Abstract
Protein tyrosine phosphatase 1B (PTP1B) contributes to leptin resistance by inhibiting intracellular leptin receptor signaling. Mice with whole body or neuron-specific deletion of PTP1B are hypersensitive to leptin and resistant to diet-induced obesity. Here we report a significant increase in PTP1B protein levels in the mediobasal hypothalamus (P = 0.003) and a concomitant reduction in leptin sensitivity following 28 days of high-fat (HF) feeding in rats. A significant increase in PTP1B mRNA levels was also observed in rats chronically infused with leptin (3 μg/day icv) for 14 days (P = 0.01) and in leptin-deficient ob/ob mice infused with leptin (5 μg/day sc for 14 days; P = 0.003). When saline-infused ob/ob mice were placed on a HF diet for 14 days, an increase in hypothalamic PTP1B mRNA expression was detected (P = 0.001) despite the absence of circulating leptin. In addition, although ob/ob mice were much more sensitive to leptin on a low-fat (LF) diet, a reduction in this sensitivity was still observed following exposure to a HF diet. Taken together, these data indicate that hypothalamic PTP1B is specifically increased during HF diet-induced leptin resistance. This increase in PTP1B is due in part to chronic hyperleptinemia, suggesting that hyperleptinemia is one mechanism contributing to the development of leptin resistance. However, these data also indicate that leptin is not required for the increase in hypothalamic PTP1B or the development of leptin resistance. Therefore, additional, leptin-independent mechanisms must exist that increase hypothalamic PTP1B and contribute to leptin resistance.
Keywords: high-fat diet, food intake, protein tyrosine phosphatase 1B
obesity is associated with increased circulating leptin and resistance to exogenous leptin administration (4, 10, 22, 23). Although the existence of leptin resistance is well accepted, the cellular mechanisms that contribute to changes in leptin sensitivity are not as well characterized (27). Protein tyrosine phosphatase 1B (PTP1B) is a cellular protein implicated in the regulation of cellular insulin and leptin signaling. Initial work focused on PTP1B as a regulator of insulin signaling, since it was shown that PTP1B directly dephosphorylates and inhibits signaling from the insulin receptor (11, 33, 36) in a variety of peripheral tissues (8, 14, 15, 41) and that whole body PTP1B deletion increases insulin sensitivity and protects against diet-induced obesity and insulin resistance (16, 20). A direct link between PTP1B and leptin signaling was subsequently noted, based on evidence that PTP1B dephosphorylates Janus Kinase 2 (Jak2), the initial tyrosine kinase mediating leptin signaling (9, 28, 40) and that mice with whole body PTP1B deletion are hypersensitive to leptin (9, 19, 40).
In addition to being expressed in peripheral tissues, PTP1B is also expressed in the brain, including areas of the hypothalamus (40). Neuron-specific deletion of PTP1B recapitulates many phenotypes of the whole body knockout, including the increased leptin sensitivity and resistance to diet-induced obesity (3). More recent experiments have shown that knockdown of PTP1B within the hypothalamus enhances leptin (and insulin) sensitivity and protects against diet-induced obesity (30). PTP1B levels also appear to be elevated in specific physiological or pathophysiological settings of leptin resistance, since available data indicate that levels of PTP1B mRNA, protein, and activity increase in settings of leptin resistance associated with age, high-fat diet, or acute inflammation (25, 30, 42). The current work provides additional evidence indicating that hypothalamic PTP1B is increased in settings of high-fat diet-induced leptin resistance and tests the hypothesis that chronic hyperleptinemia underlies both the increase in PTP1B and the development of leptin resistance.
MATERIALS AND METHODS
Animals.
All procedures were performed in accordance with the National Institutes of Health guidelines for the care and use of animals and were approved by the Animal Care and Use Committee of Pennington Biomedical Research Center. Unless otherwise noted, ∼10-wk-old male Long Evans rats (Harlan Laboratories, Indianapolis, IN) or 8-wk-old wild-type or leptin-deficient ob/ob mice on the C57BL6 background (Jackson Labs, Bar Harbor, ME) were housed singly and maintained on a 12:12-h light-dark cycle with ad libitum access to standard rat chow and water unless otherwise noted. For third ventricular (icv) cannulation (24, 26), rats were anesthetized and placed in a stereotaxic device. With the use of aseptic techniques, the skull was exposed, head screws were inserted, and a 22-gauge stainless steel cannula was implanted at coordinates −2.2 from bregma and −7.5 from dura (3rd cerebroventricle). After the cannula was anchored and all skull openings were sealed with dental acrylic, the incision was sutured and a 28-gauge obdurator was placed in the cannula. After surgery, animals were treated with analgesics and allowed to recover at least 1 wk before further study.
Experiment 1: Effect of a HF diet on central leptin resistance and hypothalamic PTP1B.
Rats bearing an intracerebroventricular cannula were placed on a high-fat (HF) diet (60% fat diet-D12492; Research Diets, New Brunswick, NJ) or the corresponding control low-fat (LF; 10% fat D12450B) diet for 28 days. On day 28 of HF feeding, rats were randomly assigned to receive an intracerebroventricular injection of either leptin (3 μg; National Hormone and Peptide Program, Dr. A. F. Parlow) or vehicle (saline) ∼2 h before lights off, and 4- and 24-h food intake was recorded. To assess levels of hypothalamic PTP1B, a separate group of uncannulated Long Evans rats was placed on the HF or LF diet as above for 28 days. On day 28, rats were rapidly decapitated, and brains were blocked and frozen on crushed dry ice for subsequent protein extraction and Western Blot analysis.
Experiment 2: Effects of chronic hyperleptinemia on PTP1B expression.
Two studies were conducted. In the first, rats were implanted with indwelling intracerebroventricular cannulas as described above and 1 wk later were implanted with Alzet osmotic minipumps (Durect, Cupertino, CA) delivering leptin (3 μg/day) or saline for 14 days. On day 14, minipumps were disconnected, and rats were allowed to recover for an additional 5 days (day 19). On day 4, day 14, or day 19 (5 days postinfusion), subgroups of rats were killed, and brains were collected and processed for PTP1B mRNA expression. Epididymal and retroperitoneal fat pads were removed and weighed as a measure of body adiposity. In addition to this primary study, an additional study was also conducted that was similar to that described above, except that rats were infused for only 10 days with either saline or leptin (3 μg/day icv) before death.
Experiment 3: Effects of HF diet on PTP1B expression and leptin sensitivity in ob/ob mice.
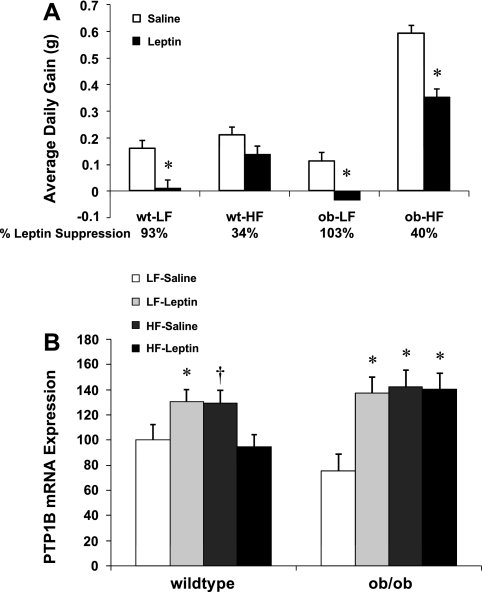
To test the effects of leptin and diet on PTP1B mRNA expression, 8-wk-old male ob/ob mice and wild-type controls on C57BL6 background were randomly assigned to either a LF or HF diet and consumed the diet for 14 days. At the onset of diet treatments, mice were also implanted with subcutaneous osmotic minipumps delivering either leptin (5 μg/day) or saline. After 14 days of infusion, body weight gain (average daily gain) was determined, mice were killed, and hypothalami were isolated to assess PTP1B mRNA expression via real-time PCR.
To test the effects of HF diet on leptin sensitivity in ob/ob mice, a separate group of wild-type and ob/ob mice was injected with varying doses of leptin to establish doses producing roughly equivalent decreases in food intake following intraperitoneal leptin injection. Once equivalent doses were identified on a LF diet, these mice were placed on a HF diet for 14 days and were then treated with leptin or saline, and 24 h food intake was recorded. On the day of individual study, food was pulled ∼3 h after lights on, injections were made ∼2 h before lights off, food was returned 1 h later, and food intake over the subsequent 24 h was assessed.
RNA extraction and real-time PCR analysis.
PTP1B mRNA expression was measured in wedges of mediobasal hypothalamus, defined caudally by the mammillary bodies, rostrally by the optic chiasm, laterally by the optic tract, and dorsally by the apex of the third ventricle (24, 25). These punches contain principally arcuate nucleus, but we cannot rule out the inclusion of other brain areas and for this reason refer to them as mediobasal hypothalamus. Specifically, it is possible that portions of the ventromedial nucleus, superchiasmatic nucleus, and premammillary areas may also be included. Mediobasal hypothalamic wedges were excised, and total RNA was isolated using Tri-Reagent (Molecular Research Center, Cincinnati, OH) and quantified spectrophotometrically. Confirmation of intact 18S and 28S RNA bands was achieved by ethidium bromide staining after electrophoresis of samples in 1% agarose/formaldehyde gels. mRNA was reversed transcribed into cDNA, and mRNA expression was determined using the SYBR green methodology in optical 384-well plates in an ABI PRISM 7900 sequence detector (Applied Biosystems, Branchburg, NJ). All expression data were normalized to cyclophilin or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression levels. Primers were designed using the PrimerExpress Software (Applied Biosystems). Primers are as follows: rat Ptp1b (ptpn1), forward: cgagggtgcaaagttcatcat, reverse: ggtcttcatgggaaagctcctt; Mouse PTP1B (ptpn1), forward: gaaggcagagcccagtcaag, reverse: catggatgtggtacggaaggt; rat cyclophilin, forward: ggagatggcacaggaggaaa, reverse: cccatagtgcttcagcttgaagtt; mouse cyclophilin, forward: catggatgtggtacggaaggt, reverse: tgacatccttcagtggcttgtc; rat GAPDH, forward: aacgaccccttcattgac, reverse: tccacgacatactcagcac.
Protein extraction and Western blot.
Frozen brain blocks containing hypothalamus were placed on a cryostat and trimmed on each side to reproducible anatomical points just before and after the hypothalamic arcuate nucleus (∼3 mm thick). A single punch was made over each arcuate nucleus (2 punches/section) using a 0.8-mm-diameter punch in a slightly overlapping pattern along the midline (over the 3rd ventricle). As above, these punches contain principally arcuate nucleus, but we cannot rule out the inclusion of other brain areas and for this reason refer to them as punches of mediobasal hypothalamus. Punches were placed in lysis buffer [1% Triton X-100, 100 mmol/l Tris (pH 7.4), 100 mmol/l sodium pyrophosphate, 100 mmol/l sodium fluoride, 10 mmol/l EDTA, 10 mmol/l sodium vanadate, 2 mmol/l phenylmethylsufonyl fluoride, and 0.1 g/l aprotinin] and snap-frozen using liquid nitrogen. Subsequently, the extracts were sonicated and centrifuged to remove insoluble material. Total protein (50 μg) was electrophoretically separated on an SDS-PAGE and transferred to a nitrocellulose membrane. Blots were blocked in 5% nonfat dry milk or 1% BSA and probed with a commercially available PTP1B antibody (1:1,000; Upstate Biotechnology, Lake Placid, NY). Positive signal was imaged using a chemiluminescent substrate (ECL Reagent; Amersham Biosciences, Piscataway, NJ) and exposure to autoradiograph film, and afterward the blot was stripped and reprobed for levels of β-actin.
Statistical analysis.
Data were analyzed using the SAS software package (SAS V8; SAS Institute, Cary, NC) using a two-tailed t-test or ANOVA using the general linear model procedure. When experiment wide tests were significant, post hoc comparisons were made using the LSMEANS statement with the PDIFF option, and thus represent least-significant difference tests for preplanned comparisons. In some studies, animal numbers were spread across two independent replicates, with replicate included in the statistical model. For Western blot analysis, levels of PTP1B were normalized to β-actin, whereas for PCR levels were normalized to cyclophilin or GAPDH. All data are expressed as means ± SE, with a probability value of <0.05 considered statistically significant.
RESULTS
Experiment 1: HF diets induce central leptin resistance and increase hypothalamic PTP1B.
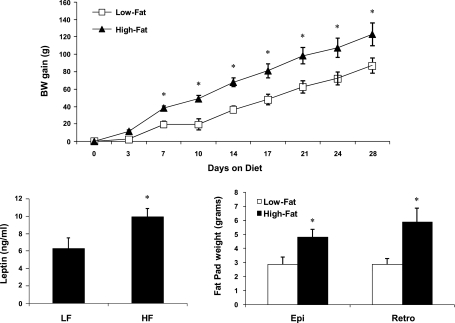
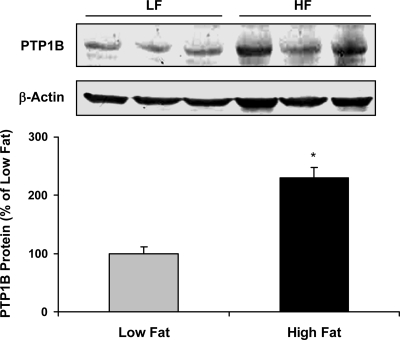
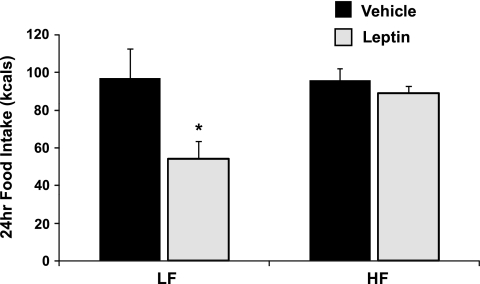
In response to 28 days of HF feeding, rats exhibited increased body weight, body adiposity, and circulating leptin levels (Fig. 1). In addition, PTP1B protein levels within the mediobasal hypothalamus were significantly higher in HF-fed rats compared with LF-fed rats (P = 0.003; Fig. 2). These increases in body adiposity, circulating leptin, and hypothalamic PTP1B are also associated with the development of central leptin resistance, since animals consuming HF diet for 28 days failed to respond to leptin (P = 0.36; Fig. 3), even though this dose is sufficient to significantly suppress food intake in LF-fed rats (P = 0.03). Consistent with previous observations (25, 30), these data indicate that hypothalamic PTP1B levels are increased in a setting of leptin resistance and likely contribute to the reduction of leptin sensitivity.
Fig. 1.
Effects of a high-fat (HF) diet on body weight, body adiposity, and circulating leptin. Male Long Evans rats were placed on a low-fat (LF) or HF diet for 28 days (5–6 rats/group). Body weight gain was measured throughout the experiment, and on day 28 rats were killed and serum leptin levels and epididymal and retroperitoneal fat pad weights were assessed. Compared with LF-fed controls, rats consuming a HF diet weighed significantly more (P = 0.01) with greater circulating leptin (P = 0.03) and larger fat pads (P = 0.03) on day 28.
Fig. 2.
HF diets increase protein tyrosine phosphatase 1 B (PTP1B) protein within the mediobasal hypothalamus. Mediobasal hypothalami were collected from rats described in Fig. 1, total protein was extracted, and levels of PTP1B protein were measured via Western Blot. Animals consuming a HF diet had significantly higher levels of PTP1B protein compared with rats consuming a LF diet (P = 0.003).
Fig. 3.
Consumption of a HF diet induces central leptin resistance. Rats bearing icv cannulas were fed a LF or HF diet for 28 days (9 rats/group). On day 28, rats were injected icv with either saline or leptin (3 μg), and 24-h food intake was assessed. Leptin significantly suppressed food intake in LF-fed rats (P = 0.03) but had no effect in rats consuming HF (P = 0.36).
Experiment 2: Effects of chronic hyperleptinemia on hypothalamic PTP1B expression.
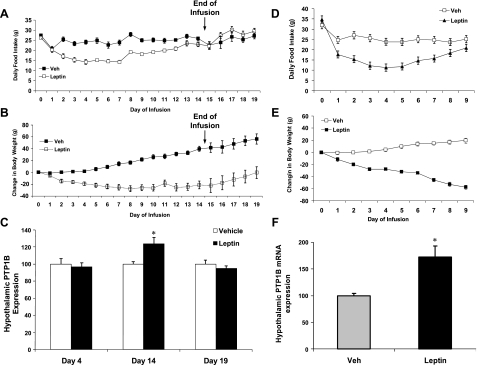
Because obesity is associated with a chronic elevation of circulating leptin, we tested the hypothesis that chronic hyperleptinemia increases PTP1B. To test this hypothesis, two experiments were conducted to determine whether chronic intracerebroventricular leptin infusion (3 μg/day) is sufficient to increase hypothalamic PTP1B expression. In the first study, leptin was continuously infused for 14 days, and rats were killed on days 4, 14, and 19 to generate groups collected during the maximal effect of leptin on food intake (day 4), when the effects of leptin on food intake were diminished (day 14), and then following the cessation of leptin infusion when body weight is recovering (day 19). As expected, leptin infusion significantly decreased both body weight and food intake (P < 0.01; Fig. 4, A and B), although the effects of leptin on food intake waned over time such that food intake was largely normalized by day 14 (Fig. 4A). Leptin also markedly reduced retroperitoneal and epididymal fat pads in a time-dependent fashion, with retroperitoneal fat pad weights being numerically but not significantly lower on day 4 (1.08 ± 0.1 vs. 0.82 ± 0.2 g; P > 0.26), markedly decreased on day 14 (1.8 ± 0.2 vs. 0.2 ± 0.06 g; P < 0.001), and beginning to normalize by day 19 (1.2 ± 0.2 g vs. 0.55 ± 0.2 g; P < 0.05). Hypothalamic PTP1B mRNA levels were significantly increased on day 14 (P = 0.02; Fig. 4C). However, no effect of leptin on PTP1B mRNA expression was detected on days 4 or 19, indicating that the increase in PTP1B mRNA expression requires chronic leptin exposure and readily normalizes following the cessation of leptin infusion.
Fig. 4.
Effect of chronic icv leptin infusion on food intake, body weight, and hypothalamic PTP1B mRNA expression. Two separate experiments were conducted to assess and replicate the effects of icv leptin infusion on hypothalamic PTP1B mRNA expression. In the first (A–C), rats bearing icv cannulas were infused with leptin (3 μg/day) or saline for 14 days. Daily food intake (A) and body weight (B) were assessed throughout the study, and on days 4 and 14 of infusion, as well as on day 19 (5 days following the cessation of infusion), subgroups of animals (6–8 rats/group) were killed, and levels of PTP1B mRNA expression were determined via real-time PCR. Leptin infusion significantly decreased both food intake and body weight compared with saline-infused controls (P < 0.01). Leptin infusion also increased levels of hypothalamic PTP1B mRNA on day 14 (P = 0.02) but had no effect on PTP1B mRNA either on days 4 or 19 (P > 0.05). A second experiment (D–F) was conducted as above, except rats were infused with leptin (3 μg/day) or saline for 10 days (5 rats/group) and killed on day 10, and levels of PTP1B mRNA in the mediobasal hypothalamus were determined via real-time PCR. Again chronic icv leptin infusion significantly suppressed food intake and body weight (P = 0.003), and PTP1B mRNA levels were significantly higher in leptin-infused rats compared with controls (P = 0.01).
In addition to this primary study, an additional group of rats were implanted with indwelling osmotic minipumps and infused with leptin (3 μg/day icv) or saline for 10 days to replicate the observed effect of chronic leptin administration. As before, leptin treatment persistently reduced body weight but only transiently reduced food intake (Fig. 4, D and E). Levels of PTP1B mRNA were significantly increased in leptin-infused rats compared with vehicle-infused controls on day 10 (P = 0.01; Fig. 4F), replicating the previous observation and indicating that hyperleptinemia is sufficient to increase hypothalamic PTP1B, independent of a change in body adiposity or exposure to dietary fat.
Experiment 3: HF diet increases PTP1B and induces leptin resistance in the absence of hyperleptinemia.
The above experiments suggest that leptin is sufficient to increase hypothalamic PTP1B, but they do not test whether hyperleptinemia is necessary for diet-induced increases in PTP1B or the development of leptin resistance. To test the necessity of hyperleptinemia, leptin-deficient ob/ob mice or wild-type controls (C57BL6 background) were placed on a HF or LF diet for 14 days and simultaneously infused with leptin (5 μg/day) via subcutaneous osmotic minipumps. In response to a HF diet, ob/ob mice exhibited a marked increase in their rate of body weight gain compared with wild-type mice, with wild-type mice increasing the rate of body weight gain by 31% on the HF diet but with ob/ob mice increasing weight gain by 737% on the HF diet. Leptin infusion reduced body weight gain by 93% in wild-type mice on a LF diet (P = 0.007; Fig. 5A), but by only 34% in wild-type mice on the HF diet (P = 0.09; Fig. 5A). In ob/ob mice, leptin significantly suppressed weight gain on both the LF and the HF diet (P < 0.01). However, the efficacy of this effect was varied, since leptin infusion suppressed body weight gain by 103% in mice on the LF diet but only by 40% in ob/ob mice on the HF diet. These data are consistent with the possibility that HF diets reduce the efficacy of leptin signaling, even in the absence of leptin.
Fig. 5.
Effects of diet and leptin on body weight gain and hypothalamic PTP1B in leptin-deficient mice. Leptin-deficient ob/ob mice were placed on a LF or HF diet for 14 days (7–8 mice/group) and were also simultaneously implanted with sc osmotic minipumps delivering either saline or leptin (5 μg/day). On day 14, mice were killed, and levels of hypothalamic PTP1B mRNA were determined via real-time PCR. A: leptin infusion reduced body weight gain in both wild-type and ob/ob mice on a LF diet (*P < 0.01). However, the effect of leptin to reduce body weight gain was attenuated in mice on a HF diet. B: both leptin infusion and exposure to a HF diet increased hypothalamic PTP1B mRNA expression in LF-fed wild-type mice, consistent with the prior rat experiments. Additionally, these same effects were also replicated in ob/ob mice, indicating that HF diets are sufficient to increase PTP1B even in the absence of leptin (*P < 0.05 and †P < 0.10 compared with LF-saline).
After 14 days of treatment, mice were killed, and levels of PTP1B in the mediobasal hypothalamus were determined via real-time PCR. In wild-type mice, chronic leptin treatment increased PTP1B mRNA expression (P = 0.048; Fig. 5B), whereas exposure to a HF diet alone also increased hypothalamic PTP1B mRNA expression (P = 0.069; Fig. 5B). In ob/ob mice, chronic leptin infusion also increased hypothalamic PTP1B mRNA expression compared with saline-infused ob/ob mice on a LF diet (P < 0.001; Fig. 5B). In addition, exposure to a HF diet alone also increased PTP1B mRNA levels in ob/ob mice (P < 0.001; Fig. 5B). Although this HF-induced increase in PTP1B replicates our prior observations in rats, this increase cannot be dependent on hyperleptinemia because it occurs in a leptin-deficient background.
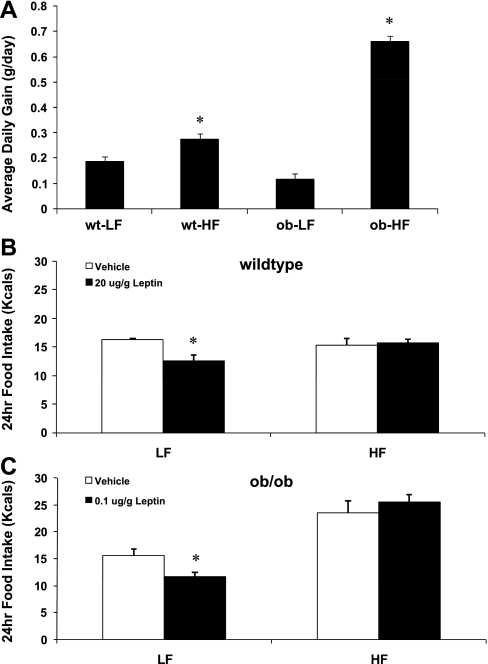
If HF diet-induced increases in PTP1B do not depend on hyperleptinemia, then it is possible that the induction of HF diet-induced leptin resistance also does not depend on hyperleptinemia. To directly test this hypothesis, leptin sensitivity was determined in wild-type (C57BL6) and ob/ob mice on a LF or HF diet. There were again marked differences in the effect of HF diet on body weight gain between wild-type and ob/ob mice, with wild-type mice increasing average daily gain by 46% on the HF diet and ob/ob mice increasing average daily gain by 564% (Fig. 6A). On a LF diet, wild-type mice required a substantially larger dose of leptin intraperitoneally to produce an equivalent reduction in food intake compared with ob/ob mice (Fig. 6, B and C), indicating that the absence of leptin markedly enhances the sensitivity to exogenous leptin. However, when placed on a HF diet for 14 days, both ob/ob mice and wild-type mice exhibited a decrease in leptin sensitivity, since doses that significantly suppressed food intake on LF (P < 0.05; Fig. 6, B and C) did not significantly decrease food intake in animals fed a HF diet (P > 0.05). Thus hyperleptinemia is not required for HF diet-induced increases in PTP1B or decreases in leptin sensitivity.
Fig. 6.
Effect of HF diet on body weight gain and leptin sensitivity in control and leptin-deficient mice. Male, leptin-deficient (ob/ob) mice and wild-type controls were fed either a LF or HF diet for 14 days (5 mice/group). On day 14, mice were injected ip with leptin or saline, and 24-h food intake was recorded. A: as noted in the prior study, ob/ob were highly sensitive to the HF diet, increasing body weight gain ∼564% (P = 0.001) when placed on the HF diet compared with an ∼40% increase in body weight gain in wild-type mice on the HF diet (P = 0.004). B: in wild-type mice on a LF diet, leptin (20 μg/g) significantly suppressed 24-h food intake (P = 0.02), but this same dose failed to significantly reduced food intake after 14 days of HF feeding (P = 0.75). C: in ob/ob mice on a LF diet, leptin also suppressed food intake, but at a much lower dose (0.1 μg/g; P = 0.02). However, after 14 days of HF feeding, this dose also failed to significantly suppress food intake (P = 0.40).
DISCUSSION
Exposure to a HF diet leads to increased body adiposity and a syndrome of leptin resistance (23, 27). Yet, whether leptin resistance is primarily a cause or a consequence of diet-induced obesity remains a matter of debate (35). Leptin resistance involves at least two separate mechanisms, the first being reduced transport across the blood-brain barrier (2) and the second a reduced capacity for cellular leptin signaling within target neurons (27). This latter resistance is evident in the attenuated response to direct brain leptin injections (which bypass the blood-brain barrier), both in terms of food intake and activation of intracellular signaling.
PTP1B is a phosphatase that binds to and dephosphorylates Jak2, the initial tyrosine kinase mediating leptin receptor signaling (9, 40). Overexpression of PTP1B in vitro dampens leptin receptor signaling (19), and global or neuron-specific deletion of PTP1B produces mice that are lean, hypersensitive to leptin, and resistant to diet-induced obesity (3, 9, 40). The primary objective of the current work was to determine whether PTP1B is a dynamic regulator of leptin signaling, whether increased PTP1B contributes to the acute loss in leptin sensitivity that accompanies a HF diet, and to identify potential mediators of this change.
To test this overall hypothesis, we first determined whether levels of PTP1B were increased within leptin-sensitive brain areas in settings of leptin resistance. The current work demonstrates that resistance to intracerebroventricular leptin is evident within 28 days of feeding a HF diet, and this leptin resistance is associated with a significant increase in PTP1B protein within the mediobasal hypothalamus. These observations are consistent with our prior evidence for increased PTP1B in a model of age-associated leptin resistance (25), since in both cases we detect increased PTP1B protein levels in leptin-resistant animals compared with leptin-sensitive animals. These observations are also consistent with recent publications showing similar increases in PTP1B mRNA or protein in animals consuming a HF diet (30, 42). These publications also suggest that increases in PTP1B protein reflect transcriptional activation and increased PTP1B mRNA (42) and that the increase in PTP1B protein is associated with a complementary increase in PTPT1B activity (30). Therefore, although our data only capture changes in PTP1B mRNA or protein, these and other data suggest a strong correlation between PTP1B activity and mRNA and protein levels (7, 13, 32, 37, 39). Last, considering that mouse genetic models clearly implicate PTP1B in the regulation of leptin signaling (3, 9, 19, 40), these data suggest that PTP1B is not simply a constitutive regulator of leptin signaling but instead that PTP1B contributes to the reduction in leptin sensitivity that occurs following exposure to a HF diet.
If increased PTP1B contributes to the development of leptin resistance, then identifying mechanisms underlying this increase may provide an opportunity to prevent leptin resistance. PTP1B is increased in models of age- and diet-induced obesity, and each of these settings is associated with changes in body adiposity and circulating leptin levels. Therefore, it is possible that increased body adiposity, increased circulating leptin, or both might contribute to the observed increases in PTP1B. To test whether hyperleptinemia is sufficient to increase PTP1B in the absence of obesity, leptin was chronically infused in the third cerebroventricle of normal, chow-fed rats. As expected, leptin persistently suppressed body weight but exerted a biphasic effect on food intake that was marked by an initial decrease in food intake followed by an eventual waning of this effect. This weakening of the feeding response following prolonged leptin infusion has been termed leptin-induced leptin resistance (29, 34). Interestingly, no difference was observed in hypothalamic PTP1B levels at the early time point (day 4), when leptin was maximally suppressing food intake. Similarly, no effect of leptin was detected on day 19, five days following the cessation of leptin infusion. However, a significant increase in PTP1B mRNA was detected at day 14, coincident with the period when leptin's anorectic effects were diminished.
To replicate this effect of chronic leptin treatment, a second study was conducted in which rats were infused chronically with leptin intracerebroventricularly for 10 days. Leptin again exerted a biphasic effect on food intake but a persistent reduction of body weight, and again a significant increase in PTP1B mRNA expression was observed during the period in which leptin's suppression of food intake was diminished (day 10). Thus this second experiment validates the first and suggests that chronic exposure to leptin is sufficient to increase hypothalamic PTP1B. It should be noted that both studies involved intracerebroventricular infusions of leptin at doses that are likely pharmacological. As such, these data do not definitively prove that obesity-induced hyperleptinemia contributes to the observed increases in PTP1B in rats (and mice) on a HF diet. It is also not clear why the increase in PTP1B was only observed following prolonged exposure to leptin, and this delay in the leptin effect suggests that the increase in PTP1B may not be due to a direct effect of leptin. In both studies, the increase in PTP1B was observed only when leptin-dependent suppression of food intake waned, leading to the speculation that the increase in PTP1B might contribute to the observation of leptin-induced leptin resistance. In addition, following removal of this elevated leptin, PTP1B levels normalize in association with normalized leptin sensitivity, as has been demonstrated in animals that are fed a HF diet but then returned to a chow diet (18). In summary, these data suggest that prolonged exposure to high levels of leptin is sufficient to increase PTP1B and that chronic hyperleptinemia may contribute to the effects of HF diet on hypothalamic PTP1B levels.
The above experiments suggest that chronic hyperleptinemia is sufficient to increase hypothalamic PTP1B expression. To determine whether this hyperleptinemia is necessary for the increase in PTP1B following a HF diet, the effects of a HF diet on hypothalamic PTP1B were tested in ob/ob mice, since this approach removes any effect of leptin. In wild-type mice, exposure to a HF diet attenuated the effect of leptin to reduce body weight gain, consistent with the hypothesis that HF diets induce leptin resistance. In addition, exposure of wild-type mice to either chronic leptin infusion or to a HF diet led to increases in hypothalamic PTP1B expression. Thus these observations are consistent with the experiments in rats, and indicate that either chronic hyperleptinemia or a HF diet is sufficient to increase hypothalamic PTP1B expression. In ob/ob mice, chronic leptin infusion again increased PTP1B mRNA expression within the hypothalamus. In addition, hypothalamic PTP1B mRNA was also increased in saline-infused ob/ob mice on a HF diet. Although this observation is consistent with previous experiments, it is also unique considering that the HF diet is increasing PTP1B in the absence of hyperleptinemia. Therefore, while chronic hyperleptinemia appears to be sufficient to increase PTP1B within the hypothalamus, it appears to not be necessary for HF diet-induced increases in PTP1B.
In addition to the observed changes in PTP1B, it should also be noted that ob/ob mice exhibited a marked increase in body weight gain compared with wild-type mice on a HF diet. This effect was observed in both studies, with the HF diet increasing weight gain by 30–40% in wild-type mice but increasing weight gain by 500–700% in ob/ob mice. These data provide compelling evidence that the absence of leptin exacerbates HF diet-induced obesity and by extension are also consistent with the hypothesis that loss of leptin signaling (leptin resistance) similarly exacerbates the response to a HF diet. These observations are consistent with previous work indicating that manipulations which inhibit leptin signaling result in increased weight gain when animals are placed on a HF diet (43). Thus these data collectively support a model in which physiological leptin signaling provides a protection against HF diet-induced weight gain, although this protection is incomplete and insufficient to prevent some weight gain. Yet, because this protection exists, individuals with reduced (leptin resistant) or absent (ob/ob mice) leptin signaling exhibit an exacerbated response to a HF diet.
If levels of PTP1B are increased in HF-fed ob/ob mice, then is it possible that leptin resistance also develops in the absence of hyperleptinemia? To test this hypothesis, we first established doses of leptin that produce equivalent changes in food intake between wild-type and ob/ob mice on a LF diet. On a LF diet, ob/ob mice were substantially more sensitive to intraperitoneal leptin administration, reinforcing the concept that leptin inhibits its own sensitivity. However, despite the high baseline leptin sensitivity, doses of leptin that suppressed food intake in LF-fed ob/ob mice failed to significantly suppress food intake following 14 days on a HF diet. Thus these data not only demonstrate that HF diets reduce leptin sensitivity; they also indicate that hyperleptinemia is not necessary for this loss of sensitivity.
The current work provides evidence for four major conclusions regarding the relationship between HF diets, hyperleptinemia, hypothalamic PTP1B levels, and leptin resistance. The first is that PTP1B levels are dynamically regulated and increased within the mediobasal hypothalamus in settings of leptin resistance, consistent with prior observations (30, 42). The implication therefore is that PTP1B is not simply a static regulator of leptin signaling but instead that alterations in PTP1B levels or activity are positioned to contribute to fluctuations in leptin sensitivity. A second major conclusion of the current work is that chronic exposure to leptin is sufficient to increase PTP1B mRNA levels within the mediobasal hypothalamus. This observation is consistent with evidence supporting the existence of leptin-induced leptin resistance (29, 34) and suggests that periods of chronic hyperleptinemia may induce leptin resistance via an induction of PTP1B. If so, these observations raise the possibility that PTP1B acts as a negative feedback regulator for leptin signaling, similar to Socs3 (5). Socs3 and PTP1B both inhibit leptin receptor signaling, although they impinge on different sites on the leptin receptor. Socs3 binds to specific tyrosine residues of the leptin receptor and attenuates leptin activation of Stat3 and perhaps other signaling systems (1, 6). Contrastingly, PTP1B dephosphorylates Jak2 (9, 40) and thus would be predicted to inhibit all aspects of downstream leptin signaling. It should be noted that, in the two current studies involving chronic leptin infusion, the increase in PTP1B was only detected following an extended period of leptin infusion. This is in contrast to the direct and rapid induction of Socs3 that occurs following leptin treatment (5, 17). Thus it remains unclear whether the increase in PTP1B is directly due to leptin signaling in the hypothalamus or indirectly due to a secondary effect of leptin infusion.
A third major conclusion from the current work is that HF diets also increase PTP1B via a mechanism that is leptin independent. The basis for this conclusion is the observation that PTP1B levels increase in leptin-deficient ob/ob mice following exposure to a HF diet and thus is by definition leptin independent. Currently, the identity of this leptin-independent mechanism is unknown. To date, there is relatively little information regarding cellular mechanisms regulating PTP1B expression, although there is evidence that leptin and other cytokine receptors stimulate PTP1B expression or activity (7, 21). One possibility is insulin, since there is evidence that insulin regulates PTP1B activity (12, 31, 38). However, in the current study, we did not detect a significant difference in insulin levels between HF and LF rats (LF: 5.13 ± 0.93; HF: 5.95 ± 0.92 ng/ml, P = 0.56), despite the increase in PTP1B. In addition to insulin, a very recent publication indicates that inflammation upregulates PTP1B mRNA and protein levels in a variety of tissues, including the hypothalamus (42), primarily via the activation of tumor necrosis factor-α and nuclear factor-κB. Obesity is often associated with an increase in inflammatory markers, and as such it is possible that HF diets increase tumor necrosis factor or some other inflammatory signal, leading to leptin-independent increases in PTP1B.
Last, the fourth major conclusion is that leptin resistance can develop in the absence of hyperleptinemia. This conclusion extends from observations demonstrating that the efficacy of leptin to suppress body weight gain and food intake is attenuated in ob/ob mice consuming a HF diet. Again, because these observations are made in the absence of leptin, the conclusion is that these changes must be leptin independent. In addition, the congruency between hypothalamic PTP1B levels and leptin sensitivity is consistent with a leptin-independent increase in PTP1B contributing to the induction of HF diet-induced leptin resistance in ob/ob mice. These issues of leptin dependence and independence have significant implications for the understanding of leptin resistance and its contribution to the development of obesity. If the development of leptin resistance were to depend on hyperleptinemia and thus require the preexistence of obesity, then leptin resistance would by necessity be primarily a consequence of obesity. As such, the observation that chronic hyperleptinemia is sufficient to induce PTP1B and leptin resistance indicates that at least a portion of the leptin resistance that is observed in obesity is likely a consequence of the excess leptin production. Nevertheless, we also observed increases in PTP1B and the induction of leptin resistance in the absence of hyperleptinemia. These data are therefore also consistent with the possibility that leptin resistance develops before obesity (hyperleptinemia) and as such contributes to its development.
In summary, the data described herein demonstrate that exposure to a HF diet is sufficient to reduce responsiveness to intracerebroventricular leptin administration in rats and that this loss of leptin sensitivity is associated with increases in PTP1B within the mediobasal hypothalamus. The current data also provide support for both a leptin-dependent and leptin-independent mechanism underlying this increase, with the leptin-dependent mechanism illustrated in the increases in PTP1B mRNA levels in response to chronic leptin infusion and the leptin-independent mechanism illustrated by the increased PTP1B and induction of leptin resistance that occurs in ob/ob mice on a HF diet.
GRANTS
This work was supported in part by National Institutes of Health (NIH) Grants NS-051570 and RR-021945 and the Pennington Biomedical Research Foundation to C. D. Morrison, and by the Molecular Mechanisms, Genomics or Cell Biology & Bioimaging core facilities that are supported in part by a Clinical Nutrition Research Unit (NIH 1P30-DK-072476) center grant.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Banks AS, Davis SM, Bates SH, Myers MG Jr. Activation of downstream signals by the long form of the leptin receptor. J Biol Chem 275: 14563–14572, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Banks WA, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity is acquired and reversible. Am J Physiol Endocrinol Metab 285: E10–E15, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med 12: 917–924, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Berthoud HR, Morrison C. The brain, appetite, obesity. Annu Rev Psychol 59: 55–92, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Bjorbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem 274: 30059–30065, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Bjorbak C, Lavery HJ, Bates SH, Olson RK, Davis SM, Flier JS, Myers MG Jr. SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J Biol Chem 275: 40649–40657, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Callero MA, Perez GM, Vittori DC, Pregi N, Nesse AB. Modulation of protein tyrosine phosphatase 1B by erythropoietin in UT-7 cell line. Cell Physiol Biochem 20: 319–328, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Wertheimer SJ, Lin CH, Katz SL, Amrein KE, Burn P, Quon MJ. Protein-tyrosine phosphatases PTP1B and syp are modulators of insulin-stimulated translocation of GLUT4 in transfected rat adipose cells. J Biol Chem 272: 8026–8031, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Cheng A, Uetani N, Simoncic PD, Chaubey VP, Lee-Loy A, McGlade CJ, Kennedy BP, Tremblay ML. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev Cell 2: 497–503, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334: 292–295, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Dadke S, Chernoff J. Interaction of protein tyrosine phosphatase (PTP) 1B with its substrates is influenced by two distinct binding domains. Biochem J 364: 377–383, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dadke S, Kusari A, Kusari J. Phosphorylation and activation of protein tyrosine phosphatase (PTP) 1B by insulin receptor. Mol Cell Biochem 221: 147–154, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Dadke SS, Li HC, Kusari AB, Begum N, Kusari J. Elevated expression and activity of protein-tyrosine phosphatase 1B in skeletal muscle of insulin-resistant type II diabetic Goto-Kakizaki rats. Biochem Biophys Res Commun 274: 583–589, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Delibegovic M, Bence KK, Mody N, Hong EG, Ko HJ, Kim JK, Kahn BB, Neel BG. Improved glucose homeostasis in mice with muscle-specific deletion of protein-tyrosine phosphatase 1B. Mol Cell Biol 27: 7727–7734, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egawa K, Maegawa H, Shimizu S, Morino K, Nishio Y, Bryer-Ash M, Cheung AT, Kolls JK, Kikkawa R, Kashiwagi A. Protein-tyrosine phosphatase-1B negatively regulates insulin signaling in l6 myocytes and Fao hepatoma cells. J Biol Chem 276: 10207–10211, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 283: 1544–1548, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron 23: 775–786, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab 5: 181–194, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Kaszubska W, Falls HD, Schaefer VG, Haasch D, Frost L, Hessler P, Kroeger PE, White DW, Jirousek MR, Trevillyan JM. Protein tyrosine phosphatase 1B negatively regulates leptin signaling in a hypothalamic cell line. Mol Cell Endocrinol 195: 109–118, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, Moghal N, Lubkin M, Kim YB, Sharpe AH, Stricker-Krongrad A, Shulman GI, Neel BG, Kahn BB. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol 20: 5479–5489, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam NT, Lewis JT, Cheung AT, Luk CT, Tse J, Wang J, Bryer-Ash M, Kolls JK, Kieffer TJ. Leptin increases hepatic insulin sensitivity and protein tyrosine phosphatase 1B expression. Mol Endocrinol 18: 1333–1345, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, and et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1: 1155–1161, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Morrison CD Leptin resistance and the response to positive energy balance. Physiol Behav 94: 660–663, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison CD, Morton GJ, Niswender KD, Gelling RW, Schwartz MW. Leptin inhibits hypothalamic Npy and Agrp gene expression via a mechanism that requires phosphatidylinositol 3-OH-kinase signaling. Am J Physiol Endocrinol Metab 289: E1051–E1057, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Morrison CD, White CL, Wang Z, Lee SY, Lawrence DS, Cefalu WT, Zhang ZY, Gettys TW. Increased hypothalamic protein tyrosine phosphatase 1B contributes to leptin resistance with age. Endocrinology 148: 433–440, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrison CD, Xi X, White CL, Ye J, Martin RJ. Amino acids inhibit Agrp gene expression via an mTOR-dependent mechanism. Am J Physiol Endocrinol Metab 293: E165–E171, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 70: 537–656, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Myers MP, Andersen JN, Cheng A, Tremblay ML, Horvath CM, Parisien JP, Salmeen A, Barford D, Tonks NK. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J Biol Chem 276: 47771–47774, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Pal R, Sahu A. Leptin signaling in the hypothalamus during chronic central leptin infusion. Endocrinology 144: 3789–3798, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Picardi PK, Calegari VC, Prada Pde O, Moraes JC, Araujo E, Marcondes MC, Ueno M, Carvalheira JB, Velloso LA, Saad MJ. Reduction of hypothalamic protein tyrosine phosphatase improves insulin and leptin resistance in diet-induced obese rats. Endocrinology 149: 3870–3880, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravichandran LV, Chen H, Li Y, Quon MJ. Phosphorylation of PTP1B at Ser(50) by Akt impairs its ability to dephosphorylate the insulin receptor. Mol Endocrinol 15: 1768–1780, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Ropelle ER, Pauli JR, Prada PO, de Souza CT, Picardi PK, Faria MC, Cintra DE, Fernandes MF, Flores MB, Velloso LA, Saad MJ, Carvalheira JB. Reversal of diet-induced insulin resistance with a single bout of exercise in the rat: the role of PTP1B and IRS-1 serine phosphorylation. J Physiol 577: 997–1007, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salmeen A, Andersen JN, Myers MP, Tonks NK, Barford D. Molecular basis for the dephosphorylation of the activation segment of the insulin receptor by protein tyrosine phosphatase 1B. Mol Cell 6: 1401–1412, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Scarpace PJ, Matheny M, Zhang Y, Shek EW, Prima V, Zolotukhin S, Tumer N. Leptin-induced leptin resistance reveals separate roles for the anorexic and thermogenic responses in weight maintenance. Endocrinology 143: 3026–3035, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Scarpace PJ, Zhang Y. Elevated leptin: consequence or cause of obesity? Front Biosci 12: 3531–3544, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Seely BL, Staubs PA, Reichart DR, Berhanu P, Milarski KL, Saltiel AR, Kusari J, Olefsky JM. Protein tyrosine phosphatase 1B interacts with the activated insulin receptor. Diabetes 45: 1379–1385, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Taghibiglou C, Rashid-Kolvear F, Van Iderstine SC, Le-Tien H, Fantus IG, Lewis GF, Adeli K. Hepatic very low density lipoprotein-ApoB overproduction is associated with attenuated hepatic insulin signaling and overexpression of protein-tyrosine phosphatase 1B in a fructose-fed hamster model of insulin resistance. J Biol Chem 277: 793–803, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Tao J, Malbon CC, Wang HY. Insulin stimulates tyrosine phosphorylation and inactivation of protein-tyrosine phosphatase 1B in vivo. J Biol Chem 276: 29520–29525, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Wu Y, Ouyang JP, Wu K, Wang SS, Wen CY, Xia ZY. Rosiglitazone ameliorates abnormal expression and activity of protein tyrosine phosphatase 1B in the skeletal muscle of fat-fed, streptozotocin-treated diabetic rats. Br J Pharmacol 146: 234–243, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, Haj F, Wang Y, Minokoshi Y, Kim YB, Elmquist JK, Tartaglia LA, Kahn BB, Neel BG. PTP1B regulates leptin signal transduction in vivo. Dev Cell 2: 489–495, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Zabolotny JM, Haj FG, Kim YB, Kim HJ, Shulman GI, Kim JK, Neel BG, Kahn BB. Transgenic overexpression of protein-tyrosine phosphatase 1B in muscle causes insulin resistance, but overexpression with leukocyte antigen-related phosphatase does not additively impair insulin action. J Biol Chem 279: 24844–24851, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Zabolotny JM, Kim YB, Welsh LA, Kershaw EE, Neel BG, Kahn BB. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J Biol Chem 283: 14230–14241, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J, Matheny MK, Tumer N, Mitchell MK, Scarpace PJ. Leptin antagonist reveals that the normalization of caloric intake and the thermic effect of food after high-fat feeding are leptin dependent. Am J Physiol Regul Integr Comp Physiol 292: R868–R874, 2007. [DOI] [PubMed] [Google Scholar]