specific T cells following infection.
Recall response to HIV-1 Gag
The proof of principle in all vaccine studies is a challenge model. To assess the effect of IFN-β on the development of long-term memory cells, a key requirement for any vaccine, we intramuscularly (i.m.) immunized mice with 106 ffu of IFN(+) or IFN(−). It has been previously shown that the peripheral injection of BNSP based vaccine vectors is not pathogenic in immune competent mice (McGettigan et al., 2003b). Likewise, no clinical signs of rabies were detected following i.m. immunization with the IFN(+) or IFN(−) vectors (data not shown). Immunized BALB/c mice were rested for at least 60 days and then challenged intraperitoneally (i.p) with 106 plaque forming units (pfu) of vaccinia virus expressing HIV-1 Gag (vv-Gag). The HIV-1 Gag specific recall response was analyzed 5 days later by evaluating expression of Gag specific T cell receptors (TCR) with an AMQMLKETI-H2Kd specific tetramer. Additionally, the functionality of the memory cells was determined by intracellular cytokine staining.
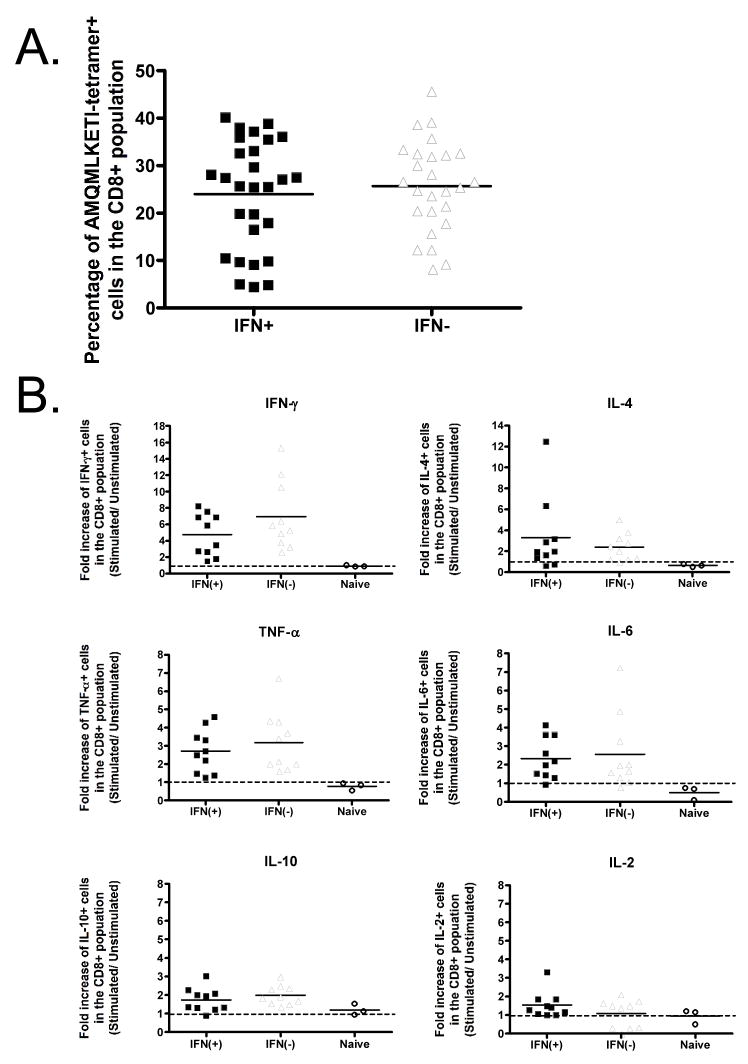
There was no significant difference in the number of tetramer positive CD8+ T cells present following immunization with IFN(+) or IFN(−) RV (Figure 8A). However, the IFN(+) immunized mice seemed to fall into 2 groups: high responders and low responders (percentage of tetramer+ CD8+ cells less than 10%). When considering the functionality the CD8+ T cells, we saw no difference in the induction of cytokines between IFN(+) and IFN(−) immunized animals (Figure 8B). The expression of IFN-γ and IL-4 was induced at the highest level, while very little IL-2 an IL-10 was induced. Taken together, these results indicate that although IFN-β enhances the primary immune response (Figure 3), the adjuvant effect of IFN-β in a viral vaccine vector only induces minimal long-term benefits as reflected by the long-term memory response.
FIGURE 8. The effect of IFN-β on HIV-1 Gag specific recall response following intramuscular immunization with either IFN(+) or IFN(−) vaccine vector.
Mice immunized intramuscularly with either the IFN(+) or IFN(−) virus were rested for approximately 60 days and then challenged with vaccinia expressing HIV-1 Gag (VV-Gag). Splenocytes and ovaries were harvested 5 days post challenge. (A) The percentage of tetramer positive cells in the CD8+ population in mice primed with the IFN(+) or IFN(−) viruses is not significantly different. (B) Splenocytes were stimulated for 16h with HIV-1 Gag peptide AMQMLKETI, and then a panel of cytokines was analyzed. Data is shown as fold increase over the unstimulated sample. Horizontal lines indicate the average value.
DISCUSSION
Here we have analyzed the effect of IFN-β on viral replication, primary CD8+ T cell activation, and memory T cell development in order to assess its use as a molecular adjuvant in vaccine development. We see that IFN-β expression efficiently decreases the viral titer and reduces vector pathogenicity (Figure 2 and 3A). Therefore, IFN-β could be added to RV-based vaccine vectors to reduce vector-associated pathogenicity and to increase safety. Furthermore, the anti-viral activity of IFN-β could potentially lead to a self-limiting infection, making it more appealing for use in immuno-compromised individuals. Increased expression of type I IFN during priming has a significant effect on the cellular immune response. Despite having significantly less viral antigen, there was a significantly greater percentage of activated CD8+ T cells in the spleen at 12 dpi (Figure 3). This highlights the potential strength for using IFN-β as an adjuvant in vaccine development. Although there is a significant effect on the primary immune response, the memory immune response seems to be unaffected by the addition of IFN-β into the RV vaccine vector (Figure 8). We hypothesize that the anti-viral activity of IFN-β during priming counterbalanced the immune-enhancing capability of IFN-β and thus no significant increase on memory cell formation was achieved.
The RV vaccine vector has already been shown to be safe (McGettigan et al., 2003b; McKenna et al., 2007), but here we show that the addition of IFN-β into the genome can work to further attenuate the virus. The recombinant IFN(+) and IFN (−) viruses were attenuated compared to the parental BNSP-Gag as seen by the 10-fold reduction in titer at all time points in the growth curve (Figure 2). Viral replication was significantly decreased in an IFN-β dependent manner, reaffirming the antiviral effects of type I IFN on negative-sense RNA viruses (Randall and Goodbourn, 2008). Both the addition of a second gene into the RV genome and the anti-viral activity of IFN-β attenuated the RV vaccine vector. This attenuation was seen in vivo as an increased survival of IFN(+) immunized mice (Figure 3A).
In addition to attenuating the virus, we saw that IFN-β was able to significantly increase the cellular immune response following immunization. Surprisingly, we saw that at 12 dpi CD8+ T cell activation was significantly greater following infection with the IFN(+) virus, despite the noted 100-fold decrease in viral load. Furthermore, with 1000-fold less virus in the brain at 8 dpi, there was still an equal activated CD8+ T cell response. Both of these facts indicate that IFN-β is a potent adjuvant for cellular immunity. Furthermore, the studies in IFNAR−/− mice indicate that the differences seen here are type I IFN dependent. This data supports the use of IFN-β to enhance the immune response to vaccine vectors that are given even at a low MOI.
In order to further understand the adjuvant properties of IFN-β we sought to determine the mechanism by which IFN-β was increasing the primary CD8+ T cell response following immunization with the IFN(+) virus. In our hands, APC activation does not seem to be affected by increased levels of IFN-β. Infection with RV however is not the only viral infection that induces APC maturation in a type I IFN independent manner. Similarly, DC maturation following Sendai virus infection has been shown to work independently of the IFN pathway (Lopez et al., 2003). Although a strong correlation between DC maturation and type I IFN induction was observed, it was determined using anti-IFN antibodies that the secreted IFN was neither necessary, nor sufficient to induce full DC maturation (Lopez et al., 2003). Furthermore, it was seen that, following adenovirus infection, type I IFN was necessary for DC to undergo full maturation. However, both wildtype and IFNAR−/− mice could mount a strong antigen-specific CD8+ T cell response (Hensley et al., 2005). Therefore, it is not surprising that the differences we saw in CD8+ T cell activation in the presence or absence of exogenous IFN-β were not functions of DC and macrophage activation.
Instead, the immune-enhancing activity of IFN-β in our vaccine strategy seems to directly impact CD8+ T cells. Although our transfer experiment does not definitively prove that IFN-β is needed to sustain a population of antigen-specific CD8+ T cells, the experiment supports this conclusion. Therefore, it is likely that IFN-β signaling through a receptor on a CD8+ T cell is required either as a signal to promote differentiation of effector cells or as a signal to resist activation-induced cell death. It was seen that IFN-α or IL-12 treatment was required during naïve CD8+ cell priming to induce proliferation and cytolytic activity. Beads coated with MHC I-peptide and CD80 co-stimulatory ligand are unable to sufficiently prime naïve CD8+ T cells. However, T cells stimulated by the same beads in the presence of IFN-α/β are capable of cytolytic function and IFN-γ production (Curtsinger et al., 2005). On the other hand, IFN-α/β can also prevent activation-induced cell death of T cells following non-specific activation with staphylococcal enterotoxin B (Marrack, Kappler, and Mitchell, 1999). Our model does not allow us to distinguish between these two possibilities; however, it is in support of a direct role for IFN-β during priming. This piece of evidence is important and should be considered during vaccine development.
Despite the undeniable adjuvant effect induced by IFN-β that we saw during the primary immune response, we did not see a significant increase in antigen-specific cells following vv-Gag challenge months later. However, there was much less antigen at the site of viral replication (the brain) in these mice. It has been suggested that there exists a diverse repertoire of T cells to a single epitope and that antigen load can influence in vitro recall responses (Naumov et al., 2006). Furthermore, the antigen dose can influence the quality of the CD8+ T cell that is induced following infection. In a study done by Alexander-Miller it was seen that higher avidity cells were induced at lower peptide concentrations. However, it was also observed that lower avidity T cells preferentially survived into memory cells (Alexander-Miller, 2000). Therefore, since memory cell differentiation is also impacted by the amount of antigen, the immune-enhancing effects of IFN-β may be counter-balanced by low antigenic loads, thus yielding a minimal change to the overall memory cell development. Thus, IFN-β may be a better adjuvant choice in a DNA or subunit vaccine where the vaccine vector is not deleteriously impacted by the cytokine.
Another consideration to be made is that type I IFN has been reported to have different effects on T cells in various activation states. The result of IFN-α/β signaling in an activated T cells is different then the response in a naïve T cell. Naïve human CD4+ T cells that were pre-treated with IFN-a had a delayed entry into the cell cycle. On the other hand, the presence of IFN-a following non-specific stimulation of human CD4+ T cells with anti-CD3/anti-CD28 antibodies did not inhibit T cell proliferation (Dondi et al., 2003). This study did not however consider how type I IFN effects CD8+ T cells or memory precursor T cells. Thus, despite the positive impact that IFN-β had on effector cells during priming, little can be concluded as to whether or not IFN-β had the same effect on memory precursor cells.
MATERIALS AND METHODS
Plasmid construction
The gene encoding mouse IFN-β, pORF-mIFNB (Invitrogen), was used as a template and was amplified by PCR using Vent polymerase (New England Biolabs Inc.) and the primers 5′-TTTCGTACGATCATGAACAACAGGTGGATCCTC-3′ and 5′-AAAGCTAGCTC AGTTTTGGAAGTTTCTGGTAAG-3′ containing the BsiWI or NheI restriction sites (underlined). The resulting PCR fragment was gel purified and digested with BsiWI and NheI. The digestion products were ligated into a plasmid encoding recombinant RV vaccine vector, pSPBN (McGettigan et al., 2003b), which had been previously digested with BsiWI and NheI. The resulting plasmid was designated pSPBN-IFN(+). Alternatively, mouse IFN-β was amplified by PCR using the primers 5′-TTTCGTACGATCAACAACAGGTGGATCC TCCAC-3′ and 5′-AAAGCTAGCTCAGTTTTGGAAGTTT CTGGTAAG-3′ containing the BsiWI or NheI restriction sites (underlined) and lacking the ATG start codon. The PCR fragment was gel purified, BsiWI and NheI digested, and ligated into the BsiWI/NheI- digested pSPBN. The resulting plasmid was designated pSPBN-IFN(−). The plasmid encoding a recombinant RV vaccine vector expressing HIV-1 Gag (pBNSP-Gag) has been described previously (McGettigan et al., 2001). The pSPBN-IFN(+) and pSPBN-IFN(−) plasmids were digested with Pml I and Nco I. The digestion fragment containing either the IFN(+) or IFN(−) gene was ligated into pBNSP-Gag previously digested with Pml I and Nco I. The generated plasmids were designated IFN(+) and IFN (−), respectively. The introduced sequence for each plasmid was confirmed by sequencing.
Recovery of recombinant viruses
Recombinant RV virions were recovered by a system described previously (Tan et al., 2007). Briefly, FuGENE 6 Transfection Reagent (Roche Diagnostics) was used to co-transfect 6 plasmids into BSR cells (a BHK-21 cell clone) in 6-well plates. The concentration of plasmids (per plate) were as follows: 2.5ug recombinant RV cDNA, 1.25ug RV N, 0.75 ug T7 polymerase, 0.63ug RV P and RV L, and 0.5ug RV G. The plasmids encoding the recombinant RV cDNA and RV N, P, G, and L were under the control of the T7 promoter (Finke, Mueller-Waldeck, and Conzelmann, 2003); the plasmid encoding the T7 polymerase was under the control of the chicken actin promoter with an enhancer from the CMV immediate early gene. 3–7 days after transfection the supernatant was collected from each well and BSR cells were stained with a fluorescein isothiocynate (FITC)- conjugated anti-RV N antibody (Centacor, Inc.) to identify wells with infectious virus. Supernatant from RV-positive wells was used to infect a T25 flask of BSR cells and increase the viral yield.
Immunostaining
BSR cells were infected at a multiplicity of infection (MOI) of 0.01 with either BNSP, IFN(+) or IFN(−) for 48 hours (h). The media was then aspirated, and the cells were fixed with 80% acetone for 30 minutes (min) at 4°C. Cells were washed with PBS and stained with a human monoclonal antibody for HIV-1 p24 (NIH AIDS Research & Reference Reagent Program, antibody 71–31) for 60 min at RT. Cells were washed with PBS and then stained with the secondary antibody Cy2-conjugated donkey anti-human IgG (Jackson Immunoresearch) at a final concentration of 5 μg/ml. Alternatively, infected cells were fixed with 80% acetone and stained with FITC-conjugated anti RV-N (Centacor, Inc) for 1 h at 37°C. Stained cells were washed with PBS and fluorescence was observed under a UV microscope.
Inhibition of vesicular stomatitis virus (VSV) replication by type I IFN
BSR cells were infected at an MOI of 0.1 with either BNSP, IFN(+) or IFN(−) for 72 h or left uninfected. Supernatant was collected, spun at 1,600 rpm for 10 min to remove any cellular debris, and then UV-inactivated (115V, 60Hz.16A) for 40 min. UV-inactivated viral supernatant from BNSP, IFN(+) or IFN(−) infected cells was diluted in RPMI-1640 1:10 or 1:1000 and added to neuroblastoma (NA) cells. Following the 24 h pre-treatment, NA cells were infected with VSV-GFP at an MOI of 5 for 5 h. VSV replication was determined by fluorescence under a UV light source.
One-step and multi-step growth curves
BSR and NA cells were infected with BNSP-Gag, IFN(+) or IFN(−) in serum-free media at a MOI of 10 (for the one-step growth curve) or of 0.01 (for the multi-step growth curve). Following 90 min incubation at 37°C, the virus was aspirated, and cells were washed twice with PBS to remove any virus that had not yet infected the cells. Serum-containing media was then added to the cells, and, at indicated time points, 0.3ml of supernatant was removed and stored at 4°C. The aliquots were titered in duplicate on BSR cells.
Mice
6–8 week old BALB/c mice or IFNAR−/− mice on the BALB/c background (Martinez-Sobrido et al., 2006) were maintained at the Thomas Jefferson University Animal Facilities. Mice were infected either intramuscularly or intranasally with sucrose purified IFN(+) or IFN(−) virus. The weight of the mice was monitored daily, and the animals were euthanized after losing 25% of their body weight and/or showing severe clinical signs of rabies. Alternately, immunized mice were euthanized at 8, 12, or 15 days post infection (dpi) to analyze viral loads and parameters of the immune response. Serum, brain, and spleen were harvested at each time point for further analysis.
Mice in the recall response experiments were rested at least 60 days after intramuscular immunization with IFN(+) or IFN(−) and then were challenged intraperitoneally (I.P.) with 106 pfu of recombinant vaccinia-expressing HIV-1 Gag. Mice were euthanized 5 days after challenge, and their spleens were harvested for further analysis.
For the transfer experiments CD3+ cells were isolated from the spleens of BALB/c or IFNAR−/− mice by a Dynal T cell negative isolation kit (Invitrogen) according to the manufacturers protocol. The purity of CD3+ cells was assessed by flow cytometry and determined to be >90%. 3×106 CD3+ cells were transferred into Nude mice I.P. One day after transfer, mice were infected with 106 ffu IFN(+) intramuscularly. Mice were euthanized 12 days after immunization, and their spleens were harvested for further analysis.
IFN-β and RV-G enzyme-linked immunosorbent assays (ELISA)
For the IFN-β ELISA, BSR cells were infected with IFN(+) or IFN(−) at an MOI of 0.5 or left uninfected for 48 h. Supernatant was collected and spun at 1,600 rpm to remove any cellular debris. Viral supernatant was diluted 1:1000, 1:2000, 1:4000, 1:8000, or 1:16,000 and the amount of IFN-β in each sample was quantified using a mouse IFN-β ELISA kit (PBL Biomedical Laboratories) according to the manufacturer’s protocol. RV-G ELISA was done as described previously (McGettigan et al., 2001). Briefly, Maxisorb plates (Nunc) were coated with 200ng/ml purified RV-G protein diluted in Na2CO3 at 4°C O/N. Plates were washed with 0.1%Tween PBS and blocked with 5% milk/PBS for 30 min at RT. Serum collected from RV-infected mice at various time points post infection was diluted in PBS and added to the plate in duplicate. Following a 1 h incubation at RT, the plate was washed with 0.1% Tween PBS. Goat anti-mouse IgG conjugated-HRP (Southern Biotech) was added at a final concentration of 200 ng/ml, and plates were incubated at 37°C for 30min. Plates were washed with 0.1% Tween PBS and developed with o-phenylenediamine dihydrochloride fast tablet peroxidase substrate (Sigma) for 20 min. The colorimetric reaction was stopped with 3M H2SO4, and the absorbance for each well was determined at 490nm.
Quantitative real-time PCR
Virus load in the brains of infected mice was determined by TaqMan probe-based real-time PCR as described previously (Tan et al., 2007) with the following modification: For genomic RNA, samples were standardized with the equation (y = −3.2211x + 38.394, R2 = 0.9999), and for RV messenger RNA samples were standardized with the equation (y = −3.1406x + 36.764, R2= 0.9996). The copy number was normalized to 1μg/μl total RNA.
Flow cytometry
For surface staining of splenocytes, spleens from infected mice were homogenized, and a single-cell suspension was prepared. Erythrocytes were removed with ACK lysing buffer (Sigma Inc), and splenocytes were counted by trypan blue exclusion. Cells were washed in FACS buffer (2% BSA/PBS) and blocked on ice for 1 h with 2μl rat anti-mouse CD16/CD32 (Fc block) (BD Biosciences Pharmigen) and 3.3μl unconjugated streptavidin in 100μl FACS. Cells were washed in FACS buffer and then stained with fluorescent antibodies for 30 min at RT. After staining, cells were washed with FACS buffer and fixed with Cytofix (BD Biosciences) for 16–18 hours at 4°C.
For internal staining of splenocytes, samples were stimulated with 10μg/ml of AMQMLKETI peptide or left unstimulated for 16 h. GolgiBlock (BD Bioscience) was added for 6 h and then cells were stained as above for any surface molecules. Following fixation, cells were washed twice in Perm/Wash Buffer (BD Bioscience). They were then stained for 30 min at room temperature for internal molecules.
For staining of the APC cell lines, at various time points post infection (MOI=10) cells were collected and blocked on ice for 30 min with 2μl Fc block (BD Biosciences Pharmigen) in 100μl FACS. Cells were washed twice in Perm/Wash Buffer (BD Bioscience) and then stained with fluorescent antibodies for 30 min at RT. After staining, cells were washed with FACS buffer and fixed with Cytofix (BD Biosciences) for 16–18 hours at 4°C
Antibodies used include
PE-AMQMLKETI tetramer (Becton Dickinson), FITC-CD44, PerCP-CD8α, APC-CD62L, FITC-CD4, PE-CD80, PE-CD86, FITC-IFN-γ, APC-TNF-α, APC-IL-2, PE-IL-4, PE-IL-6, FITC-IL-10 (BD Biosciences Pharmingen), and RV-N (Centacor, Inc). All samples were analyzed on BD FACS Calibur 100,000–150,000 events were counted for splenocyte samples and 50,000 events were counted for APC cell lines.
Acknowledgments
We thank John Hiscott (Montreal, Canada) for providing the recombinant VSV – expressing GFP, Joan Durbin (Columbus, Ohio) for providing the IFNAR −/− mice and the AIDS Research and Reference Reagent Program (ARRRP), Division of AIDS, NIAID, NIH for providing the recombinant vaccinia viruses expressing HIV-1 Gag protein.
This study was supported by NIH/NIAID R01 A1049153 to M.J.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander-Miller MA. Differential expansion and survival of high and low avidity cytotoxic T cell populations during the immune response to a viral infection. Cell Immunol. 2000;201(1):58–62. doi: 10.1006/cimm.1999.1632. [DOI] [PubMed] [Google Scholar]
- Brzozka K, Finke S, Conzelmann KK. Identification of the rabies virus alpha/beta interferon antagonist: phosphoprotein P interferes with phosphorylation of interferon regulatory factor 3. J Virol. 2005;79(12):7673–81. doi: 10.1128/JVI.79.12.7673-7681.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzozka K, Finke S, Conzelmann KK. Inhibition of interferon signaling by rabies virus phosphoprotein P: activation-dependent binding of STAT1 and STAT2. J Virol. 2006;80(6):2675–83. doi: 10.1128/JVI.80.6.2675-2683.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174(8):4465–9. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- Dietzschold B, Faber M, Schnell MJ. New approaches to the prevention and eradication of rabies. Expert Rev Vaccines. 2003;2(3):399–406. doi: 10.1586/14760584.2.3.399. [DOI] [PubMed] [Google Scholar]
- Dondi E, Rogge L, Lutfalla G, Uze G, Pellegrini S. Down-modulation of responses to type I IFN upon T cell activation. J Immunol. 2003;170(2):749–56. doi: 10.4049/jimmunol.170.2.749. [DOI] [PubMed] [Google Scholar]
- Faber M, Lamirande EW, Roberts A, Rice AB, Koprowski H, Dietzschold B, Schnell MJ. A single immunization with a rhabdovirus-based vector expressing severe acute respiratory syndrome coronavirus (SARS-CoV) S protein results in the production of high levels of SARS-CoV-neutralizing antibodies. J Gen Virol. 2005;86(Pt 5):1435–40. doi: 10.1099/vir.0.80844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finke S, Mueller-Waldeck R, Conzelmann KK. Rabies virus matrix protein regulates the balance of virus transcription and replication. J Gen Virol. 2003;84(Pt 6):1613–21. doi: 10.1099/vir.0.19128-0. [DOI] [PubMed] [Google Scholar]
- Heer AK, Shamshiev A, Donda A, Uematsu S, Akira S, Kopf M, Marsland BJ. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J Immunol. 2007;178(4):2182–91. doi: 10.4049/jimmunol.178.4.2182. [DOI] [PubMed] [Google Scholar]
- Hensley SE, Giles-Davis W, McCoy KC, Weninger W, Ertl HC. Dendritic cell maturation, but not CD8+ T cell induction, is dependent on type I IFN signaling during vaccination with adenovirus vectors. J Immunol. 2005;175(9):6032–41. doi: 10.4049/jimmunol.175.9.6032. [DOI] [PubMed] [Google Scholar]
- Kindred B. Nude mice in immunology. Prog Allergy. 1979;26:137–238. [PubMed] [Google Scholar]
- Kishihara K, Yoshikai Y, Matsuzaki G, Mak TW, Nomoto K. Functional alpha and beta T cell chain receptor messages can be detected in old but not in young athymic mice. Eur J Immunol. 1987;17(4):477–82. doi: 10.1002/eji.1830170407. [DOI] [PubMed] [Google Scholar]
- Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202(5):637–50. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letvin NL. Progress and obstacles in the development of an AIDS vaccine. Nat Rev Immunol. 2006;6(12):930–9. doi: 10.1038/nri1959. [DOI] [PubMed] [Google Scholar]
- Lopez CB, Garcia-Sastre A, Williams BR, Moran TM. Type I interferon induction pathway, but not released interferon, participates in the maturation of dendritic cells induced by negative-strand RNA viruses. J Infect Dis. 2003;187(7):1126–36. doi: 10.1086/368381. [DOI] [PubMed] [Google Scholar]
- Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189(3):521–30. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Sobrido L, Gitiban N, Fernandez-Sesma A, Cros J, Mertz SE, Jewell NA, Hammond S, Flano E, Durbin RK, Garcia-Sastre A, Durbin JE. Protection against respiratory syncytial virus by a recombinant Newcastle disease virus vector. J Virol. 2006;80(3):1130–9. doi: 10.1128/JVI.80.3.1130-1139.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGettigan JP, Koser ML, McKenna PM, Smith ME, Marvin JM, Eisenlohr LC, Dietzschold B, Schnell MJ. Enhanced humoral HIV-1-specific immune responses generated from recombinant rhabdoviral-based vaccine vectors co-expressing HIV-1 proteins and IL-2. Virology. 2006;344(2):363–77. doi: 10.1016/j.virol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- McGettigan JP, Naper K, Orenstein J, Koser M, McKenna PM, Schnell MJ. Functional human immunodeficiency virus type 1 (HIV-1) Gag-Pol or HIV-1 Gag-Pol and env expressed from a single rhabdovirus-based vaccine vector genome. J Virol. 2003a;77(20):10889–99. doi: 10.1128/JVI.77.20.10889-10899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGettigan JP, Pomerantz RJ, Siler CA, McKenna PM, Foley HD, Dietzschold B, Schnell MJ. Second-generation rabies virus-based vaccine vectors expressing human immunodeficiency virus type 1 gag have greatly reduced pathogenicity but are highly immunogenic. J Virol. 2003b;77(1):237–44. doi: 10.1128/JVI.77.1.237-244.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGettigan JP, Sarma S, Orenstein JM, Pomerantz RJ, Schnell MJ. Expression and immunogenicity of human immunodeficiency virus type 1 Gag expressed by a replication-competent rhabdovirus-based vaccine vector. J Virol. 2001;75(18):8724–32. doi: 10.1128/JVI.75.18.8724-8732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna PM, Koser ML, Carlson KR, Montefiori DC, Letvin NL, Papaneri AB, Pomerantz RJ, Dietzschold B, Silvera P, McGettigan JP, Schnell MJ. Highly attenuated rabies virus-based vaccine vectors expressing simian-human immunodeficiency virus89.6P Env and simian immunodeficiency virusmac239 Gag are safe in rhesus macaques and protect from an AIDS-like disease. J Infect Dis. 2007;195(7):980–8. doi: 10.1086/512243. [DOI] [PubMed] [Google Scholar]
- McKenna PM, Pomerantz RJ, Dietzschold B, McGettigan JP, Schnell MJ. Covalently linked human immunodeficiency virus type 1 gp120/gp41 is stably anchored in rhabdovirus particles and exposes critical neutralizing epitopes. J Virol. 2003;77(23):12782–94. doi: 10.1128/JVI.77.23.12782-12794.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebatsion T, Konig M, Conzelmann KK. Budding of rabies virus particles in the absence of the spike glycoprotein. Cell. 1996;84:941–951. doi: 10.1016/s0092-8674(00)81072-7. [DOI] [PubMed] [Google Scholar]
- Mebatsion T, Weiland F, Conzelmann KK. Matrix protein of rabies virus is responsible for the assembly and budding of bullet-shaped particles and interacts with the transmembrane spike glycoprotein G. Journal of Virology. 1999;73(1):242–50. doi: 10.1128/jvi.73.1.242-250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya M, Edwards MJ, Reid DM, Borrow P. Rapid activation of spleen dendritic cell subsets following lymphocytic choriomeningitis virus infection of mice: analysis of the involvement of type 1 IFN. J Immunol. 2005;174(4):1851–61. doi: 10.4049/jimmunol.174.4.1851. [DOI] [PubMed] [Google Scholar]
- Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264(5167):1918–21. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- Naumov YN, Naumova EN, Clute SC, Watkin LB, Kota K, Gorski J, Selin LK. Complex T cell memory repertoires participate in recall responses at extremes of antigenic load. J Immunol. 2006;177(3):2006–14. doi: 10.4049/jimmunol.177.3.2006. [DOI] [PubMed] [Google Scholar]
- Parrino J, Graham BS. Smallpox vaccines: Past, present, and future. J Allergy Clin Immunol. 2006;118(6):1320–6. doi: 10.1016/j.jaci.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollara G, Jones M, Handley ME, Rajpopat M, Kwan A, Coffin RS, Foster G, Chain B, Katz DR. Herpes simplex virus type-1-induced activation of myeloid dendritic cells: the roles of virus cell interaction and paracrine type I IFN secretion. J Immunol. 2004;173(6):4108–19. doi: 10.4049/jimmunol.173.6.4108. [DOI] [PubMed] [Google Scholar]
- Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89(Pt 1):1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- Santodonato L, D’Agostino G, Nisini R, Mariotti S, Monque DM, Spada M, Lattanzi L, Perrone MP, Andreotti M, Belardelli F, Ferrantini M. Monocyte-derived dendritic cells generated after a short-term culture with IFN-alpha and granulocyte-macrophage colony-stimulating factor stimulate a potent Epstein-Barr virus-specific CD8+ T cell response. J Immunol. 2003;170(10):5195–202. doi: 10.4049/jimmunol.170.10.5195. [DOI] [PubMed] [Google Scholar]
- Siler CA, McGettigan JP, Dietzschold B, Herrine SK, Dubuisson J, Pomerantz RJ, Schnell MJ. Live and killed rhabdovirus-based vectors as potential hepatitis C vaccines. Virology. 2002;292(1):24–34. doi: 10.1006/viro.2001.1212. [DOI] [PubMed] [Google Scholar]
- Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, Knowles S, Marius R, Reynard J, Poliquin L, Atkins H, Brown EG, Durbin RK, Durbin JE, Hiscott J, Bell JC. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4(4):263–75. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- Tan GS, Preuss MA, Williams JC, Schnell MJ. The dynein light chain 8 binding motif of rabies virus phosphoprotein promotes efficient viral transcription. Proc Natl Acad Sci U S A. 2007;104(17):7229–34. doi: 10.1073/pnas.0701397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier MD, Jr, Palian BM, Reiss CS. VSV replication in neurons is inhibited by type I IFN at multiple stages of infection. Virology. 2005;333(2):215–25. doi: 10.1016/j.virol.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Vidy A, Chelbi-Alix M, Blondel D. Rabies virus P protein interacts with STAT1 and inhibits interferon signal transduction pathways. J Virol. 2005;79(22):14411–20. doi: 10.1128/JVI.79.22.14411-14420.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidy A, El Bougrini J, Chelbi-Alix MK, Blondel D. The nucleocytoplasmic rabies virus P protein counteracts interferon signaling by inhibiting both nuclear accumulation and DNA binding of STAT1. J Virol. 2007;81(8):4255–63. doi: 10.1128/JVI.01930-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenner CA, Guler ML, Macatonia SE, O’Garra A, Murphy KM. Roles of IFN-gamma and IFN-alpha in IL-12-induced T helper cell-1 development. J Immunol. 1996;156(4):1442–7. [PubMed] [Google Scholar]
- Yoshikai Y, Reis MD, Mak TW. Athymic mice express a high level of functional gamma-chain but greatly reduced levels of alpha- and beta-chain T-cell receptor messages. Nature. 1986;324(6096):482–5. doi: 10.1038/324482a0. [DOI] [PubMed] [Google Scholar]