Abstract
We examined the effect of early exercise training (Ex) on glucose kinetics, basal, and insulin-stimulated skeletal muscle (SKM) plasma membrane (PM) GLUT4 in pre- and/or postnatal nutrient-restricted adult rat offspring compared with sedentary (Sed) state. Pregestational control female (Ex CON vs. Sed CON) and offspring exposed to prenatal (Ex IUGR vs. Sed IUGR), postnatal (Ex PNGR vs. Sed PNGR), or pre- and postnatal (Ex IUGR + PNGR vs. Sed IUGR + PNGR) nutrient restriction were studied. The combined effect of exercise and pre/postnatal nutrition in the Ex IUGR demonstrated positive effects on basal and glucose-stimulated plasma insulin response (GSIR) with suppression of endogenous hepatic glucose production (HGP) compared with sedentary state. Ex PNGR was hyperglycemic after glucose challenge with no change in glucose-stimulated insulin production or HGP compared with sedentary state. Ex IUGR + PNGR remained glucose tolerant with unchanged glucose-stimulated insulin production but increased endogenous HGP compared with sedentary state. Basal SKM PM-associated GLUT4 was unchanged by exercise in all four groups. Whereas Ex PNGR and Ex IUGR + PNGR insulin responsiveness was similar to that of Ex CON, Ex IUGR remained nonresponsive to insulin. Early introduction of regular Ex in the pregestational female offspring had a positive effect on hepatic adaptation to GSIR and HGP in IUGR and IUGR + PNGR, with no effect in PNGR. Change in insulin responsiveness of SKM GLUT4 translocation was observed in exercised IUGR + PNGR and PNGR but not in exercised IUGR.
Keywords: glucose tolerance test, metabolic programming, glucose transporter 4, insulin-responsive glucose transporter 4 translocation
restriction of early growth prenatally or postnatally is linked to adult-onset insulin resistance, visceral adiposity, and type 2 diabetes mellitus (T2DM) (1–4). Animal models consisting of fetal and/or postnatal malnutrition as either global (19, 36, 41) or selective nutrient restriction (17) causing intrauterine growth restriction (IUGR) and/or postnatal growth restriction (PNGR) predispose the adult offspring to selective tissue-specific insulin resistance (17, 19, 36). Prenatal nutrient restriction with IUGR enhances glucose-induced insulin response (19) and causes hyperinsulinemia (17, 19), hepatic insulin resistance per hyperglycemic euglycemic clamp experiments (22), and insulin resistance of skeletal muscle (SKM) (32, 41) and adipose tissue (17) GLUT4 translocation. In contrast, postnatal nutrient restriction (PNGR) programs adult females toward unsuppressed hepatic glucose production, hypoinsulinemia with a lean body habitat, and relative hepatic insulin resistance (19) but partially retained insulin sensitivity of SKM GLUT4 translocation (41). The glucose metabolic adaptations and this insulin-resistant phenotype in the IUGR (19, 22) are transgenerationally inherited and amplified in the next generation (7, 9, 39).
Acute exercise or endurance training lowers circulating insulin concentrations and increases counterregulatory hormones. Exercise overcomes glucose intolerance by attenuating hepatic glucose production via suppression of glycogenolysis (13) and enhancing SKM glucose utilization (5, 11). Exercise improves insulin sensitivity by augmenting insulin signaling and non-insulin-mediated alternative signaling mechanisms (37). Exercise and muscle contraction overcome insulin resistance by increasing adenosine 5′-monophosphate kinase (AMPK) enzyme activity that in turn increases cellular glucose uptake. This occurs by AMPK-mediated SKM GLUT4 translocation to the plasma membrane (PM), thereby circumventing the insulin resistance of GLUT4 translocation (20).
On the basis of this information, we hypothesized that early introduction of submaximal exercise training (ET) will ameliorate certain forerunning features of selective insulin resistance in the adult IUGR and/or PNGR pregestational female offspring. To test this hypothesis, we subjected postweaned IUGR or PNGR female rats to a moderate exercise regimen for ∼6 wk and determined its effect on hepatic glucose kinetics and SKM subcellular GLUT4 protein distribution.
RESEARCH DESIGN AND METHODS
Animals
Sprague-Dawley rats (Charles River Laboratories, Hollister, CA) were housed in individual cages, exposed to 12:12-h light-dark cycles at 21–23°C, and allowed ad libitum access to standard rat chow (composition carbohydrate 63.9%, fat 4.5%, and protein 14.5%). The National Institutes of Health guidelines were followed as approved by the Animal Research Committee of the University of California Los Angeles.
Maternal Nutrient Restriction Model
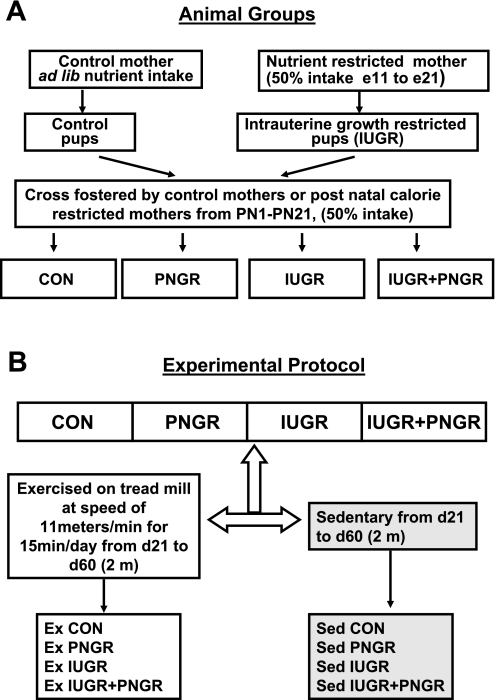
Pregnant rats received 50% of their daily food intake (11 g/day) beginning from day 11 through day 21 of gestation, which constitutes mid- to late gestation, compared with their control (CON) counterparts that received ad libitum access to rat chow (22 g/day). Both groups had ad libitum access to drinking water. At birth, the litter size was culled to six to ensure no interlitter nutritional variability. Postnatally, the cross-fostering of animals generated four experimental groups, as described previously by us (19). Briefly, the newborn pups born to ad libitum feeding CON mothers were reared either by mothers on seminutrient restriction from PN1–PN21 (PNGR) or by CON mothers (Fig. 1A). The intrauterine semi-nutrient-restricted progeny was fed either by CON mothers with ad libitum access to nutrients representing intrauterine nutrient restriction (IUGR) alone or by semi-nutrient-restricted mothers representing a combination of intrauterine and postnatal nutrient restriction (IUGR + PNGR) (Fig. 1A). After being weaned from the mother, all animals had ad libitum access to food and water.
Fig. 1.
A: study design demonstrating the control (CON) group and nutrient restriction achieved by cross-fostering postnatal rat pups. Nutrient-restricted mothers received 50% of daily nutrient intake from mid- to late pregnancy [embryonic days 11 (e11) to 21 (e21)] through lactation [postnatal days 1 (PN1) to 21 (PN21)]. B: experimental protocol for exercise (Ex) in 4 groups is shown. The CON and nutrient-restricted groups of animals either underwent supervised exercise or remained sedentary (Sed) from PN day 21 (d21) to PN day 60 (d60) (2 mo). Ad lib, ad libitum; IUGR, intrauterine growth restricted; PNGR, postnatal growth restricted.
Moderate ET
Postweaned animals in all four groups underwent daily supervised ET, whereas another set of postweaned animals in each group were maintained under sedentary conditions from PN21 to PN60 (∼2 mo). The ET comprised of running on a motorized treadmill at a speed of 11 m/min for 15 min/day, spanning 5 days/wk and lasting for a total of 6 wk (Fig. 1B). This moderate exercise regimen was devised to accommodate the perinatally energy-restricted animal groups in our study.
Surgical Catheter Placement
The adult females were anesthetized using an anesthetic cocktail of ketamine HCl (50 mg/kg) and xylazine (4.8 mg/kg) by intraperitoneal injection (19). Jugular vein catheters were inserted aseptically and maintained patent with heparinized saline, as described previously (19). All animals were allowed full recovery from the surgical procedure prior to glucose tolerance tests (GTTs) being conducted.
Intravenous GTT
All tests were performed in the resting state 48–72 h after ET was completed following an overnight fast. The awake animals received 1 g/kg body wt of the 1:1 mixture of [2-2H]- and [6,6-2H2]glucose via the surgically placed jugular vein catheters. Blood (500 μl) was obtained at 0, 5, 15, 30, 60, and 120 min for assessment of glucose and insulin concentrations and isotopomer enrichment (19).
Insulin Tolerance Test
Awake adult females received 0.75 U/kg of human insulin via the jugular venous catheter, and blood was obtained at 0, 15, 30, and 60 min subsequently to measure glucose concentration (41).
Plasma Assays
Plasma was separated and aliquots stored for measurement of glucose by the glucose oxidase method (sensitivity = 0.1 mM; Sigma Diagnostics, St. Louis, MO). Insulin was quantified by enzyme-linked immunoabsorbent assays using rat standards and anti-rat insulin antibody (sensitivity: insulin = 0.2 ng/ml; Linco Research, St. Charles, MO). Homeostasis model of insulin resistance (HOMA-IR) was calculated on the basis of these measured values.
Gas Chromatography-Mass Spectrometry Analysis
Glucose was analyzed by modified gas chromatography-mass spectrometry method, as described previously (19, 43, 44). All isotopomeric determinations were performed using a Hewlett-Packard gas chromatograph (model 6890) connected to a mass selective detector (model 5973A) (Hewlett-Packard, Palo Alto, CA). Electron impact ionization was used to characterize glucose positional isotopomers of [6,6-2H2]glucose at mass-to-charge ratio (m/z) of 187 for C3–C6 of [2-2H]glucose at m/z of 242 for C1–C4 fragments (19, 44).
Analysis and Interpretation of Glucose Tolerance Test
Mass isotopomer distribution was determined using the method of Lee et al. (27). Briefly, disappearance of the two isotopes [2-2H]- and [6,6-2H2]glucose was determined for the M1 label that represented [2-2H]glucose and the M2 label that represented the [6,6-2H2]glucose (19, 43, 44). The difference between disappearance rates of M1 and M2 was used as a measurement of futile cycling (i.e., glucose to glucose 6-phosphate and back) (19, 27).
SKM AMPK Studies
To validate our exercise regimen, SKM AMPK enzyme activity was initially measured after an acute 15-min bout of similar treadmill training in control animals and compared with their respective sedentary controls. To guard against detecting remnant effects from the last 15-min bout of acute exercise that can potentially contaminate the chronic effects of exercise lasting over 6 wk duration, we measured SKM total AMPK, phosphorylated AMPK (pAMPK) (32), and AMPK enzyme activity (28, 42) in all four exercise (Ex) groups 48–72 h after their last 15-min bout of exercise and compared them with their respective Sed groups.
Western Blot Analysis
The animals were deeply anesthetized with inhalational isoflurane to maintain organ blood flow, and SKM was rapidly separated from surrounding tissues, quickly snap-frozen in liquid nitrogen, and stored at −70°C. Fifty micrograms of prepared SKM homogenates were separated on SDS-PAGE and subjected to Western blot analysis, as described previously (32). The primary antibodies consisted of one raised against total AMPK (1:1,000 dilution, H-300; Santa Cruz Biotechnology, Santa Cruz, CA) or pAMPK (1:1,000 dilution; Cell Signaling Technology, Boston, MA) both collectively comprised of AMPKα1 and -α2 isoforms. Protein bands were visualized by using the enhanced chemiluminescence method (Amersham Biosciences, Piscataway, NJ). The quantification of protein bands was performed by densitometry using the Scion Image software (32).
AMPK Enzyme Activity
Five milligrams of skeletal muscle homogenate supernatants were incubated overnight at 4°C with protein A/G Sepharose beads prebound for 2 h at 4°C (32) at a 1:500 dilution to antibodies raised against either total AMPK (containing α1 and α2 isoforms, H-300 AMPK IgG; Santa Cruz Biotechnology) or specifically against either nonconserved rat AMPKα1 peptide (amino acids 231–251) or rat AMPKα2 peptide (amino acids 351–366) (Cell Signaling Technology) (28, 42). Immunocomplexes were washed three times with PBS and suspended in 40 mM HEPES, 0.2 mM SAMS (peptide substrate for AMPK), 0.2 mM AMP, 80 mM NaCl, 8% glycerol, 0.8 mM EDTA, 0.8 mM dithiothreitol, 5 mM MgCl2, and 0.2 mM ATP (+2 μCi [32P]ATP), pH 7.0, in a final volume of 40 μl for 10 min at 37°C. At the end of incubation, an aliquot was spotted on Whatman P81 filter paper. The unbound [32P]ATP was removed with six washes of 1% phosphoric acid and one wash of acetone. The filter papers were air-dried, and radioactivity was quantified using a scintillation counter (42).
Skeletal Muscle GLUT4 Studies
To investigate the effect of exercise, skeletal muscle homogenates from all four Ex and Sed groups were employed. To examine insulin responsiveness of SKM GLUT4 translocation, 2-mo-old female animals from all four Ex groups received either vehicle or insulin (8 U/kg by intraperitoneal injection). After 20 min, the predetermined optimal time point hindlimb SKM was harvested, and subcellular fractions were prepared. PM and low-density microsomal (LDM) subfractions were isolated as described previously, and the relative purity was determined by marker enzyme enrichment (40, 41). The homogenate, fractionated sacrolemmal PM, and LDM samples were subjected to Western blot analysis (40, 41). The affinity-purified rabbit anti-rat GLUT4 antibody (1:2,500 dilution) was used as the primary antibody (40, 41).
Data Analysis
All data are expressed as means ± SE. Effect of two interventions (exercise and nutrition) was compared simultaneously using the two-way ANOVA, and the F values were determined. Independent effects of exercise and pre/postnatal nutrition and their interaction were also determined. Intergroup differences were established by the post hoc Holm-Sidak method. Significance was assigned when P values were <0.05.
RESULTS
Anthropometric Changes, Basal Glucose and Insulin Concentrations, and HOMA-IR
ET did not affect body weight but significantly increased nose-to-tail length in Ex CON, Ex IUGR, and Ex IUGR + PNGR groups (P < 0.0001). Body length in sedentary (Sed) PNGR was significantly greater than all other sedentary groups and did not change significantly after exercise. There was a significant exercise × pre/postnatal nutritional effect on nose-tail length (F = 18.66, P < 0.001), a pre/postnatal nutrition effect alone (F = 43.58, P < 0.001), and an exercise effect alone (F = 226.7, P < 0.001). Similarly, heart weights revealed a significant effect of exercise (F = 32.13, P < 0.001) and pre/post nutrition (F = 3.55, P < 0.02) but no effect of Ex × pre/postnatal nutrition (F = 0.556, P < 0.64). Heart weights increased after ET in Ex CON, Ex IUGR, and Ex IUGR + PNGR groups (P < 0.001, Holm-Sidak test), whereas brain weights increased in Ex CON, Ex IUGR, Ex PNGR, and Ex IUGR + PNGR groups vs. their Sed counterparts (P < 0.001, Holm-Sidak test). Brown adipose tissue weight was no different, but abdominal white adipose tissue increased in Ex CON and Ex IUGR compared with corresponding Sed counterparts (P < 0.001 and P < 0.001, Holm-Sidak test; Table 1).
Table 1.
NT length and organ weights in all Ex and Sed groups: CON, IUGR, PNGR, and IUGR + PNGR
| Groups | Body Weight, g | NT Length, cm | Heart, g | Kidney, g | Liver, g | Brain, g | BAT, g | WAT, g |
|---|---|---|---|---|---|---|---|---|
| Ex CON (5) | 282.5±7.8 | 41.8±0.49a | 1.09±0.02b | 1.94±0.02c | 9.54±0.35 | 1.95±0.04b | 0.35±0.04 | 10.42±1.4b |
| Sed CON (8) | 271.6±9.7 | 37.75±0.25 | 0.87±0.02 | 2.13±0.07 | 9.87±0.31 | 1.73±0.06 | 0.29±0.03 | 5.86±0.81 |
| Ex PNGR (6) | 272.3±4.6 | 43.3±0.42 | 1.08±0.05 | 1.79±0.02 | 8.73±0.3b | 1.9±0.03b | 0.29±0.04 | 6.26±1.16# |
| Sed PNGR (7) | 277.3±4.8 | 42.57±0.29* | 0.97±0.08 | 1.95±0.02** | 10.61±0.67 | 1.74±0.02 | 0.21±0.05 | 6.98±0.92 |
| Ex IUGR (6) | 290.5±7.0 | 42.5±0.34a | 1.08±0.04b | 1.92±0.07 | 9.63±0.31 | 1.94±0.04a | 0.33±0.02 | 9.42±1.01a |
| Sed IUGR (8) | 293.7±12.7 | 36.88±0.35 | 0.896±0.05 | 1.88±0.06 | 8.51±0.49 | 1.67±0.04 | 0.28±0.03 | 4.33±0.53 |
| Ex IUGR + PNGR (6) | 256±11.6 | 41.84±0.4a | 0.986±0.51b | 1.59±0.08 | 8.21±0.3 | 1.87±0.04a | 0.21±0.04 | 3.63±0.53# |
| Sed IUGR + PNGR (8) | 246.8±10.5 | 36.1±0.44 | 0.79±0.08 | 1.54±0.04* | 7.88±0.37* | 1.52±0.04* | 0.3±0.04 | 1.68±0.15* |
Data are shown as means ± SE; n is shown in parentheses. NT, nose-tail; Ex, exercise; Sed, sedentary; CON, control; IUGR, intrauterine growth restricted; PNGR, postnatal growth restricted; BAT, brown adipose tissue; WAT, white adipose tissue.
P < 0.0001, P < 0.001, and P < 0.03, respectively, for Ex vs. Sed states in the pre/postnatal nutrition groups (Holm-Sidak test).
P < 0.001 and
P < 0.02, Sed pre/postnatal nutrition groups vs. Sed CON (Holm-Sidak test).
P < 0.0001, Ex pre/postnatal nutrition groups vs. Ex CON (Holm-Sidak test).
ET did not alter fasting plasma glucose concentration (Table 2); a significant decrease in fasting plasma insulin concentration was observed by exercise alone (F = 11.16, P < 0.002) and by pre/postnatal nutrition alone (F = 3.69, P < 0.02), but there was no effect of exercise × pre/postnatal nutrition (F = 1.26, P < 0.3). The fasting plasma insulin concentration decreased in Ex CON and Ex IUGR groups (P < 0.03 and P < 0.008 respectively, Holm-Sidak Test; Table 2) compared with their sedentary counterparts. Similarly, HOMA-IR decreased significantly with exercise alone (F = 11.34, P < 0.002) and with pre/postnatal nutrition alone (F = 3.56, P < 0.02) but not with exercise × pre/postnatal nutrition (F = 1.74, P < 0.18). HOMA-IR values decreased significantly in Ex CON and Ex IUGR (P < 0.03, Holm-Sidak test), supporting improved insulin sensitivity vs. their Sed counterparts (Table 2). The Ex PNGR and Ex IUGR + PNGR groups remained unchanged from the sedentary insulin-sensitive state.
Table 2.
Baseline plasma glucose and insulin concentrations in all Ex and Sed groups: CON, IUGR, PNGR, and IUGR + PNGR
| Baseline Glucose, mmol/l | Baseline Insulin, nmol/l | HOMA-IR | |
|---|---|---|---|
| Ex CON (6) | 5.2±0.5 | 10.3±2.7a | 15.8±4.1a |
| Sed CON (6) | 6.0±0.2 | 13.6±2.2 | 28.6±4.7 |
| Ex PNGR (5) | 6.3±0.3 | 6.1±0.6 | 13.1±1.3 |
| Sed PNGR (5) | 5.6±0.2 | 6.9±0.7* | 14.7±1* |
| Ex IUGR (6) | 6.0±0.2 | 7.0±0.6b | 14.3±0.9c |
| Sed IUGR (6) | 6.1±0.4 | 12.9±2.7 | 29.9±7.3 |
| Ex IUGR + PNGR (6) | 5.3±0.4 | 5.7±0.9 | 11.4±2.1 |
| Sed IUGR + PNGR (6) | 6.6±0.3 | 8.2±0.7 | 15.9±2.8 |
Data are shown as means ± SE. HOMA-IR, homeostasis model assessment of insulin resistance.
P < 0.03, P < 0.008, and P < 0.003, respectively, for Ex vs. Sed states in the pre/postnatal nutrition groups (Holm-Sidak test).
P < 0.01, Sed pre/postnatal nutrition groups vs. Sed CON (Holm-Sidak test).
GTT and Glucose-Stimulated Insulin Release
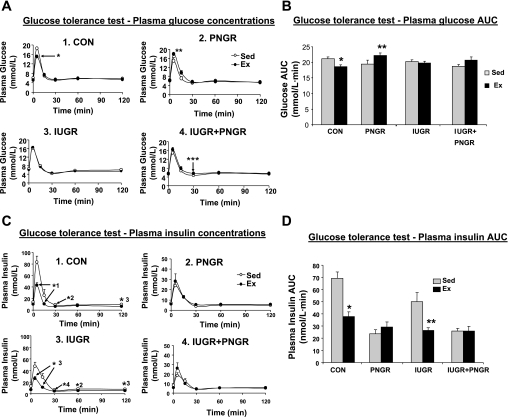
An intravenous glucose challenge led to a lower peak plasma glucose concentration at 5 min in Ex CON (Holm-Sidak test, P < 0.007; Fig. 2A, graph 1) compared with that of Sed CON. The plasma glucose concentration was higher at 5 and 15 min in Ex PNGR (Holm-Sidak test, P < 0.02 and P < 0.05 respectively; Fig. 2A, graph 2) vs. Sed PNGR, but Ex IUGR (Fig. 2A, graph 3) was similar to Sed IUGR. The interactive effect of exercise × pre/postnatal nutrition resulted in a reduced glucose area under the curve (AUC) in Ex CON (P < 0.04 by Holm-Sidak test; Fig. 2B) but significantly increased AUC in Ex PNGR (P < 0.03 by Holm-Sidak test). Euglycemia during GTT was maintained by a significant lowering of the glucose-stimulated insulin release (GSIR) at 5, 15, and 30 min (Fig. 2C, graphs 1 and 3) and a lower insulin AUC (P < 0.001 by Holm-Sidak test; Fig. 2D) in Ex Con and Ex IUGR groups. These findings support improved insulin sensitivity. The GSIR in Sed PNGR and Sed IUGR + PNGR was decreased vs. Sed CON but did not change further after ET (Fig. 2C, graphs 2 and 4).
Fig. 2.
A: plasma glucose concentration at 0, 5, 15, 30, 60, and 120 min after intravenous glucose challenge is shown in Ex and Sed states in individual graphs (graph 1, CON; graph 2, PNGR; graph 3, IUGR; graph 4, IUGR + PNGR). *P < 0.006, **P < 0.02, ***P < 0.05, Ex vs. Sed counterpart. B: area under the curve (AUC) for plasma glucose concentration during glucose tolerance test (GTT) is shown for all groups. Two-way ANOVA revealed a significant effect of exercise × pre/postnatal nutrition (F = 4.07, P = 0.013) but no effect of exercise alone (F = 0.491, P = 0.488) or pre/postnatal nutrition (F = 0.653, P = 0.586) alone. Holm-Sidak test showed *P < 0.04, Ex CON vs. Sed CON; **P < 0.03, Ex PNGR vs. Sed PNGR. C: plasma insulin concentration at 0, 5, 15, 30, 60, and 120 min after an intravenous glucose challenge is shown in Ex and Sed states in individual graphs (graph 1, CON; graph 2, PNGR; graph 3, IUGR; graph 4, IUGR + PNGR). The scale for plasma insulin is adjusted from 0 to 50 nmol/l to show the lower values in PNGR (graph 2) and IUGR + PNGR (graph 4). Holm-Sidak test shows a significant decrease at 5, 15, 30, 60, and 120 min in Ex CON and Ex IUGR compared with their respective Sed counterparts (*1P < 0.017, *2P < 0.005, *3P < 0.004, *4P < 0.03). D: AUC for plasma insulin concentration during GTT is shown for all groups. Two-way ANOVA revealed significant effect of exercise (F = 13.926, P = <0.001), pre/postnatal nutrition (F = 14.843, P = <0.001), and exercise × pre/postnatal nutrition (F = 6.94, P = <0.001). Holm-Sidak test revealed significant changes in Ex CON and Ex IUGR groups (*P < 0.0001, **P < 0.001) compared with their respective Sed states; n = 6 for all study groups, except n = 5 each for Sed and Ex PNGR groups alone.
Insulin Tolerance Test Reflecting Insulin-Stimulated Glucose Uptake
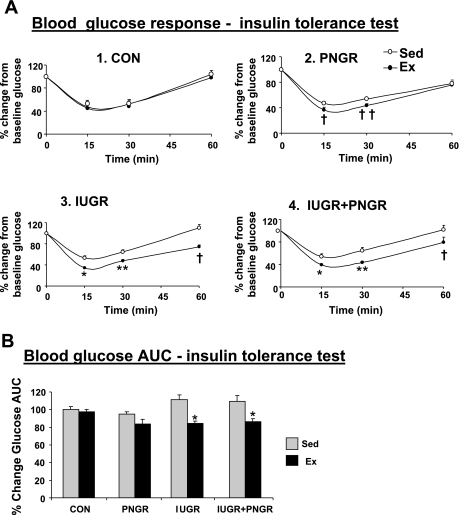
The percent decrease in plasma glucose concentration from the zero time value was greater at 15 and 30 min in Ex PNGR, Ex IUGR, and Ex IUGR + PNGR groups and at 60 min in Ex IUGR and Ex IUGR + PNGR (Fig. 3A, graphs 1–4) vs. their Sed counterparts. This resulted in decreased percent glucose AUC in Ex IUGR and Ex IUGR + PNGR after ET (P < 0.00001 by Holm-Sidak test; Fig. 3B).
Fig. 3.
A: %change in plasma glucose concentration from the “0” time point value during insulin tolerance test is shown in Ex and Sed states in individual graphs (graph 1, CON; graph 2, PNGR; graph 3, IUGR; graph 4, IUGR + PNGR). Holm-Sidak test shows significant response in Ex PNGR, Ex IUGR, and Ex IUGR + PNGR groups (†P < 0.01, ††P < 0.03, *P < 0.0001, **P < 0.0004) compared with corresponding Sed group at the same time point. B: AUC for %change in plasma glucose concentration during the insulin tolerance test for all Ex and Sed groups is shown. Two-way ANOVA revealed a significant effect of exercise alone (F = 34.793, P < 0.001) and exercise × pre/postnatal nutrition (F = 3.16, P < 0.03) but not of pre/postnatal nutrition alone (F = 2.763, P = 0.057). Holm-Sidak test demonstrated a difference at *P < 0.0001 between Ex IUGR and Ex IUGR + PNGR vs. their corresponding Sed groups; n = 6 for all study groups, except n = 5 each for Sed and Ex PNGR groups alone.
Glucose Metabolic Adaptations After a Glucose Challenge
Hepatic glucose production.
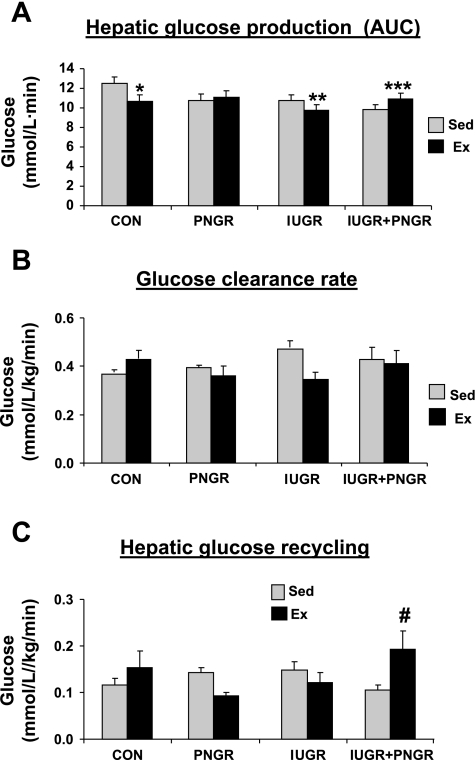
ET resulted in greater suppression of endogenous hepatic glucose production (HGP) during the GTT in Ex CON and Ex IUGR at various time points (5, 15, 30, and 60 min) compared with that of Sed CON and Sed IUGR, respectively. Sixty minutes after the glucose challenge, HGP in Ex IUGR was suppressed to a greater extent than that observed in Ex CON, Ex PNGR, and Ex IUGR + PNGR (P < 0.04 by Holm-Sidak test). This translated into a greater suppression of HGP AUC in Ex CON and Ex IUGR (P < 0.05 by Holm-Sidak test; Fig. 4A). The HGP AUC in Ex PNGR did not change, whereas it increased by 11% in Ex IUGR + PNGR from their sedentary counterparts (Fig. 4A). These findings support improved insulin sensitivity only in the Ex IUGR vs. the Sed IUGR being similar in range to that achieved by the Ex CON vs. Sed CON.
Fig. 4.
A: AUC for endogenous hepatic glucose production during GTT is shown in all Ex and Sed groups. Two-way ANOVA revealed a significant effect of pre/postnatal nutrition alone (F = 3.338, P = 0.03) and exercise × pre/postnatal nutrition (F = 4.64, P < 0.008) but no effect of exercise alone (F = 1.053, P = 0.311). Holm-Sidak test demonstrated differences between the Ex and Sed counterparts at *P < 0.02, **P < 0.04, and ***P < 0.05. B: total glucose clearance (mg·kg−1·min−1) is shown for all Ex and Sed groups. Two-way ANOVA did not demonstrate an effect of exercise (F =1.497, P = 0.229), pre/postnatal nutrition (F = 0.480, P = 0.698), or exercise × pre/postnatal nutrition (F = 2.298, P = 0.094). C: hepatic glucose recycling or glucose futile cycling is shown for all Ex and Sed groups. Two-way ANOVA revealed a significant effect of exercise × pre/postnatal nutrition (F = 3.714, P = 0.02) but no effect of exercise (F = 0.695, P = 0.410) or pre/postnatal nutrition alone (F = 0.581, P = 0.631). Holm-Sidak test demonstrated a difference between the Ex IUGR + PNGR vs. the corresponding Sed group at #P < 0.015; n = 6 for all study groups, except n = 5 each for Sed and Ex PNGR groups alone.
Glucose clearance and hepatic glucose recycling.
The resting glucose clearance rate was unchanged after ET (Fig. 4B). Hepatic glucose futile cycling (GFC) increased in Ex IUGR + PNGR compared with the nonexercised state, an adaptation geared toward meeting the glucose requirements. Hepatic glucose recycling in Ex CON, Ex PNGR, and Ex IUGR groups was similar to that of their respective sedentary group.
SKM Studies
AMPK protein concentrations and enzyme activity.
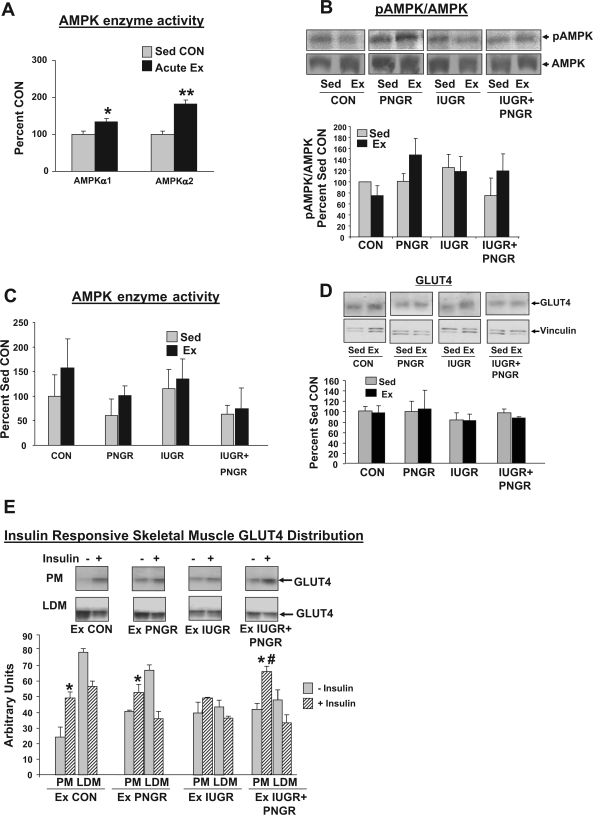
The AMPKα1 and AMPKα2 activity was increased in Ex CON 15 min after an acute bout of exercise compared with Sed CON (P < 0.04 and P < 0.01, respectively; Fig. 5A). In contrast, when pAMPK and total AMPK protein concentrations were examined 48–72 h after cessation of the chronic exercise regimen, there was no statistical difference in all experimental groups between Sed and exercised rat SKM (Fig. 5B). Separate and combined AMPKα1 and -α2 enzyme activity in all exercised groups was also not significantly different from that of the sedentary counterparts (Fig. 5C).
Fig. 5.
A: skeletal muscle AMPKα1 and -α2 isoform-specific activities measured 15 min after acute Ex (n = 4) or in a Sed state (n = 3) and expressed as a percent of the sedentary CON state are shown in a subgroup of control animals. *P < 0.04, acute Ex vs. Sed CON; **P < 0.01, acute Ex vs. Sed CON. B: skeletal muscle pAMPK and total AMPK protein concentrations measured 48–72 h after last bout of exercise training (n = 3–4 each for all groups) did not significantly change when compared with the Sed counterpart (n = 3–4 each for all groups). Representative Western blots are shown at top, and the densitometric quantification of pAMPK/total AMPK expressed as a percent of Sed CON is shown as a bar graph. C: skeletal muscle AMPK (AMPKα1 and -α2 isoforms) enzyme activity measured 48–72 h after the last bout of exercise training and expressed as a percent of Sed CON did not show any significant difference when compared to the sedentary counterpart (n = 3–4 each for all Ex and Sed groups). D: skeletal muscle total GLUT4 protein concentrations were no different between the Sed and Ex groups (n = 3 each for all groups). Representative Western blots of GLUT4 and vinculin (internal control) are shown at top, and the densitometric quantification of total GLUT4/vinculin expressed as a percent of Sed CON is shown as a bar graph. E: basal and insulin-responsive skeletal muscle GLUT4 distribution in plasma membrane (PM) and low-density microsomes (LDM) of Ex CON, Ex PNGR, Ex IUGR, and Ex IUGR + PNGR is shown; n = 3 each for PM and LDM in all experimental groups. Top: representative Western blots of GLUT4 in PM and LDM subfractions in the presence (+) and absence (−) of insulin administration in the 4 Ex groups (Ex CON, Ex PNGR, Ex IUGR, and Ex IUGR + PNGR). The densitometric quantification is expressed in arbitrary units as a bar graph. PM GLUT4 concentrations increased after insulin administration (+) vs. the corresponding basal state (−) in Ex CON, Ex PNGR, and Ex IUGR + PNGR (*P < 0.05) but not in Ex IUGR. PM GLUT4 concentrations in the Ex IUGR + PNGR were higher than Ex CON (#P < 0.05). Sed state results were reported previously (41) and not shown.
Total and subcellular GLUT4 protein distribution.
Examination of total GLUT4 protien revealed no differences between Ex and Sed states in all four experimental groups (Fig. 5D). We have previously reported increased SKM basal PM-associated GLUT4 concentrations in Sed IUGR and Sed IUGR + PNGR groups, which was unresponsive to exogenous insulin administration (41). After ET, the basal SKM PM- and LDM-associated GLUT4 protein concentrations in Ex IUGR, Ex PNGR, and Ex IUGR + PNGR groups were no different from each other, being similar to that of Ex CON (Fig. 5E). Insulin-responsive SKM GLUT4 translocation to PM from LDM was observed in Ex IUGR + PNGR (P < 0.05) and Ex PNGR (P < 0.05) groups, the former being more and the latter being less to that seen in Ex CON, but was absent in Ex IUGR (Fig. 5E). Similar to Ex CON, the attaining of insulin-responsive SKM GLUT4 protein translocation from LDM to PM over that not present in the previously reported sedentary counterpart (41) was observed only in the Ex IUGR + PNGR group that overshot the Ex CON group (P < 0.05).
DISCUSSION
Effect of Exercise
The positive effect of exercise on glucose kinetics, GSIR, and skeletal muscle PM GLUT4 association was observed in the CON group as anticipated. Most animal investigations link IUGR to adult-onset glucose intolerance and tissue-specific insulin resistance, lending credence to the Barker hypothesis (1, 2). The IUGR adult female develops gestational diabetes (7) and poses additional risk of transgenerational inheritance of the insulin-resistant phenotype (9, 39). Therefore, we focused the present study on the critical pregestational phase by initiating submaximal exercise training prior to the appearance of gestational diabetes with the onset of pregnancy (7). Exercise training in IUGR offspring improved insulin sensitivity by suppressing glucose-stimulated insulin production and hepatic glucose production. It also altered baseline skeletal muscle PM GLUT4 concentrations similar to that seen in Ex CON but did not further affect the insulin-responsive PM association of GLUT4.
Epidemiological human studies indicate that premature birth and catchup growth are associated with insulin resistance and higher susceptibility to adult chronic diseases (4, 33, 34). Further poor growth during infancy also predisposes toward glucose intolerance and type 2 diabetes mellitus (6). Contrary to our expectation in the pregestational PNGR group, early exercise training resulted in hyperglycemia with a 20% increase in glucose AUC without perturbations in basal glucose or basal and GSIR compared with the sedentary counterpart and Ex CON. This was despite partial insulin responsiveness of skeletal muscle PM GLUT4 association. Inadequacy of basal energy stores (fat and glycogen) and the superimposed increased metabolic demands of early exercise training may cause a shortfall in replenishing depleted stores. This in turn may cause a dependency on unsuppressed hepatic glucose production to meet the exercise-induced metabolic demands. The resultant hyperglycemia may have decreased hepatic glucose recycling.
Early implementation of exercise training in the Ex IUGR + PNGR group improved insulin sensitivity and maintained euglycemia due to the adaptive decrease in GSIR and increase in HGP, GFC, and insulin responsiveness of skeletal muscle PM GLUT4 association (41). Thus early exercise training in the pregestational state may prove protective when exposed to additional metabolic stress as encountered in a pregnant state.
These results indicate that the exercise-induced glucose metabolic adaptations are specifically tailored to the preexisting metabolic phenotype. Although these adaptations ameliorate hepatic insulin resistance in IUGR, they have no effect in PNGR. Thus, when considering exercise regimen in the prevention of T2DM, the lack of an effect in a preexisting PNGR phenotype is a distinct possibility.
Insulin Sensitivity
Benefits of acute exercise and endurance training in human and rat consist of lowered insulin concentrations, improved glucose tolerance, and insulin sensitivity. The latter is achieved by decreasing hepatic glucose production and enhancing skeletal muscle glucose utilization (8, 11, 14, 20, 21, 30). In the adult female IUGR offspring, we have previously observed increased GSIR and diminished HGP during GTT (19). Several studies demonstrate that modest weight loss and physical activity can reduce the risk of diabetes (16, 26, 31). Human studies show that moderate levels of endurance and resistance training protect adult males and females with impaired glucose tolerance from developing type 2 diabetes (26). Furthermore, regular exercise protects adult human males and females born with a low birth weight (16). Similar to these observations, our present results demonstrate beneficial effects of exercise training in the presence of preexisting conditions such as IUGR that are high risk for adult-onset diabetes mellitus. T2DM is a progressive disease where the β-islets fail in the face of increasing insulin resistance, which in turn is linked to obesity and sedentary lifestyle (29). The acquisition of these phenotypic features can be prevented by regular exercise and physical activity (10, 15, 18, 23, 24, 45). Moderate exercise prior to overt symptoms may preserve pancreatic β-islet cell function and prevent the onset of chronic insulin resistance. Our present results demonstrated enhanced insulin-responsive glucose uptake during insulin tolerance test in all exercised nutrient-restricted groups (Ex IUGR, Ex PNGR, and Ex IUGR + PNGR) compared with their corresponding sedentary counterparts. These responses further support that moderate exercise training prior to developing glucose intolerance or obesity improved insulin sensitivity in the IUGR female offspring.
Anthropometric Effects
Unlike the various adult studies reported, our studies are novel in that they consist of early postweaning exercise training in growing animals that have not yet developed insulin resistance, diabetes, or obesity. This early intervention compared with sedentary counterparts did not affect total body weight but had a positive anabolic effect on nose-tail length and certain organ/tissue weights in all four groups, improved glucose tolerance, and decreased GSIR without affecting glucose clearance or recycling. Thus, although an increase in brain and heart weights was observed, more surprising was the increase in abdominal white adipose tissue weight despite improved insulin sensitivity in the Ex CON, Ex IUGR, and Ex IUGR + PNGR vs. the respective sedentary counterparts. These changes may collectively reflect a positive growth-promoting effect of exercise when introduced early in life at a time when cellular growth in different organs/tissues translates into exponential growth and development. Our present observations confirm the positive effects of early exercise training in pre- and postnatal nutrient-restricted rat phenotypes, similar to previous reports in healthy human adults (11, 30).
Skeletal Muscle
Skeletal muscle is the predominant site of insulin- and exercise-dependent glucose disposal. Insulin and exercise have additive effects on skeletal muscle glucose uptake mediated by GLUT4 proteins via differing signaling cascades (25, 35). In this study we implemented a submaximal treadmill exercise regimen that was validated by an increase in skeletal muscle AMPK activity (28, 42). Additionally, exercise training-induced increase in skeletal muscle GLUT4 expression is well described in human and animal studies (8). However, no information exists in the sedentary IUGR and IUGR + PNGR regarding the effect of exercise training on reversing preexisting perturbed skeletal muscle subcellular GLUT4 distribution (41). Six weeks of exercise training led to partial reversal in Ex IUGR by bringing basal PM GLUT4 concentration closer to that of Ex CON. Therefore, although this submaximal exercise training improved basal insulin sensitivity of skeletal muscle PM GLUT4 association in Ex IUGR, no further response to exogenous insulin administration was observed. Such disparity in the exercise-induced response of glucose homeostasis was previously described to depend on the severity of the preexisting metabolic perturbation. This includes an example of improved glucose tolerance in a mild state of diabetes (20), with no effect in the severe insulin-deficient state (21). Along similar lines, perhaps exogenous insulin in IUGR has no further effect on chronically exercised skeletal muscle PM GLUT4 concentrations while affecting that of IUGR + PNGR. An alternate explanation may be that, in the IUGR, exercise had a maximal impact with no further room for an added insulin effect. In contrast, the IUGR + PNGR group while exercised still retained some insulin sensitivity of the skeletal muscle GLUT4. At the same time, intertissue differences between the effects of exercise on liver vs. skeletal muscle within the same experimental group (IUGR or IUGR + PNGR) may relate to end-organ specificity and sensitivity. Whether a more intense exercise regimen than the one we employed would demonstrate insulin responsiveness of skeletal muscle PM GLUT4 association in Ex IUGR remains unknown. Since skeletal muscle GLUT4 expression in insulin-resistant obese Zucker rat parallels the intensity of the exercise regimen instituted (12), this is a possibility in the Ex IUGR as well. Total glucose clearance in Ex CON was similar to Ex IUGR, Ex PNGR, and Ex IUGR + PNGR. These observations support a previous report of unaltered skeletal muscle glucose uptake due to moderate exercise of endurance training in female rats (38).
Summary
Early exercise training in the nutrient-restricted offspring that positively regulates the growth potential impacts tissue-specific adaptations involved in nutritional programming of glucose kinetics. This occurs in a phenotype-specific manner, proving advantageous to the IUGR and IUGR + PNGR, but without affecting the PNGR adult female offspring.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grants HD-41230, HD-25024, HD-046979, and HD-33997 (to S. U. Devaskar).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Barker DJ The malnourished baby and infant. Br Med Bull 60: 69–88, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ Adult consequences of fetal growth restriction. Clin Obstet Gynecol 49: 270–283, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X) relation to reduced fetal growth. Diabetologia 36: 62–67, 1993. [DOI] [PubMed] [Google Scholar]
- 4.Bazaes RA, Mericq V. Premature birth and insulin resistance. N Engl J Med 352: 939–940, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Bernard JR, Crain AM, Rivas DA, Herr HJ, Reeder DW, Yaspelkis BB 3rd. Chronic aerobic exercise enhances components of the classical and novel insulin signaling cascades in Sprague-Dawley rat skeletal muscle. Acta Physiol Scand 183: 357–366, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Bhargava SK, Sachdev HS, Fall C, Osmond C, Lakshmy R, Barker DJ, Biswas SK, Stat M, Ramji S, Prabhakaran D, Reddy KS. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med 350: 865–875, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boloker J, Gertz SJ, Simmons RA. Gestational diabetes leads to the development of diabetes in adulthood in the rat. Diabetes 51: 1499–1506, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Brozinick JT, Etgen GJ Jr, Yaspelkis BB 3rd, Kang HY, Ivy JL. Effects of exercise training on muscle GLUT4 protein content and translocation in obese Zucker rats. Am J Physiol Endocrinol Metab 265: E419–E427, 1993. [DOI] [PubMed] [Google Scholar]
- 9.Burt BE, Hess BW, Nathanielsz PW, Ford SP. Flock differences in the impact of maternal dietary restriction on offspring growth and glucose tolerance in female offspring. Soc Reprod Fertil Suppl 64: 411–424, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Carroll S, Dudfield M. What is the relationship between exercise and metabolic abnormalities? A review of the metabolic syndrome. Sports Med 34: 371–418, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Coggan AR, Swanson SC, Mendenhall LA, Habash DL, Kien CL. Effect of endurance training on hepatic glycogenolysis and gluconeogenesis during prolonged exercise in men. Am J Physiol Endocrinol Metab 268: E375–E383, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Cortez MY, Torgan CE, Brozinick JT Jr, Ivy JL. Insulin resistance of obese Zucker rats exercise trained at two different intensities. Am J Physiol Endocrinol Metab 261: E613–E619, 1991. [DOI] [PubMed] [Google Scholar]
- 13.Donovan CM, Sumida KD. Training improves glucose homeostasis in rats during exercise via glucose production. Am J Physiol Regul Integr Comp Physiol 258: R770–R776, 1990. [DOI] [PubMed] [Google Scholar]
- 14.Donovan CM, Sumida KD. Training enhanced hepatic gluconeogenesis: the importance for glucose homeostasis during exercise. Med Sci Sports Exerc 29: 628–634, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Ebeling P, Bourey R, Koranyi L, Tuominen JA, Groop LC, Henriksson J, Mueckler M, Sovijarvi A, Koivisto VA. Mechanism of enhanced insulin sensitivity in athletes. Increased blood flow, muscle glucose transport protein (GLUT4) concentration, and glycogen synthase activity. J Clin Invest 92: 1623–1631, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eriksson JG, Yliharsila H, Forsen T, Osmond C, Barker DJ. Exercise protects against glucose intolerance in individuals with a small body size at birth. Prev Med 39: 164–167, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Twinn DS, Wayman A, Ekizoglou S, Martin MS, Hales CN, Ozanne SE. Maternal protein restriction leads to hyperinsulinemia and reduced insulin-signaling protein expression in 21-mo-old female rat offspring. Am J Physiol Regul Integr Comp Physiol 288: R368–R373, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara CM, McCrone SH, Brendie D, Ryan AS, Goldberg AP. Metabolic effects of the addition of resistive to aerobic exercise in older men. Int J Sport Nutr Exerc Metab 14: 73–80, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Garg M, Thamotharan M, Rogers L, Bassilian S, Lee WN, Devaskar SU. Metabolic adaptations in the intrauterine growth-restricted adult female rat offspring. Am J Physiol Endocrinol Metab 290: E1218–E1226, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Goodyear LJ, Hirshman MF, Horton ES. Exercise-induced translocation of skeletal muscle glucose transporters. Am J Physiol Endocrinol Metab 261: E795–E799, 1991. [DOI] [PubMed] [Google Scholar]
- 21.Goodyear LJ, Hirshman MF, Knutson SM, Horton ED, Horton ES. Effect of exercise training on glucose homeostasis in normal and insulin-deficient diabetic rats. J Appl Physiol 65: 844–851, 1988. [DOI] [PubMed] [Google Scholar]
- 22.Holness MJ Impact of early growth retardation on glucoregulatory control and insulin action in mature rats. Am J Physiol Endocrinol Metab 270: E946–E954, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Holten MK, Zacho M, Gaster M, Juel C, Woktaszewski JF, Dela F. Strength training increases insulin-mediated glucose uptake, GLUT4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes 53: 294–305, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Hughes VA, Fiatarone MA, Fielding RA, Kahn BB, Ferrara CM, Shepherd P, Fisher EC, Wolfe RR, Elahi D, Evans WJ. Exercise increases muscle GLUT4 levels and insulin action in subjects with impaired glucose tolerance. Am J Physiol Endocrinol Metab 264: E855–E862, 1993. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy JW, Hirshman MF, Gervino EV, Ocel JV, Forse RA, Hoenig SJ, Aronson D, Goodyear LJ, Horton ES. Acute exercise induces GLUT4 translocation in skeletal muscle of normal human subjects and subjects with type 2 diabetes. Diabetes 48: 1192–1197, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Laaksonen DE, Lindstrom J, Lakka TA, Eriksson JG, Niskanen L, Wikstrom K, Aunola S, Keinanen-Kiukaanniemi S, Laakso M, Valle TT, Ilanne-Parikka P, Louheranta A, Hamalainen H, Rastas M, Salminen V, Cepaitis Z, Hakumaki M, Kaikkonen H, Harkonen P, Sundvall J, Tuomilehto J, Uusitupa M. Physical activity in the prevention of type 2 diabetes: the Finnish diabetes prevention study. Diabetes 54: 158–165, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Lee WN, Byerley LO, Bergner EA, Edmond J. Mass isotopomer analysis: theoretical and practical considerations. Biol Mass Spectrom 20: 451–458, 1991. [DOI] [PubMed] [Google Scholar]
- 28.Lessand SJ, Chen ZP, Watt MJ, Hashem M, Reid JJ, Febbraio MA, Kemp BE, Hawley JA. Chronic rosiglitazone treatment restores AMPKα2 activity in insulin-resistant rat skeletal muscle. Am J Physiol Endocrinol Metab 290: E251–E257, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Marcovecchio M, Mohn A, Chiarelli F. Type 2 diabetes mellitus in children and adolescents. J Endocrinol Invest 28: 853–863, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Mendenhall LA, Swanson SC, Habash DL, Coggan AR. Ten days of exercise training reduces glucose production and utilization during moderate-intensity exercise. Am J Physiol Endocrinol Metab 266: E136–E143, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Nishida Y, Higaki Y, Tokuyama K, Fujimi K, Kiyonaga A, Shindo M, Sato Y, Tanaka H. Effect of mild exercise training on glucose effectiveness in healthy men. Diabetes Care 24: 1008–1013, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Oak SA, Tran C, Pan G, Thamotharan M, Devaskar SU. Perturbed skeletal muscle insulin signaling in the adult female intrauterine growth-restricted rat. Am J Physiol Endocrinol Metab 290: E1321–E1330, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. Br Med J 320: 967–971, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Regan FM, Cutfield WS, Jefferies C, Robinson E, Hofman PL. The impact of early nutrition in premature infants on later childhood insulin sensitivity and growth. Pediatrics 118: 1943–1949, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Saengsirisuwan V, Perez FR, Kinnick TR, Henriksen EJ. Effects of exercise training and antioxidant R-ALA on glucose transport in insulin-sensitive rat skeletal muscle. J Appl Physiol 92: 50–58, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes 50: 2279–2286, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Sriwijitkamol A, Ivy JL, Christ-Roberts C, DeFronzo RA, Mandarino LJ, Musi N. LKB1-AMPK signaling in muscle from obese insulin-resistant Zucker rats and effects of training. Am J Physiol Endocrinol Metab 290: E925–E932, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Sumida KD, Donovan CM. Endurance training fails to inhibit skeletal muscle glucose uptake during exercise. J Appl Physiol 76: 1876–1881, 1994. [DOI] [PubMed] [Google Scholar]
- 39.Thamotharan M, Garg M, Oak S, Rogers LM, Pan G, Sangiorgi F, Lee PW, Devaskar SU. Transgenerational inheritance of the insulin-resistant phenotype in embryo-transferred intrauterine growth-restricted adult female rat offspring. Am J Physiol Endocrinol Metab 292: E1270–E1279, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Thamotharan M, McKnight RA, Thamotharan S, Kao DJ, Devaskar SU. Aberrant insulin-induced GLUT4 translocation predicts glucose intolerance in the offspring of a diabetic mother. Am J Physiol Endocrinol Metab 284: E901–E914, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Thamotharan M, Shin BC, Suddirikku DT, Thamotharan S, Garg M, Devaskar SU. GLUT4 expression and subcellular localization in the intrauterine growth-restricted adult rat female offspring. Am J Physiol Endocrinol Metab 288: E935–E947, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol Endocrinol Metab 270: E299–E304, 1996. [DOI] [PubMed] [Google Scholar]
- 43.Xu J, Chang V, Joseph SB, Trujillo C, Bassilian S, Saad MF, Lee WN, Kurland IJ. Peroxisomal proliferator-activated receptor alpha deficiency diminishes insulin-responsiveness of gluconeogenic/glycolytic/pentose gene expression and substrate cycle flux. Endocrinology 145: 1087–1095, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Xu J, Lee WN, Xiao G, Trujillo C, Chang V, Bianco L, Hernandez F, Chung B, Makabi S, Ahmed S, Bassilian S, Saad M, Kurland IJ. Determination of a glucose-dependent futile cycling rate constant from an intraperitoneal glucose tolerance test. Anal Biochem 315: 238–246, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Zinman B, Ruderman N, Campaigne BN, Devlin JT, Schneider SH. Physical activity/exercise and diabetes mellitus. Diabetes Care 26, Suppl 1: S73–S77, 2003. [DOI] [PubMed] [Google Scholar]