Abstract
Controversy exists as to whether endogenous cortisol production is associated with visceral obesity and insulin resistance in humans. We therefore quantified cortisol production and clearance rates, abdominal fat depots, insulin sensitivity, and adipocyte gene expression in a cohort of 24 men. To test whether the relationships found are a consequence rather than a cause of obesity, eight men from this larger group were studied before and after weight loss. Daily cortisol production rates (CPR), free cortisol levels (FC), and metabolic clearance rates (MCR) were measured by stable isotope methodology and 24-h sampling; intra-abdominal fat (IAF) and subcutaneous fat (SQF) by computed tomography; insulin sensitivity (SI) by frequently sampled intravenous glucose tolerance test; and adipocyte 11β-hydroxysteroid dehydrogenase-1 (11β-HSD-1) gene expression by quantitative RT-PCR from subcutaneous biopsies. Increased CPR and FC correlated with increased IAF, but not SQF, and with decreased SI. Increased 11β-HSD-1 gene expression correlated with both IAF and SQF and with decreased SI. With weight loss, CPR, FC, and MCR did not change compared with baseline; however, with greater loss in body fat than lean mass during weight loss, both CPR and FC increased proportionally to final fat mass and IAF and 11β-HSD-1 decreased compared with baseline. These data support a model in which increased hypothalamic-pituitary-adrenal activity in men promotes selective visceral fat accumulation and insulin resistance and may promote weight regain after diet-induced weight loss, whereas 11β-HSD-1 gene expression in SQF is a consequence rather than cause of adiposity.
Keywords: obesity, metabolic clearance rate, disposition index
excess accumulation of abdominal fat occurs in men and is a risk factor for both type 2 diabetes and cardiovascular disease (9, 11, 22). A number of specific mechanisms that could mediate selective uptake of fat into the visceral depot have been proposed, including both dysregulation of central pituitary hormone secretion and alterations in fat cell- and depot-specific gene and enzyme activities (6, 23, 27).
Hypercortisolemia, or Cushing's syndrome, can lead to central obesity and type 2 diabetes. Many studies of subjects selected only for obesity have found that while cortisol production rates (CPR) are increased compared with those in lean control subjects, this difference is no longer significant after adjusting for body size (usually body surface area) and that levels of total and free cortisol are not different between lean and obese groups (29, 36–38). Therefore, the balance of evidence does not support a role of increased cortisol secretion contributing to generalized adiposity (29, 41). It is still possible, however, that increased CPR and peripheral cortisol levels contribute to central and visceral obesity (independent of total adiposity) and insulin resistance in humans, although recent studies using surrogate outcome measures of cortisol production (such as urinary cortisol excretion) and visceral obesity have yielded mixed results (39, 41, 48).
Using a direct measurement of cortisol production by steady-state isotope infusion and mass spectroscopy with 24-h blood sampling, we recently reported (29) that daily CPR and free cortisol levels (FC) are higher in men than in women. Since men have, on average, greater amounts of visceral fat than women (24) and visceral fat increases in men with aging (9), we hypothesized that increased CPR and blood cortisol levels might play a role in increased expression of visceral adiposity and insulin resistance in this group.
MATERIALS AND METHODS
Research subjects.
Twenty-four men aged 18–70 yr were recruited through campus advertisements, local newspapers, and clinics at the University of Washington to represent a spectrum of body weights. All subjects were otherwise healthy, at their lifetime maximal weight, and free of active medical diseases (29). Eight of these subjects who were obese underwent a diet-induced weight loss program and were studied again during a period of weight stability. Additional exclusion criteria were psychiatric illness or medication for a psychiatric diagnosis, smoking, consumption of more than two alcoholic beverages per day, substance abuse, untreated hypothyroidism, or exercise >30 min three times a week. As previously reported (29), the mean age of the group was 44 yr (range 19–70 yr) and the mean body mass index (BMI) was 33 kg/m2 (range 20–64 kg/m2). The Human Subjects Review Committee at the University of Washington approved all procedures, and consent was obtained from each subject before study entry.
Body composition.
All subjects had measurement of percent fat (% fat), total body fat mass (FM), and total body fat-free mass (FFM) at the time of their inpatient stay by the method of underwater weighing (15). Because of weight or size limitations of the computerized tomography (CT) scanner, only 21 of the 24 subjects had quantification of intra-abdominal fat (IAF) and subcutaneous fat (SQF) that was read by a single, blinded observer with a single CT image obtained at the level of the umbilicus (34).
Cortisol production rates.
Subjects were admitted to the University of Washington General Clinical Research Center (GCRC) the evening before study for measurement of CPR. Two separate peripheral intravenous lines were started, one for infusion of the deuterium-labeled cortisol isotope (d3-cortisol, [9,12,12-2H3]cortisol, 99.8 atom %, Cambridge Isotope Labs, Andover, MA), and the other for drawing blood. Starting at 0200, a continuous infusion of deuterium-labeled cortisol (20 μg/h) was administered at a constant rate over the next 30 h (until 0800 of the final study day). Five-milliliter blood samples were drawn every 30 min from the second intravenous line during the final 24 h of the deuterated cortisol infusion (0800–0800). The total amount of deuterated cortisol infused over 24 h (480 μg) was <5% of a normal endogenous 24-h CPR. During each stay, subjects consumed a diet provided by the GCRC Bionutrition staff that had a macronutrient content of 30% of total calories as fat, 15% as protein, and 55% as carbohydrate. During the baseline study subjects consumed their meals ad libitum, while at the follow-up study after weight loss caloric intake was determined for each participant by the GCRC Bionutrition staff to maintain stable weight. Breakfast was consumed at 0800, lunch at 1200, and dinner at 1730.
Plasma was separated, and 50 μl from each sample was combined into a 24-h pooled sample for mass spectrophotometric analysis. Plasma from the pooled sample (0.5 ml) then underwent an extraction and derivatization process to form fluoroacyl derivatives of cortisol as previously described to enhance detection by gas chromatography-negative ion chemical ionization mass spectrometry (GC-NICIMS) (7). The areas under the curves for the total selected ion chromatogram of fluoroacyl derivatives of cortisol [d0, mass-to-charge ratio (m/z) 782] and d3-cortisol (d3, m/z 785) were used to determine the isotopic dilution ratio (d3/d0) in 24-h pooled plasma samples (PS). Retention times of the fluoroacyl derivatives were verified by authentic standards. CPR was then calculated from the product infusion rate (IR) and the ratio of the isotopic enrichment (IE) to isotopic dilution in plasma [CPR = IR × (IE/PS)]. Standard, control, and subject samples were run in triplicate. The intra-assay variability [5 pools taken from the same 24-h samples and measured 5 times in the same gas chromatography-mass spectrometry (GC-MS) run] was 9%. The interassay variability (5 separate pools taken from the same 24-h samples and measured 5 separate times by GC-MS) was 5%. The biological variation in the same individual studied on five separate occasions over the span of 16 mo was 12%.
Free cortisol, cortisol binding globulin, and metabolic clearance rate of cortisol.
Total cortisol levels were measured in the individual 30-min timed samples by a two-site chemiluminescent immunometric assay (Nichols Institute Diagnostic, San Juan Capistrano, CA) in the core laboratory of the Oregon Health and Science University GCRC. Intra-assay coefficients of variation were 3–5%, and interassay coefficients of variation were 6–10%. Assay sensitivity was 0.8 μg/dl. All samples were run in duplicate.
Separate 24-h pooled aliquots were made as described above to determine the 24-h FC by the method of 18-h equilibrium dialysis and radioimmunoassay (RIA) of the dialysate (14). Cortisol binding globulin (CBG) was quantified by RIA (Quest Diagnostics, Nichols Institute, San Juan Capistrano, CA) in the same sample (31). The intra-assay coefficient of variation, interassay coefficient of variation, and assay sensitivity of the free cortisol assay were 9.8%, 12.6%, and 0.03 μg/dl, respectively. The intra-assay coefficient of variation, interassay coefficient of variation, and assay sensitivity of the CBG assay were 5.8%, 9.2%, and 0.01 mg/l, respectively.
The metabolic clearance rate (MCR) of cortisol was determined with the formula MCR = CPR/FC (49). The mean (range) of MCR in all 24 men was 326 (133–570) l/day, which is in close agreement with previous studies (36, 45).
Insulin sensitivity, acute insulin response to glucose, and disposition index.
The morning before CPR measurement and after an overnight fast, a tolbutamide-modified frequently sampled intravenous glucose tolerance test (IVGTT) was performed in all subjects as previously described (4). Briefly, glucose (11.4 g/m2) was injected at time 0 as a bolus over 60 s, and tolbutamide (125 mg/m2) was injected at the 20-min time point after the glucose injection over 30 s; blood samples for glucose and insulin measurements were drawn at 32 time points over 4 h. Insulin sensitivity (SI) was quantified with Bergman's minimal model of glucose kinetics. The acute insulin response to glucose (AIRglu) was computed from the IVGTT results as the mean increment above baseline between 2 and 10 min after glucose injection. The disposition index (DI), a measure of the ability of the islet β-cell to compensate in response to differences in insulin sensitivity, was calculated as DI = SI × AIRglu (18).
Fat biopsy, cell sizing, and 11β-hydroxysteroid dehydrogenase-1 mRNA gene expression.
A subcutaneous fat tissue biopsy was performed at the level of the posterior, superior iliac crest (representing truncal fat stores) by aspiration under local anesthesia. Fat samples were immediately processed for fat cell sizing as previously described (33) or frozen at −80°C for measurement of gene expression. Gene expression for 11β-hydroxysteroid dehydrogenase-1 (11β-HSD-1) was measured in the Molecular and Genetics Core at the University of Washington. Total RNA was isolated, and quantitative PCR was performed on an Mx4000 Multiplex QPCR System (Stratagene, La Jolla, CA) with samples loaded in triplicate with 50 ng of total RNA per reaction. Quantitative PCR was run in a 25-μl reaction with the Stratagene Brilliant Single-Step QRT-PCR Kit (2.5 μl of 10× core RT-PCR buffer, 5.0 mM MgCl2, each primer at 300 nM, 200 nM probe, each dNTP at 0.2 mM, 75 nM passive reference dye, 1.56 U Stratascript RT, 1.25 U SureStart TaqDNA-polymerase) with PCR cycling conditions of 45°C for 30 min, 95°C for 10 min, and 40 cycles of 95°C for 30 s, 60°C for 1 min. Pooled total RNA from patient samples was used for standard curves as 1:3 serial dilutions. The standard curve for HSD-1 showed an efficiency of 77% (SD 5%). Resulting threshold cycle (CT) values were converted to nanograms and normalized to the values of total RNA quantified on the Mx4000 Multiplex QPCR System in duplicate wells with the RiboGreen RNA Quantitation Kit (Molecular Probes, Eugene, OR) with standards supplied by the manufacturer.
Weight loss diet.
After completion of the baseline studies, eight obese subjects (Table 1) met with GCRC bionutritionists for weight loss counseling. Subjects then followed a low-fat, high-protein, liquid formula diet of 1,000 kcal/day provided by the Bionutrition Unit for 3 mo, supplemented with daily multivitamins and minerals for 3 mo. After this period, subjects were transitioned over 2 wk to a solid-food diet containing 30% fat, 15% protein, and 55% carbohydrates. The total number of calories was adjusted to stabilize weight, and subjects were taught to continue this process at home in order to maintain a stable, reduced weight for another 3 mo. Throughout the 6 mo of the program of weight loss and stabilization, subjects returned two or three times per week to the Bionutrition Unit to be weighed with the same scale and to pick up their prepared food or receive diet counseling.
Table 1.
Characteristics of dieting group before and after weight loss
| Pre | Post | P Value (Pre → Post) | |
|---|---|---|---|
| Age, yr | 44.5 (28–54) | NA | |
| BMI, kg/m2 | 38 (29–54) | 32 (24–47) | <0.001 |
| %Fat | 37 (29–47) | 29 (19–41) | 0.002 |
| Fat mass, kg | 47 (25–79) | 32 (14–65) | <0.001 |
| Fat cell volume, μg TG/cell | 0.79 (0.55–1.14) | 0.55 (0.41–0.700) | <0.001 |
| Fat-free mass, kg | 77 (60–105) | 72 (57–94) | 0.012 |
| IAF, cm2 | 182 (122–295) | 90 (35–209) | <0.001 |
| SQF, cm2 | 436 (224–638) | 289 (108–499) | 0.002 |
| Leptin, ng/ml | 19 (7.6–44) | 12 (3.2–33) | 0.002 |
| Glucose, mg/dl | 78 (65–91) | 94 (84–108) | 0.004 |
| Insulin, μU/ml | 19 (4.4–30) | 10 (4.5–19) | 0.02 |
| SI, min−1·μU −1·ml−1 (× 10−4) | 1.2 (0.5–3.1) | 2.4 (1.0–3.9) | 0.002 |
| CPR, mg/day | 20 (11–26) | 18 (12–24) | 0.21 |
| MCR, l/day | 375 (231–472) | 355 (246–611) | 0.68 |
| CBG, mg/l | 21.5 (17–28) | 21.6 (17–26) | 0.91 |
| Free cortisol, μg/dl–day | 0.53 (0.37–0.66) | 0.52 (0.40–0.66) | 0.59 |
| 11β-HSD-1 mRNA, AU | 1.78 (0.65–2.29) | 0.99 (0.005–1.78) | 0.02 |
Values are means (range); n = 8 subjects. Pre, before weight loss; Post, after weight loss; BMI, body mass index; TG, triglyceride; IAF, intra-abdominal fat. SQF, abdominal subcutaneous fat; SI, insulin sensitivity; CPR, daily cortisol production rate; MCR, metabolic clearance rate of cortisol; CBG, cortisol binding globulin; 11β-HSD-1, 11β hydroxysteroid dehydrogenase-1; AU, arbitrary units; NA, not applicable.
Statistical analysis.
Correlational relationships were tested with linear regression. When appropriate, nonnormally distributed variables were natural log transformed before linear regression analysis. Area under the curve (AUC) cortisol levels were calculated with the trapezoidal method for the entire 24 h and in blocks of 6 h beginning at 12 midnight based on previous observations of increased levels occurring just past midnight and noon in older men and women (43). ANOVA on ranks was used for comparison of study outcomes between IAF tertile groups. Before and after results were compared with paired t-testing. A value of P < 0.05 was considered significant.
RESULTS
Cortisol production rates, cortisol levels, and abdominal fat depots.
In these men, 24-h FC increased as a function of increasing CPR (r = 0.56, P = 0.004), which remained significant even after adjustment for body surface area (CPR/BSA, r = 0.58, P = 0.003). Twenty-four-hour FC were, however, independent of variations in CBG levels (r = 0.29, P = 0.17).
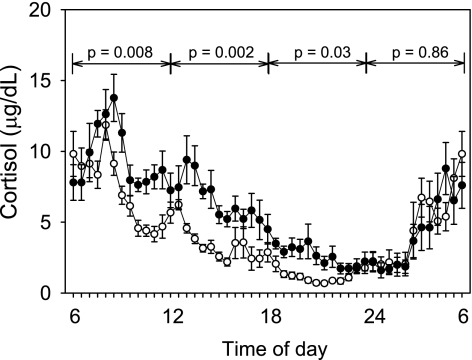
With the exception of CBG, all cortisol parameters correlated with fat cell volume (fat cell volume vs. CPR, r = 0.71, P < 0.001; vs. CPR/BSA, r = 0.50, P = 0.03; vs. 24-h FC, r = 0.59, P = 0.007) and IAF (Fig. 1, top) but not SQF (data not shown). Because of the significant relationship between higher 24-h FC from the pooled samples and increasing IAF, subjects were divided by tertiles of IAF to determine at which time points during the circadian appearance of total cortisol significant differences between those with the least and the greatest amount of IAF occurred (Fig. 2). Subjects in the first tertile of IAF had a mean IAF of 64 cm2 and a waist-to-hip ratio (WHR) of 0.86; those in the third tertile had a mean IAF of 226 cm2 and a WHR of 1.0. Median 24-h AUC total cortisol levels increased significantly in each tertile of IAF (1st tertile 6,120 μg-day/dl, 2nd tertile 7,930 μg-day/dl, 3rd tertile 8,330 μg-day/dl; P = 0.007). When divided into 6-h blocks, cortisol levels were significantly higher during the day, from 0600 to midnight in the third vs. first tertiles of IAF, but not overnight (Fig. 2). In contrast, MCR of cortisol did not significantly differ by tertile (P = 0.14).
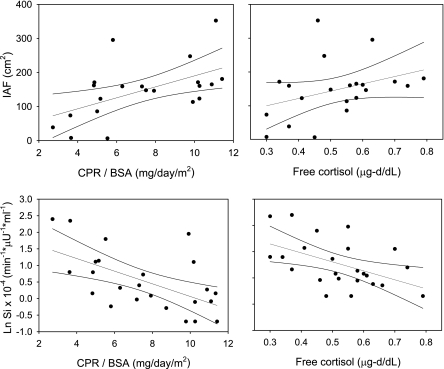
Fig. 1.
Top: linear regression analysis with 95% confidence intervals for 24-h cortisol production rates adjusted for body surface area (CPR/BSA) vs. intra-abdominal fat (IAF) (r = 0.53, P = 0.01; left) and 24-h plasma free cortisol levels vs. IAF (r = 0.45, P = 0.04; right). CPR vs. IAF, r = 0.59, P = 0.005 (graph not shown). Bottom: linear regression analysis with 95% confidence intervals for CPR/BSA vs. insulin sensitivity (SI) (r = −0.58, P = 0.003; left) and 24-h plasma free cortisol levels vs. SI (r = −0.50, P = 0.01; right). CPR vs. SI, r = −0.70, P < 0.001 (graph not shown).
Fig. 2.
Mean ± SE plasma total cortisol levels sampled every 30 min over 24 h in the subjects with the least amount of IAF (1st tertile, ○; n = 7) and the subjects with the greatest amount of IAF (3rd tertile, •; n = 7). Median 24-h area under the curve (AUC): total cortisol 6,120 μg-day/dl vs. 8,330 μg-day/dl, 1st vs. 3rd tertile, P = 0.01. Lines and P values signify AUC values during 6-h time blocks that are different in the 1st vs. 3rd tertiles.
Cortisol and glucose metabolism.
Subjects exhibited broad ranges of SI from 0.5 to 11 × 10−4 min−1·μU−1·ml−1 and fasting insulin levels from 2.3 to 47 μU/ml.
SI correlated negatively with CPR (r = −0.66, P < 0.001), CPR/BSA (r = −0.58, P = 0.003), and 24-h FC (r = −0.50, P = 0.01) (Fig. 1, bottom). Fasting insulin levels correlated positively with CPR (r = 0.63, P = 0.001) and CPR/BSA (r = 0.47, P = 0.02) but not 24-h FC (r = 0.17, P = 0.43). AIRglu was not associated with any cortisol outcome (data not shown). DI correlated inversely with CPR (r = −0.52, P = 0.009) and 24-h FC (r = −0.43, P = 0.04). Finally, CBG was not associated with any parameters of glucose metabolism (fasting glucose levels, fasting insulin levels, SI, AIRglu, or DI) (data not shown).
Adipocyte 11β-HSD-1 mRNA.
Adipocyte 11β-HSD-1 gene expression correlated with % fat (r = 0.54, P = 0.02), IAF (r = 0.54, P = 0.02), SQF (r = 0.49, P = 0.04), fat cell volume (r = 0.66, P = 0.002), and leptin (r = 0.58, P = 0.01) but not age, BMI, FM, FFM, CPR, MCR, CBG, or FC. 11β-HSD-1 gene expression also correlated with SI (r = −0.58, P = 0.01) but not glucose levels (P = 0.56), insulin levels (P = 0.06), AIRglu (P = 0.07), or DI (P = 0.28).
Effect of weight loss on body composition, cortisol, and 11β-HSD-1.
Mean weight loss during the 6-mo intervention was 19.7 kg (16%). All fat parameters significantly decreased, with proportionally larger losses of IAF (−51%) than SQF (−34%) (Table 1). FFM was relatively spared (−6% compared with baseline) compared with FM (−32% compared with baseline) (Table 1).
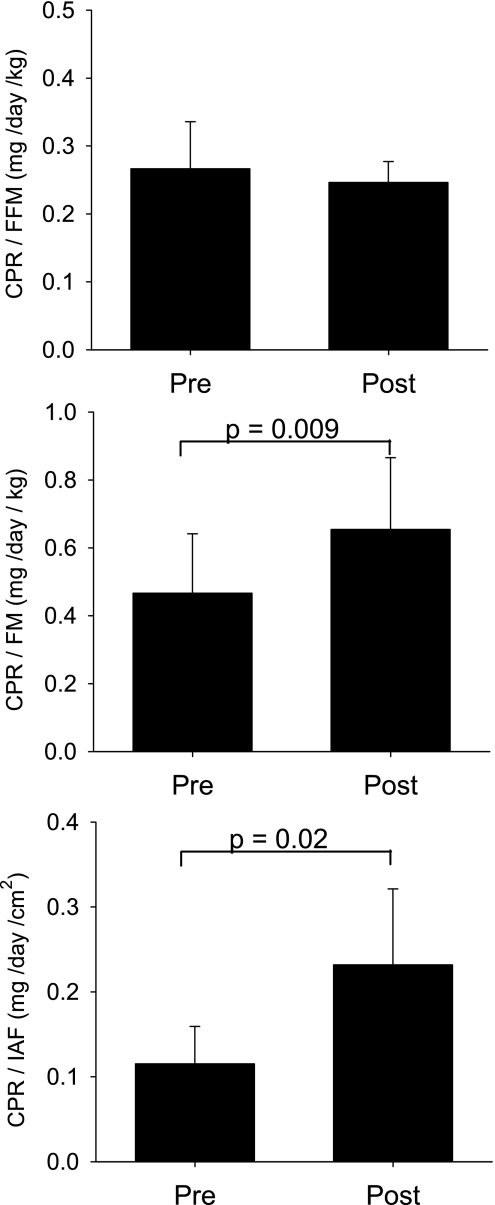
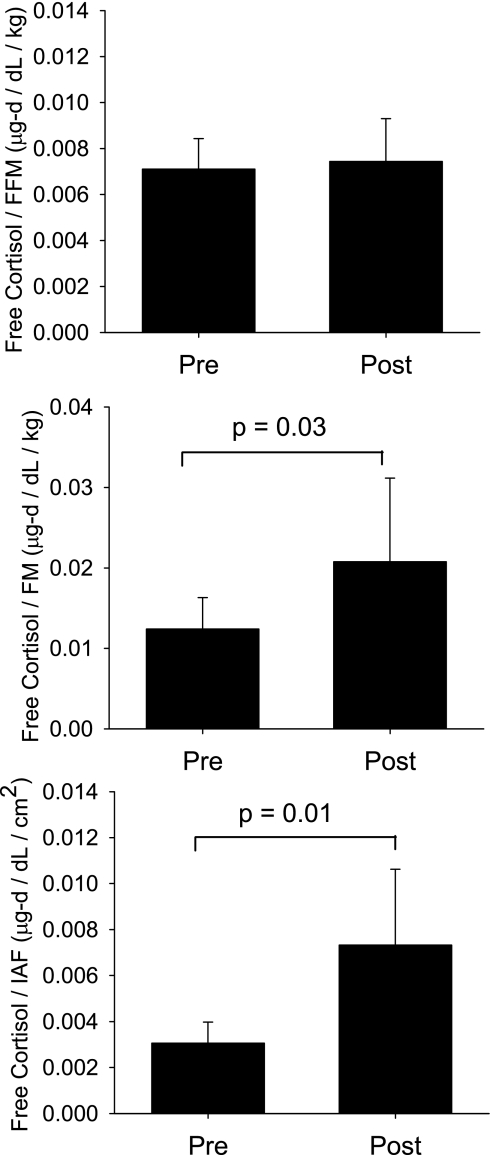
CPR, FC, CBG levels, and MCR of cortisol did not change with weight loss (Table 1). Typically, CPR is expressed as a ratio to BSA to adjust for the relationship between production rates and body size between individuals. Previous studies had suggested that CPR in men most closely reflects FFM (36). However, we previously showed (29) that CPR is significantly related to both FM (r = 0.65, P = 0.003) and FFM (r = 0.53, P = 0.02) in these men. Therefore, using measures of FM, FFM, and IAF, we tested the adjusted (or proportional) effects of weight loss on CPR in these subjects. CPR/FFM was unchanged with weight loss (P = 0.41), but CPR/FM and CPR/IAF increased 40% (P = 0.009) and 100% (P = 0.02), respectively (Fig. 3). Similarly, FC adjusted for FFM (P = 0.51) did not differ before or after weight loss but was proportionally higher in proportion to FM (67%, P = 0.03) and IAF (125%, P = 0.01) after weight loss (Fig. 4).
Fig. 3.
CPR in relationship to fat-free mass (FFM), fat mass (FM), and IAF before (Pre) and after (Post) a period of caloric restriction-induced weight loss.
Fig. 4.
Twenty-four-hour free cortisol levels in relationship to FFM, FM, and IAF, before (Pre) and after (Post) a period of caloric restriction-induced weight loss.
Unlike the other measures of cortisol metabolism, adipocyte 11β-HSD-1 gene expression significantly decreased with weight loss (Table 1). Levels of fasting insulin and leptin also significantly decreased and insulin sensitivity significantly increased with weight loss (Table 1).
DISCUSSION
Previous studies have shown that compared with women, men have increased CPR (29), cortisol levels (29, 44), and visceral adiposity (9, 13). Given that hypercortisolemia can induce central obesity in disease states such as Cushing's syndrome, elevated endogenous cortisol secretion has been considered a potential mechanism that contributes to the expression of visceral adiposity in humans. However, of four previous reports that used 24-h urinary excretion rates of cortisol as a surrogate for cortisol production, only one found significant relationships between urinary secretion of total glucocorticoids, truncal fat, and insulin sensitivity in men and women (39), while three other studies in men have failed to show associations between urinary glucocorticoid secretion and either WHR (16, 26) or visceral fat (48). These studies, however, did not measure cortisol production directly, did not include blood FC, and did not test for differences in circadian variations of blood levels of cortisol, and in only one study was visceral fat specifically measured.
Using steady-state infusion of a tracer amount of a stable cortisol isotope over 24 h, we report for the first time in men that increased endogenous daily CPR and FC in the blood correlate selectively with increased amounts of visceral fat, not abdominal SQF, and with reduced SI. Importantly, these relationships remained significant even after adjustment for differences in body size (CPR/BSA) and could not be accounted for by enhanced cortisol metabolic rates.
The 24-h cortisol profile demonstrated that the higher total cortisol levels in men with greater visceral obesity occurred during the hours from late morning to early evening, but not during the “trough” period between midnight and 0200 typically associated with Cushing's disease (20, 28). Wallerius et al. (47) described a similar pattern, using salivary FC measurements in men with higher vs. lower WHR during a limited daytime sampling period. Although we did not measure ACTH levels in this study to verify simultaneously enhanced pituitary signaling, Ljung et al. (26) reported increased ACTH levels during this same late morning-early afternoon time period in men with higher compared with lower WHR. Nutrients are known to increase cortisol production through mechanisms that include extrasplanchnic sources (2). It is therefore possible that increased food consumption by heavier men contributed to the relationship between higher CPR and increased visceral adiposity that we report here. However, the relationships between CPR, IAF, and SI remained significant even after adjustment for body size, which is a rough surrogate for higher caloric intake, and CPR was not significantly reduced when the men were studied after weight loss, when caloric intake is obligatorily reduced. Therefore, this potential effect of nutrient regulation on hypothalamic-pituitary-adrenal (HPA) axis activity (5) could not fully explain the selective correlation with increased visceral fat reported here.
Glucocorticoids can also adversely affect glucose metabolism (21), and indeed we found significant associations between increased CPR and FC with decreased SI and DI, the latter a measure of the capacity of the islet β-cell to increase insulin secretion in response to diminished SI (18). Because of the cross-sectional nature of the baseline study, however, we could not exclude the possibility that these associations between cortisol and glucose metabolism are direct effects that are, for example, independent of visceral fat. But whether by direct or indirect mechanisms, these data support a model in which enhanced CPR and FC promote selective visceral fat accumulation, decreased SI, and toxic effects on β-cell function in men (19).
An additional cortisol production pathway that may contribute to visceral fat is the intracellular conversion of cortisone to cortisol in fat tissue through enhanced oxoreductase activity of 11β-HSD-1 (8). In previous publications, in vitro 11β-HSD-1 oxoreductase activity paralleled gene expression in the same tissue (25, 46), and in the present study 11β-HSD-1 gene expression was significantly and positively associated with fat cell volume, percent body fat, and both visceral and subcutaneous fat and inversely associated with SI. This nonspecific association of 11β-HSD-1 gene expression with both the abdominal visceral and subcutaneous fat depots differs from the CPR and FC data above. This difference in specificity with increased IAF between these variables may possibly be explained by the strong association of 11β-HSD-1 gene expression with fat cell volume, for fat cell volume was also highly correlated with % fat (r = 0.84, P < 0.001), SQF (r = 0.75, P < 0.001), IAF (r = 0.81, P < 0.001), and SI (r = −0.83, P < 0.001) in the present study. Therefore, although FC is also associated with fat cell volume, this relationship is stronger for 11β-HSD-1 gene expression and could result in “colinear” associations between 11β-HSD-1 gene expression, abdominal fat accumulation, and insulin resistance. One way to test this hypothesis, as discussed below, is to induce weight loss and determine whether CPR, FC, and 11β-HSD-1 gene expression are altered in parallel with changes in body fat, which would suggest that the fat variable (fat mass, fat cell volume, etc.) is influencing the cortisol metabolism measurement and not vice versa (see below). It should be pointed out, however, that the biopsy site in this study was the posterior truncal region, about the level of the umbilicus. This tissue is representative of the abdominal-truncal subcutaneous depot and may not reflect activity in the visceral depot. Even if 11β-HSD-1 gene expression and oxoreductase activity were increased in visceral tissue in these men, it is unlikely, however, that this would account for the increase in CPR that we report here since splanchnic bed cortisol production was shown previously to not correlate with visceral adiposity (3).
With weight loss, CPR, MCR, and FC did not change, whereas fat cell volume, IAF, SQF, leptin, and 11β-HSD-1 gene expression all decreased. Our original hypothesis, based on animal studies that demonstrated reductions in ACTH and corticosterone levels in rodents given injections of leptin (1, 35), was that a fall in leptin with weight loss would trigger an increase in HPA axis activity and CPR in these men, which would then contribute to the weight regain commonly seen after diet-induced weight loss (32, 42). We clearly did not see a rise in CPR, but we did not see a reduction either, which is in contrast to a recent report of reduced total glucocorticoid secretion following weight loss in men and women by Tomlinson et al. (39). This discrepancy may be accounted for by several possible explanations. First, we measure CPR directly, using blood samples, whereas urinary excretion may be influenced by changes in renal hemodynamics with weight loss. In addition, urinary collections represent incomplete samplings of whole body cortisol secretion since they do not include cortisol metabolized and excreted into bile by the liver. Second, the study by Tomlinson et al. resampled subjects only 1 wk after completion of the hypocaloric phase, whereas our subjects were 3-mo weight stable before restudy so as to avoid potential confounding influences of active weight loss on study outcomes.
Of interest is that despite the reduced overall body weight, the relative sparing of FFM during the diet weight loss phase may have led to maintenance of MCR and, in turn, CPR. Indeed, both CPR and FC as a proportion of FFM levels were identical before and after weight loss. In contrast, when CPR or FC levels were expressed as a proportion of FM or IAF, values increased significantly. This raises an interesting implication for obese men who attempt repeated weight loss by low-calorie, protein-sparing diets alone: the failure to fully compensate CPR and FC in proportion to the new fat mass may contribute to preferential central weight gain (30) or impaired glucose tolerance (17) as reported in some studies of weight cycling. On the other hand, 11β-HSD-1 gene expression decreased with weight loss in parallel with decreases in fat cell volume and other parameters of fat mass. This finding contrasts with other recent weight loss studies in which 11β-HSD-1 gene expression either did not change (12) or increased (40) with weight loss. Again, a possible explanation for these discrepancies is that subjects in the previous studies were studied shortly (1 wk or less) after completing their weight loss phase, when acute starvation-state physiology may still be playing a role in the in the body's metabolic response. We conclude from our findings that 11β-HSD-1 gene expression is most probably the result of, rather than the cause of, changes in fat cell volume and FM.
In summary, we found in men that increased CPR and circulating FC are associated with accumulation of IAF, but not SQF, and with insulin resistance and impaired islet β-cell compensation (DI). In addition, with protein-sparing weight loss, CPR and FC remain unchanged and may contribute to adverse fat distribution on weight regain. In contrast, 11β-HSD-1 gene expression from a subcutaneous abdominal-truncal fat depot correlated nonspecifically with both IAF and SQF and decreased in parallel with reductions in fat cell volume and FM. Together, these findings support a role for activation of the HPA axis and abnormal cortisol secretion in determining body fat (visceral fat) distribution and predisposing these men to type 2 diabetes. Our data do not rule out a role for visceral adipose tissue 11β-HSD-1 enzyme activity in the expression of central distribution of fat in men but do suggest that subcutaneous 11β-HSD-1 gene expression is more likely a consequence rather than a cause of the general obese state and, more specifically, fat cell volume.
GRANTS
This work was conducted through the General Clinical Research Center (GCRC) of the University of Washington (M01-RR-00037) and the Oregon Clinical and Translational Research Institute (OCTRI), grant number UL1-RR-024140-01 from the National Center for Research Resources, National Institutes of Health (NIH), and NIH Roadmap for Medical Research. These studies were performed while J. Q. Purnell was a Clinical Associate Physician investigator on the University of Washington GCRC RR37 and were supported in part by NIH Grants DK-02456, K23-DK-002689, and R01-DK-068146 (J. Q. Purnell). Additional support was provided by K24-DK-02456 (J. D. Brunzell) and by the Medical Research Service of the Department of Veterans Affairs (S. E. Kahn). Gene expression analyses were performed in the Molecular and Genetics Core of the Diabetes and Endocrinology Research Center, Grant P30-DK-17047.
Acknowledgments
We thank Holly Callahan in the University of Washington GCRC Bionutrition Unit for her invaluable services.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature 382: 250–252, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Basu R, Singh R, Basu A, Johnson CM, Rizza RA. Effect of nutrient ingestion on total-body and splanchnic cortisol production in humans. Diabetes 55: 667–674, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Basu R, Singh RJ, Basu A, Chittilapilly EG, Johnson MC, Toffolo G, Cobelli C, Rizza RA. Obesity and type 2 diabetes do not alter splanchnic cortisol production in humans. J Clin Endocrinol Metab 90: 3919–3926, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Beard JC, Bergman RN, Ward WK, Porte D Jr. The insulin sensitivity index in nondiabetic man. Correlation between clamp-derived and IVGTT-derived values. Diabetes 35: 362–369, 1986. [DOI] [PubMed] [Google Scholar]
- 5.Benedict C, Hallschmid M, Scheibner J, Niemeyer D, Schultes B, Merl V, Fehm HL, Born J, Kern W. Gut protein uptake and mechanisms of meal-induced cortisol release. J Clin Endocrinol Metab 90: 1692–1696, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Bjorntorp P The regulation of adipose tissue distribution in humans. Int J Obes Relat Metab Disord 20: 291–302, 1996. [PubMed] [Google Scholar]
- 7.Brandon DD, Isabelle LM, Samuels MH, Kendall JW, Loriaux DL. Cortisol production rate measurement by stable isotope dilution using gas chromatography-negative ion chemical ionization mass spectrometry. Steroids 64: 372–378, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Bujalska IJ, Kumar S, Stewart PM. Does central obesity reflect “Cushing's disease of the omentum”? Lancet 349: 1210–1213, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Cefalu WT, Wang ZQ, Werbel S, Bell-Farrow A, Crouse JR 3rd, Hinson WH, Terry JG, Anderson R. Contribution of visceral fat mass to the insulin resistance of aging. Metabolism 44: 954–959, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Chen KW, Boyko EJ, Bergstrom RW, Leonetti DL, Newell-Morris L, Wahl PW, Fujimoto WY. Earlier appearance of impaired insulin secretion than of visceral adiposity in the pathogenesis of NIDDM. 5-Year follow-up of initially nondiabetic Japanese-American men. Diabetes Care 18: 747–753, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Engeli S, Bohnke J, Feldpausch M, Gorzelniak K, Heintze U, Janke J, Luft FC, Sharma AM. Regulation of 11beta-HSD genes in human adipose tissue: influence of central obesity and weight loss. Obes Res 12: 9–17, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Enzi G, Gasparo M, Biondetti PR, Fiore D, Semisa M, Zurlo F. Subcutaneous and visceral fat distribution according to sex, age, and overweight, evaluated by computed tomography. Am J Clin Nutr 44: 739–746, 1986. [DOI] [PubMed] [Google Scholar]
- 14.Geiseler D, Ritter M. On the validity of free hormone measurements. Anal Biochem 132: 174–182, 1983. [DOI] [PubMed] [Google Scholar]
- 15.Goldman RF, Buskirk ER. Body volume measurement by underwater weighing: description of a method. In: Techniques for Measuring Body Composition, edited by Brozek J and Henschel A. Washington, DC: National Academy of Sciences, 1961, p. 78–89.
- 16.Hautanen A, Adlercreutz H. Pituitary-adrenocortical function in abdominal obesity of males: evidence for decreased 21-hydroxylase activity. J Steroid Biochem Mol Biol 58: 123–133, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Holbrook TL, Barrett-Connor E, Wingard DL. The association of lifetime weight and weight control patterns with diabetes among men and women in an adult community. Int J Obes 13: 723–729, 1989. [PubMed] [Google Scholar]
- 18.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP, Porte D Jr. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects: EVIDENCE for a hyperbolic function. Diabetes 42: 1663–1672, 1993. [DOI] [PubMed] [Google Scholar]
- 19.Kalhan SC, Adam PA. Inhibitory effect of prednisone on insulin secretion in man: model for duplication of blood glucose concentration. J Clin Endocrinol Metab 41: 600–610, 1975. [DOI] [PubMed] [Google Scholar]
- 20.Krieger DT, Allen W. Relationship of bioassayable and immunoassayable plasma ACTH and cortisol concentrations in normal subjects and in patients with Cushing's disease. J Clin Endocrinol Metab 40: 675–687, 1975. [DOI] [PubMed] [Google Scholar]
- 21.Lambillotte C, Gilon P, Henquin JC. Direct glucocorticoid inhibition of insulin secretion. An in vitro study of dexamethasone effects in mouse islets. J Clin Invest 99: 414–423, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsson B, Svardsudd K, Welin L, Wilhelmsen L, Bjorntorp P, Tibblin G. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br Med J (Clin Res Ed) 288: 1401–1404, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lefebvre AM, Laville M, Vega N, Riou JP, van Gaal L, Auwerx J, Vidal H. Depot-specific differences in adipose tissue gene expression in lean and obese subjects. Diabetes 47: 98–103, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Lemieux S, Prud'homme D, Bouchard C, Tremblay A, Despres JP. Sex differences in the relation of visceral adipose tissue accumulation to total body fatness. Am J Clin Nutr 58: 463–467, 1993. [DOI] [PubMed] [Google Scholar]
- 25.Lindsay RS, Wake DJ, Nair S, Bunt J, Livingstone DE, Permana PA, Tataranni PA, Walker BR. Subcutaneous adipose 11beta-hydroxysteroid dehydrogenase type 1 activity and messenger ribonucleic acid levels are associated with adiposity and insulinemia in Pima Indians and Caucasians. J Clin Endocrinol Metab 88: 2738–2744, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Ljung T, Holm G, Friberg P, Andersson B, Bengtsson BA, Svensson J, Dallman M, McEwen B, Bjorntorp P. The activity of the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system in relation to waist/hip circumference ratio in men. Obes Res 8: 487–495, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Montague CT, Prins JB, Sanders L, Zhang J, Sewter CP, Digby J, Byrne CD, O'Rahilly S. Depot-related gene expression in human subcutaneous and omental adipocytes. Diabetes 47: 1384–1391, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Papanicolaou DA, Yanovski JA, Cutler GB, Chrousos GP, Nieman LK. A single midnight serum cortisol measurement distinguishes Cushing's syndrome from pseudo-Cushing's states. J Clin Endocrinol Metab 83: 1163–1167, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Purnell JQ, Brandon DD, Isabelle LM, Loriaux DL, Samuels MH. Association of 24-hour cortisol production rates, cortisol-binding globulin, and plasma-free cortisol levels with body composition, leptin levels, and aging in adult men and women. J Clin Endocrinol Metab 89: 281–287, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Rodin J, Radke-Sharpe N, Rebuffe-Scrive M, Greenwood MR. Weight cycling and fat distribution. Int J Obes 14: 303–310, 1990. [PubMed] [Google Scholar]
- 31.Rosner W, Polimeni S, Khan MS. Radioimmunoassay for human corticosteroid-binding globulin. Clin Chem 29: 1389–1391, 1983. [PubMed] [Google Scholar]
- 32.Safer DJ Diet, behavior modification, and exercise: a review of obesity treatments from a long-term perspective. South Med J 84: 1470–1474, 1991. [PubMed] [Google Scholar]
- 33.Schwartz RS, Brunzell JD. Increase of adipose tissue lipoprotein lipase activity with weight loss. J Clin Invest 67: 1425–1430, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shuman WP, Morris LL, Leonetti DL, Wahl PW, Moceri VM, Moss AA, Fujimoto WY. Abnormal body fat distribution detected by computed tomography in diabetic men. Invest Radiol 21: 483–487, 1986. [DOI] [PubMed] [Google Scholar]
- 35.Stephens TW, Basinski M, Bristow PK, Bue-Valleskey JM, Burgett SG, Craft L, Hale J, Hoffmann J, Hsiung HM, Kriauciunas A, MacKellar W, Rosteck PR Jr, Schoner B, Smith D, Tinsley FC, Zhang XY, Heiman M. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature 377: 530–532, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Strain GW, Zumoff B, Kream J, Strain JJ, Levin J, Fukushima D. Sex difference in the influence of obesity on the 24 hr mean plasma concentration of cortisol. Metabolism 31: 209–212, 1982. [DOI] [PubMed] [Google Scholar]
- 37.Strain GW, Zumoff B, Strain JJ, Levin J, Fukushima DK. Cortisol production in obesity. Metabolism 29: 980–985, 1980. [DOI] [PubMed] [Google Scholar]
- 38.Szenas P, Pattee CJ. Studies on adrenocortical function in obesity. J Clin Endocrinol Metab 19: 344–350, 1959. [DOI] [PubMed] [Google Scholar]
- 39.Tomlinson JW, Finney J, Hughes BA, Hughes SV, Stewart PM. Reduced glucocorticoid production rate, decreased 5alpha-reductase activity, and adipose tissue insulin sensitization after weight loss. Diabetes 57: 1536–1543, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomlinson JW, Moore JS, Clark PM, Holder G, Shakespeare L, Stewart PM. Weight loss increases 11beta-hydroxysteroid dehydrogenase type 1 expression in human adipose tissue. J Clin Endocrinol Metab 89: 2711–2716, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomlinson JW, Walker EA, Bujalska IJ, Draper N, Lavery GG, Cooper MS, Hewison M, Stewart PM. 11Beta-hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr Rev 25: 831–866, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Tsai AG, Wadden TA. Systematic review: an evaluation of major commercial weight loss programs in the United States. Ann Intern Med 142: 56–66, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab 81: 2468–2473, 1996. [DOI] [PubMed] [Google Scholar]
- 44.Van Cauter E, Shapiro ET, Tillil H, Polonsky KS. Circadian modulation of glucose and insulin responses to meals: relationship to cortisol rhythm. Am J Physiol Endocrinol Metab 262: E467–E475, 1992. [DOI] [PubMed] [Google Scholar]
- 45.Vierhapper H, Nowotny P, Waldhausl W. Production rates of cortisol in obesity. Obes Res 12: 1421–1425, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Wake DJ, Rask E, Livingstone DE, Soderberg S, Olsson T, Walker BR. Local and systemic impact of transcriptional up-regulation of 11beta-hydroxysteroid dehydrogenase type 1 in adipose tissue in human obesity. J Clin Endocrinol Metab 88: 3983–3988, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Wallerius S, Rosmond R, Ljung T, Holm G, Bjorntorp P. Rise in morning saliva cortisol is associated with abdominal obesity in men: a preliminary report. J Endocrinol Invest 26: 616–619, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Westerbacka J, Yki-Jarvinen H, Vehkavaara S, Hakkinen AM, Andrew R, Wake DJ, Seckl JR, Walker BR. Body fat distribution and cortisol metabolism in healthy men: enhanced 5beta-reductase and lower cortisol/cortisone metabolite ratios in men with fatty liver. J Clin Endocrinol Metab 88: 4924–4931, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Zumoff B, Fukushima DK, Hellman L. Intercomparison of four methods for measuring cortisol production. J Clin Endocrinol Metab 38: 169–175, 1974. [DOI] [PubMed] [Google Scholar]