Abstract
There is limited in vivo data in humans evaluating myocardial fat utilization during increased heart work. This study was done to determine myocardial free fatty acid (FFA) metabolism during rest and atrial pacing, which increases cardiac work without changing arterial substrate concentration. We studied seven healthy men and women (age = 49.7 ± 3.9 yr, BMI = 23.4 ± 1.1 kg/m2, V̇o2max = 35.5 ± 3.0 ml·kg−1·min−1, ejection fraction = 68 ± 3%). After 3 days of dietary control, coronary sinus, femoral arterial and venous, and peripheral venous catheters were placed. Subjects received [13C]bicarbonate followed by a continuous infusion of [1-13C]palmitate through the end of the study. Arterial and coronary sinus blood sampling and measurements of resting coronary sinus blood flow were made during rest and atrial pacing to 120 beats/min. MV̇o2 increased (P < 0.05) from rest to atrial pacing. Coronary sinus FFA concentration was significantly lower than arterial through rest and atrial pacing (P = 0.007). Isotopically measured myocardial palmitate uptake increased significantly from rest to atrial pacing (P = 0.03). Approximately one-third of palmitate delivery was extracted by the myocardium during rest and atrial pacing. Myocardial V13CO2 production and palmitate oxidation increased significantly from rest (P < 0.01) to atrial pacing. Net glycerol balance was significantly greater than zero during rest (P = 0.04) but not different from zero during atrial pacing (P = 0.13). These data suggest that myocardial lipid uptake and oxidation increase with greater heart work during atrial pacing, with a similar relative proportion of fat oxidation to total myocardial energy expenditure.
Keywords: free fatty acid, stable isotopes, myocardial substrate utilization
myocardial substrate utilization can influence myocardial energy efficiency (8), tissue survival during ischemia (19), and cardiac efficiency (30). Additionally, alteration of myocardial substrate utilization has been reported and may be implicated in decreased ventricular performance in heart failure (2, 36), obesity (30), and type 1 (4) and type 2 diabetes (35). Therefore, understanding myocardial substrate utilization is important to elucidate potential derangements in myocardial metabolic control that may contribute to disease states.
Much of the knowledge on myocardial free fatty acid (FFA) utilization comes from studies on isolated heart preparations. It is well established that the majority of myocardial energy supply in the resting state comes from the oxidation of FFAs (6, 38), with arterial substrate concentration influencing myocardial uptake (32). Some data indicate that the isolated working heart increases reliance on carbohydrate-derived fuels more than β-oxidation (15), whereas others have suggested increased reliance on circulating FFAs with a decrease in glucose utilization (26). Others have suggested increased reliance on both glucose and FFA oxidation during exercise compared with rest in catheterized dogs (25).
Data from in vivo human studies largely corroborate findings from work in the isolated heart. The human myocardium in the basal state relies mostly on FFAs for fuel (18, 22). Similarly to the isolated working heart, arterial substrate concentration plays a large role in dictating myocardial substrate utilization (18, 22). During increased heart work induced by whole body exercise, there is a shift in myocardial substrate utilization. Most studies investigating myocardial FFA utilization increased heart work using whole body exercise, which results in changes in arterial substrate concentration (18, 22, 44). Substrate concentration, especially FFA, influences myocardial substrate utilization (18, 22, 44). Therefore, these studies do not allow investigators to determine myocardial substrate preferences independent of changing arterial substrate supply during increased heart work. Atrial pacing increases heart work independent of changes in arterial substrate concentration and may be used to study metabolic demands of the working heart in vivo. Several investigators reported data using atrial pacing to increase heart work in normal humans (3, 9, 17, 34). However, only one of these publications used isotopic tracers to accurately determine simultaneous myocardial substrate uptake, release, and oxidation (17). As a result, there are limited data available on myocardial FFA utilization during increased heart work without changes in arterial substrate supply induced by peripheral muscular work.
This study was done to determine myocardial palmitate uptake and oxidation during rest and increased heart work performed without a change in arterial substrate concentration. An increase in cardiac work was achieved with atrial pacing instead of whole body exercise or pharmacological intervention. This allowed us to measure myocardial substrate utilization during increased cardiac work without the influence of alterations in arterial substrate supply. We hypothesized that palmitate uptake and oxidation would increase during atrial pacing on an absolute basis, with no significant changes in uptake relative to total myocardial work.
METHODS
Subjects.
Seven healthy men and women were recruited for this study. Subjects gave informed consent and were excluded if they smoked, had diabetes, hyperlipidemia, or liver or kidney disease, or were taking medications that affect glucose or lipid metabolism and/or regularly engaged in vigorous exercise (>2 h/wk). Subjects were excluded if they had a body mass index (BMI) of <20 or >30 kg/m2. Women were taking oral contraceptives and were studied during the midfollicular phase of their menstrual cycle to minimize effects of menstrual cycle phase on substrate metabolism. This study was approved by the Colorado Multiple Institution Review Board at the University of Colorado Denver.
General experimental design.
After preliminary testing, subjects participated in one metabolic trial. Diet was controlled for 3 days prior to the study via the metabolic kitchen on the General Clinical Research Center (GCRC). During the metabolic trial, a right heart catheterization was combined with the measurement of palmitate uptake and oxidation at rest and under conditions of increased cardiac work with atrial pacing.
Preliminary testing.
Subjects reported to the GCRC for the screening procedures following a 12-h overnight fast. They were given a health and physical exam followed by a fasting blood draw. Body composition was determined using dual-energy X-ray absorptiometry analysis (Lunar DPX-IQ; Lunar, Madison, WI). Resting metabolic rate was measured using indirect calorimetry via a metabolic cart system (Sensormedics 2900; Sensormedics, Yorba Linda, CA). Subjects rested supine for 30 min; then a ventilated canopy was placed over their heads, and measurements were continued for 15–20 min. Metabolic rate was calculated from the flow rate of expired air in conjunction with measuring differences in the oxygen (O2) and carbon dioxide (CO2) concentrations using standard equations (43). Subjects were included in the study if they had normal fasting glucose, defined by the American Diabetes Association as fasting serum glucose concentration <100 mg/dl. On a separate screening day, subjects arrived on the GCRC and completed a maximal oxygen uptake (V̇o2max) test using the Balke treadmill test. A resting echocardiogram was also performed to exclude subjects with myocardial or valvular disease and left ventricular hypertrophy (Sonos 5500; Royal Philips Electronics) and to verify a normal left ventricular ejection fraction.
Diet control.
Three-day diet records were analyzed for each subject to determine macronutrient and energy content of their diets. Dieticians used the resting metabolic rate with an activity factor of 1.4, combined with each subject's typical dietary intake, to make a 3-day diet, which was provided to each subject prior to the metabolic study. The diet followed the American Heart Association recommendations for macronutrient composition (30% fat, 15% protein, and 55% carbohydrate). Subjects were asked to maintain normal daily physical activity during the period of dietary control and refrain from planned exercise for 3 days prior to the metabolic study. Prestudy nutritional control ensured that subjects were close to energy balance, and therefore, differences in energy status and glycogen stores prior to testing were controlled for between subjects. Subjects spent the evening before the metabolic study in the GCRC at University of Colorado Hospital to ensure compliance with the overnight fast.
Metabolic study.
Subjects were taken to the cardiac catheterization laboratory on the morning of the metabolic study after an overnight fast, where catheters were placed into the right internal jugular vein for coronary sinus access (6 Fr, Cordis Multipurpose A-2; Johnson and Johnson, Piscataway, NJ) and Doppler flow (0.014 Doppler Flow Wire, size 300; Cardiometrics, Mountain View, CA), femoral artery for arterial blood (Cordis 4 Fr arterial sheath; Johnson and Johnson), right femoral vein for pulmonary artery access (8Fr venous sheath; Boston Scientific, Natick, MA, and 7.5 Fr VIP thermodilution pulmonary artery catheter; Baxter-Edwards, Deerfield, IL), right femoral vein for atrial pacer wire (6 Fr venous sheath; Boston Scientific, and Cordis 6Fr atrial pacing wire; Johnson and Johnson), and a peripheral venous catheter for isotope infusion using standard catheterization techniques. Heparin was administered at 15 U/h to maintain patency of the arterial line during the 3-h study. After insertion of the coronary sinus catheter, an angiogram was performed using 30 ml of an nonionic contrast agent to measure the diameter of the coronary sinus and to determine the position of the multipurpose catheter for both blood sampling and positioning of the Doppler flow wire. This angiogram was performed prior to the initiation of the isotope infusion, and there was an interval of ≥60 min between the angiogram and the measurement of coronary sinus flow velocity with the Doppler wire. Initial cardiac hemodynamic measurements were made with a pulmonary artery catheter, with the determination of cardiac output by thermodilution and Fick methods and the recording of right atrial, pulmonary artery, and pulmonary capillary wedge pressures. Systemic arterial pressure was determined from the femoral arterial sheath. Determination of rate pressure product, mean arterial pressure, and systemic and pulmonary vascular resistances was calculated by standard methods. Following these measurements, blood was withdrawn from the arterial and coronary sinus catheters for background palmitate and blood 13CO2 enrichment. Expired air was collected for background breath 13CO2 enrichment. Then, through the peripheral venous catheter, a priming bolus of [1-14C]acetate, NaH13CO3, and NaH14CO3 containing 1.5 μmol/kg NaH13CO3 and 10 nCi/kg NaH14CO3 was initiated. A continuous infusion of [1-13C]palmitate was started at 0.012 mg·kg−1·min−1 and [1-14C]acetate at 0.2 nCi·kg−1·min−1 and continued until the end of the study. We followed methods for the acetate correction factor that have been published previously (33). Additionally, we infused [3,3,3-2H]lactate and [6,6-2H]glucose to measure myocardial glucose and lactate exchange, which are reported separately. Measurements of resting coronary sinus blood flow were determined from Doppler flow velocities in the coronary sinus, and hemodynamic measurements were made with the pulmonary artery catheter at minutes 30 and 45 of the infusion. Indirect calorimetry was performed for 15 min starting at 50 min of the infusion. Simultaneous arterial and coronary sinus blood sampling was performed at 60, 70, 80, and 90 min of rest. Atrial pacing was then started at a heart rate of 120 beats/min in an attempt to standardize myocardial oxygen consumption (MV̇o2). Measurements of paced coronary sinus blood flow, cardiac output by the thermodilution and Fick methods, and intracardiac filling pressures were made at minutes 15 and 55 of pacing. Simultaneous arterial and coronary sinus blood samples were obtained following 20, 30, 40, and 50 min of atrial pacing. Blood was obtained for glucose, lactate, FFA, glycerol, plasma catecholamines, insulin, glucagon, isotopic enrichment of palmitate, arterial blood gasses, hemoglobin and hematocrit, and O2 content. Another angiogram of the coronary sinus was performed with intravenous contrast during atrial pacing after completion of all coronary sinus Doppler flow velocity measurements and blood sampling. At the completion of the study, all catheters were removed and the subjects returned to the GCRC for 7 h of observation before discharge. Three of the subjects were tested when the [1-14C]acetate was not available for infusion; therefore, the NaH14CO3 prime was also not given. Mean values for acetate correction were used to estimate the acetate correction factor in these three subjects.
Metabolite and hormone analyses.
Insulin (42) (Clinical Assays γ-Coat RIA) and glucagon (1) were determined by radioimmunoassay. Catecholamines were determined by high-performance liquid chromatography with electrochemical detection (10). Standard enzymatic assays were used to measure lactate (Sigma Kit no. 826), glycerol (Boehringer Mannheim Diagnostics), and FFA (NEFA kit; Wako).
Gas chromatography-mass spectroscopy methods.
Metabolite isotopic enrichment was measured using gas chromatography-mass spectrometry (GC model 5890 series II and MS model 5989A; Hewlett-Packard). The [1-13C]palmitate isotopic enrichments were measured by derivatization to the fatty acid methyl ester to allow easy volatilization by gas chromatography. The instrumentation measured total FFA concentration by monitoring oleate, palmitate, stearate, linoleate, and palmitoleate (98% of FFA content) and isotopic enrichment of palmitate by gas chromatography-mass spectrometry. Measurement of 13CO2 enrichments was determined by isotope ratio mass spectroscopy. Measurement of breath 14CO2-specific activity was performed via liquid scintillation counting (Beckman LS 6000TA).
Calculations.
The rates of appearance (Ra) and disappearance (Rd) of palmitate were calculated using equations defined by Steele and modified for stable isotopes (45):
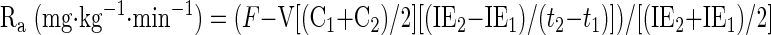 |
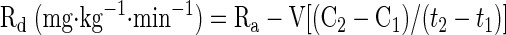 |
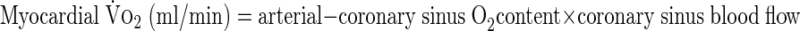 |
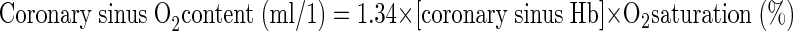 |
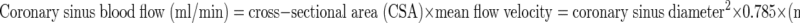 |
The mean flow velocity was determined by the area under the curve of the CS Doppler flow velocity
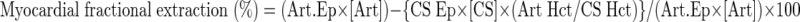 |
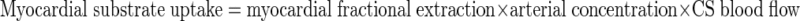 |
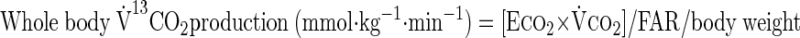 |
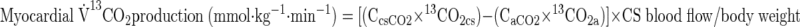 |
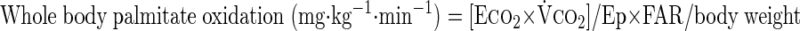 |
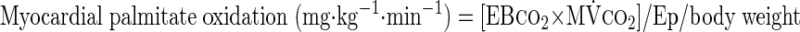 |
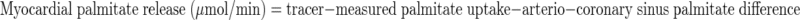 |
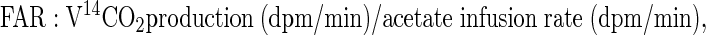 |
where F represents isotope infusion rate, IE1 and IE2 are isotopic enrichments at sampling time points 1 (t1) and 2 (t2), respectively, C1 and C2 are metabolite concentrations at t1 and t2, V is the estimated volume distribution of palmitate (40 ml/kg) that has been published previously (14), SAco2 is the specific activity of CO2, Eco2 is the isotopic enrichment of breath CO2, EBco2 is the isotopic enrichment of blood CO2, V̇co2 is whole body breath CO2 production, MV̇o2 is myocardial CO2 production, Ep is the plasma palmitate enrichment, CcsCO2 is the coronary sinus CO2 content, 13CO2cs is the enrichment of coronary sinus CO2, CaCO2 is the arterial CO2 content, 13CO2a is the enrichment of arterial CO2, and FAR is the fractional acetate recovery. All isotopic enrichments were corrected for background enrichments from blood samples taken before isotope infusion.
Myocardial exchange of glycerol and triglyceride was determined by the arterio-coronary sinus difference corrected for differences in hematocrit × coronary blood flow.
Blood Pco2, Po2, pH, and hemoglobin were measured on both arterial and venous samples and used in the calculations by Douglas et al. (11) for determination of blood CO2 content. CO2 solubility and apparent dissociation constant were estimated from the equations of Kelman (20). Hemoglobin concentration, saturation, and content of O2 were directly measured using the OSM-3 Hemocytometer (Radiometer, Copenhagen, Denmark). Blood O2 content was calculated with hemoglobin concentration, and saturation was determined from an equation by Nunn (29), as described previously (5).
Myocardial respiratory quotient (RQ) was calculated from the ratio of coronary sinus minus arterial CO2 difference and arterial minus coronary sinus O2 difference. When RQ was <0.70 or >1.0, a theoretical limit was assumed. Myocardial oxygen extraction ratio (OER, %) was used to calculate the relative proportion of tracer-measured FFA uptake to MV̇o2, as described by Lassers et al. (21).
Statistics.
Data are presented as means ± SE. Differences between rest and atrial pacing measures were analyzed using a repeated-measures ANOVA. Differences in arterial and coronary sinus metabolite concentrations were analyzed using paired Student's t-test (SPSS, Chicago, IL). Linear regression analyses were performed to correlate arterial substrate concentration and myocardial uptake or release. An α-level of 0.05 was used throughout.
RESULTS
Subject characteristics.
Anthropometric data for subjects are reported in Table 1. Subjects were weight stable in the 6 mo prior to participation in this research study. The men and women in this study were in the healthy BMI range, with a mean age of 49.7 ± 3.9 yr. V̇o2max was average for this age range, and left ventricular size and ejection fraction were also normal as determined by echocardiography.
Table 1.
Subject demographics
| Values | |
|---|---|
| n (M/W) | 7 (5/2) |
| Age, yr | 49.7±3.9 |
| Weight, kg | 72.6±4.5 |
| Height, cm | 175.9±2.2 |
| BMI, kg/m2 | 23.4±1.1 |
| %Body fat | 28.7±1.5 |
| V̇o2max, ml·kg−1·min−1 | 35.5±3.0 |
| LV ejection fraction, % | 68±3 |
Values are means ± SE. M, men; W, women; BMI, body mass index; V̇o2max, maximal oxygen uptake; LV, left ventricular.
Hemodynamics.
Cardiac output was 5.3 ± 0.2 l/min at rest and did not change significantly with atrial pacing (Table 2). Heart rate increased significantly from 70 ± 4 to 108 ± 5 beats/min from rest to atrial pacing. Coronary sinus oxygen saturation (31 ± 1%) and arterial-coronary sinus oxygen content difference (4.1 ± 0.4 %vol) also did not change significantly from rest to atrial pacing. There were no significant changes in cardiac filling pressures, systemic blood pressure, or vascular resistance during atrial pacing compared with rest.
Table 2.
Hemodynamics and concentrations of hormones and substrates during rest and atrial pacing in men and women
| Rest | Atrial Pacing | |
|---|---|---|
| Heart rate, beats/min | 70±4 | 108±5§ |
| Cardiac output, l/min (thermodilution) | 5.3±0.25 | 5.6±0.44 |
| Coronary sinus blood flow, ml/min | 196±34 | 430±102§ |
| MV̇O2, ml/min | 24±2 | 65±18§ |
| Glucose, mg/dl | 83.5±2.4 | 83.9±2.5 |
| Lactate, mmol/l | 0.67±0.03 | 0.75±0.1 |
| Glycerol, umol/l | 108.7±30.3 | 114.6±28.6 |
| Triglyceride, mg/dl | 95.8±12.3 | 100.8±12.3§ |
| Epinephrine, pg/ml | 61.4±12.4 | 62.6±16.1 |
| Noriepinephrine, pg/ml | 398.6±86.6 | 391.7±96.5 |
| Insulin, pg/ml | 4.8±0.5 | 4.6±0.7 |
| Glucagon, pg/ml | 68.2±4.7 | 72.2±4.7§ |
Values are means ± SE. MV̇O2, myocardial oxygen consumption.
Significantly different from rest at P < 0.05.
Indirect calorimetry.
Whole body oxygen consumption, CO2 production, and respiratory exchange ratio were not significantly different from rest to atrial pacing. MV̇o2 increased significantly from rest to atrial pacing (P < 0.05). Myocardial RQ was also not significantly different during rest (0.83 ± 0.03) compared with atrial pacing (0.78 ± 0.04).
Substrate concentration.
Atrial pacing did not significantly change glycerol, epinephrine, norepinephrine, and insulin concentrations compared with rest (Table 2). However, triglyceride and glucagon concentrations increased during atrial pacing compared with rest (P < 0.05).
Cardiac hemodynamic measurements.
Atrial pacing increased coronary sinus blood flow from rest to atrial pacing (P < 0.05). There was an expected significant increase in heart rate with atrial pacing. Although a target heart rate of 120 beats/min was sought, three subjects developed intermittent atrioventricular nodal Wenkebach, resulting in a slight decrease in mean pacing heart rate for the group. There were no significant changes with atrial pacing in systemic arterial pressure, right atrial and pulmonary capillary wedge pressure, or cardiac output. Since heart rate increased without a change in cardiac output, mean stroke volume decreased from 77 ± 7 ml at rest to 53 ± 7 ml with atrial pacing (P < 0.05). Systemic vascular resistance was unchanged, but there was a 61% increase in the rate pressure product with atrial pacing. The rate pressure product, an indicator of myocardial O2 consumption, was 9,587 ± 640 mmHg-beats/min at rest and 15,419 ± 1,131 mmHg-beats/min with pacing (P < 0.005). There was a slight decrease in systolic pulmonary artery pressure with pacing. There were no significant changes in mixed venous O2 saturation, arteriovenous O2 content difference, coronary sinus O2 saturation, and arterial-coronary sinus O2 content difference with pacing.
Tracer kinetics.
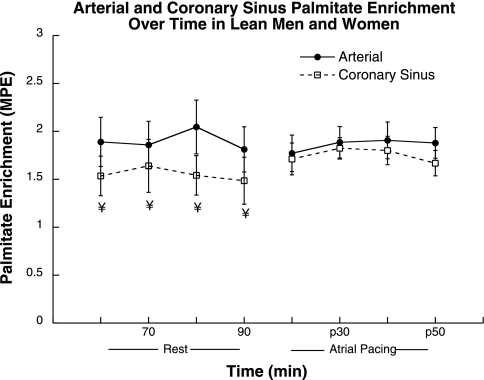
Arterial palmitate enrichment was stable over time during rest and atrial pacing (Fig. 1). Coronary sinus palmitate enrichment was significantly decreased compared with arterial palmitate enrichment at rest (P = 0.01) but was not significantly different during atrial pacing. Whole body palmitate appearance did not change significantly during the study, with Ra at 2.9 ± 0.6 μmol·kg−1·min−1 at rest and 2.8 ± 0.3 μmol·kg−1·min−1 during atrial pacing.
Fig. 1.
Arterial and coronary sinus palmitate enrichment during rest and atrial pacing. Values are means ± SE. ¥Significantly different from arterial, P < 0.05. MPE, moles percent excess.
Palmitate metabolism.
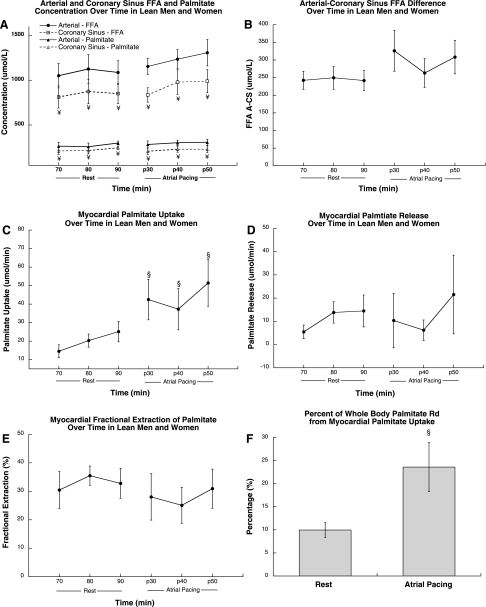
Arterial and coronary sinus FFA and palmitate concentrations did not change significantly during rest and atrial pacing (Fig. 2A). Throughout rest and atrial pacing, coronary sinus FFA and palmitate concentrations were significantly lower than arterial concentration (P < 0.001). Arterial-coronary sinus FFA concentration difference did not change significantly during atrial pacing compared with rest (Fig. 2B). Figure 2C shows isotopically measured myocardial palmitate uptake, which increased significantly from rest to atrial pacing (P = 0.04). The myocardium released palmitate at rest, which did not change significantly during atrial pacing (Fig. 2D). Approximately one-third of palmitate delivery was extracted by the myocardium at rest, which did not change significantly during atrial pacing (Fig. 2E). The heart accounted for a significantly greater proportion of whole body palmitate disappearance during atrial pacing compared with rest (P = 0.04; Fig. 2F). The relative proportion of tracer-measured FFA uptake to myocardial oxygen consumption (OER) was 48 ± 11% at rest and 43 ± 11% during atrial pacing.
Fig. 2.
Arterial and coronary sinus palmitate concentration (A), arterial-coronary sinus palmitate difference (B), and myocardial net palmitate uptake (C), myocardial palmitate release (D), myocardial palmitate fractional extraction (E), and %palmitate rate of disappearance (Rd) from myocardial palmitate uptake (F) during rest and atrial pacing in men and women. Values are means ± SE. ¥Significantly different from arterial, P < 0.05; §significantly different from rest, P < 0.05. FFA, free fatty acid.
Palmitate oxidation.
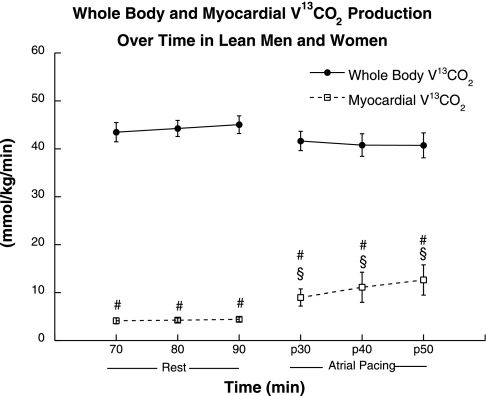
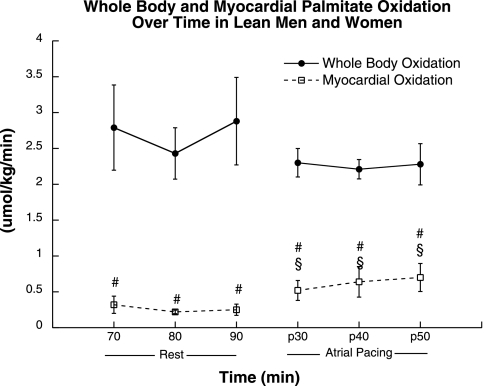
Whole body and myocardial acetate recovery factor was determined in four of the seven subjects in this study. Average acetate recovery factor data were used for the subjects in which an individual recovery factor was not determined. The acetate correction factor used was 72.2% at rest and 87.4% during atrial pacing. Whole body V13CO2 production was stable at rest and did not change significantly during atrial pacing (Fig. 3). Approximately 100% of the [1-14C]acetate label was recovered across the heart during rest and atrial pacing, so tissue label recovery was not applied. Myocardial V13CO2 production was significantly lower than whole body values during rest (P = 0.0001; Fig. 3) and atrial pacing (P = 0.0002). Myocardial V13CO2 production was stable at rest and increased significantly (P = 0.04) during atrial pacing. Similarly, whole body palmitate oxidation was stable at rest and did not change significantly during atrial pacing (Fig. 4). Myocardial palmitate oxidation was significantly lower than whole body values throughout rest (P = 0.004) and atrial pacing (P = 0.002). Myocardial palmitate oxidation was stable at rest and increased significantly during atrial pacing (P = 0.05).
Fig. 3.
Whole body and myocardial V13CO2 oxidation during rest and atrial pacing. Values are means ± SE. §Significantly different from rest, P < 0.05; #significantly different from whole body, P < 0.05.
Fig. 4.
Whole body and myocardial palmitate oxidation during rest and atrial pacing. Values are means ± SE. §Significantly different from rest, P < 0.05; #significantly different from whole body, P < 0.05.
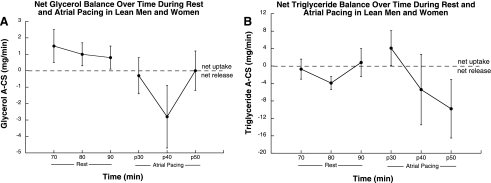
Glycerol and triglyceride net balance.
Net glycerol release was significantly greater than zero during rest (P = 0.04) and was not significantly different from zero during atrial pacing (P = 0.13). Net glycerol balance was nonsignificantly decreased during atrial pacing (P = 0.08; Fig. 5). These data indicate that the myocardium was taking up glycerol on a net basis during rest but not during atrial pacing. There was no significant net triglyceride exchange during rest (P = 0.43) and no significant change in net triglyceride balance across the heart during atrial pacing compared with rest (P = 0.36).
Fig. 5.
Net myocardial glycerol (A) and triglyceride (B) balance during rest and atrial pacing. Values are means ± SE.
DISCUSSION
We performed this study to test the hypothesis that myocardial palmitate uptake and oxidation would increase from rest to atrial pacing on an absolute basis, with similar relative proportion to total substrate uptake by the heart. These data support our hypothesis and suggest that myocardial lipid uptake and oxidation increase with an increase in heart work during atrial pacing, with a similar relative proportion of fat oxidation to total myocardial energy expenditure. The similar proportion of energy expenditure from lipid likely reflects unchanged concentration of substrates delivered to the myocardium.
There were no changes in whole body oxygen consumption or respiratory exchange ratio during the period of atrial pacing compared with rest. Therefore, atrial pacing was successful in increasing heart work only, without a change in whole body energy expenditure, substrate utilization, or arterial concentration of glucose, lactate, and FFA. Furthermore, systemic sympathetic nervous system activity, as determined by plasma catecholamine concentrations, was not significantly different during atrial pacing.
Myocardial RQ and oxygen extraction ratio data suggest the relative proportion of lipid to total energy expenditure was not significantly different during atrial pacing compared with rest. These data are similar to data published by Camici et al. (9), who reported unchanged myocardial RQ during atrial pacing at a similar heart rate. In their study, myocardial RQ increased only during maximal atrial pacing at a heart rate of 159 beats/min despite similar arterial substrate concentration. Therefore, changes in myocardial substrate use during atrial pacing may be intensity dependent. However, others reported that myocardial RQ was not significantly different at the end of 2 h of whole body exercise compared with rest with alterations in substrate concentration (18, 22). Therefore, during moderate intensity exercise or atrial pacing, RQ data from this study and others suggest that the relative proportions of carbohydrate and fat to total myocardial energy expenditure are similar compared with rest. However, the sources of carbohydrate and lipids may be different with increased contributions from lactate, and potentially from intramyocellular triglyceride (IMTG), which is discussed below.
Myocardial palmitate extraction in the current study was similar to others reporting 40–74% isotopically measured fractional extraction in resting human myocardium (18, 40, 44). Fractional extraction of FFA has been reported to be unchanged during atrial pacing (34) and to either not change (22) or decrease during whole body exercise (18, 22). Therefore, our data showing similar myocardial extraction during pacing compared with rest are consistent with the literature. Unlike others (22), we did not find a significant relationship between arterial FFA concentration and FFA uptake or arterial-coronary sinus difference FFA difference during either rest or atrial pacing. This is likely due to the small variation in fasting FFA concentration in the current study.
As during rest (7, 44), myocardial substrate utilization during whole body exercise in humans is heavily influenced by arterial substrate concentration (22, 24). Lassers et al. (22) found that changes in myocardial FFA utilization during 2 h of submaximal exercise were directly proportional to alterations in FFA concentration. They infused nicotinic acid to decrease FFA concentration and found a proportional decrease in FFA uptake along with increased myocardial carbohydrate oxidation. Similarly, Kaijser et al. (18) reported that the relative proportions of myocardial substrate utilization changed during 2 h of cycle ergometry at 50% of V̇o2max relative to the changes in substrate concentration. At the beginning of exercise, lactate concentration increased and FFA concentration decreased, which was reflected in their myocardial uptake. By the end of the 2 h of exercise, glucose concentration had decreased significantly along with myocardial glucose uptake. Arterial FFA and lactate concentrations were increased at the end of exercise relative to rest, whereas the uptake of lactate was doubled and that of FFA determined isotopically decreased. Myocardial RQ was not significantly different during exercise compared with rest, suggesting that increased lactate uptake replaced the contribution of glucose, and decreased FFA uptake during exercise was compensated for by increased reliance on intramyocellular lipids (18). These data indicate that arterial substrate concentration plays an important role in myocardial substrate utilization during rest and increased heart work. Additionally, duration of increased heart work may influence substrate selection. Unlike our data, others (9, 27) reported unchanged plasma FFA but increased glucose uptake during short-duration atrial pacing (6–16 min) with similar arterial substrate concentrations. This comparison suggests that an increase in heart work may stimulate FFA uptake only after a sustained period of time, with short changes in heart work fueled by alterations in carbohydrate utilization. Therefore, our data are consistent with the literature and reveal that, when heart work is increased in isolation by atrial pacing, the absolute uptake of FFA increases proportionally to myocardial energy requirements.
Some (18, 22), but not all, previous studies (9) have reported net glycerol release across the myocardium. Kaijser et al. (18) found that net glycerol release was not different from zero at rest but switched to significant net glycerol release during cycle ergometry at 50% of maximum workload. These authors suggested IMTG was the source of glycerol release during exercise. Net glycerol release during atrial pacing was not reported by Camici et al. (9). This may be due to the different pacing strategy used by Camici et al., which was performed for only 4 min/step and for 16 total min. By comparison, we performed atrial pacing for 50 min at one pacing stress and were able to achieve steady state. Our data showed net myocardial glycerol uptake during rest, which was not significantly different than zero during atrial pacing. Therefore, our data are unclear as to the role of intramyocardial triglyceride utilization during increased heart work. If the myocardium increases reliance on IMTG during prolonged increases in heart work, this would be opposite of that reported for skeletal muscle IMTG, where decreased IMTG utilization has been reported in humans during prolonged exercise (41).
Another explanation for net glycerol uptake during rest, but not during atrial pacing, may be due to glycerol release from lipoprotein-derived triglyceride hydrolysis during pacing. Triglyceride extraction by the heart has been reported during rest and atrial pacing (9). More recently, Nelson et al. (28) reported tracer-measured myocardial triglyceride utilization in six individuals, most of whom were diagnosed with coronary artery disease, that accounted for 17% of myocardial fatty acid uptake. So it is possible that changes in lipoprotein-mediated triglyceride uptake may explain variability in our net glycerol release data. Others reported increased net glycerol release across the myocardium during exercise at 50% V̇o2max compared with rest without a change in net triglyceride balance (18, 22). Our triglyceride balance data shown in Fig. 5B argue against plasma triglyceride degradation contributing to myocardial net glycerol balance. Therefore, IMTG degradation may explain the switch from net glycerol uptake to no net glycerol exchange during atrial pacing. Myocardial glycerol release has been reported in an isolated perfused rat heart model that did not contain triglycerides in the perfusion medium (12). This glycerol release likely originated from degradation of IMTG. Those authors also reported that isotopically measured palmitate uptake was greater than net chemical uptake, providing evidence that myocardial FFA release may originate from IMTG. Thus, the literature is unclear regarding degradation of intramyocardial triglyceride stores. Future studies measuring myocardial isotopic glycerol and triglyceride exchange during rest and atrial pacing and/or exercise are needed to determine the relative importance of circulating triglyceride vs. IMTG stores to energy expenditure.
There are several limitations to our study. During atrial pacing we observed a 118% increase in coronary sinus blood flow but no increase in cardiac output compared with the resting state. Despite the increase in heart rate, stroke volume decreased due to Starling forces, a concept that was demonstrated in early studies with atrial pacing in humans (31). Therefore, although atrial pacing increases myocardial oxygen consumption and does not influence arterial concentration, it is not a completely physiological model since increased heart work is performed without an increase in cardiac output. However, the lack of increased systemic blood flow and the lack of skeletal muscle and adipose tissue activity are responsible for the unchanged arterial FFA concentrations observed in this study. In a similar metabolically controlled model with increased cardiac work from either exercise or pharmacological stimulation, the findings may be different. Furthermore, we increased heart rate to approximately double myocardial oxygen consumption. It is unknown whether our data would be similar with more dramatic increases in heart rate and thus myocardial work. However, our data suggest that if arterial substrate concentrations are unchanged, the further increase in myocardial work would also be achieved without a significant change in the relative proportion of substrates to total myocardial energy demand. We infused a small amount of heparin to maintain patency of the arterial line in each study. The amount of heparin used to mobilize lipoprotein lipase, which would increase FFA concentration, is ∼60 U/kg, or 4,400 units for the average subject in this study (16). We infused only 15 U/h, and therefore, we did not observe changes in FFA concentration outside the normal increase with duration of fasting. Only one-half of our subjects received the [1-14C]acetate infusion to quantify the whole body and myocardial acetate correction factor in this study. We applied mean values to the three subjects who did not receive this infusion. However, since we recovered all of the acetate across the heart, as has been reported before for working muscle (39), the lack of agreement between mean and individual acetate correction factors would affect only the rates of whole body fat oxidation in this study.
The determination of FFA substrate utilization by the human myocardium under a variety of physiological conditions may have important clinical relevance to disease states such as heart failure, diabetes, and coronary artery disease. Plasma FFAs are elevated in patients with heart failure, and there is enhanced total body FFA turnover and oxidation (23). There also appears to be enhanced myocardial β-oxidation of FFA in patients with cardiomyopathy (13). However, there is a greater oxygen cost in the production of ATP when FFAs are used preferentially to glucose and lactate for energy metabolism. Despite this apparent increased myocardial oxygen cost with FFA utilization, pharmacological approaches that decrease serum FFA levels have been associated with impaired cardiac performance in patients with heart failure (37). Thus the role of FFA utilization in patients with cardiac dysfunction has yet to be elucidated; however, it is clear that the pathophysiology of myocardial dysfunction is associated with abnormalities in myocardial lipid metabolism. Determination of the fate of FFAs in the normal human myocardium under a variety of physiological conditions is an important step in pursuing the metabolic derangements and potential therapeutic approaches in cardiac muscle disease.
Conclusions.
These data suggest that, in normal healthy humans, increased heart work in vivo without a change in arterial substrate concentration results in a similar proportion of FFA utilization relative to myocardial energy expenditure. The similar proportion of energy expenditure from fat sources during rest and atrial pacing likely reflects unchanged arterial concentration of substrates delivered to the myocardium.
GRANTS
This work was partially supported by National Institutes of Health GCRC Grant RR-00036, National Institute of Diabetes and Digestive and Kidney Diseases grants to B. C. Bergman (DK-059739), and a National Institutes of Health Clinical Nutrition Research Unit pilot project grant from the University of Colorado Denver.
Acknowledgments
We thank Dr. Ronald Zolty for assistance during some of the invasive metabolic studies.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aguilar-Parada E, Eisentraut AM, Unger RH. Pancreatic glucagon secretion in normal and diabetic subjects. Am J Med Sci 257: 415–419, 1969. [DOI] [PubMed] [Google Scholar]
- 2.Ashrafian H, Frenneaux MP, Opie LH. Metabolic mechanisms in heart failure. Circulation 116: 434–448, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Avogaro A, Nosadini R, Doria A, Fioretto P, Velussi M, Vigorito C, Sacca L, Toffolo G, Cobelli C, Trevisan R, Duner E, Razzolini R, Crepaldi G. Myocardial metabolism in insulin-deficient diabetic humans without coronary artery disease. Am J Physiol Endocrinol Metab 258: E606–E618, 1990. [DOI] [PubMed] [Google Scholar]
- 4.Avogaro A, Nosadini R, Doria A, Fioretto P, Velussi M, Vigorito C, Saccà L, Toffolo G, Cobelli C, Trevisan R, Duner E, Razzolini R, Rengo F, Crepaldi G. Myocardial metabolism in insulin-deficient diabetic humans without coronary artery disease. Am J Physiol Endocrinol Metab 258: E606–E618, 1990. [DOI] [PubMed] [Google Scholar]
- 5.Bergman BC, Butterfield GE, Wolfel EE, Lopaschuk GD, Casazza GA, Horning MA, Brooks GA. Muscle net glucose uptake and glucose kinetics after endurance training in men. Am J Physiol Endocrinol Metab 277: E81–E92, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Bing RJ Cardiac metabolism. Physiol Rev 45: 171–213, 1965. [DOI] [PubMed] [Google Scholar]
- 7.Bing RJ, Siegel A, Ungar I, Gilbert M. Metabolism of the human heart. II. Studies on fat, ketone and amino acid metabolism. Am J Med 16: 504–515, 1954. [DOI] [PubMed] [Google Scholar]
- 8.Burkhoff D, Weiss RG, Schulman SP, Kalil-Filho R, Wannenburg T, Gerstenblith G. Influence of metabolic substrate on rat heart function and metabolism at different coronary flows. Am J Physiol Heart Circ Physiol 261: H741–H750, 1991. [DOI] [PubMed] [Google Scholar]
- 9.Camici P, Marraccini P, Marzilli M, Lorenzoni R, Buzzigoli G, Puntoni R, Boni C, Bellina CR, Klasen GA, L'Abbate A, Ferrannini E. Coronary hemodynamics and myocardial metabolism during and after pacing stress in normal humans. Am J Physiol Endocrinol Metab 257: E309–E317, 1989. [DOI] [PubMed] [Google Scholar]
- 10.Causon RC, Carruthers ME, Rodnight R. Assay of plasma catecholamines by liquid chromatography with electrochemical detection. Anal Biochem 116: 223–226, 1981. [DOI] [PubMed] [Google Scholar]
- 11.Douglas A, Jones N, Reed J. Calculation of whole blood CO2 content. J Appl Physiol 65: 473–477, 1988. [DOI] [PubMed] [Google Scholar]
- 12.Evans JR, Opie LH, Shipp JC. Metabolism Of palmitic acid in perfused rat heart. Am J Physiol 205: 766–770, 1963. [DOI] [PubMed] [Google Scholar]
- 13.Feinendegen LE, Henrich MM, Kuikka JT, Thompson KH, Vester EG, Strauer B. Myocardial lipid turnover in dilated cardiomyopathy: a dual in vivo tracer approach. J Nucl Cardiol 2: 42–52, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Friedlander AL, Casazza GA, Horning MA, Usaj A, Brooks GA. Endurance training increases fatty acid turnover, but not fat oxidation, in young men. J Appl Physiol 86: 2097–2105, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Goodwin GW, Taylor CS, Taegtmeyer H. Regulation of energy metabolism of the heart during acute increase in heart work. J Biol Chem 273: 29530–29539, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Imamura S, Kobayashi J, Nakajima K, Sakasegawa S, Nohara A, Noguchi T, Kawashiri MA, Inazu A, Deeb SS, Mabuchi H, Brunzell JD. A novel method for measuring human lipoprotein lipase and hepatic lipase activities in postheparin plasma. J Lipid Res 49: 1431–1437, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaijser L, Ericsson M, Walldius G. Fatty acid turnover in the ischaemic compared to the non-ischaemic human heart. Mol Cell Biochem 88: 181–184, 1989. [DOI] [PubMed] [Google Scholar]
- 18.Kaijser L, Lassers BW, Wahlqvist ML, Carlson LA. Myocardial lipid and carbohydrate metabolism in fasting men during prolonged exercise. J Appl Physiol 32: 847–858, 1972. [DOI] [PubMed] [Google Scholar]
- 19.Kantor PF, Dyck JR, Lopaschuk GD. Fatty acid oxidation in the reperfused ischemic heart. Am J Med Sci 318: 3–14, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Kelman G Digital computer procedure for the conversion of PCO2 into blood CO2 content. Respir Physiol 3: 111–115, 1967. [DOI] [PubMed] [Google Scholar]
- 21.Lassers BW, Kaijser L, Carlson LA. Myocardial lipid and carbohydrate metabolism in healthy, fasting men at rest: studies during continuous infusion of 3 H-palmitate. Eur J Clin Invest 2: 348–358, 1972. [DOI] [PubMed] [Google Scholar]
- 22.Lassers BW, Wahlqvist ML, Kaijser L, Carlson LA. Effect of nicotinic acid on myocardial metabolism in man at rest and during exercise. J Appl Physiol 33: 72–80, 1972. [DOI] [PubMed] [Google Scholar]
- 23.Lommi J, Kupari M, Yki-Jarvinen H. Free fatty acid kinetics and oxidation in congestive heart failure. Am J Cardiol 81: 45–50, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Lopaschuk G, Belke D, Gamble J, Itoi T, Schönekess B. Regulation of fatty acid oxidation in the mammalian heart in health and disease. Biochim Biophys Acta 1213: 263–276, 1994. [DOI] [PubMed] [Google Scholar]
- 25.Miller HI, Yum KY, Durham BC. Myocardial free fatty acid in unanesthetized dogs at rest and during exercise. Am J Physiol 220: 589–596, 1971. [DOI] [PubMed] [Google Scholar]
- 26.Neely JR, Bowman RH, Morgan HE. Effects of ventricular pressure development and palmitate on glucose transport. Am J Physiol 216: 804–811, 1969. [DOI] [PubMed] [Google Scholar]
- 27.Neglia D, De Caterina A, Marraccini P, Natali A, Ciardetti M, Vecoli C, Gastaldelli A, Ciociaro D, Pellegrini P, Testa R, Menichetti L, L'Abbate A, Stanley WC, Recchia FA. Impaired myocardial metabolic reserve and substrate selection flexibility during stress in patients with idiopathic dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 293: H3270–H3278, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Nelson RH, Prasad A, Lerman A, Miles JM. Myocardial uptake of circulating triglycerides in nondiabetic patients with heart disease. Diabetes 56: 527–530, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Nunn JF Nunn's Applied Respiratory Physiology. Boston, MA: Butterworth-Heineman, 1993.
- 30.Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, Dence C, Klein S, Marsala J, Meyer T, Gropler RJ. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation 109: 2191–2196, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Ross J Jr, Linhart JW, Brauwald E. Effects of changing heart rate in man by electrical stimulation of the right atrium. Studies at rest, during exercise, and with isoproterenol. Circulation 32: 549–558, 1965. [DOI] [PubMed] [Google Scholar]
- 32.Saddik M, Lopaschuk GD. Myocardial triglyceride turnover and contribution to energy substrate. J Biol Chem 266: 8162–8170, 1991. [PubMed] [Google Scholar]
- 33.Sidossis LS, Coggan AR, Gastaldelli A, Wolfe RR. A new correction factor for use in tracer estimations of plasma fatty acid oxidation. Am J Physiol Endocrinol Metab 269: E649–E656, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Simonsen S, Kjekshus JK. The effect of free fatty acids on myocardial oxygen consumption during atrial pacing and catecholamine infusion in man. Circulation 58: 484–491, 1978. [DOI] [PubMed] [Google Scholar]
- 35.Stanley WC, Lopaschuk GD, McCormack JG. Regulation of energy substrate metabolism in the diabetic heart. Cardiovasc Res 34: 25–33, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85: 1093–1129, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Tuunanen H, Engblom E, Naum A, Nagren K, Hesse B, Airaksinen KE, Nuutila P, Iozzo P, Ukkonen H, Opie LH, Knuuti J. Free fatty acid depletion acutely decreases cardiac work and efficiency in cardiomyopathic heart failure. Circulation 114: 2130–2137, 2006. [DOI] [PubMed] [Google Scholar]
- 38.van der Vusse GJ, Glatz JF, Stam HC, Reneman RS. Fatty acid homeostasis in the normoxic and ischemic heart. Physiol Rev 72: 881–940, 1992. [DOI] [PubMed] [Google Scholar]
- 39.van Hall G, Sacchetti M, Rådegran G. Whole body and leg acetate kinetics at rest, during exercise and recovery in humans. J Physiol 542: 263–272, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vyska K, Meyer W, Stremmel W, Notohamiprodjo G, Minami K, Machulla HJ, Gleichmann U, Meyer H, Korfer R. Fatty acid uptake in normal human myocardium. Circ Res 69: 857–870, 1991. [DOI] [PubMed] [Google Scholar]
- 41.Watt MJ, Heigenhauser GJ, Dyck DJ, Spriet LL. Intramuscular triacylglycerol, glycogen and acetyl group metabolism during 4 h of moderate exercise in man. J Physiol 541: 969–978, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wide L, Porath J. Radioimmunoassay of proteins with the use of Sephadex-coupled antibodies. Biochim Biophys Acta 130: 257–260, 1966. [Google Scholar]
- 43.Weir JB New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1–9, 1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wisneski JA, Gertz EW, Neese RA, Mayr M. Myocardial metabolism of free fatty acids: studies with 14C-labeled substrates in humans. J Clin Invest 79: 359–366, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolfe R Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis. New York: Wiley-Liss, 1992.