Abstract
Islet transplantation is a promising therapeutic approach for type 1 diabetes. However, current success rates are low due to progressive graft failure in the long term and inability to monitor graft development in vivo. Other limitations include the necessity of initial invasive surgery and continued immunosuppressive therapy. We report an alternative transplantation strategy with the potential to overcome these problems. This technique involves transplantation of embryonic pancreatic tissue into recipients’ subcutaneous space, eliminating the need for invasive surgery and associated risks. Current results in mouse models of type 1 diabetes show that embryonic pancreatic transplants in the subcutaneous space can normalize blood glucose homeostasis and achieve extensive endocrine differentiation and vascularization. Furthermore, modern imaging techniques such as two-photon excitation microscopy (TPEM) can be employed to monitor transplants through the intact skin in a completely noninvasive manner. Thus, this strategy is a convenient alternative to islet transplantation in diabetic mice and has the potential to be translated to human clinical applications with appropriate modifications.
Keywords: therapies for type 1 diabetes, embryonic pancreatic tissue, subcutaneous site, stem cell-derived islet tissue
type 1 diabetes is a serious disease that affects about two million Americans, causing life-threatening complications in over 100,000 patients per year (32). Patients with type 1 diabetes require lifelong insulin replacement therapy administered via daily injections or insulin pump. Not only is this inconvenient, but intensive exogenous insulin therapy could lead to complications from hypoglycemia unawareness. A promising approach for more permanent and long-term insulin replacement is islet transplantation (50, 53). However, this approach has numerous limitations, such as the need for millions of donor islets, need for initial invasive surgery and prolonged immunosuppressive therapy, poor graft survival, and lack of suitable techniques to monitor transplant development in vivo. Such problems greatly limit the long-term success rate of islet transplantation (3, 6–8, 14, 50, 53). The ideal transplantation strategy for type 1 diabetes should include minimally invasive placement of transplants, no need for immunosuppression, long-term reversal of diabetes, and the possibility for noninvasive in vivo monitoring of transplants. We report considerable success in achieving these goals in diabetic mouse models through subcutaneous transplantation of GFP-expressing embryonic pancreatic tissue into recipient mice with type 1 diabetes followed by noninvasive graft monitoring with two-photon excitation microscopy (TPEM).
Even though the techniques for intrahepatic embolization of islets through portal vein are being progressively refined, life-threatening complications, such as portal hemorrhage, portal hypertension, and thrombosis, can still occur (3, 6, 9, 50). The subcutaneous space is a superficial and easily accessible site where transplants can be performed in a minimally invasive manner with no risk and little stress to recipients. In addition to eliminating risks associated with portal vein cannulation, the subcutaneous site has the added advantage of extensive surface area and the option for repeated transplants if necessary. However, this site is limited by poor vascularaization potential, resulting in low success rates in previous attempts at islet transplantation (19, 28, 54, 63). Although different techniques are being developed to improve graft vascularization in the subcutaneous space, these require complex manipulations and/or multiple surgeries for the same transplant (10, 29, 42, 59, 63). Transplantation of embryonic tissue as opposed to mature islets is a simpler solution for maintaining graft survival and vascularization in the subcutaneous space. Being a built-in source of angiogenic factors, embryonic tissue may achieve better vascularization than mature islets (44–46). It is possible that embryonic tissue may also resist rejection due to its immune-privileged nature (16–18, 26, 31, 34, 41), although the immunogenicity of embryonic pancreatic tissue has not yet been demonstrated. Embryonic pancreas from mice expressing green fluorescent protein (GFP) under the regulation of insulin 1 promoter (MIP-GFP mice) emit easily detectable bright fluorescence from their β-cells after embryonic day E13.5 (20, 21), providing an excellent model for in vivo graft monitoring through the intact skin.
The current study demonstrates successful endocrine differentiation, vacularization, and significant improvement of blood glucose homeostasis following subcutaneous transplantation of embryonic pancreata into immune-deficient diabetic mice. In addition to normalization of glucose tolerance, subcutaneous transplants also enabled diabetic mice to gain back the lost adipose tissue and enhanced overall well-being. Considering that these outcomes occurred without specialized maneuvers, it is predictable that better graft survival rates in non-immune-deficient animals may be achieved through simple facilitation techniques such as addition of exogenous growth factors or anti-inflammatory agents. Thus, the current results demonstrate a successful first step toward correction of type 1 diabetes through noninvasive transplantation, which can be refined and customized for human patients through appropriate modifications such as the use of stem cell-derived islet tissue.
METHODS
Animals
Donor embryonic pancreata were obtained from MIP-GFP mice, originally donated by Dr. Graeme Bell at the University of Chicago and now maintained in our colony (20, 21). Recipients were immune-deficient NCRNU-M-M nude mice (Taconic) (60), and NOD-SC-M-M mice (Taconic) (61) rendered diabetic with streptozotocin (125 mg/kg ip, repeated every 2 wk as necessary until diabetes induced). All recipients were males aged 2–4 mo. Animals were fed standard laboratory chow and were cared for according to the guidelines of the Vanderbilt Institutional Animal Care and Use Committee.
Isolation of Embryonic Pancreata
Pregnant females carrying MIP-GFP embryos (E14.5-E16.5) were used. Gestational age E14.5-E16.5 was selected by comparing the relative success of preliminary transplants at different ages. The mice were anesthetized with ketamine-xylazine (110/10 mg/kg ip). A bilateral subcostal incision was made and extended by a midline transverse incision to expose the abdominal cavity. Uterine horns were exposed one at a time. Starting near the ovary, a longitudinal incision was made along the uterine horn. Embryos were removed and placed in sterile, ice-cold saline. The mice were then quickly killed by cervical dislocation. The embryos were rapidly dissected with Dumont forceps, and the embryonic pancreatic buds were removed and placed in sterile, ice-cold saline and transplanted into recipients as quickly as possible.
Transplantation of Embryonic Pancreata
Pancreata isolated from MIP-GFP embryos were transplanted into NCRNU nude mice or NOD-SC mice. The transplant sites were 1) under the skin of the earlobe or the dorsal surface of the body and 2) underneath the renal capsule. The untreated diabetic control group received mock transplants without pancreatic tissue. Two to four embryonic pancreata were transplanted at each site, depending on the size of the available pancreata. Surgeries were performed under general anesthesia with ketamine-xylazine (110/10 mg/kg ip), and postoperative anlegesia was provided with buprenorphine 0.1 mg·kg−1·day−1 SQ as necessary.
Renal subcapsular transplants.
A dorsal incision was made just over the kidney, and the kidney was exposed by gently pressing below it. A small nick was made in the kidney capsule, and pancreata were placed underneath the capsule with a Dumont forceps and guided in with a blunt-ended microspatula. The kidney was wetted with saline if necessary to prevent drying. After the transplant, the body wall was closed up with Vicryl suture for muscle and nylon for skin. Sutures were removed 7 days post-op.
Subcutaneous transplants.
Through a small (1–2 mm) incision, a subcutaneous pocket was made by blunt dissection. Pancreata were introduced into the pocket with Dumont forceps and guided in with a blunt-ended microspatula. The incision was closed by firm pressure without sutures.
Metabolic Parameters
Streptozotocin-treated mice with fasting blood glucose levels over 300 mg/dl were selected as transplant candidates. Blood samples were collected from a tail snip under isoflurane-oxygen anesthesia, for glucose, insulin, and adiponectin measurements. Intraperitoneal glucose tolerance tests (IPGTT) on 6-h-fasted mice were performed 1 wk before the transplant and then every month after the transplant until euthanasia for tissue collection. IPGTT involves blood collection prior to and 15, 30, 60, and 120 min after intraperitoneal injection of sterile glucose (Sigma; 2 g/kg body wt under isofluorane-oxygen anesthesia). Blood glucose was measured immediately with an Ascencia Contour glucose meter (Bayer). Insulin and adiponectin measurements were performed at the Vanderbilt Hormone Assay Core Facility, with standard radioimmunoassay and Luminex assay techniques.
Transplant Monitoring
Anesthetized mice were placed on a customized stage on the LSM510 META confocal microscope (Zeiss). The approximate area of GFP fluorescence was first detected with confocal microscopy (excitation 488 nm, emission 500- to 550-nm bandpass filter). Deeper and more detailed images of the transplant were then taken with TPEM using a Coherent Chameleon laser (excitation 900 nm, emission 500–550 nm). To distinguish the transplants from nonspecific fluorescence emitting from autofluorscent structures, the emission spectrum of each fluorescent structure was measured with narrow spectral bands by use of the META detector of a Zeiss LSM510. These emission spectra were then compared with the emission spectra of true GFP in isolated islets from MIP-GFP mice.
Histological Studies
Mice were killed 3–5 mo after transplantation, and the transplants were excised. Excised tissue samples were fixed in 4% paraformaldehyde for 2 h and washed several times in 1× phosphate-buffered saline in preparation for histological sectioning. Sections (5 μm) of transplants were analyzed by immunostaining for insulin and CD34 (counterstained with Alexa fluor 488 and Alexa fluor 568, respectively) to verify differentiation of β-cells and vascular endothelial cells. Other sections were stained for glucagon for further verification of endocrine differentiation and with bromodeoxyuridine (BrDU) to check for neoplastic transformation (both secondary stained with Alexa fluor 568 in separate sections). Sectioning and staining were performed at the Vanderbilt Immnunohistochemistry Core Facility.
Statistical Analysis
Values are expressed as means ± SE; n denotes the number of animals in each group. Groups were compared using paired Student's t-test.
RESULTS
Normal body weight of NCRNU nude mice (8–16 wk of age) ranged from 25 to 30 g, averaging 27.6 ± 1.04 g. Treatment with streptozotocin resulted in weight loss of up to 10% of body weight and development of other clinical symptoms diabetes such as polyuria, polydipsia, and polyphagia. As shown in Table 1 and Fig. 1, subcutaneous transplants reversed streptozotocin-induced weight loss, exceeding prediabetic body weight. Clinical observations indicated reduced symptoms of diabetes in the transplant group. As expected, the renal subcapsular transplant group also regained the adipose tissue lost due to streptozotocin treatment, although they did not exceed prediabetic weight (Table 1). The untreated diabetic control group, which did not receive pancreatic transplants, continued to lose weight, up to 20% of body weight, and exhibited worsening symptoms until the time of euthanasia.
Table 1.
Changes of body weight before and 3 mo after transplants in each group
| Group | Body weight, g |
|---|---|
| Normal NCRNU mice | 27.6±1.04 |
| Diabetic pretransplant | 25.6±0.82 |
| Subcutaneous pransplants | 28.23±1.07* |
| Renal subcapsular pransplants | 26.35±0.35 |
| Untreated diabetic controls | 22.2±0.74 |
P < 0.05 when subcutaneous transplant group was compared with diabetic pretransplant conditions and/or untreated diabetic controls.
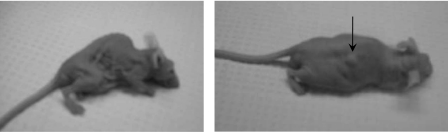
Fig. 1.
Visible differences in body weight in NCRNU-M-M nude mice with and without embryonic pancreatic transplants. Left: untreated diabetic control. Right: 10 wk after subcutaneous transplant. Arrow indicates subcutaneous transplant under the skin of the dorsal body surface (DBS).
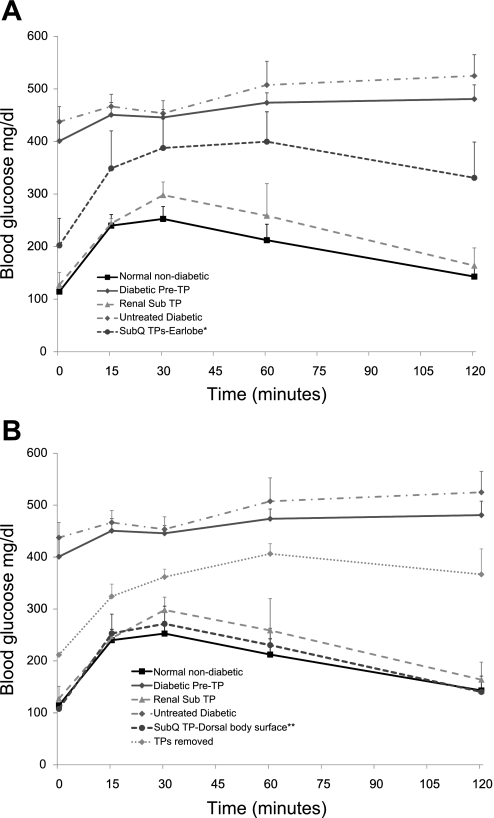
Figure 2 shows the effects of transplants on glucose tolerance in NCRNU nude mice. Transplants were performed on streptozotocin-treated mice that showed fasting glucose levels over 300 mg/dl. In some animals, diabetes was not induced by a single injection of streptozotocin, and two or three biweekly injections were necessary. Once induced, diabetes was persistent and progressive, causing severe impairment of glucose tolerance (Fig. 2). As expected, renal subcapsular transplants produced a significant improvement of glucose tolerance, bringing it back to normal. Subcutaneous transplants were first placed in the earlobe, as this was a better site for parallel imaging studies. Fifty percent of the earlobe transplants were successful in normalizing glucose tolerance, whereas the remaining 50% were unsuccessful (Fig. 2A). In contrast, all of the subcutaneous transplants placed in the dorsal body surface produced complete reversal of diabetes, with a 100% success rate at 3 mo after transplantation (Fig. 2B), which lasted until the time of euthanasia at 5 or 6 mo posttransplant. The performance of successful subcutaneous transplants was comparable to those of normal nondiabetic mice or renal subcapsular transplants, bringing glucose tolerance back to normal. To eliminate the possibility of false positives, we compared these results with those of an untreated diabetic control group that did not receive pancreatic transplants, which group continued to exhibit severely impaired glucose tolerance. For further verification, three of the visible subcutaneous transplants on the dorsal body surface were surgically excised, and mice were allowed to recover. IPGTT results showed that these animals reverted to diabetic status 2 wk later. Transplants placed in the earlobes were not easy to distinguish from the surrounding tissue, and therefore were difficult to excise. Figure 2A compares the average performance of the earlobe subcutaneous transplants with those of positive and negative control groups (including normal mice, diabetic pretransplant condition, untreated diabetic group, and renal subcapsular transplant group), while Fig. 2B compares the subcutaneous transplants in the dorsal body surface with the positive and negative control groups.
Fig. 2.
Subcutaneous transplants normalize glucose tolerance in streptozotocin-treated NCRNU nude mice. TP, transplant; SubQ, subcutaneous; Renal sub, renal subscapular; DBS, dorsal body surface. IPGTT was performed under different conditions, as noted, by injection of sterile glucose (2 g/kg ip) and blood collection at denoted time points under isoflurane-oxygen. Stretpozotocin treatment resulted in severe impairment of glucose tolerance (•-solid line, n = 11) with drastic deviation from normal situation (▪-solid line, n = 11). The renal subcapsular transplant group (positive control) brought glucose tolerance back to normal (▴-dashed line, n = 4), whereas the untreated diabetic control group, which did not receive pancreatic transplants (negative control), continued to show progressive impairment of glucose tolerance (▪-broken line, n = 6). A: subcutaneous transplants of embryonic days E15.5–E16.5 embryonic pancreas placed in the earlobe site produced significant improvement of glucose tolerance compared with negative controls (•-dashed line, n = 6; *P < 0.05 at every time point except 30 min), with a 50% success rate (3 of 6). B: subcutaneous transplants placed in the DBS (•-dashed line, n = 5) produced complete normalization of glucose tolerance similar to normal nondiabetic group or renal subcapsular transplant group. Excision of subcutaneous transplants also resulted in progressive impairment of glucose tolerance (⧫-dotted line, n = 3). Changes in glucose tolerance were first observed at 3 wk posttrasnplant and have been followed up to 6 mo so far. Representative data at 12 wk posttransplant placement are shown here. **P < 0.001 for each time point vs. corresponding time point of any negative control.
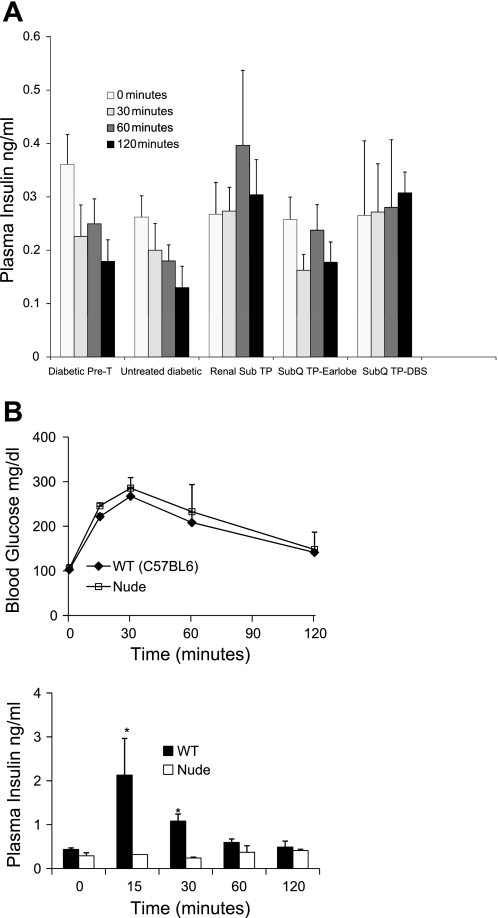
As expected, the insulin response to a glucose challenge was drastically impaired in streptozotocin-treated diabetic nude mice (Fig. 3A, left, 2 negative control groups), and the renal subcapuslar transplant group shows some recovery in the insulin response (Fig. 3A, middle). Unexpectedly, the subcutaneous transplant groups did not exhibit an overt insulin response to the glucose challenge, except for a significant improvement at the 2-h time point in the dorsal body surface transplants (Fig. 3A, right) compared with the corresponding time point in the pretransplant condition or the untreated diabetic group. One possible reason for the poor systemic insulin response is the insulin produced in the subcutaneous transplants not getting properly distributed into the systemic circulation. To explore this, we measured insulin levels in the inflammatory exudates at the subcutaneous transplant site in earlobes (no exudates were present on the dorsal body surface). The exudates, when present, contained 0.25–0.35 ng/ml insulin, averaging 0.3 ng/ml. These levels were comparable to or higher than their plasma levels, confirming endocrine differentiation and functionality of the transplants. Another possible explanation for the unusual insulin data is the overall weak insulin response characteristic to this mouse strain (Fig. 3B). Normal nondiabetic NCRNU nude mice maintain glucose tolerance comparable to wild type mice (Fig. 3B). However, their basal insulin levels are rather low compared those of with wild-type mice, and, unlike with wild-type mice, their plasma insulin level does not rise until 1 h after glucose challenge (albeit to a small degree) (Fig. 3B). Thus, it appears that the NCRNU strain can maintain normal glucose tolerance with considerably low plasma insulin levels, presumably through high sensitivity to insulin in peripheral tissues. Recent studies show evidence of specific adipokines that enhance insulin sensitivity in the peripheral tissues in the absence of increased levels of insulin (24). Furthermore, considering that the subcutaneous transplants enable a robust recovery of lost adipose tissue, it is likely that adipokines may contribute to the improved glucose homeostasis in addition to insulin in the subcutaneous transplant group. We provide more evidence for this possibility in subsequent sections.
Fig. 3.
A: insulin response to glucose challenge during IPGTT in Fig. 2 from selected groups of streptozotocin-treated NCRNU nude mice. Plasma insulin levels at different time points before and after injection of sterile glucose (2 g/kg body wt ip) are shown before and 12 wk after transplant placement. None of the groups showed a significant increase of plasma insulin in response to glucose challenge compared with 0 time point in each group. The subcutaneous transplant group on DBS showed significantly higher insulin secretion at the 2-h time point compared with the corresponding time point of the diabetic pretransplant control or untreated diabetic group (P < 0.05). B: comparison of glucose tolerance (top) and insulin response (bottom) between nondiabetic NCRNU nude mice and C57/BL6J wild-type mice. IPGTT was performed in the 2 mouse strains as noted, by injection of sterile glucose (2 g/kg ip) and blood collection at the denoted time points under isoflurane-oxygen. Plasma insulin levels at different time points before and after glucose injection are measured. Although there is no significant difference in glucose tolerance between the 2 strains, insulin response is comparably lower in NCRNU nude mice, with a significant difference (*P < 0.05) in 15- and 30-min time points compared with wild type mice. NCRNU nude mice showed no significant increase of insulin in response to glucose challenge (15-, 30-, and 60-min time points vs. 0 time point), whereas the wild-type mice did. For each strain, n = 6.
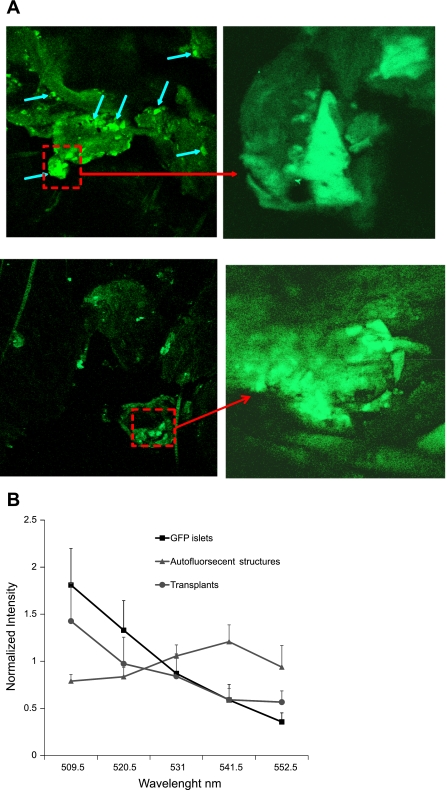
In streptozotocin-treated NOD-SC mice, both IPGTT and insulin responses were significantly improved following subcutaneous transplants placed in the earlobes (Fig. 4). Endocrine differentiation of subcutaneous transplants was monitored noninvasively through the intact skin by use of TPEM. Although TPEM is an excellent method for monitoring transplant development in live animals, having to image through several layers of tissue reduces the clarity and resolution of images compared with in vitro imaging. Subcutaneous transplants in the earlobes are covered by epithelial and connective tissue at a depth of 100–150 μm. These were detectable by TPEM, whereas transplants in the dorsal body surface were undetectable so far due to excessive thickness of adipose tissue. During the first few weeks following placement, earlobe transplants could be easily located by their GFP fluorescence, which progressively spread out into the surrounding tissue (Fig. 5A), indicating transplant growth and expansion. One difficulty encountered in transplant monitoring was the presence of autofluorescent structures with high brightness, such as hair follicles. True GFP fluorescence emitted from transplants can be distinguished from autofluorescence by measuring the emission spectrum of each fluorescent area with a spectral detector (Fig. 5B). We know that that GFP emits light from 500 to 550 nm, with peak emission at 514 nm. Autofluorescent structures also emitted light in the range of 500–550 nm (and beyond), so were indistinguishable from GFP. To distinguish GFP from autofluorescent structures, we measured the peak emission of each fluorescent structure within the range of 500–550 nm. GFP had peak emission between 510–520 nm, whereas other structures had peak emission elsewhere (∼540 nm in this case) (Fig. 5B). These results were later confirmed by immunostaining for insulin.
Fig. 4.
Subcutaneous transplants improve glucose tolerance and insulin response to glucose challenge in streptozotocin-treated NOD-SC mice. IPGTT was performed by injection of sterile glucose (2 g/kg ip) and blood collection at denoted time points under isoflurane-oxygen. A: glucose tolerance. Stretpozotocin treatment resulted in severe impairment of glucose tolerance (○). Significant improvement of glucose tolerance resulted from subcutaneous transplants of E14.5 embryonic pancreas in the earlobe site (•). Representative data at 12 wk posttransplant placement are shown here. *P < 0.05 when average blood glucose levels at each time point in the subcutaneous transplant group was compared with corresponding time points of the untreated diabetic group. B: corresponding insulin response to glucose challenge during IPGTTS in A. Insulin response is impaired in untreated controls (open bars) and shows some improvement in the subcutaneous transplant group (filled bars). Significant increase of insulin response seen at the 2-h time point in the subcutaneous transplant group. *P < 0.05 vs. corresponding time point in the diabetic control group.
Fig. 5.
A: two-photon images of embryonic pancreas (E14.5) transplanted in earlobe of streptozotocin-treated NOD-SC mice. Excitation 900 nm, emission 500–550 nm. Top: 5 days posttransplantation. Blue arrows indicate green fluorescence from developing β-cell clusters in the graft. Region indicated by red square is enlarged on right. Scale, 600 μm left; 100 μm right. Bottom: 4 wk after transplantation. Scale, 600 μm left; 120 μm right. B: comparison of emission spectra of true GFP from islets isolated from MIP-GFP mice (▪), subcutaneous transplants (•), and other fluorescent structures in earlobes (▴) to distinguish transplants from nonspecific fluorescence. Emission spectra were obtained with narrow spectral bands using the META detector of Zeiss LSM510. GFP emits light from 500 to 550 nm, with peak emission at 514 nm. To distinguish GFP from autofluorescent structures that also emitted in range of 500–550 nm, peak emission of each fluorescent structure was determined. GFP had peak emission between 510 and 520 nm, whereas other structures had peak emission ∼540 nm.
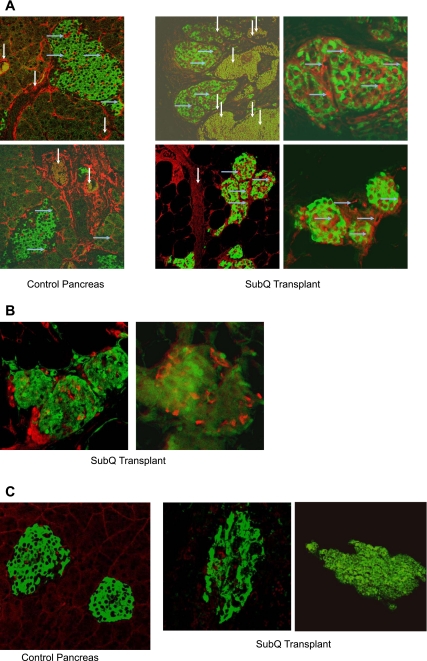
Transplant vascularization was verified by simultaneous immnuostaining for insulin and CD34, an endothelial marker. Unlike the earlobe transplants, the transplants placed under the skin of the dorsal body surface were detectable by naked eye (Fig. 1) and could be removed as a whole for immunohistochemistry (Fig. 6). The transplants excised after 4 mo contained well-formed islets similar to those in control pancreas, as well as scattered, separate, insulin-staining cells. Islets in transplants ranged fro 25 to 200 μm in diameter, comparable to control pancreatic islets. The excised transplants showed extensive neovascularization as indicated by CD34 staining (Fig. 6), comparable to control islets from normal pancreas. Improved vascularization was observed when the transplanted embryonic pancreata were from day E15.5 or later and when transplants were excised at 4 mo or later. Endocrine differentiation was further confirmed by the presence of glucagon-containing cells (Fig. 6B). No neoplastic transformation was detected, as indicated by BrDU staining (Fig. 6C), which was similar to control pancreas.
Fig. 6.
Endocrine differentiation and neovascularization of transplants verified by immunohistochemistry. A: control pancreas showing islets (left 2 panels) and different areas of subcutaneous (SubQ) transplant on DBS of NCRNU nude mouse (E16.5, excised after 4 mo; right 4 panels); immunostained for insulin (green, counterstain Alexa fluor 488) and CD34 (red, counterstain Alexa fluor 568). Transplant has differentiated into distinguishable islets similar to those in control pancreas. Vascularization of islets is indicated by CD34 staining inside islets. Large blood vessels are seen in vicinity of islets. Dimensions of structures are as follows. Islets in control pancreas: 250 × 160 μm top; 200 × 90 μm bottom. Islets in SubQ transplant: top left 90 × 180 and 125 × 80 μm; top right 125 × 90 μm; bottom left 80 × 60, 55 × 75, and 65 × 45 μm; bottom right 50 × 50, 25 × 25, and 40 × 40 μm. Large blood vessels (white vertical arrows) around islets: control pancreas 40–60 μm in diameter; SubQ transplant 60–200 μm in diameter. Small blood vessels inside islets (blue horizontal arrows): 1–12 μm in diameter in both control pancreas and SubQ transplant. B: glucagon staining in islets of subcutaneous transplant, indicating further endocrine differentiation. Green, GFP fluorsescence in β-cells; red, glucagon, secondary stain with Alexa fluor 568. Islet diameter: 50, 80, and 60 μm in left; 80 × 65 μm in right. C: minimal bromodeoxyuridine (BrDU) staining in islets of control pancreas and subcutaneous transplant, indicating absence of neoplastic transformation. Green, insulin, secondary stain with Alexa fluor 488; red, BrDU, secondary stain with Alexa fluor 568. Islet diameter: 150 and 100 μm in control pancreas, 100 and 200 μm in subcutaneous transplant.
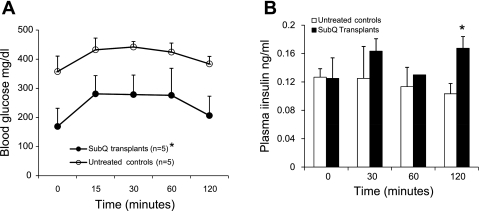
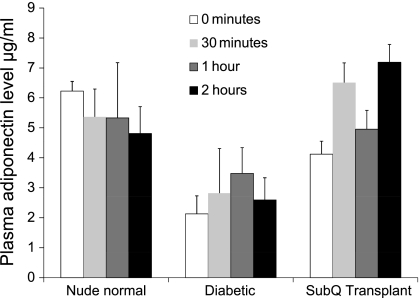
Since the normalization of glucose tolerance in the subcutaneous transplant group of NCRNU mice was not accompanied by a corresponding increase of plasma insulin, we performed additional experiments to explore a possible role in adipokines in glucose control. Plasma adiponectin levels were compared in the prediabetic, diabetic, and posttrasnplant groups. As Fig. 7 shows, streptozotocin-treated diabetic mice had significantly lower adiponectin levels compared with normal nondiabetic mice, and these levels did not increase following a glucose challenge. The subcutaneous transplant group had significantly higher adiponectin levels, which, unlike in the normal group, continued to increase in response to the glucose challenge. Thus, it appears that adiponectin plays an active role in maintaining glucose homeostasis following subcutaneous placement of embryonic tissue.
Fig. 7.
Comparison of plasma adiponectin concentrations in NCRNU nude mice under normal conditions, strepzotocin-induced diabetes, and with subcutaneous pancreatic transplants. Blood samples were collected at denoted intervals during standard IPGTT before and after injection of 2 g/kg sterile glucose. Adiponectin was measured in plasma using Luminex assays; n = 5 for normal and transplant groups, n = 4 for diabetic group. Adiponectin levels were significantly different (P < 0.005) between the diabetic group and both other groups at 0 and 2-h time points. In the SubQ group, 30-min and 2-h time points were significantly different from 0 time point (P < 0.05).
DISCUSSION
Although islet transplantation is a commonly used treatment for type 1 diabetes with great success in the short term, up to 90% of patients are reported to revert to insulin dependence within 5 years (50). The success rate also varies widely with the quality of available donor tissue as well as the experience of the center performing the transplant (53). Portal vein cannulation, a requirement for traditional islet transplantation, carries the risk of life-threatening complications from hemorrhage, thrombosis, and portal hypertension (3, 6, 9, 53). Transplantation at a superficial and more accessible site such as the subcutaneous space would eliminate surgical complications, minimize stress associated with transplant placement, and enable better graft monitoring and addressing potential problems in the long term. However, previous attempts at islet transplantation at the subcutaneous site have not been very successful (19, 28, 54, 63) and/or required specialized manipulations (10, 29, 42, 59, 63). A recent study reported successful reversal of diabetes and minimally invasive graft monitoring with islet transplantation in the anterior chamber of the mouse eye (55). Although this compartment could become an excellent research model with demonstrated success, the eye is not a suitable therapeutic transplant site in a clinical setting, and repeated imaging carries the potential for tissue damage. Thus, the need remains for noninvasive transplant strategies that are customizable for clinical situations.
We have shown that an alternative technique for minimally invasive transplantation with potential long-term success is to use embryonic pancreatic tissue in the subcutaneous space. This superficial site offers many advantages such as no-risk transplant placement, noninvasive imaging, and ability to perform repeated transplants if necessary. In addition, the use of embryonic tissue eliminates the need for large numbers of donor islets and can be customized for human patients by using stem cell-derived islet-like cell clusters (27, 30, 33, 52, 56). In the current experiments with immune-deficient nude mice and NOD-SC mice, embryonic pancreata survived in the subcutaneous space with no additional facilitation techniques and produced remarkable improvement of glucose homeostasis and body weight. The possibility of false positive results due to potential spontaneous reversal of streptozotocin-induced diabetes was eliminated by excision of some transplants as well as maintaining an untreated diabetic control group for comparison.
An interesting finding was that the improvement of glucose tolerance occurred without a significant increase in plasma insulin response. Although this is somewhat unusual, NCRNU nude mice appear to have higher sensitivity to insulin, enabling them to maintain normal glucose tolerance with lower insulin levels. Although there was no information on this strain in the literature, our data show that they maintain normal glucose tolerance with considerably low insulin levels in their normal nondiabetic status (Fig. 3B). This may be a possible effect of higher insulin sensitivity at the peripheral tissue level unique to this strain, that merits further investigation. As several recent studies have reported, hormones from adipose tissue play an important role in sensitizing the peripheral tissues to insulin (4, 11, 23–25, 51, 58, 64–66). Adiponectin descreases blood glucose levels and increases insulin sensitivity without increasing insulin levels in diabetic swine (11, 24, 64), and may well have a role in enabling the mice in the current study to function without a detectable increase in insulin.
As Fig. 1 and Table 1 show, the subcutaneous transplant group showed increased body weight, gaining back all the adipose tissue lost following streptozotocin treatment and surpassing the pre-streptozotocin weight. Although successful treatment of type 1 diabetes is generally associated with recovery of body weight, there was also a significant and clinically visible increase in the adipose tissue in our subcutaneous transplant group compared with the renal subcapuslar transplant group. Thus, it is possible that that the glucose-lowering effect was partially independent of insulin and produced by adiponectin (or other adipokines) from the adipose tissue instead. To verify this, we tested plasma adiponectin levels in nondiabetic, diabetic, and transplant groups.
As Fig. 7 shows, streptozotocin-treated diabetic mice have significantly lower adiponectin levels compared with normal, nondiabetic mice, and these levels do not increase following a glucose challenge. The subcutaneous transplant group has significantly higher adiponectin levels, which, unlike in the normal group, continue to increase in response to the glucose challenge. Thus, it appears that adiponectin plays an active role in maintaining glucose homeostasis following subcutaneous placement of embryonic tissue. This is not surprising, since adiponectin as well as several other adipokines are reported to exert strong effects on plasma glucose homeostasis. While some adipokines, such as resistin and retinol-binding protein-4, tend to exacerbate hyperglycemia (11, 23, 51, 58), a number of adipokines exert beneficial properties in glucose homeostasis, either directly decreasing blood glucose or enhancing sensitivity to insulin (4, 11, 23–25, 64–66). Particularly important among these are adiponectin, visfatin, and leptin. Visfatin is reported to exert insulin-like properties and lower blood glucose levels by binding to insulin receptors (25). Leptin was previously reported to improve glucose homeostasis through downregulation of resistin (4), while a recent study shows that leptin administration alone can lead to reversal of type 1 diabetes without insulin replacement (66). Adiponectin, whose levels decrease in diabetes (11, 64), has been known to increase insulin sensitivity in peripheral tissues in the absence of an increase of plasma insulin levels (24). Thus, adiponectin is a likely candidate that contributes to maintaining glucose homeostasis NCRNU nude mice whose insulin levels are low (Figs. 3B and 7). It is rather curious that the presence of subcutaneous embryonic pancreatic transplants appears to enable adiponectin to play a more active role in glucose homeostasis compared with the normal nondiabetic situation (Fig. 7); the mechanisms are unclear and merit further investigation. Considering the many different adipokines recently reported to play a role in glucose homeostasis, it is likely that several other factors from the adipose tissue are involved in the normalization of glucose tolerance produced by the subcutaneous embryonic pancreatic transplants. The next steps of our investigation include identifying these factors as well as exploring possible structural and functional alterations in the adipose tissue brought about by the presence of transplants.
Considerable success from embryonic pancreas transplantation has been reported before (47–49), where successful endocrine differentiation and long-term reversal of diabetes occurred upon transplantation of pig pancreatic anlagae of specific gestational ages into the omentum, mesentery, or renal capsule of nonimmune suppressed diabetic rats and rhesus monkeys. Studies by Dafoe's group (1, 2, 13, 57) reported success in transplantation of fetal pancreas in relatively superficial sites such as muscle with the aid of growth factors or simultaneous transplantation of fetal liver tissue. Our study confirms the value of embryonic pancreatic tissue as a superior source of insulin-producing cells and demonstrates the potential advantages of the subcutaneous space as opposed to traditional, deeper sites for transplantation. Another important advantage of placing transplants in the subcutaneous space is the ability to monitor transplant survival and development in a noninvasive manner. Monitoring transplants in traditional sites such as the omentum and renal subcapular space require techniques such as skin fold chamber preparations or repeated surgery (35–39, 62), which are traumatic to the subject, inconvenient to the researcher, and unfeasible in a clinical setting. Unlike the traditional deeper transplants, subcutaneous transplants can be monitored noninvasively through the intact skin with modern imaging techniques (5, 12, 15, 22, 40, 43). We have used TPEM to monitor endocrine differentiation through GFP expression. Aided by histological verification (Fig. 6 ), we are in the process of optimizing TPEM parameters for progressive imaging of transplant vascularization in vivo.
Considering the positive results obtained without specialized manipulations, this study demonstrates the potential therapeutic value of subcutaneous transplantation of embryonic pancreatic tissue as a convenient alternative to traditional islet transplantation in deeper sites and points to an intriguing alternative mechanism of glycemic regulation through adipokines in addition to or instead of insulin. Our future directions include a thorough examination of such alternative glycemic control through measurements of different adipokines in normal, diabetic, and transplant conditions; exploration of possible structural and functional changes produced in adipose tissue by transplants; and verifying whether type 1 diabetes can be corrected through administration of specific adipokines or transplantation of adipose tissue alone. The current success of subcutaneous transplants with immune-deficient mouse strains is encouraging, and we hope to verify whether similar results can be achieved in nonimmune-deficient animals with or without facilitation techniques such as adding exogenous growth factors or anti-inflammatory compounds, culturing embryonic pancreas in different media prior to transplantation, and/or using specific gestational ages optimal for immune tolerance. Long-term goals are to customize this strategy for humans and companion animals by using stem cell-derived islet tissue.
GRANTS
This work was supported by the Vanderbilt University Medical Center, the US Department of Defense Medical Free-Electron Laser Program, and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-53434 (to D. W. Piston).
Acknowledgments
We are grateful to Steven Head for technical and administrative support and Drs. Al Powers and Lara Nyman for providing part of the MIP-GFP breeder colony. The MIP-GFP mice were originally provided to us by Drs. Graeme Bell and Manami Hara at the University of Chicago and Dr. Mark Magnuson at Vanderbilt University. Some of the assays and histological studies were performed by Vanderbilt Research Core Facilities.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adams GA, Maestri M, Squiers EC, Alfrey EJ, Starzl TE, Dafoe DC. Augmenter of liver regeneration enhances the success rate of fetal pancreas transplantation in rodents. Transplantation 65: 32–36, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams GA, Wang X, Lee LK, Piercy CE, Alfrey EJ, Dafoe DC. Insulin-like growth factor-I promotes successful fetal pancreas transplantation in the intramuscular site. Surgery 116: 751–757, 1994. [PubMed] [Google Scholar]
- 3.Allen RD, Nankivell BJ, Hawthorne WJ, O'Connell PJ, Chapman JR. Pancreas and islet transplantation: an unfinished journey. Transplant Proc 33: 3485–3488, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Asensio C, Cettour-Rose P, Theander-Carrillo C, Rohner-Jeanrenaud F, Muzzin P. Changes in glycemia by leptin administration or high-fat feeding in rodent models of obesity/type 2 diabetes suggest a link between resistin expression and control of glucose homeostasis. Endocrinology 145: 2206–2213, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Benninger RKP, Hao MM, Piston DW. Multi-photon excitation imaging of dynamic processes in living cells and tissues. Rev Physiol Biochem Pharmacol 160: 71–92, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Bucher P, Mathe Z, Bosco D, Becker C, Kessler L, Greget M, Benhamou PY, Andres A, Oberholzer J, Buhler L, Morel P, Berney T. Morbidity associated with intraportal islet transplantation. Transplant Proc 36: 1119–1120, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Campbell PM, Senior PA, Salam A, Labranche K, Bigam DL, Kneteman NM, Imes S, Halpin A, Ryan EA, Shapiro AM. High risk of sensitization after failed islet transplantation. Am J Transplant 7: 2217–2218, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Campbell PM, Salam A, Ryan EA, Senior P, Paty BW, Bigam D, McCready T, Halpin A, Imes S, Al Saif F, Lakey JR, Shapiro AM. Pretransplant HLA antibodies are associated with reduced graft survival after clinical islet transplantation. Am J Transplant 7: 1242–1248, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Casey JJ, Lakey JR, Ryan EA, Paty BW, Owen R, O'Kelly K, Nanji S, Rajotte RV, Korbutt GS, Bigam D, Kneteman NN, Shapiro AM. Portal venous pressure changes after sequential clinical islet transplantation. Transplantation 74: 913–915, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Zhang X, Larson CS, Baker MS, Kaufman DB. In vivo bioluminescence imaging of transplanted islets and early detection of graft rejection. Transplantation 81: 1421–1427, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Choi SH, Kwak SH, Youn BS, Lim S, Park YJ, Lee H, Lee N, Cho YM, Lee HK, Kim YB, Park KS, Jang HC. High plasma retinol binding protein-4 and low plasma adiponectin concentrations are associated with severity of glucose intolerance in women with previous gestational diabetes mellitus. J Clin Endocrinol Metab 93: 3142–3148, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Denk W, Piston DW, Webb WW. Two-photon molecular excitation in laser-scanning microscopy. In: Handbook of Biological Confocal Microscopy (2nd ed.), edited by Pawley JB. New York: Plenum 1995, p. 445–458.
- 13.Desai DM, Adams GA, Wang X, Alfrey EJ, Sibley RK, Dafoe DC. The influence of combined trophic factors on the success of fetal pancreas grafts. Transplantation 68: 491–496, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Emamaullee JA, Shapiro AM. Factors influencing the loss of beta-cell mass in islet transplantation. Cell Transplant 16: 1–8, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Evgenov NV, Medarova Z, Dai G, Bonner-Weir S, Moore A. In vivo imaging of islet transplantation. Nat Med 12: 144–148, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Eventov-Friedman S, Katchman H, Shezen E, Aronovich A, Tchorsh D, Dekel B, Freud E, Reisner Y. Embryonic pig liver, pancreas, and lung as a source for transplantation: optimal organogenesis without teratoma depends on distinct time windows. Proc Natl Acad Sci USA 102: 2928–2933, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eventov-Friedman S, Tchorsh D, Katchman H, Shezen E, Aronovich A, Hecht G, Dekel B, Rechavi G, Blazar BR, Feine I, Tal O, Freud E, Reisner Y. Embryonic pig pancreatic tissue transplantation for the treatment of diabetes. PLoS Med 3: e215, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fändrich F, Dresske B, Bader M, Schulze M. Embryonic stem cells share immune-privileged features relevant for tolerance induction. J Mol Med 80: 343–350, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Figliuzzi M, Cornolti R, Plati T, Rajan N, Adobati F, Remuzzi G, Remuzzi A. Subcutaneous xenotransplantation of bovine pancreatic islets. Biomaterials 26: 5640–5647, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Gunawardana SC, Hara M, Bell GI, Head WS, Magnuson MA, Piston DW. Analysis of pancreatic development in real-time using transgenic mice with green fluorescent protein-labeled pancreatic beta cells. In Vitro Cell Devel Biol Animal 41: 7–11, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Hara M, Wang X, Kawamura T, Bindokas VP, Dizon RF, Alcoser SY, Magnuson MA, Bell GI. Transgenic mice with green fluorescent protein-labeled pancreatic β-cells. Am J Physiol Endocrinol Metab 284: E177–E183, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Helmchen F, Denk W. New developments in multiphoton microscopy. Curr Opin Neurobiol 12: 593–601, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Hivert MF, Sullivan LM, Fox CS, Nathan DM, D'Agostino Sr RB, Wilson PW, Meigs JB. Associations of adiponectin, resistin, and TNF(alpha) with insulin resistance. J Clin Endocrinol Metab 93: 3165–3172, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu X, She M, Hou H, Li Q, Shen Q, Luo Y, Yin W. Adiponectin decreases plasma glucose and improves insulin sensitivity in diabetic Swine. Acta Biochim Biophys Sin (Shanghai) 39: 13113–13116, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Hug C, Lodish HF. Visfatin: a new adipokine. Science 307: 426–430, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Hullett DA, Falany JL, Love RB, et al. Human fetal pancrease—a potential source for transplantation. Transplant 43: 18–22, 1987. [DOI] [PubMed] [Google Scholar]
- 27.Jiang J, Au M, Lu K, Eshpeter A, Korbutt G, Fisk G, Majumdar AS. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells 25: 1940–1953, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Juang JH, Hsu BR, Kuo CH. Islet transplantation at subcutaneous and intramuscular sites. Transplant Proc 37: 3479–3481, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Kawakami Y, Iwata H, Gu YJ, Miyamoto M, Murakami Y, Balamurugan AN, Imamura M, Inoue K. Successful subcutaneous pancreatic islet transplantation using an angiogenic growth factor-releasing device. Pancreas 23: 375–381, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Kim D, Gu Y, Ishii M, Fujimiya M, Qi M, Nakamura N, Yoshikawa T, Sumi S, Inoue K. In vivo functioning and transplantable mature pancreatic islet-like cell clusters differentiated from embryonic stem cell. Pancreas 27: e34–e41, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Koch KS, Son KH, Maehr R, Pellicciotta I, Ploegh HL, Zanetti M, Sell S, Leffert HL. Immune-privileged embryonic Swiss mouse STO and STO cell-derived progenitor cells: major histocompatibility complex and cell differentiation antigen expression patterns resemble those of human embryonic stem cell lines. Immunology 119: 98–115, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laffel L Acute complications of diabetes. Endocrinol Metab Clin 29: 707–726, 2000. [Google Scholar]
- 33.Lee DD, Grossman E, Chong AS. Cellular therapies for type 1 diabetes. Horm Metab Res 40: 147–154, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li L, Baroja ML, Majumdar A, Chadwick K, Rouleau A, Gallacher L, Ferber I, Lebkowski J, Martin T, Madrenas J, Bhatia M. Human embryonic stem cells possess immune-privileged properties. Stem Cells 22: 448–456, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Merchant FA, Aggarwal SJ, Diller KR, Bovik AC. In-vivo analysis of angiogenesis and revascularization of transplanted pancreatic islets using confocal microscopy. J Microsc 176: 262–275, 1994. [DOI] [PubMed] [Google Scholar]
- 36.Merchant FA, Diller KR, Aggarwal SJ, Bovik AC. Angiogenesis in cultured and cryopreserved pancreatic islet grafts. Transplantation 15;63: 1652–1660, 1997. [DOI] [PubMed] [Google Scholar]
- 37.Menger MD, Jager S, Walter P, Hammersen F, Messmer K. A novel technique for studies on the microvasculature of transplanted islets of Langerhans in vivo. Int J Microcirc Clin Exp 9: 103–117, 1990. [PubMed] [Google Scholar]
- 38.Menger MD, Wolf B, Hobel R, Schorlemmer HU, Messmer K. Microvascular phenomena during pancreatic islet graft rejection. Langenbecks Arch Chir 376: 214–221, 1991. [DOI] [PubMed] [Google Scholar]
- 39.Menger MD, Yamauchi J, Vollmar B. Revascularization and microcirculation of freely grafted islets of Langerhans. World J Surg 25: 509–515, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Medarova Z, Evgenov NV, Dai G, Bonner-Weir S, Moore A. In vivo multimodal imaging of transplanted pancreatic islets. Nature Protocols 1: 429–435, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Mullen YS, Clark WR, Molnar IG, Brown J. Complete reversal of experimental diabetes mellitus in rats by a single fetal pancreas. Science 195: 68–70, 1977. [DOI] [PubMed] [Google Scholar]
- 42.Pileggi A, Molano RD, Ricordi C, Zahr E, Collins J, Valdes R, Inverardi L. Reversal of diabetes by pancreatic islet transplantation into a subcutaneous, neovascularized device. Transplantation 81: 1318–1324, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Piston DW Imaging living cells and tissues by two-photon excitation microscopy. Trends Cell Biol 9: 66–69, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Risau W, Ekblom P. Production of a heparin-binding angiogenesis factor by the embryonic kidney. J Cell Biol 103: 1101–1107, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Risau W, Ekblom P. Growth factors and the embryonic kidney. Prog Clin Biol Res 226: 147–156, 1986. [PubMed] [Google Scholar]
- 46.Risau W, Sariola H, Zerwes HG, Sasse J, Ekblom P, Kemler R, Doetschman T. Vasculogenesis and angiogenesis in embryonic-stem-cell-derived embryoid bodies. Development 102: 471–478, 1988. [DOI] [PubMed] [Google Scholar]
- 47.Rogers SA, Chen F, Talcott MR, Faulkner C, Thomas JM, Thevis M, Hammerman MR. Long-term engraftment following transplantation of pig pancreatic primordia into non-immunosuppressed diabetic rhesus macaques. Xenotransplantation 14: 591–602, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Rogers SA, Chen F, Talcott M, Liapis H, Hammerman MR. Glucose tolerance normalization following transplantation of pig pancreatic primordia into non-immunosuppressed diabetic ZDF rats. Transpl Immunol 16: 176–184, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Rogers SA, Liapis H, Hammerman MR. Normalization of glucose post-transplantation of pig pancreatic anlagen into non-immunosuppressed diabetic rats depends on obtaining anlagen prior to embryonic day 35. Transpl Immunol 14: 67–75, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes 54: 2060–2069, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Schreyer SA, Chua SC, Renée LeBoeuf RC C. Obesity and diabetes in TNFa receptor-deficient mice. J Clin Invest 102: 402–411, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Segev H, Fishman B, Ziskind A, Shulman M, Itskovitz-Eldor J. Differentiation of human embryonic stem cells into insulin-producing clusters. Stem Cells 22: 265–274, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Srinivasan P, Huang GC, Amiel SA, Heaton ND. Islet cell transplantation. Postgrad Med J 83: 224–229, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simeonovic CJ, Dhall DP, Wilson JD, Lafferty KJ. A comparative study of transplant sites for endocrine tissue transplantation in the pig. Aust J Exp Biol Med Sci 64: 37–41, 1986. [DOI] [PubMed] [Google Scholar]
- 55.Speier S, Nyqvist D, Cabrera O, Yu J, Molano RD, Pileggi A, Moede T, Köhler M, Wilbertz J, Leibiger B, Ricordi C, Leibiger IB, Caicedo A, Berggren PO. Noninvasive in vivo imaging of pancreatic islet cell biology. Nat Med 14: 574–578, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sumi S, Gu Y, Hiura A, Inoue K. Stem cells and regenerative medicine for diabetes mellitus. Pancreas 29: e85–e89, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Tafra L, Berezniak R, Dafoe DC. Beneficial effects of fetal liver tissue on fetal pancreatic transplantation. Surgery 108: 734–741, 1990. [PubMed] [Google Scholar]
- 58.Tamori Y, Sakaue H, Kasuga M. RBP4, an unexpected adipokine Nat Med 12: 30–31, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Tatarkiewicz K, Hollister-Lock J, Quickel RR, Colton CK, Bonner-Weir S, Weir GC. Reversal of hyperglycemia in mice after subcutaneous transplantation of macroencapsulated islets. Transplantation 67: 665–671, 1999. [DOI] [PubMed] [Google Scholar]
- 60.Taconic. [Online] http://www.taconic.com/wmspage.cfm?parm1=873 [2008].
- 61.Taconic. [Online] http://www.taconic.com/wmspage.cfm?parm1=780 [2008].
- 62.Vajkoczy P, Menger MD, Simpson E, Messmer K. Angiogenesis and vascularization of murine pancreatic islet isografts. Transplantation 60: 123–127, 1995. [PubMed] [Google Scholar]
- 63.Wang W, Gu Y, Hori H, Sakurai T, Hiura A, Sumi S, Tabata Y, Inoue K. Subcutaneous transplantation of macroencapsulated porcine pancreatic endocrine cells normalizes hyperglycemia in diabetic mice. Transplantation 76: 290–296, 2003. [DOI] [PubMed] [Google Scholar]
- 64.Worda C, Leipold H, Gruber C, Kautzky-Willer A, Knöfler M, Bancher-Todesca D. Decreased plasma adiponectin concentrations in women with gestational diabetes mellitus. Am J Ob Gyn 191: 2120–2124, 2004. [DOI] [PubMed] [Google Scholar]
- 65.Xu A, Lam MC, Chan KW, Wang Y, Zhang J, Hoo RL, Xu JY, Chen B, Chow WS, Tso AW, Lam KS. Angiopoietin-like protein 4 decreases blood glucose and improves glucose tolerance but induces hyperlipidemia and hepatic steatosis in mice. Proc Natl Acad Sci USA 26;102: 6086–6091, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu X, Park BH, Wang MY, Wang ZV, Unger RH. Making insulin-deficient type 1 diabetic rodents thrive without insulin. PNAS 105: 37, 14070–14075, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]